Air Pollution: Another Threat to HDL Function
Abstract
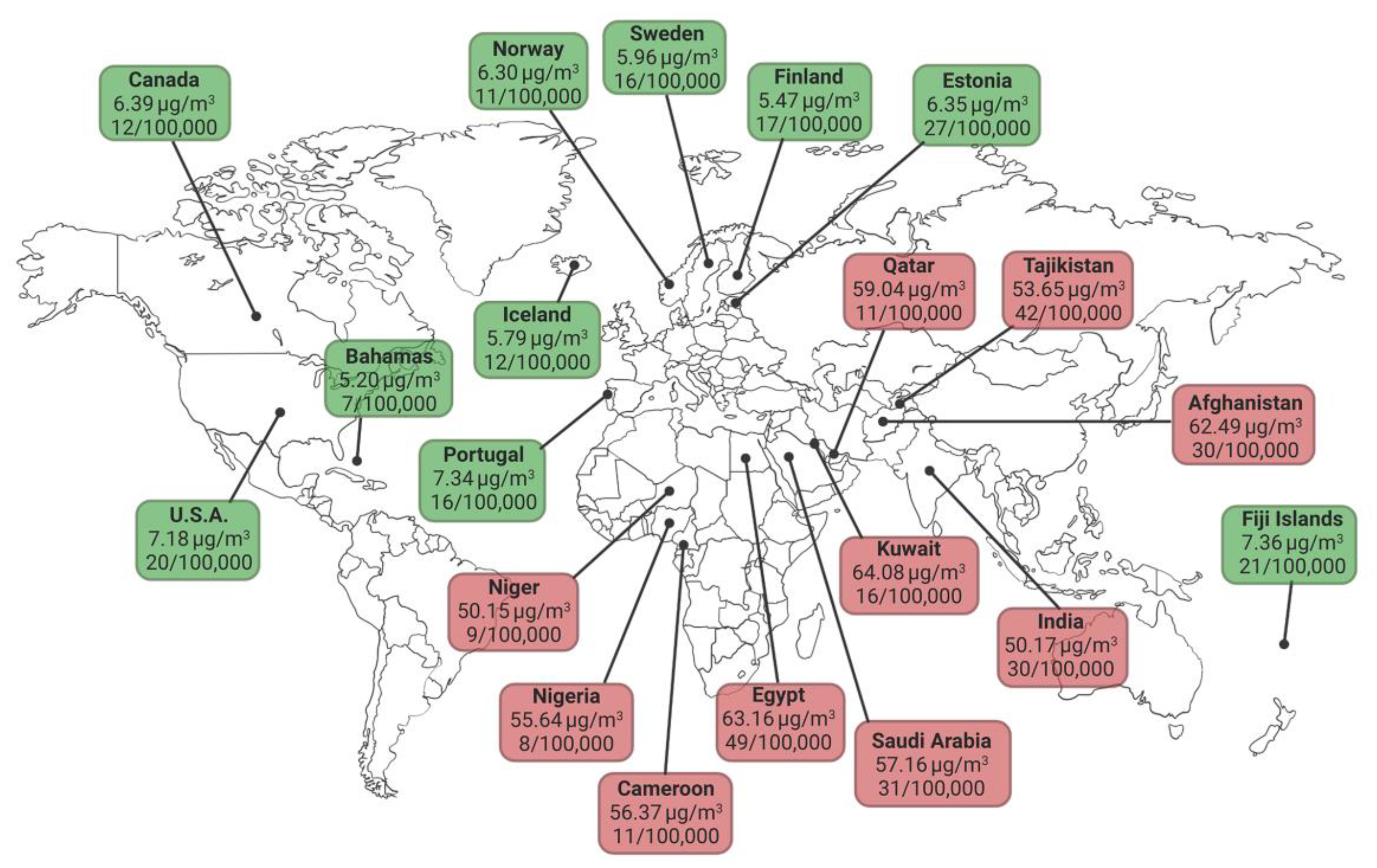
1. Air Pollution as a Cause of Cardiovascular Diseases
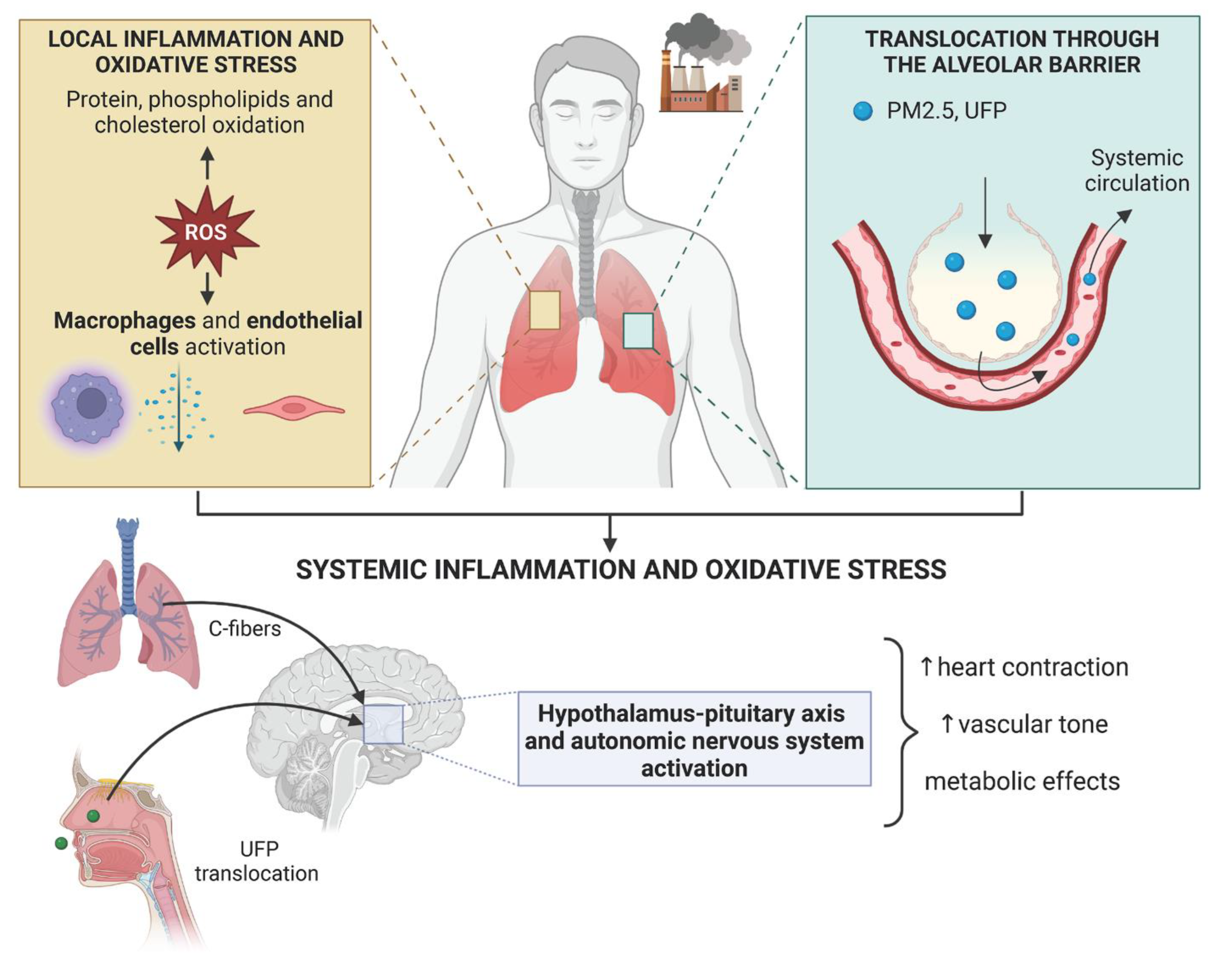
2. Mechanisms of Action
3. Effects of Air Pollution on HDL-Cholesterol Levels
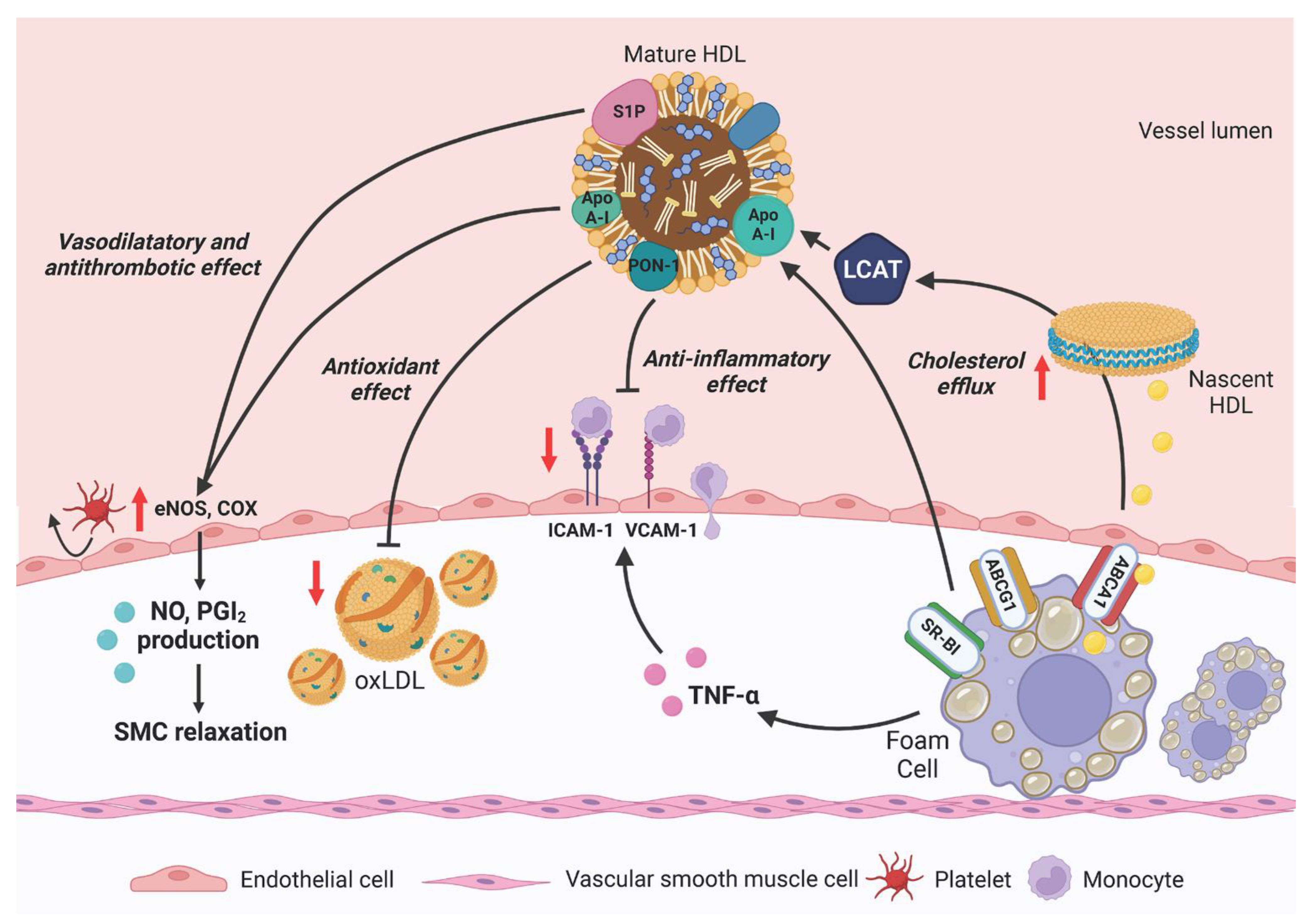
4. Cardiovascular Protection by HDL
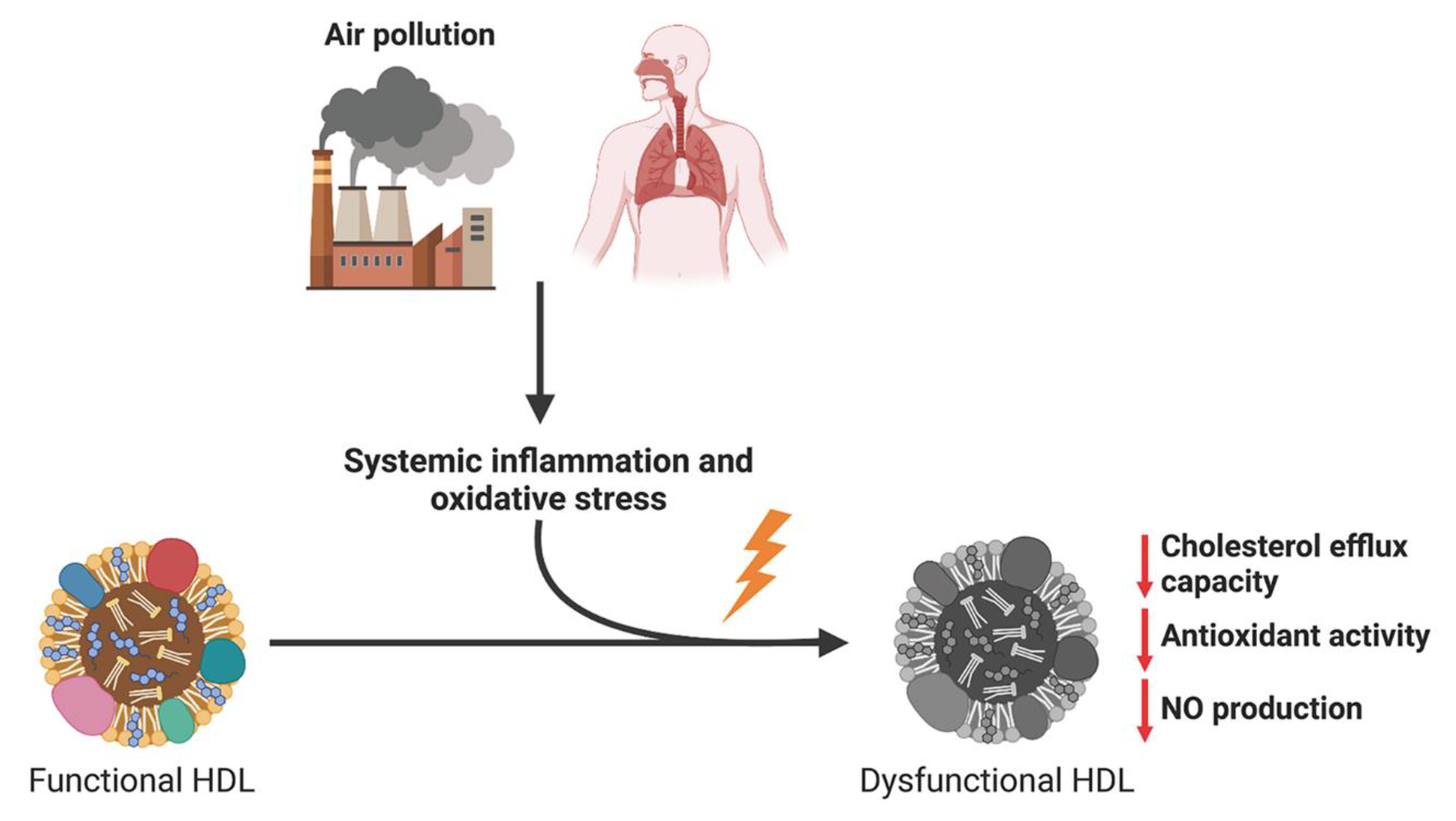
5. Effects of Air Pollution on HDL Function
6. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Bevan, G.H.; Al-Kindi, S.G.; Brook, R.; Rajagopalan, S. Ambient Air Pollution and Atherosclerosis: Recent Updates. Curr. Atheroscler. Rep. 2021, 23, 63. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Weagle, C.L.; Snider, G.; Li, C.; van Donkelaar, A.; Philip, S.; Bissonnette, P.; Burke, J.; Jackson, J.; Latimer, R.; Stone, E.; et al. Global Sources of Fine Particulate Matter: Interpretation of PM2.5 Chemical Composition Observed by SPARTAN using a Global Chemical Transport Model. Environ. Sci. Technol. 2018, 52, 11670–11681. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Miller, M.R.; Newby, D.E. Air pollution and cardiovascular disease: Car sick. Card. Res. 2020, 116, 279–294. [Google Scholar] [CrossRef]
- Newby, D.E.; Mannucci, P.M.; Tell, G.S.; Baccarelli, A.A.; Brook, R.D.; Donaldson, K.; Forastiere, F.; Franchini, M.; Franco, O.H.; Graham, I.; et al. Expert position paper on air pollution and cardiovascular disease. Eur. Heart J. 2015, 36, 83–93b. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Brauer, M.; Bhatnagar, A.; Bhatt, D.L.; Brook, J.R.; Huang, W.; Münzel, T.; Newby, D.; Siegel, J.; Brook, R.D. Personal-Level Protective Actions Against Particulate Matter Air Pollution Exposure: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e411–e431. [Google Scholar] [CrossRef]
- Cesaroni, G.; Forastiere, F.; Stafoggia, M.; Andersen, Z.J.; Badaloni, C.; Beelen, R.; Caracciolo, B.; de Faire, U.; Erbel, R.; Eriksen, K.T.; et al. Long term exposure to ambient air pollution and incidence of acute coronary events: Prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ 2014, 348, f7412. [Google Scholar] [CrossRef]
- Wolf, K.; Hoffmann, B.; Andersen, Z.J.; Atkinson, R.W.; Bauwelinck, M.; Bellander, T.; Brandt, J.; Brunekreef, B.; Cesaroni, G.; Chen, J.; et al. Long-term exposure to low-level ambient air pollution and incidence of stroke and coronary heart disease: A pooled analysis of six European cohorts within the ELAPSE project. Lancet Planet. Health 2021, 5, e620–e632. [Google Scholar] [CrossRef]
- de Bont, J.; Jaganathan, S.; Dahlquist, M.; Persson, Å.; Stafoggia, M.; Ljungman, P. Ambient air pollution and cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. J. Intern. Med. 2022, 291, 779–800. [Google Scholar] [CrossRef]
- Bevan, G.H.; Al-Kindi, S.G.; Brook, R.D.; Münzel, T.; Rajagopalan, S. Ambient Air Pollution and Atherosclerosis: Insights Into Dose, Time, and Mechanisms. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Mudway, I.S.; Kelly, F.J. Ozone and the lung: A sensitive issue. Mol. Asp. Med. 2000, 21, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.A.; Crapo, J.D. Biology of disease: Free radicals and tissue injury. Lab. Investig. 1982, 47, 412–426. [Google Scholar]
- Kurtz, M.L.; Astort, F.; Lezon, C.; Ferraro, S.A.; Maglione, G.A.; Orona, N.S.; Friedman, S.M.; Boyer, P.M.; Tasat, D.R. Oxidative stress response to air particle pollution in a rat nutritional growth retardation model. J. Toxicol. Environ. Health A. 2018, 81, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Kampfrath, T.; Maiseyeu, A.; Ying, Z.; Shah, Z.; Deiuliis, J.A.; Xu, X.; Kherada, N.; Brook, R.D.; Reddy, K.M.; Padture, N.P.; et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ. Res. 2011, 108, 716–726. [Google Scholar] [CrossRef]
- Guan, L.; Geng, X.; Stone, C.; Cosky, E.E.P.; Ji, Y.; Du, H.; Zhang, K.; Sun, Q.; Ding, Y. PM2.5 exposure induces systemic inflammation and oxidative stress in an intracranial atherosclerosis rat model. Environ. Toxicol. 2019, 34, 530–538. [Google Scholar] [CrossRef]
- Kim, Y.W.; West, X.Z.; Byzova, T.V. Inflammation and oxidative stress in angiogenesis and vascular disease. J. Mol. Med. 2013, 91, 323–328. [Google Scholar] [CrossRef]
- Lawal, A.O. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathways. Toxicol. Lett. 2017, 270, 88–95. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef]
- Joshi, S.; Miller, M.R.; Newby, D.E. Air pollution and cardiovascular disease: The Paul Wood Lecture, British Cardiovascular Society 2021. Heart 2022, 108, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Raftis, J.B.; Langrish, J.P.; McLean, S.G.; Samutrtai, P.; Connell, S.P.; Wilson, S.; Vesey, A.T.; Fokkens, P.H.B.; Boere, A.J.F.; et al. Inhaled Nanoparticles Accumulate at Sites of Vascular Disease. ACS Nano 2017, 11, 4542–4552. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Xiong, W.; Fan, Q.; Wang, H.; Chen, Q.; Shen, W.; Zhang, R.; Ding, F.; Lu, L.; Tao, R. Association of decreased serum sTREM-1 level with the severity of coronary artery disease: Inhibitory effect of sTREM-1 on TNF-α- and oxLDL-induced inflammatory reactions in endothelial cells. Medicine 2016, 95, e4693. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A.; Barajas, B.; Kleinman, M.; Wang, X.; Bennett, B.J.; Gong, K.W.; Navab, M.; Harkema, J.; Sioutas, C.; Lusis, A.J.; et al. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ. Res. 2008, 102, 589–596. [Google Scholar] [CrossRef]
- Yin, F.; Lawal, A.; Ricks, J.; Fox, J.R.; Larson, T.; Navab, M.; Fogelman, A.M.; Rosenfeld, M.E.; Araujo, J.A. Diesel exhaust induces systemic lipid peroxidation and development of dysfunctional pro-oxidant and pro-inflammatory high-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1153–1161. [Google Scholar] [CrossRef]
- Wong, J.Y.; De Vivo, I.; Lin, X.; Christiani, D.C. Cumulative PM(2.5) exposure and telomere length in workers exposed to welding fumes. J. Toxicol. Environ. Health A 2014, 77, 441–455. [Google Scholar] [CrossRef]
- Cherng, T.W.; Paffett, M.L.; Jackson-Weaver, O.; Campen, M.J.; Walker, B.R.; Kanagy, N.L. Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles. Environ. Health Perspect. 2011, 119, 98–103. [Google Scholar] [CrossRef]
- Hemmingsen, J.G.; Rissler, J.; Lykkesfeldt, J.; Sallsten, G.; Kristiansen, J.; Møller, P.P.; Loft, S. Controlled exposure to particulate matter from urban street air is associated with decreased vasodilation and heart rate variability in overweight and older adults. Part. Fibre Toxicol. 2015, 12, 6. [Google Scholar] [CrossRef]
- Ishii, K.; Koyama, A.; Kobayashi, M.; Kashiwagi, H.; Narita, M. A study of microproteinuria in patients with collagen disease. Nihon Jinzo Gakkai Shi 1989, 31, 827–837. [Google Scholar]
- Sun, Q.; Yue, P.; Kirk, R.I.; Wang, A.; Moatti, D.; Jin, X.; Lu, B.; Schecter, A.D.; Lippmann, M.; Gordon, T.; et al. Ambient air particulate matter exposure and tissue factor expression in atherosclerosis. Inhal. Toxicol. 2008, 20, 127–137. [Google Scholar] [CrossRef]
- Ying, Z.; Xie, X.; Bai, Y.; Chen, M.; Wang, X.; Zhang, X.; Morishita, M.; Sun, Q.; Rajagopalan, S. Exposure to concentrated ambient particulate matter induces reversible increase of heart weight in spontaneously hypertensive rats. Part. Fibre Toxicol. 2015, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Miller, M.R. Ambient air pollution and thrombosis. Part. Fibre Toxicol. 2018, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, T.; Liu, S.; Brook, R.D.; Feng, B.; Zhao, Q.; Song, X.; Yi, T.; Chen, J.; Zhang, Y.; et al. Extreme Levels of Air Pollution Associated With Changes in Biomarkers of Atherosclerotic Plaque Vulnerability and Thrombogenicity in Healthy Adults. Circ. Res. 2019, 124, e30–e43. [Google Scholar] [CrossRef]
- Mancini, F.R.; Laine, J.E.; Tarallo, S.; Vlaanderen, J.; Vermeulen, R.; van Nunen, E.; Hoek, G.; Probst-Hensch, N.; Imboden, M.; Jeong, A.; et al. MicroRNA expression profiles and personal monitoring of exposure to particulate matter. Environ. Pollut. 2020, 263 Pt B, 114392. [Google Scholar] [CrossRef]
- Rodosthenous, R.S.; Kloog, I.; Colicino, E.; Zhong, J.; Herrera, L.A.; Vokonas, P.; Schwartz, J.; Baccarelli, A.A.; Prada, D. Extracellular vesicle-enriched microRNAs interact in the association between long-term particulate matter and blood pressure in elderly men. Environ. Res. 2018, 167, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Vera, T.; Ochoa-Martínez, Á.C.; Pruneda-Álvarez, L.G.; Domínguez-Cortinas, G.; Pérez-Maldonado, I.N. Expression levels of circulating microRNAs-126, -155, and -145 in Mexican women exposed to polycyclic aromatic hydrocarbons through biomass fuel use. Environ. Mol. Mutagen. 2019, 60, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Daiber, A. Environmental Stressors and Their Impact on Health and Disease with Focus on Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Aryal, A.; Harmon, A.C.; Dugas, T.R. Particulate matter air pollutants and cardiovascular disease: Strategies for intervention. Pharmacol. Ther. 2021, 223, 107890. [Google Scholar] [CrossRef]
- Münzel, T.; Gori, T.; Al-Kindi, S.; Deanfield, J.; Lelieveld, J.; Daiber, A.; Rajagopalan, S. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur. Heart J. 2018, 39, 3543–3550. [Google Scholar] [CrossRef]
- Verones, B.; Oortgiesen, M. Neurogenic inflammation and particulate matter (PM) air pollutants. Neurotoxicology 2001, 22, 795–810. [Google Scholar] [CrossRef]
- Hajat, A.; Diez Roux, A.V.; Castro-Diehl, C.; Cosselman, K.; Golden, S.H.; Hazlehurst, M.F.; Szpiro, A.; Vedal, S.; Kaufman, J.D. The Association between Long-Term Air Pollution and Urinary Catecholamines: Evidence from the Multi-Ethnic Study of Atherosclerosis. Environ. Health Perspect. 2019, 127, 57007. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Chen, R.; Xia, Y.; Cai, J.; Ying, Z.; Lin, Z.; Liu, C.; Chen, C.; Peng, L.; Zhao, Z.; et al. Fine particulate matter constituents and stress hormones in the hypothalamus-pituitary-adrenal axis. Environ. Int. 2018, 119, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Corral, C.M.; Alderete, T.L.; Herting, M.M.; Habre, R.; Peterson, A.K.; Lurmann, F.; Goran, M.I.; Weigensberg, M.J.; Gilliland, F.D. Ambient air pollutants are associated with morning serum cortisol in overweight and obese Latino youth in Los Angeles. Environ. Health 2021, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Widén, C.; Gustafsson, J.A.; Wikström, A.C. Cytosolic glucocorticoid receptor interaction with nuclear factor-kappa B proteins in rat liver cells. Biochem. J. 2003, 373 Pt 1, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Asico, L.D.; Zanos, P.; Mahabeleshwar, G.H.; Gangwar, R.S.; Xia, C.; Duan, L.; Cisse, Y.-M.; Rengasamy, P.; Jose, P.A.; et al. Alpha2B-Adrenergic Receptor Overexpression in the Brain Potentiate Air Pollution-induced Behavior and Blood Pressure Changes. Toxicol. Sci. 2019, 169, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Chiarella, S.E.; Soberanes, S.; Urich, D.; Morales-Nebreda, L.; Nigdelioglu, R.; Green, D.; Young, J.B.; Gonzalez, A.; Rosario, C.; Misharin, A.V.; et al. β2-Adrenergic agonists augment air pollution-induced IL-6 release and thrombosis. J. Clin. Investig. 2014, 124, 2935–2946. [Google Scholar] [CrossRef]
- Wu, X.M.; Broadwin, R.; Basu, R.; Malig, B.; Ebisu, K.; Gold, E.B.; Qi, L.; Derby, C.; Park, S.K.; Green, S. Associations between fine particulate matter and changes in lipids/lipoproteins among midlife women. Sci. Total Environ. 2019, 654, 1179–1186. [Google Scholar] [CrossRef]
- Bell, G.; Mora, S.; Greenland, P.; Tsai, M.; Gill, E.; Kaufman, J.D. Association of Air Pollution Exposures With High-Density Lipoprotein Cholesterol and Particle Number: The Multi-Ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 976–982. [Google Scholar] [CrossRef]
- Yang, B.Y.; Bloom, M.S.; Markevych, I.; Qian, Z.M.; Vaughn, M.G.; Cummings-Vaughn, L.A.; Li, S.; Chen, G.; Bowatte, G.; Perret, J.L. Exposure to ambient air pollution and blood lipids in adults: The 33 Communities Chinese Health Study. Environ. Int. 2018, 119, 485–492. [Google Scholar] [CrossRef]
- Cai, Y.; Hansell, A.L.; Blangiardo, M.; Burton, P.R.; BioSHaRE; de Hoogh, K.; Doiron, D.; Fortier, I.; Gulliver, J.; Hveem, K.; et al. Long-term exposure to road traffic noise, ambient air pollution, and cardiovascular risk factors in the HUNT and lifelines cohorts. Eur. Heart J. 2017, 38, 2290–2296. [Google Scholar] [CrossRef]
- Zhang, A.L.; Balmes, J.R.; Lutzker, L.; Mann, J.K.; Margolis, H.G.; Tyner, T.; Holland, N.; Noth, E.M.; Lurmann, F.; Hammond, S.K.; et al. Traffic-related air pollution, biomarkers of metabolic dysfunction, oxidative stress, and CC16 in children. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, S.; Cesaroni, G.; Badaloni, C.; Michelozzi, P.; Forastiere, F.; Porta, D. Traffic-related air pollution and childhood obesity in an Italian birth cohort. Environ. Res. 2018, 160, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.H.; Yang, B.Y.; Zou, Z.Y.; Ma, J.; Jing, J.; Wang, H.J.; Dong, G.H.; Ma, Y.H.; Guo, Y.M.; Chen, Y.J. Exposure to ambient air pollution and blood lipids in children and adolescents: A national population based study in China. Environ. Pollut. 2020, 115422. [Google Scholar] [CrossRef]
- Li, J.; Yao, Y.; Xie, W.; Wang, B.; Guan, T.; Han, Y.; Wang, H.; Zhu, T.; Xue, T. Association of long-term exposure to PM2.5 with blood lipids in the Chinese population: Findings from a longitudinal quasi-experiment. Environ. Int. 2021, 151, 106454. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.C.; Waters, D.D.; et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef]
- AIM-HIGH Investigators; Boden, W.E.; Probstfield, J.L.; Anderson, T.; Chaitman, B.R.; Desvignes-Nickens, P.; Koprowicz, K.; McBride, R.; Teo, K.; Weintraub, W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 2011, 365, 2255–2267. [Google Scholar]
- Lincoff, A.M.; Nicholls, S.J.; Riesmeyer, J.S.; Barter, P.J.; Brewer, H.B.; Fox, K.A.A.; Gibson, C.M.; Granger, C.; Menon, V.; Montalescot, G.; et al. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N. Engl. J. Med. 2017, 376, 1933–1942. [Google Scholar] [CrossRef]
- Khera, A.V.; Demler, O.V.; Adelman, S.J.; Collins, H.L.; Glynn, R.J.; Ridker, P.M.; Rader, D.J.; Mora, S. Cholesterol Efflux Capacity, High-Density Lipoprotein Particle Number, and Incident Cardiovascular Events: An Analysis From the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation 2017, 135, 2494–2504. [Google Scholar] [CrossRef]
- Calabresi, L.; Gomaraschi, M.; Franceschini, G. High-density lipoprotein quantity or quality for cardiovascular prevention? Curr. Pharm. Des. 2010, 16, 1494–1503. [Google Scholar] [CrossRef]
- Basso, F.; Freeman, L.; Knapper, C.L.; Remaley, A.; Stonik, J.; Neufeld, E.B.; Tansey, T.; Amar, M.J.; Fruchart-Najib, J.; Duverger, N.; et al. Role of the hepatic ABCA1 transporter in modulating intrahepatic cholesterol and plasma HDL cholesterol concentrations. J. Lipid Res. 2003, 44, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, L.; Gomaraschi, M.; Simonelli, S.; Bernini, F.; Franceschini, G. HDL and atherosclerosis: Insights from inherited HDL disorders. Biochim. Biophys. Acta 2015, 1851, 13–18. [Google Scholar] [CrossRef]
- Rye, K.A.; Clay, M.A.; Barter, P.J. Remodelling of high density lipoproteins by plasma factors. Atherosclerosis 1999, 145, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Kozyraki, R.; Fyfe, J.; Kristiansen, M.; Gerdes, C.; Jacobsen, C.; Cui, S.; Christensen, E.I.; Aminoff, M.; de la Chapelle, A.; Krahe, R.; et al. The intrinsic factor-vitamin B12 receptor, cubilin, is a high-affinity apolipoprotein A-I receptor facilitating endocytosis of high-density lipoprotein. Nat. Med. 1999, 5, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Brewer, H.B., Jr.; Davidson, W.S.; Fayad, Z.A.; Fuster, V.; Goldstein, J.; Hellerstein, M.; Jiang, X.C.; Phillips, M.C.; Rader, D.J.; et al. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation 2012, 125, 1905–1919. [Google Scholar] [CrossRef]
- Soria-Florido, M.T.; Schröder, H.; Grau, M.; Fitó, M.; Lassale, C. High density lipoprotein functionality and cardiovascular events and mortality: A systematic review and meta-analysis. Atherosclerosis 2020, 302, 36–42. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B., Jr.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef]
- Calabresi, L.; Gomaraschi, M.; Franceschini, G. Endothelial protection by high-density lipoproteins: From bench to bedside. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1724–1731. [Google Scholar] [CrossRef]
- Sangvanich, P.; Mackness, B.; Gaskell, S.J.; Durrington, P.; Mackness, M. The effect of high-density lipoproteins on the formation of lipid/protein conjugates during in vitro oxidation of low-density lipoprotein. Biochem. Biophys. Res. Commun. 2003, 300, 501–506. [Google Scholar] [CrossRef]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. HDL in infectious diseases and sepsis. Handb. Exp. Pharmacol. 2015, 224, 483–508. [Google Scholar]
- Kontush, A.; Chantepie, S.; Chapman, M.J. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Shuhei, N.; Söderlund, S.; Jauhiainen, M.; Taskinen, M.R. Effect of HDL composition and particle size on the resistance of HDL to the oxidation. Lipids Health Dis. 2010, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Ashby, D.T.; Rye, K.A.; Clay, M.A.; Vadas, M.A.; Gamble, J.R.; Barter, P.J. Factors influencing the ability of HDL to inhibit expression of vascular cell adhesion molecule-1 in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Karathanasis, S.K.; Freeman, L.A.; Gordon, S.M.; Remaley, A.T. The Changing Face of HDL and the Best Way to Measure It. Clin. Chem. 2017, 63, 196–210. [Google Scholar] [CrossRef]
- Ossoli, A.; Pavanello, C.; Giorgio, E.; Calabresi, L.; Gomaraschi, M. Dysfunctional HDL as a Therapeutic Target for Atherosclerosis Prevention. Curr. Med. Chem. 2019, 26, 1610–1630. [Google Scholar] [CrossRef]
- Batuca, J.R.; Ames, P.R.; Amaral, M.; Favas, C.; Isenberg, D.A.; Delgado Alves, J. Anti-atherogenic and anti-inflammatory properties of high-density lipoprotein are affected by specific antibodies in systemic lupus erythematosus. Rheumatology 2009, 48, 26–31. [Google Scholar] [CrossRef]
- Mathew, A.V.; Yu, J.; Guo, Y.; Byun, J.; Chen, Y.E.; Wang, L.; Liu, M.; Bard, R.L.; Morishita, M.; Huang, W.; et al. Effect of Ambient Fine Particulate Matter Air Pollution and Colder Outdoor Temperatures on High-Density Lipoprotein Function. Am. J. Cardiol. 2018, 122, 565–570. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Xu, H.; Brook, R.D.; Liu, S.; Yi, T.; Wang, Y.; Feng, B.; Zhao, M.; Wang, X.; et al. Ambient Air Pollution Is Associated With HDL (High-Density Lipoprotein) Dysfunction in Healthy Adults. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 513–522. [Google Scholar] [CrossRef]
- Ossoli, A.; Favero, C.; Vigna, L.; Pesatori, A.C.; Bollati, V.; Gomaraschi, M. Body Mass Index Modulates the Impact of Short-Term Exposure to Air Particulate Matter on High-Density Lipoprotein Function. Antioxidants 2022, 11, 1938. [Google Scholar] [CrossRef]
- Yin, F.; Ramanathan, G.; Zhang, M.; Araujo, J.A. Prooxidative effects of ambient pollutant chemicals are inhibited by HDL. J. Biochem. Mol. Toxicol. 2013, 27, 172–183. [Google Scholar] [CrossRef]
- Mills, N.L.; Törnqvist, H.; Robinson, S.D.; Gonzalez, M.; Darnley, K.; MacNee, W.; Boon, N.A.; Donaldson, K.; Blomberg, A.; Sandstrom, T.; et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 2005, 112, 3930–3936. [Google Scholar] [CrossRef] [PubMed]
- Maiseyeu, A.; Yang, H.Y.; Ramanathan, G.; Yin, F.; Bard, R.L.; Morishita, M.; Dvonch, J.T.; Wang, L.; Spino, C.; Mukherjee, B.; et al. No effect of acute exposure to coarse particulate matter air pollution in a rural location on high-density lipoprotein function. Inhal. Toxicol. 2014, 26, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, G.; Yin, F.; Speck, M.; Tseng, C.H.; Brook, J.R.; Silverman, F.; Urch, B.; Brook, R.D.; Araujo, J.A. Effects of urban fine particulate matter and ozone on HDL functionality. Part. Fibre Toxicol. 2016, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Navab, M.; Pakbin, P.; Ning, Z.; Navab, K.; Hough, G.; Morgan, T.E.; Finch, C.E.; Araujo, J.A.; Fogelman, A.M.; et al. Ambient ultrafine particles alter lipid metabolism and HDL anti-oxidant capacity in LDLR-null mice. J. Lipid Res. 2013, 54, 1608–1615. [Google Scholar] [CrossRef]
- Alex, A.M.; Kunkel, G.; Sayles, H.; Flautero Arcos, J.D.; Mikuls, T.R.; Kerr, G.S. Exposure to ambient air pollution and autoantibody status in rheumatoid arthritis. Clin. Rheumatol. 2020, 39, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.N.; Xu, Z.; Wu, G.C.; Mao, Y.M.; Liu, L.N.; Wu, Q.; Dan, Y.L.; Tao, S.S.; Zhang, Q.; Sam, N.B.; et al. Emerging role of air pollution in autoimmune diseases. Autoimmun. Rev. 2019, 18, 607–614. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossoli, A.; Cetti, F.; Gomaraschi, M. Air Pollution: Another Threat to HDL Function. Int. J. Mol. Sci. 2023, 24, 317. https://doi.org/10.3390/ijms24010317
Ossoli A, Cetti F, Gomaraschi M. Air Pollution: Another Threat to HDL Function. International Journal of Molecular Sciences. 2023; 24(1):317. https://doi.org/10.3390/ijms24010317
Chicago/Turabian StyleOssoli, Alice, Federica Cetti, and Monica Gomaraschi. 2023. "Air Pollution: Another Threat to HDL Function" International Journal of Molecular Sciences 24, no. 1: 317. https://doi.org/10.3390/ijms24010317
APA StyleOssoli, A., Cetti, F., & Gomaraschi, M. (2023). Air Pollution: Another Threat to HDL Function. International Journal of Molecular Sciences, 24(1), 317. https://doi.org/10.3390/ijms24010317





