Sphingosine 1-Phosphate and Apolipoprotein M Levels and Their Correlations with Inflammatory Biomarkers in Patients with Untreated Familial Hypercholesterolemia
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Blood Sampling
4.3. Measurement of ApoM and S1P
4.4. TNF-α Measurement
4.5. Oxidized LDL Measurement
4.6. The sICAM-1, sVCAM-1, and sCD40L Measurements
4.7. Myeloperoxidase Measurement
4.8. Determination of PON1 Enzyme Activities
4.9. Determinations of Lipoprotein Subfractions
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paragh, G.; Harangi, M.; Karányi, Z.; Daróczy, B.; Németh, Á.; Fülöp, P. Identifying patients with familial hypercholesterolemia using data mining methods in the Northern Great Plain region of Hungary. Atherosclerosis 2018, 277, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Ferrières, J.; Lambert, J.; Lussier-Cacan, S.; Davignon, J. Coronary artery disease in heterozygous familial hypercholesterolemia patients with the same LDL receptor gene mutation. Circulation 1995, 92, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Berta, E.; Zsíros, N.; Bodor, M.; Balogh, I.; Lőrincz, H.; Paragh, G.; Harangi, M. Clinical Aspects of Genetic and Non-Genetic Cardiovascular Risk Factors in Familial Hypercholesterolemia. Genes 2022, 13, 1158. [Google Scholar] [CrossRef] [PubMed]
- Pedro-Botet, J.; Climent, E.; Benaiges, D. Familial Hypercholesterolemia: Do HDL Play a Role? Biomedicines 2021, 9, 810. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S. The High-Density Lipoprotein Puzzle: Why Classic Epidemiology, Genetic Epidemiology, and Clinical Trials Conflict? Arterioscler. Thromb. Vasc. Biol. 2016, 36, 777–782. [Google Scholar] [CrossRef][Green Version]
- Ganjali, S.; Momtazi, A.A.; Banach, M.; Kovanen, P.T.; Stein, E.A.; Sahebkar, A. HDL abnormalities in familial hypercholesterolemia: Focus on biological functions. Prog. Lipid Res. 2017, 67, 16–26. [Google Scholar] [CrossRef]
- Barter, P.J.; Rye, K.A. HDL cholesterol concentration or HDL function: Which matters? Eur. Heart J. 2017, 38, 2487–2489. [Google Scholar] [CrossRef]
- Valencia, C.S.Y.; Isaza, M.C.A.; Henao, B.J.; Beltrán, A.L.; Loango, N.; Landázuri, P. Arylesterase activity of paraoxonase 1 (PON1) on HDL. Biochem. Biophys. Rep. 2021, 26, 100971. [Google Scholar] [CrossRef]
- Idrees, M.; Siddiq, A.R.; Ajmal, M.; Akram, M.; Khalid, R.R.; Hussain, A.; Qamar, R.; Bokhari, H. Decreased serum PON1 arylesterase activity in familial hypercholesterolemia patients with a mutated LDLR gene. Genet. Mol. Biol. 2018, 41, 570–577. [Google Scholar] [CrossRef]
- Himbergen, T.M.; van Tits, L.J.; Voorbij, H.A.; de Graaf, J.; Stalenhoef, A.F.; Roest, M. The effect of statin therapy on plasma high-density lipoprotein cholesterol levels is modified by paraoxonase-1 in patients with familial hypercholesterolaemia. J. Intern. Med. 2005, 258, 442–449. [Google Scholar] [CrossRef]
- Versmissen, J.; Vongpromek, R.; Yahya, R.; van der Net, J.B.; van Vark-van der Zee, L.; Blommesteijn-Touw, J.; Wattimena, D.; Rietveld, T.; Pullinger, C.R.; Christoffersen, C.; et al. Familial hypercholesterolaemia: Cholesterol efflux and coronary disease. Eur. J. Clin. Investig. 2016, 46, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Takabe, K.; Paugh, S.W.; Milstien, S.; Spiegel, S. “Inside-out” signaling of sphingosine-1-phosphate: Therapeutic targets. Pharmacol. Rev. 2008, 60, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, K.; Thangada, S.; Michaud, J.; Oo, M.L.; Ai, Y.; Lee, Y.M.; Wu, M.; Parikh, N.S.; Khan, F.; Proia, R.L.; et al. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem. J. 2006, 397, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Cannavo, A.; Liccardo, D.; Komici, K.; Corbi, G.; de Lucia, C.; Femminella, G.D.; Elia, A.; Bencivenga, L.; Ferrara, N.; Koch, W.J.; et al. Sphingosine Kinases and Sphingosine 1-Phosphate Receptors: Signaling and Actions in the Cardiovascular System. Front. Pharmacol. 2017, 8, 556. [Google Scholar] [CrossRef]
- Schuchardt, M.; Tölle, M.; Prüfer, J.; van der Giet, M. Pharmacological relevance and potential of sphingosine 1-phosphate in the vascular system. Br. J. Pharmacol. 2011, 163, 1140–1162. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.S.; Silva, R.A.; Chantepie, S.; Lagor, W.R.; Chapman, M.J.; Kontush, A. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: Relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Frias, M.A.; Thomas, A.; Brulhart-Meynet, M.C.; Kövamees, O.; Pernow, J.; Eriksson, M.; Angelin, B.; James, R.W.; Brinck, J.W. High-density lipoprotein-associated sphingosine-1-phosphate activity in heterozygous familial hypercholesterolaemia. Eur. J. Clin. Investig. 2017, 47, 38–43. [Google Scholar] [CrossRef]
- Kingwell, B.A.; Chapman, M.J.; Kontush, A.; Miller, N.E. HDL-targeted therapies: Progress, failures and future. Nat. Rev. Drug Discov. 2014, 13, 445–464. [Google Scholar] [CrossRef]
- Harangi, M.; Szentpéteri, A.; Nádró, B.; Lőrincz, H.; Seres, I.; Páll, D.; Paragh, G. HDL subfraction distribution and HDL function in untreated dyslipidemic patients. Vessel Plus 2017, 1, 166–173. [Google Scholar] [CrossRef]
- Reddy, S.T.; Navab, M.; Anantharamaiah, G.M.; Fogelman, A.M. Searching for a successful HDL-based treatment strategy. Biochim. Biophys. Acta 2014, 1841, 162–167. [Google Scholar] [CrossRef]
- Navab, M.; Reddy, S.T.; Van Lenten, B.J.; Fogelman, A.M. HDL and cardiovascular disease: Atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol. 2011, 8, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Levkau, B. HDL-S1P: Cardiovascular functions, disease-associated alterations, and therapeutic applications. Front. Pharmacol. 2015, 6, 243. [Google Scholar] [CrossRef] [PubMed]
- Potì, F.; Simoni, M.; Nofer, J.R. Atheroprotective role of high-density lipoprotein (HDL)-associated sphingosine-1-phosphate (S1P). Cardiovasc. Res. 2014, 103, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Badimon, J.J.; Santos-Gallego, C.G. HDL Dysfunction: Is the Answer in the Sphinx’s Riddle? J. Am. Coll. Cardiol. 2015, 66, 1486–1488. [Google Scholar] [CrossRef]
- Koenig, W.; Khuseyinova, N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Therond, P.; Chapman, M.J. Sphingosine-1-phosphate: Metabolism, transport, atheroprotection and effect of statin treatment. Curr. Opin. Lipidol. 2022, 33, 199–207. [Google Scholar] [CrossRef]
- Skoura, A.; Michaud, J.; Im, D.S.; Thangada, S.; Xiong, Y.; Smith, J.D.; Hla, T. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 81–85. [Google Scholar] [CrossRef]
- Keul, P.; Polzin, A.; Kaiser, K.; Gräler, M.; Dannenberg, L.; Daum, G.; Heusch, G.; Levkau, B. Potent anti-inflammatory properties of HDL in vascular smooth muscle cells mediated by HDL-S1P and their impairment in coronary artery disease due to lower HDL-S1P: A new aspect of HDL dysfunction and its therapy. FASEB J. 2019, 33, 1482–1495. [Google Scholar] [CrossRef]
- Barter, P.J.; Nicholls, S.; Rye, K.A.; Anantharamaiah, G.M.; Navab, M.; Fogelman, A.M. Antiinflammatory properties of HDL. Circ. Res. 2004, 95, 764–772. [Google Scholar] [CrossRef]
- Camps, J.; Castañé, H.; Rodríguez-Tomàs, E.; Baiges-Gaya, G.; Hernández-Aguilera, A.; Arenas, M.; Iftimie, S.; Joven, J. On the Role of Paraoxonase-1 and Chemokine Ligand 2 (C-C motif) in Metabolic Alterations Linked to Inflammation and Disease. A 2021 Update. Biomolecules 2021, 11, 971. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Sahebkar, A. Effect of statin therapy on paraoxonase-1 status: A systematic review and meta-analysis of 25 clinical trials. Prog. Lipid Res. 2015, 60, 50–73. [Google Scholar] [CrossRef] [PubMed]
- Harangi, M.; Seres, I.; Harangi, J.; Paragh, G. Benefits and difficulties in measuring HDL subfractions and human paraoxonase-1 activity during statin treatment. Cardiovasc. Drugs Ther. 2009, 23, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Nádró, B.; Lőrincz, H.; Juhász, L.; Szentpéteri, A.; Sztanek, F.; Varga, É.; Páll, D.; Paragh, G.; Harangi, M. Determination of Serum Progranulin in Patients with Untreated Familial Hypercholesterolemia. Biomedicines 2022, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Melnes, T.; Bogsrud, M.P.; Thorsen, I.; Fossum, J.; Christensen, J.J.; Narverud, I.; Retterstøl, K.; Ulven, S.M.; Holven, K.B. What characterizes event-free elderly FH patients? A comprehensive lipoprotein profiling. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1651–1660. [Google Scholar] [CrossRef]
- Al-Rasadi, K.; Al-Waili, K.; Al-Sabti, H.A.; Al-Hinai, A.; Al-Hashmi, K.; Al-Zakwani, I.; Banerjee, Y. Criteria for Diagnosis of Familial Hypercholesterolemia: A Comprehensive Analysis of the Different Guidelines, Appraising their Suitability in the Omani Arab Population. Oman Med. J. 2014, 29, 85–91. [Google Scholar] [CrossRef]
- Fülöp, P.; Seres, I.; Lőrincz, H.; Harangi, M.; Somodi, S.; Paragh, G. Association of chemerin with oxidative stress, inflammation and classical adipokines in non-diabetic obese patients. J. Cell. Mol. Med. 2014, 18, 1313–1320. [Google Scholar] [CrossRef]
- Hoefner, D.M.; Hodel, S.D.; O’Brien, J.F.; Branum, E.L.; Sun, D.; Meissner, I.; McConnell, J.P. Development of a rapid, quantitative method for LDL subfractionation with use of the Quantimetrix Lipoprint LDL System. Clin. Chem. 2001, 47, 266–274. [Google Scholar] [CrossRef]
- Lőrincz, H.; Katkó, M.; Harangi, M.; Somodi, S.; Gaál, K.; Fülöp, P.; Paragh, G.; Seres, I. Strong correlations between circulating chemerin levels and lipoprotein subfractions in nondiabetic obese and nonobese subjects. Clin. Endocrinol. 2014, 81, 370–377. [Google Scholar] [CrossRef]
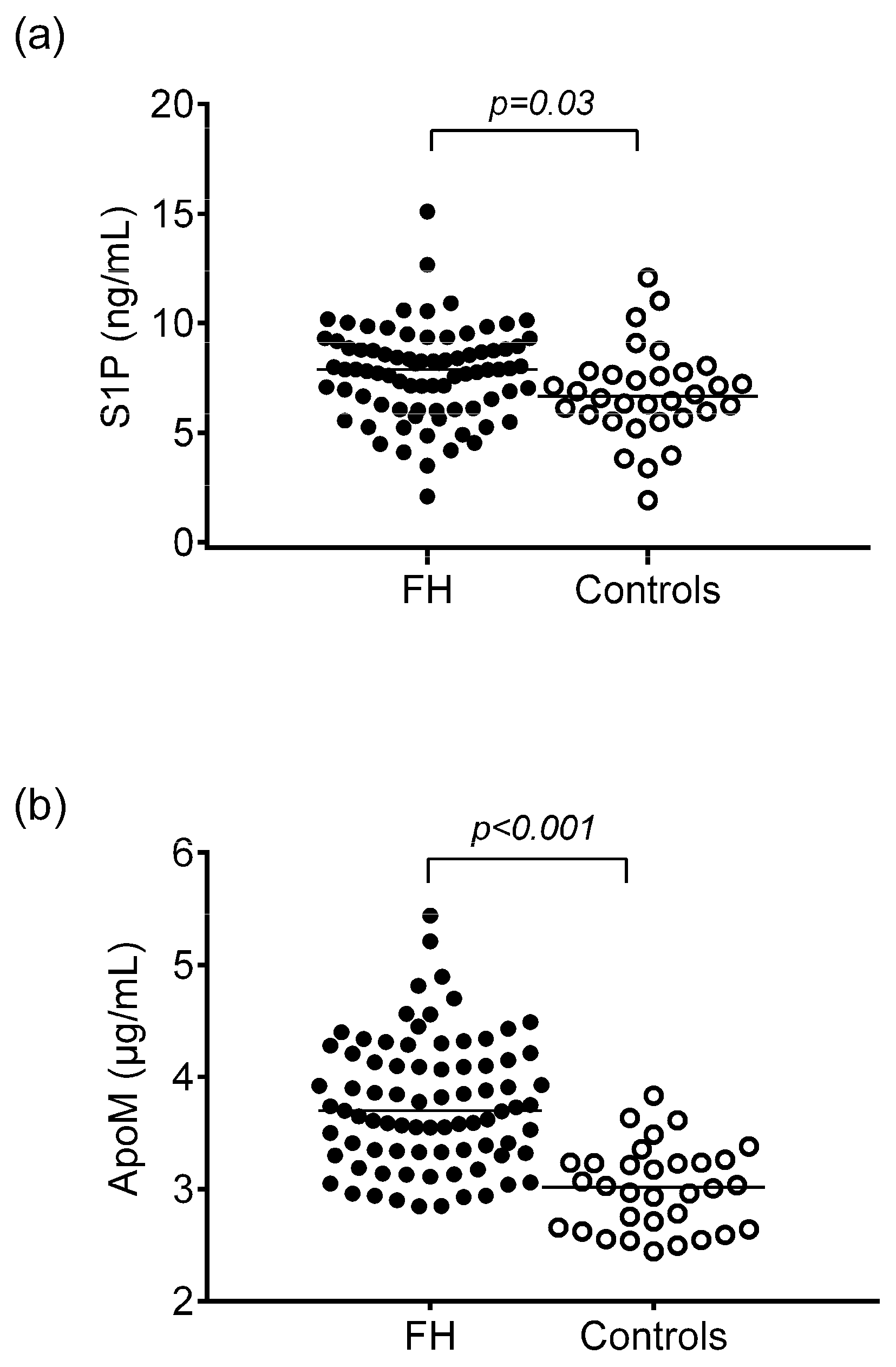

| FH Patients | Controls | p Values | |
|---|---|---|---|
| Number of subjects | 81 | 32 | |
| Male/female | 26/55 | 5/27 | ns. |
| Age (years) | 53.22 ± 14.5 | 41.8 ± 6.0 | p < 0.001 |
| Cholesterol (mmol/L) | 8.87 ± 1.47 | 5.07 ± 0.78 | p < 0.001 |
| HDL-C (mmol/L) | 1.62 ± 0.48 | 1.56 ± 0.46 | ns. |
| LDL-C (mmol/L) | 6.48 ± 1.28 | 2.93 ± 0.52 | p < 0.001 |
| Triglyceride (mmol/L) | 1.6 (1.0–2.4) | 1.0 (0.75–1.39) | p < 0.001 |
| ApoB100 (g/L) | 1.78 ± 0.38 | 0.94 ± 0.18 | p < 0.001 |
| ApoA1 (g/L) | 1.71 ± 0.28 | 1.68 ± 0.31 | ns. |
| Lp(a) (mg/L) | 179 (75–857) | 90 (30–214) | p < 0.05 |
| ApoM (μg/mL) | 3.76 ± 0.57 | 3.01 ± 0.37 | p < 0.001 |
| S1P (ng/mL) | 7.73 ± 2.07 | 6.79 ± 2.09 | p < 0.05 |
| S1P/ApoM ratio | 2120 ± 700 | 2290 ± 740 | ns. |
| hsCRP (mg/L) | 1.84 (0.70–2.90) | 1.55 (0.6–2.95) | ns. |
| PON1 paraoxonase activity (U/L) | 107.02 (43.61–166.5) | 83.0 (47.9–167.4) | ns. |
| PON1 salt-stimulated paraoxonase activity (U/L) | 183.5 (103.2–322.6) | 169.4 (97.3–297.4) | ns. |
| PON1 arylesterase activity (U/L) | 143.2 ± 25.12 | 135.4 ± 36.8 | p < 0.01 |
| Myeloperoxidase (ng/mL) | 297.7 (158.15–456.5) | 135.7 (99.4–195.1) | p < 0.001 |
| oxLDL (U/L) | 187.98 ± 71.04 | 41.1 ± 9.57 | p < 0.001 |
| sICAM-1 (ng/mL) | 270.66 ± 69.9 | 210.8 ± 32,2 | p < 0.001 |
| sVCAM-1 (ng/mL) | 573.9 ± 140.45 | 467.7 ± 106.3 | ns. |
| sCD40L (ng/mL) | 10.02 ± 4.3 | 8.22 ± 3.44 | ns. |
| TNFα (pg/mL) | 0.47 ± 0.17 | 1.66 ± 0.91 | p < 0.001 |
| FH Patients | Controls | p | |
|---|---|---|---|
| VLDL subfraction (%) | 19.76 ± 5.8 | 16.95 ± 2.2 | 0.01 |
| VLDL subfraction (mmol/L) | 1.77 ± 0.66 | 0.868 ± 0.17 | <0.001 |
| Midband (IDL) (%) | 28.89 ± 4.5 | 29.83 ± 4.9 | ns |
| Midband (IDL) (mmol/L) | 2.52 ± 0.62 | 1.505 ± 0.38 | <0.001 |
| LDL subfractions | |||
| Large LDL (%) | 27.3 ± 5.5 | 20.9 ± 5.8 | <0.001 |
| Small LDL (%) | 3.2 (1.1–11.0) | 0.5 (0–0.8) | <0.001 |
| Large LDL (mmol/L) | 2.29 (2.05–2.64) | 1.047 (0.827–1.344) | <0.001 |
| Small-density LDL (mmol/L) | 0.18 (0.05–0.79) | 0.026 (0–0.052) | <0.001 |
| Mean LDL size (nm) | 26.78 ± 0.58 | 27.26 ± 0.37 | <0.05 |
| HDL subfractions | |||
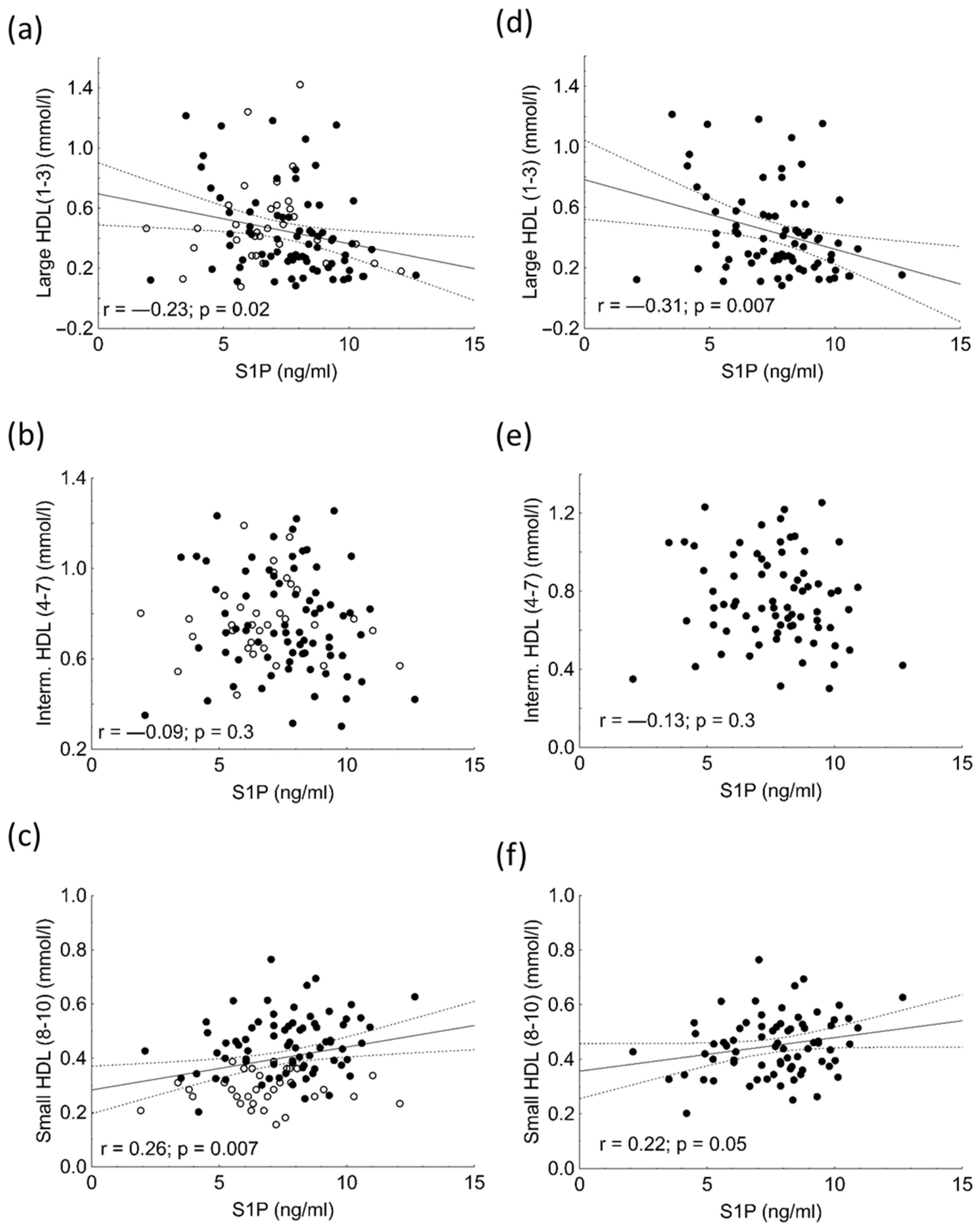
| Large HDL (%) | 24.7 ± 11.0 | 30.2 ± 8.9 | 0.02 |
| Intermediate HDL (%) | 46.0 ± 4.9 | 50.6 ± 4.7 | <0.001 |
| Small HDL (%) | 29.3 ± 10.6 | 19.2 ± 5.4 | <0.001 |
| Large HDL (mmol/L) | 0.35 (0.231–0.571) | 0.453 (0.31–0.608) | <0.001 |
| Intermediate HDL (mmol/L) | 0.72 (0.613–0.932) | 0.750 (0.659–0.853) | <0.05 |
| Small HDL (mmol/L) | 0.452 (0.374–0.523) | 0.284 (0.246–0.336) | <0.01 |
| Variable | β | p-Value |
|---|---|---|
| log triglyceride | 0.1 | 0.5 |
| large HDL (%) | 0.35 | <0.01 |
| small HDL (%) | 0.136 | 0.8 |
| sVCAM-1 | −0.13 | 0.2 |
| ApoM | −0.1 | 0.2 |
| PON1 arylesterase activity | 0.281 | <0.001 |
| MMP-9 | 0.142 | 0.3 |
| log sCD40L | −0.16 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhász, L.; Lőrincz, H.; Szentpéteri, A.; Nádró, B.; Varga, É.; Paragh, G.; Harangi, M. Sphingosine 1-Phosphate and Apolipoprotein M Levels and Their Correlations with Inflammatory Biomarkers in Patients with Untreated Familial Hypercholesterolemia. Int. J. Mol. Sci. 2022, 23, 14065. https://doi.org/10.3390/ijms232214065
Juhász L, Lőrincz H, Szentpéteri A, Nádró B, Varga É, Paragh G, Harangi M. Sphingosine 1-Phosphate and Apolipoprotein M Levels and Their Correlations with Inflammatory Biomarkers in Patients with Untreated Familial Hypercholesterolemia. International Journal of Molecular Sciences. 2022; 23(22):14065. https://doi.org/10.3390/ijms232214065
Chicago/Turabian StyleJuhász, Lilla, Hajnalka Lőrincz, Anita Szentpéteri, Bíborka Nádró, Éva Varga, György Paragh, and Mariann Harangi. 2022. "Sphingosine 1-Phosphate and Apolipoprotein M Levels and Their Correlations with Inflammatory Biomarkers in Patients with Untreated Familial Hypercholesterolemia" International Journal of Molecular Sciences 23, no. 22: 14065. https://doi.org/10.3390/ijms232214065
APA StyleJuhász, L., Lőrincz, H., Szentpéteri, A., Nádró, B., Varga, É., Paragh, G., & Harangi, M. (2022). Sphingosine 1-Phosphate and Apolipoprotein M Levels and Their Correlations with Inflammatory Biomarkers in Patients with Untreated Familial Hypercholesterolemia. International Journal of Molecular Sciences, 23(22), 14065. https://doi.org/10.3390/ijms232214065








