Triple-Networked Hybrid Hydrogels Reinforced with Montmorillonite Clay and Graphene Nanoplatelets for Soft and Hard Tissue Regeneration
Abstract
1. Introduction
2. Results and Discussion
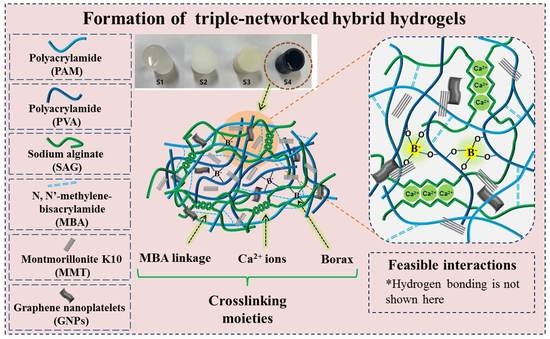
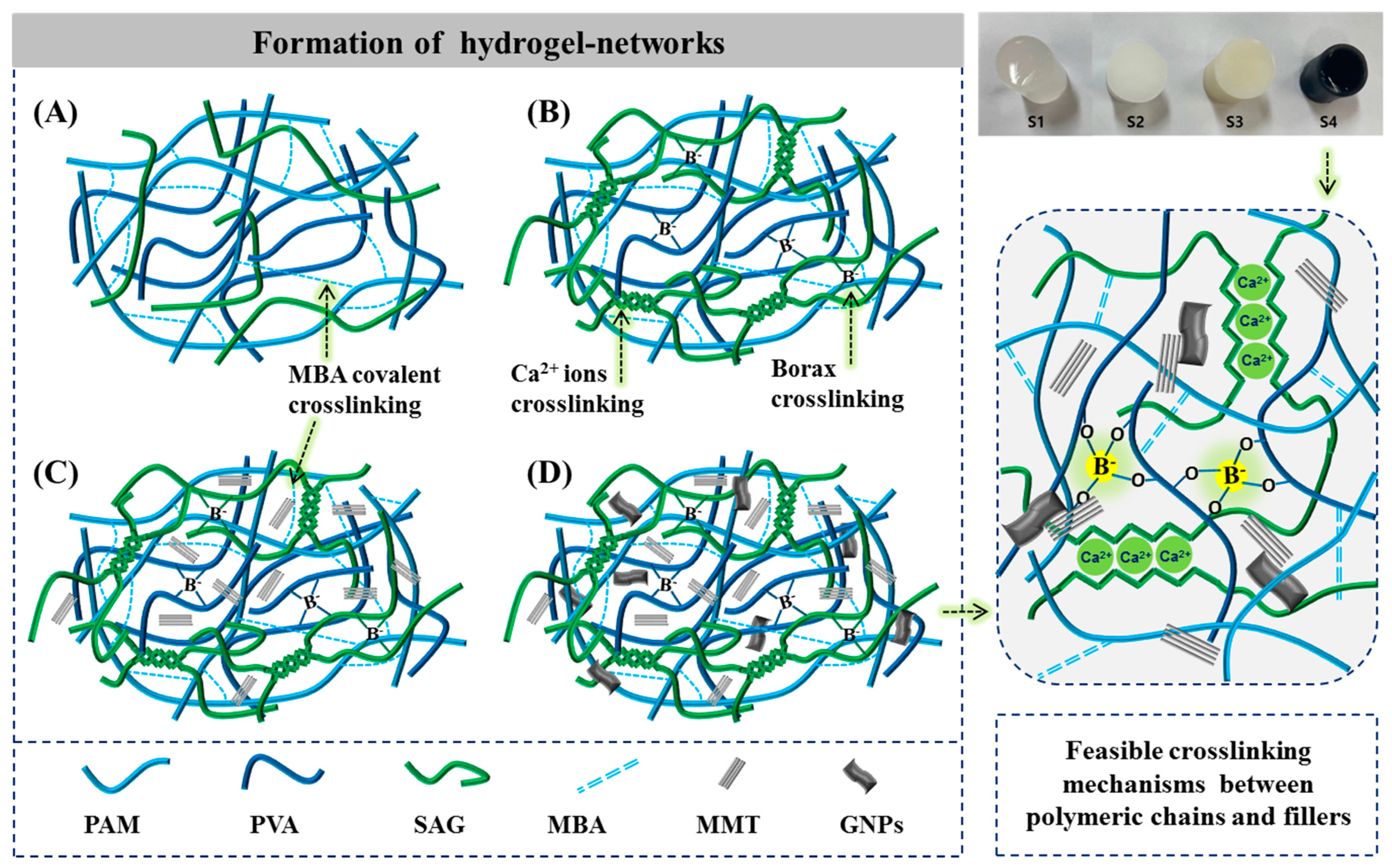
2.1. Formation of Hybrid Hydrogels
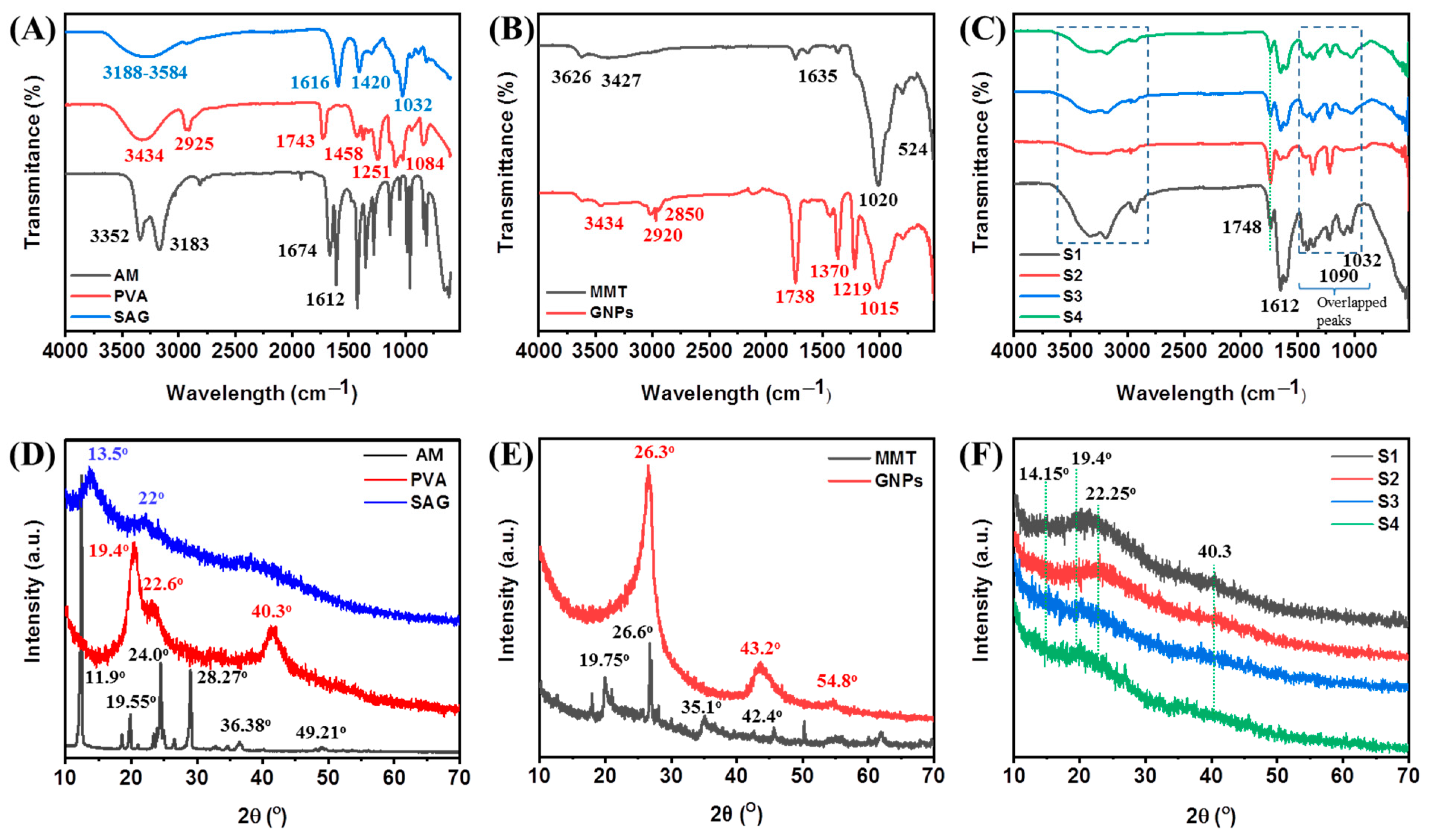
2.2. Structural Analysis
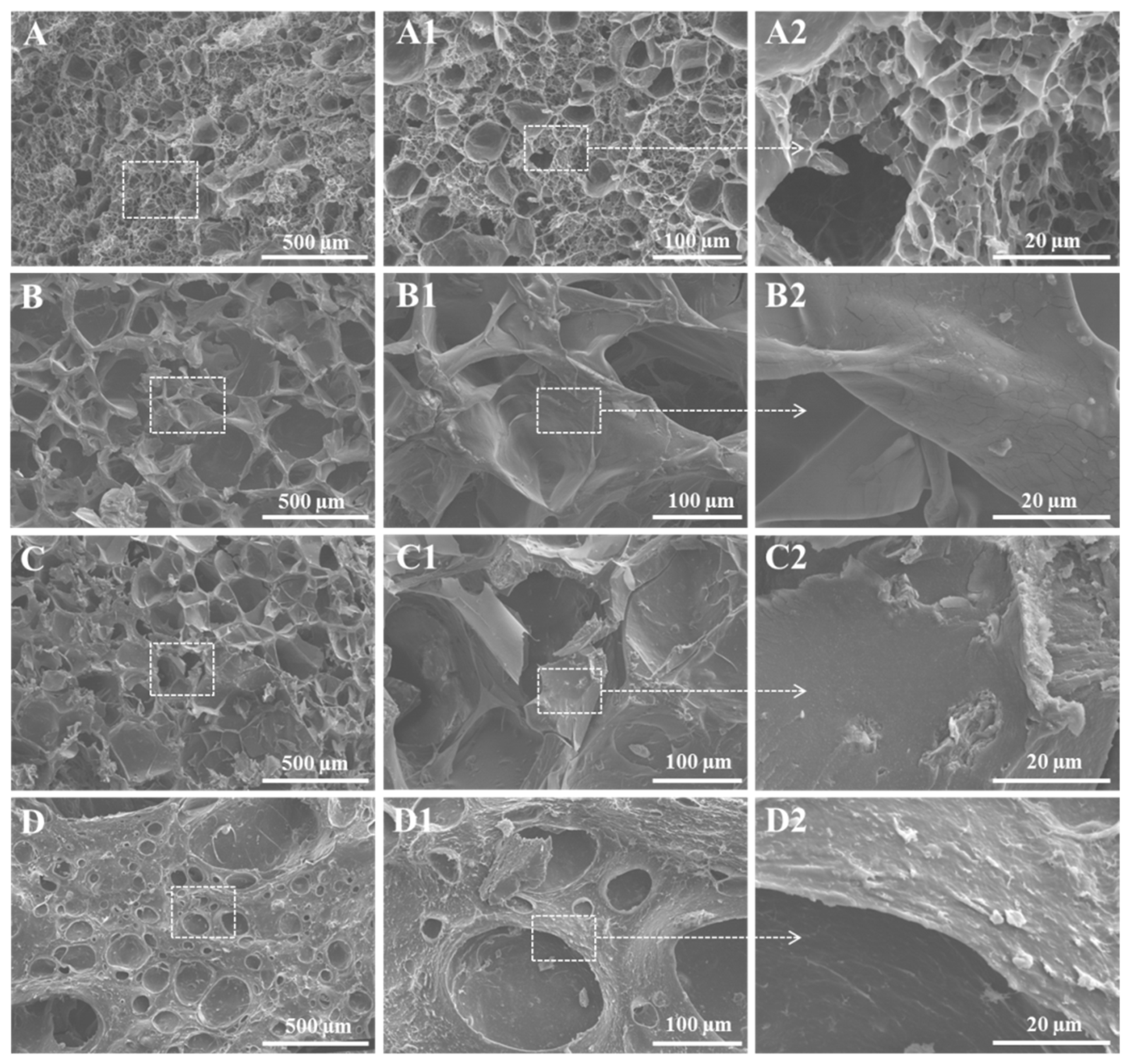
2.3. Morphological Analysis
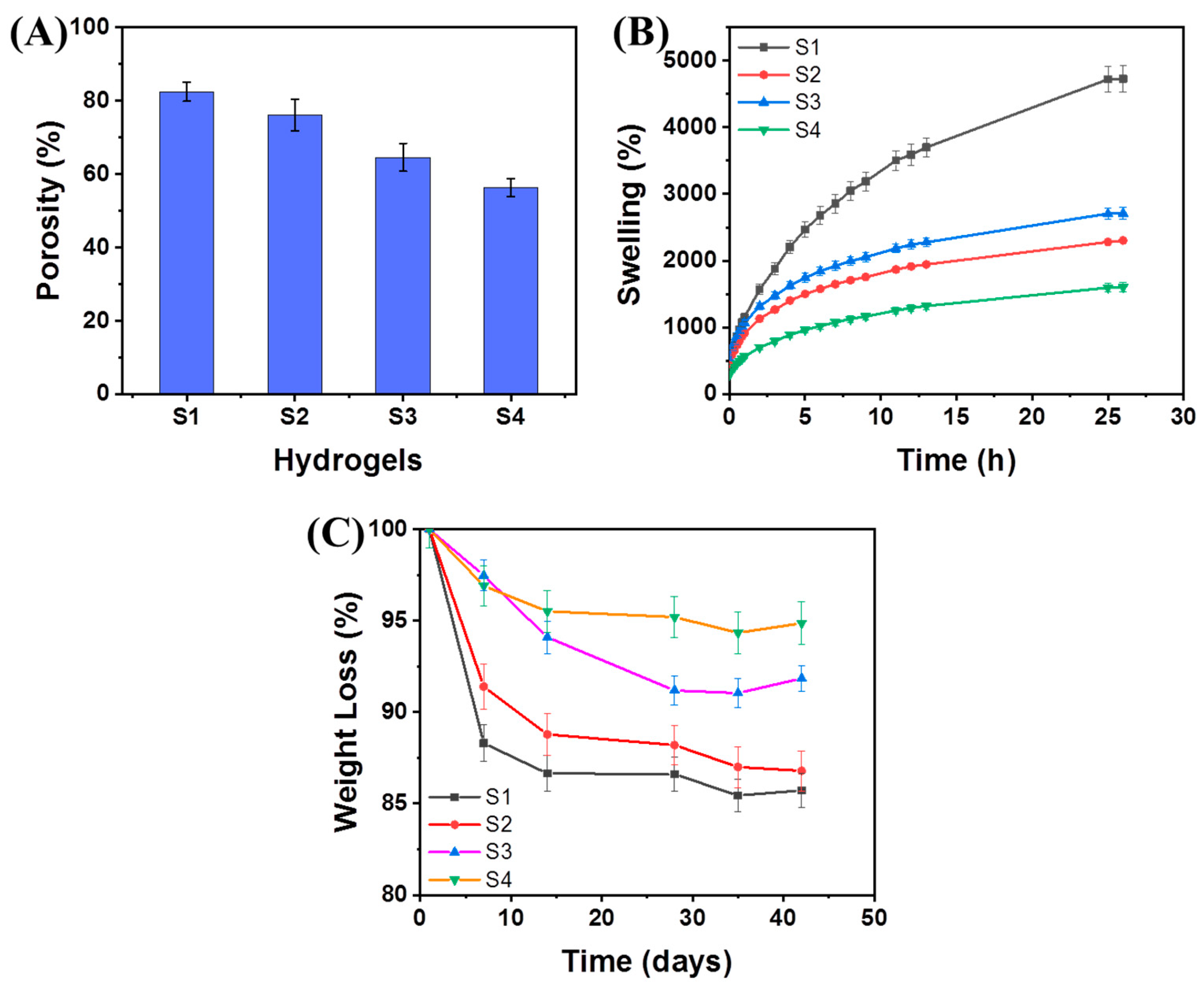
2.4. Swelling and Degradation Behaviors
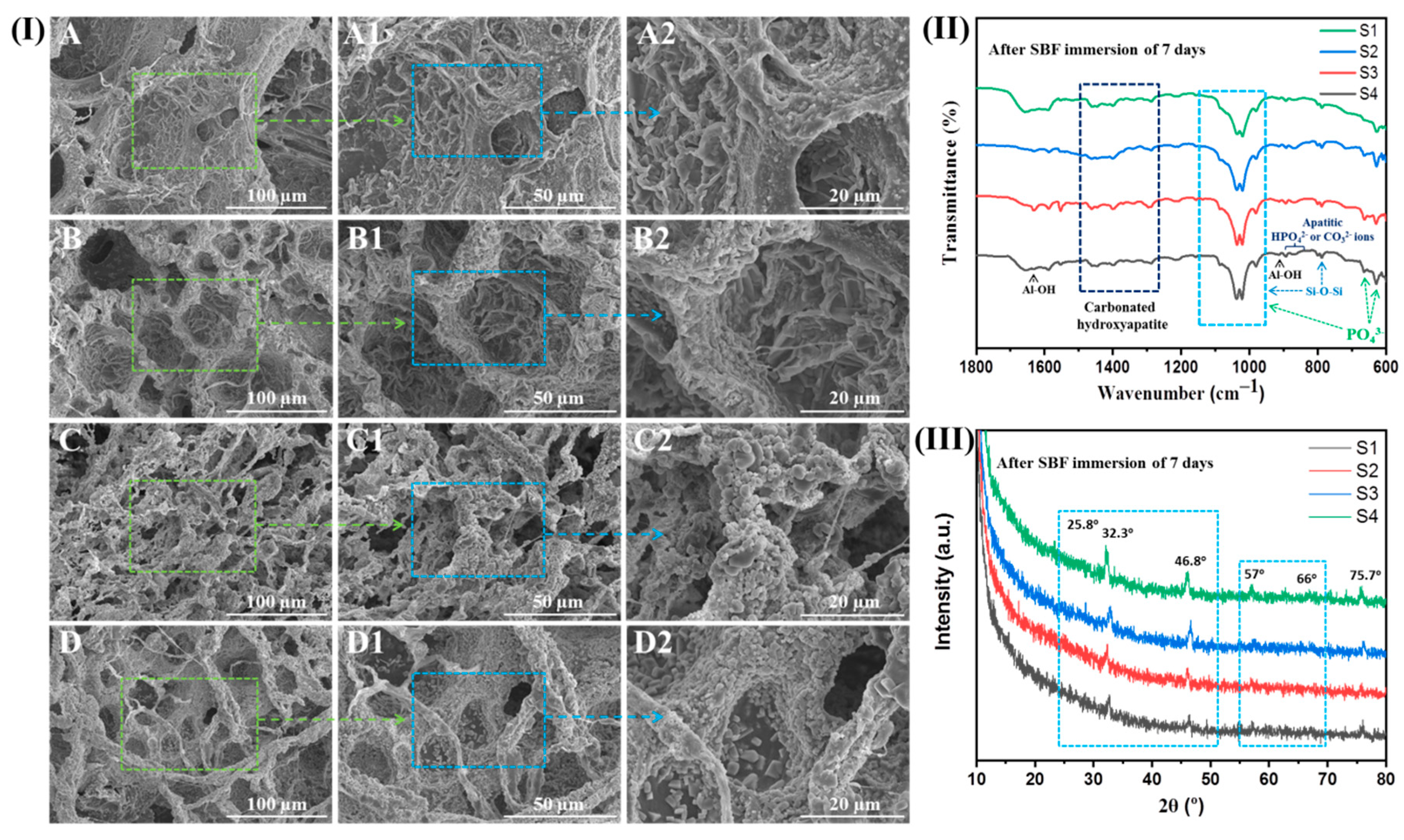
2.5. Biomineralization Activity (In Vitro)
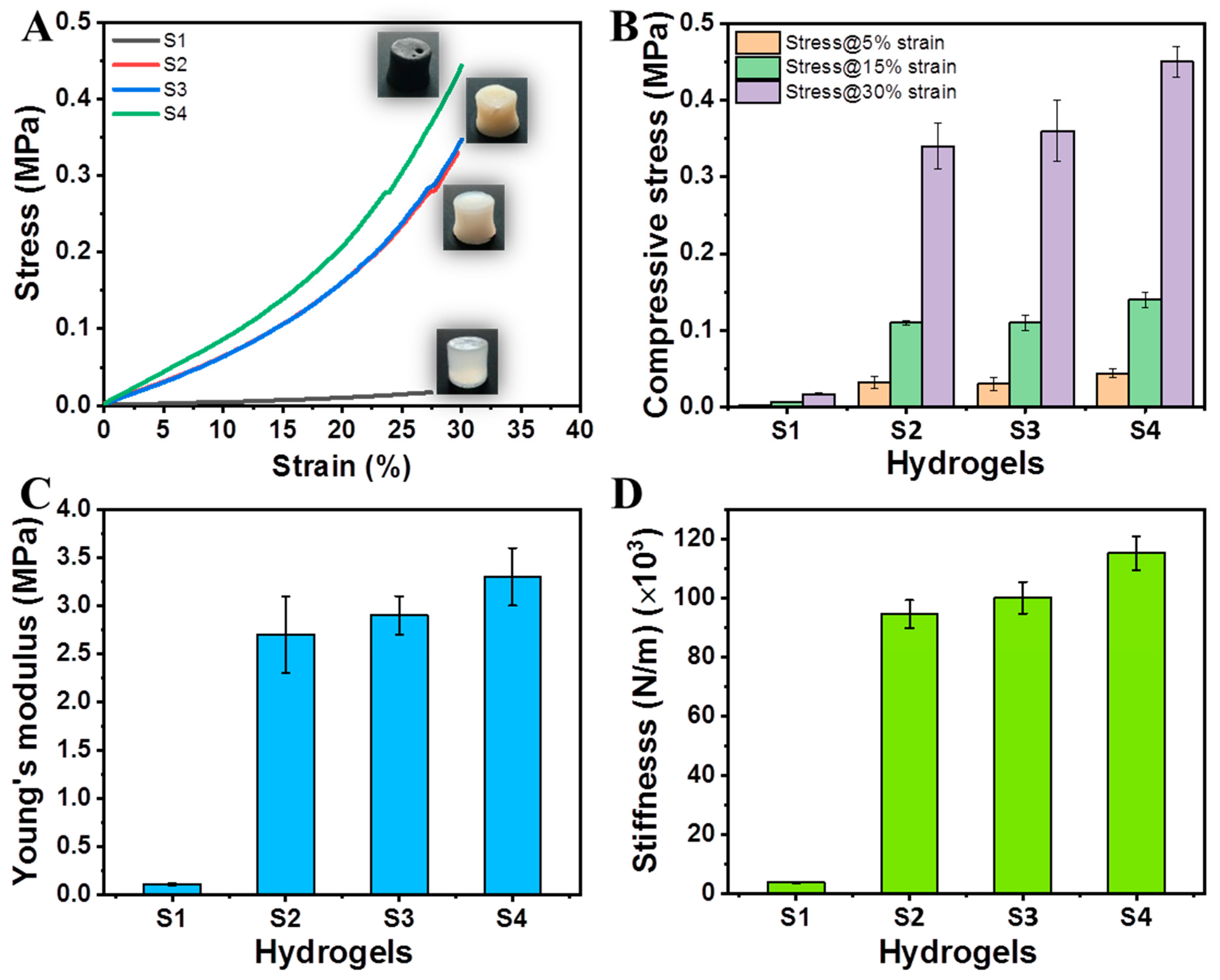
2.6. Mechanical Properties
2.7. Cytocompatibility
2.7.1. Interaction of Hydrogels with Fibroblast Cells
2.7.2. Interaction of Hydrogels with Pre-Osteoblast Cells
3. Materials and Methods
3.1. Materials
3.2. Preparation of Hybrid Hydrogels
3.3. Characterization
3.3.1. Morphology
3.3.2. Porosity
3.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.4. X-ray Diffraction (XRD)
3.3.5. Relative Swelling and Degradation Behaviors
3.3.6. Mechanical Testing
3.3.7. In Vitro Bioactivity
3.3.8. In Vitro Cytocompatibility
Cell Adhesion and Spreading
Cell Viability
3.3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, S.; Baño, M.C.; Cima, L.G.; Allcock, H.R.; Vacanti, J.P.; Vacanti, C.A.; Langer, R. Design of synthetic polymeric structures for cell transplantation and tissue engineering. Clin. Mater. 1993, 13, 3–10. [Google Scholar] [CrossRef]
- Kumar, A.; Zo, S.M.; Kim, J.H.; Kim, S.-C.; Han, S.S. Enhanced physical, mechanical, and cytocompatibility behavior of polyelectrolyte complex hydrogels by reinforcing halloysite nanotubes and graphene oxide. Compos. Sci. Technol. 2019, 175, 35–45. [Google Scholar] [CrossRef]
- Bendtsen, S.T.; Wei, M. Synthesis and characterization of a novel injectable alginate–collagen–hydroxyapatite hydrogel for bone tissue regeneration. J. Mater. Chem. B 2015, 3, 3081–3090. [Google Scholar] [CrossRef] [PubMed]
- Saberianpour, S.; Heidarzadeh, M.; Geranmayeh, M.H.; Hosseinkhani, H.; Rahbarghazi, R.; Nouri, M. Tissue engineering strategies for the induction of angiogenesis using biomaterials. J. Biol. Eng. 2018, 12, 1–15. [Google Scholar] [CrossRef]
- Lopes, D.; Martins-Cruz, C.; Oliveira, M.B.; Mano, J.F. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials 2018, 185, 240–275. [Google Scholar] [CrossRef]
- Lee, J.-H.; Parthiban, P.; Jin, G.-Z.; Knowles, J.C.; Kim, H.-W. Materials roles for promoting angiogenesis in tissue regeneration. Prog. Mater. Sci. 2021, 117, 100732. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Vernerey, F.J.; Bryant, S. The role of percolation in hydrogel-based tissue engineering and bioprinting. Curr. Opin. Biomed. Eng. 2020, 15, 68–74. [Google Scholar] [CrossRef]
- Sood, A.; Kumar, A.; Dev, A.; Gupta, V.K.; Han, S.S. Advances in Hydrogel-Based Microfluidic Blood–Brain-Barrier Models in Oncology Research. Pharmaceutics 2022, 14, 993. [Google Scholar]
- Radulescu, D.-M.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. New insights of scaffolds based on hydrogels in tissue engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef]
- Li, Q.; Niu, Y.; Xing, P.; Wang, C. Bioactive polysaccharides from natural resources including Chinese medicinal herbs on tissue repair. Chin. Med. 2018, 13, 7. [Google Scholar] [CrossRef]
- Gupta, A.; Sood, A.; Fuhrer, E.; Djanashvili, K.; Agrawal, G. Polysaccharide-Based Theranostic Systems for Combined Imaging and Cancer Therapy: Recent Advances and Challenges. ACS Biomater. Sci. Eng. 2022, 8, 2281–2306. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Han, S.S. Enhanced mechanical, biomineralization, and cellular response of nanocomposite hydrogels by bioactive glass and halloysite nanotubes for bone tissue regeneration. Mater. Sci. Eng. C 2021, 128, 112236. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sood, A.; Han, S.S. Poly (vinyl alcohol)-alginate as potential matrix for various applications: A focused review. Carbohyd. Polym. 2022, 277, 118881. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef]
- Petrova, V.A.; Elokhovskiy, V.Y.; Raik, S.V.; Poshina, D.N.; Romanov, D.P.; Skorik, Y.A. Alginate gel reinforcement with chitin nanowhiskers modulates rheological properties and drug release profile. Biomolecules 2019, 9, 291. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Cidonio, G.; Glinka, M.; Kim, Y.-H.; Kanczler, J.M.; Lanham, S.A.; Ahlfeld, T.; Lode, A.; Dawson, J.I.; Gelinsky, M.; Oreffo, R.O. Nanoclay-based 3D printed scaffolds promote vascular ingrowth ex vivo and generate bone mineral tissue in vitro and in vivo. Biofabrication 2020, 12, 035010. [Google Scholar] [CrossRef]
- Li, G.; Quan, K.; Liang, Y.; Li, T.; Yuan, Q.; Tao, L.; Xie, Q.; Wang, X. Graphene-montmorillonite composite sponge for safe and effective hemostasis. ACS Appl. Mater. Interfaces 2016, 8, 35071–35080. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.T.; Zhou, C.H.; Kabwe, F.B.; Wu, Q.Q.; Li, C.S.; Zhang, J.R. Exfoliation of montmorillonite and related properties of clay/polymer nanocomposites. Appl. Clay Sci. 2019, 169, 48–66. [Google Scholar] [CrossRef]
- Chen, K.; Guo, B.; Luo, J. Quaternized carboxymethyl chitosan/organic montmorillonite nanocomposite as a novel cosmetic ingredient against skin aging. Carbohyd. Polym. 2017, 173, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, K.; Chai, Q.; Liu, S.; Feng, C.; Xu, L.; Zhang, D. Freestanding vascular scaffolds engineered by direct 3D printing with Gt-Alg-MMT bioinks. Mater. Sci. Eng. C 2022, 133, 112658. [Google Scholar] [CrossRef]
- Menegasso, J.F.; Moraes, N.A.C.; Vásquez, T.P.; Felipetti, F.A.; Antonio, R.V.; Dutra, R.C. Modified montmorillonite-bacterial cellulose composites as a novel dressing system for pressure injury. Int. J. Biol. Macromol. 2022, 194, 402–411. [Google Scholar] [CrossRef]
- Lin, F.H.; Chen, C.H.; Cheng, W.T.; Kuo, T.F. Modified montmorillonite as vector for gene delivery. Biomaterials 2006, 27, 3333–3338. [Google Scholar] [CrossRef]
- Jayrajsinh, S.; Shankar, G.; Agrawal, Y.K.; Bakre, L. Montmorillonite nanoclay as a multifaceted drug-delivery carrier: A review. J. Drug Deliv. Sci. Technol. 2017, 39, 200–209. [Google Scholar] [CrossRef]
- Talukdar, Y.; Rashkow, J.T.; Lalwani, G.; Kanakia, S.; Sitharaman, B. The effects of graphene nanostructures on mesenchymal stem cells. Biomaterials 2014, 35, 4863–4877. [Google Scholar] [CrossRef]
- Lahiri, D.; Dua, R.; Zhang, C.; de Socarraz-Novoa, I.; Bhat, A.; Ramaswamy, S.; Agarwal, A. Graphene nanoplatelet-induced strengthening of ultrahigh molecular weight polyethylene and biocompatibility in vitro. ACS Appl. Mater. Interfaces 2012, 4, 2234–2241. [Google Scholar] [CrossRef]
- Pereira, A.T.; Henriques, P.C.; Schneider, K.H.; Pires, A.L.; Pereira, A.M.; Martins, M.C.L.; Magalhaes, F.D.; Bergmeister, H.; Gonçalves, I.C. Graphene-based materials: The key for the successful application of pHEMA as a blood-contacting device. Biomater. Sci. 2021, 9, 3362–3377. [Google Scholar] [CrossRef]
- Paydayesh, A.; Mousavi, S.R.; Estaji, S.; Khonakdar, H.A.; Nozarinya, M.A. Functionalized graphene nanoplatelets/poly (lactic acid)/chitosan nanocomposites: Mechanical, biodegradability, and electrical conductivity properties. Polym. Compos. 2022, 43, 411–421. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A.; Luo, Z. In situ deposition of hydroxyapatite on graphene nanosheets. Mater. Res. Bull. 2013, 48, 175–179. [Google Scholar] [CrossRef]
- Porwal, H.; Grasso, S.; Cordero-Arias, L.; Li, C.; Boccaccini, A.R.; Reece, M.J. Processing and bioactivity of 45S5 Bioglass®-graphene nanoplatelets composites. J. Mater. Sci. Mater. Med. 2014, 25, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Kumar, T.S.; Venkataprasanna, K.; Niranjan, R.; Kaushik, M.; Samal, D.B.; Venkatasubbu, G.D. PVA/alginate/hydroxyapatite films for controlled release of amoxicillin for the treatment of periodontal defects. Appl. Surf. Sci. 2019, 495, 143543. [Google Scholar] [CrossRef]
- Li, X.; Shu, M.; Li, H.; Gao, X.; Long, S.; Hu, T.; Wu, C. Strong, tough and mechanically self-recoverable poly (vinyl alcohol)/alginate dual-physical double-network hydrogels with large cross-link density contrast. RSC Adv. 2018, 8, 16674–16689. [Google Scholar] [CrossRef]
- Kumar, A.; Lee, Y.; Kim, D.; Rao, K.M.; Kim, J.; Park, S.; Haider, A.; Han, S.S. Effect of crosslinking functionality on microstructure, mechanical properties, and in vitro cytocompatibility of cellulose nanocrystals reinforced poly (vinyl alcohol)/sodium alginate hybrid scaffolds. Int. J. Biol. Macromol. 2017, 95, 962–973. [Google Scholar] [CrossRef]
- Xu, K.; Li, K.; Tu, D.; Zhong, T.; Xie, C. Reinforcement on the mechanical-, thermal-, and water-resistance properties of the wood flour/chitosan/poly (vinyl chloride) composites by physical and chemical modification. J. Appl. Polym. Sci. 2014, 131, 40757. [Google Scholar] [CrossRef]
- Phua, J.-L.; Teh, P.-L.; Ghani, S.A.; Yeoh, C.-K. Effect of heat assisted bath sonication on the mechanical and thermal deformation behaviours of graphene nanoplatelets filled epoxy polymer composites. Int. J. Polym. Sci. 2016, 2016, 9767183. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Xiao, Y.; Yu, X.; Li, X. PAMPS/PVA/MMT Semi-interpenetrating polymer network hydrogel electrolyte for solid-state supercapacitors. Int. J. Electrochem. Sci 2019, 14, 1817–1829. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Meng, Q.; Wang, T.; Guo, W.; Wu, G.; You, L. Preparation of high antistatic HDPE/polyaniline encapsulated graphene nanoplatelet composites by solution blending. RSC Adv. 2017, 7, 2796–2803. [Google Scholar] [CrossRef]
- Nikpour, P.; Salimi-Kenari, H.; Fahimipour, F.; Rabiee, S.M.; Imani, M.; Dashtimoghadam, E.; Tayebi, L. Dextran hydrogels incorporated with bioactive glass-ceramic: Nanocomposite scaffolds for bone tissue engineering. Carbohyd. Polym. 2018, 190, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, K.; Furtos, G. Study of apatite layer formation on SBF-treated chitosan composite thin films. Polym. Testing 2018, 71, 173–181. [Google Scholar] [CrossRef]
- Rojas-Yañez, M.-A.; Rodríguez-González, C.-A.; Martel-Estrada, S.-A.; Valencia-Gómez, L.-E.; Vargas-Requena, C.-L.; Hernández-Paz, J.-F.; Chavarría-Gaytán, M.-C.; Olivas-Armendáriz, I. Composite scaffolds of chitosan/polycaprolactone functionalized with protein of Mytilus californiensis for bone tissue regeneration. AIMS Mater. Sci. 2022, 9, 344–358. [Google Scholar] [CrossRef]
- Zheng, K.; Balasubramanian, P.; Paterson, T.E.; Stein, R.; MacNeil, S.; Fiorilli, S.; Vitale-Brovarone, C.; Shepherd, J.; Boccaccini, A.R. Ag modified mesoporous bioactive glass nanoparticles for enhanced antibacterial activity in 3D infected skin model. Mater. Sci. Eng. C 2019, 103, 109764. [Google Scholar] [CrossRef]
- Joshi, M.K.; Tiwari, A.P.; Pant, H.R.; Shrestha, B.K.; Kim, H.J.; Park, C.H.; Kim, C.S. In situ generation of cellulose nanocrystals in polycaprolactone nanofibers: Effects on crystallinity, mechanical strength, biocompatibility, and biomimetic mineralization. ACS Appl. Mater. Interfaces 2015, 7, 19672–19683. [Google Scholar] [CrossRef]
- Wang, H.; Hu, B.; Li, H.; Feng, G.; Pan, S.; Chen, Z.; Li, B.; Song, J. Biomimetic Mineralized Hydroxyapatite Nanofiber-Incorporated Methacrylated Gelatin Hydrogel with Improved Mechanical and Osteoinductive Performances for Bone Regeneration. Int. J. Nanomed. 2022, 17, 1511. [Google Scholar] [CrossRef]
- Mohammed, Z.; Tcherbi-Narteh, A.; Jeelani, S. Effect of graphene nanoplatelets and montmorillonite nanoclay on mechanical and thermal properties of polymer nanocomposites and carbon fiber reinforced composites. SN Appl. Sci. 2020, 2, 1959. [Google Scholar] [CrossRef]
- Nie, L.; Chen, D.; Suo, J.; Zou, P.; Feng, S.; Yang, Q.; Yang, S.; Ye, S. Physicochemical characterization and biocompatibility in vitro of biphasic calcium phosphate/polyvinyl alcohol scaffolds prepared by freeze-drying method for bone tissue engineering applications. Colloids Surf. B Biointerfaces 2012, 100, 169–176. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Jalota, S.; Bhaduri, S.B.; Tas, A.C. Using a synthetic body fluid (SBF) solution of 27 mM HCO3− to make bone substitutes more osteointegrative. Mater. Sci. Eng. C 2008, 28, 129–140. [Google Scholar] [CrossRef]


| Composition | AM (g) | PVA (g) | SAG (g) | MBA (g) | APS (g) | MMT (g) | GNPs (g) | BX/Ca2+ |
|---|---|---|---|---|---|---|---|---|
| S1 | 9.8 | 2 | 2 | 0.2 | 0.1 | - | ||
| S2 | 9.8 | 2 | 2 | 0.2 | 0.1 | + | ||
| S3 | 9.8 | 2 | 2 | 0.2 | 0.1 | 1.4 | + | |
| S4 | 9.8 | 2 | 2 | 0.2 | 0.1 | 1.4 | 0.14 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Won, S.-Y.; Sood, A.; Choi, S.-Y.; Singhmar, R.; Bhaskar, R.; Kumar, V.; Zo, S.M.; Han, S.-S. Triple-Networked Hybrid Hydrogels Reinforced with Montmorillonite Clay and Graphene Nanoplatelets for Soft and Hard Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 14158. https://doi.org/10.3390/ijms232214158
Kumar A, Won S-Y, Sood A, Choi S-Y, Singhmar R, Bhaskar R, Kumar V, Zo SM, Han S-S. Triple-Networked Hybrid Hydrogels Reinforced with Montmorillonite Clay and Graphene Nanoplatelets for Soft and Hard Tissue Regeneration. International Journal of Molecular Sciences. 2022; 23(22):14158. https://doi.org/10.3390/ijms232214158
Chicago/Turabian StyleKumar, Anuj, So-Yeon Won, Ankur Sood, So-Yeon Choi, Ritu Singhmar, Rakesh Bhaskar, Vineet Kumar, Sun Mi Zo, and Sung-Soo Han. 2022. "Triple-Networked Hybrid Hydrogels Reinforced with Montmorillonite Clay and Graphene Nanoplatelets for Soft and Hard Tissue Regeneration" International Journal of Molecular Sciences 23, no. 22: 14158. https://doi.org/10.3390/ijms232214158
APA StyleKumar, A., Won, S.-Y., Sood, A., Choi, S.-Y., Singhmar, R., Bhaskar, R., Kumar, V., Zo, S. M., & Han, S.-S. (2022). Triple-Networked Hybrid Hydrogels Reinforced with Montmorillonite Clay and Graphene Nanoplatelets for Soft and Hard Tissue Regeneration. International Journal of Molecular Sciences, 23(22), 14158. https://doi.org/10.3390/ijms232214158