Abstract
The MET gene, known as MET proto-oncogene receptor tyrosine kinase, was first identified to induce tumor cell migration, invasion, and proliferation/survival through canonical RAS-CDC42-PAK-Rho kinase, RAS-MAPK, PI3K-AKT-mTOR, and β-catenin signaling pathways, and its driver mutations, such as MET gene amplification (METamp) and the exon 14 skipping alterations (METex14), activate cell transformation, cancer progression, and worse patient prognosis, principally in lung cancer through the overactivation of their own oncogenic and MET parallel signaling pathways. Because of this, MET driver alterations have become of interest in lung adenocarcinomas since the FDA approval of target therapies for METamp and METex14 in 2020. However, after using MET target therapies, tumor cells develop adaptative changes, favoring tumor resistance to drugs, the main current challenge to precision medicine. Here, we review a link between the resistance mechanism and MET signaling pathways, which is not only limited to MET. The resistance impacts MET parallel tyrosine kinase receptors and signals shared hubs. Therefore, this information could be relevant in the patient’s mutational profile evaluation before the first target therapy prescription and follow-up to reduce the risk of drug resistance. However, to develop a resistance mechanism to a MET inhibitor, patients must have access to the drugs. For instance, none of the FDA approved MET inhibitors are registered as such in Chile and other developing countries. Constant cross-feeding between basic and clinical research will thus be required to meet future challenges imposed by the acquired resistance to targeted therapies.
1. Introduction
The principal hallmark of tumorigenesis is cell transformation, involving the transition of normal cells into the tumorigenic state, followed by an enhanced cell proliferation, and anchorage-independent growth, which result in cell migration, invasion, and finally metastasis. However, depending on the signaling pathways altered by the driver mutations, the tumor mass would be highly proliferative, invasive, angiogenic, and or metastatic [1]. Assuming that the driver mutations in genes such as EGFR, ALK, ROS1, HER2, and MET (among others) had demonstrated advantages favoring cell transformation, leading to the expansion of the altered clone, which could be followed by tumor formation and its evolution.
The MET gene encodes a member of the receptor tyrosine kinase (RTK) family of proteins, and, since the early 1980s, different authors have studied the effect of MET on cancer development, starting by Cooper et al., who were the pioneers in recognizing MET gene as a “driver gene” when this concept did not exist yet, describing it as a transforming gene detected in chemically transformed cells [2]. Afterwards, Tward et al. found that the METamp would be able to induce cell transformation and hepatocellular carcinoma (HCC) in mice overexpressing a wild-type allele of human MET, although the carcinoma only arose in cooperation with the constitutively active β-catenin expression [3]. Perhaps this was possible through the crosstalk signaling Met/β-catenin since the inactivation of MET transgenes induced the regression of HCC [4]. In the same way, Mi et al. speculated whether MET could have initiated tumorigenesis in mice prostates, so they tested this idea designing a conditional Met transgenic mouse that mimicked human prostate cancer through an increased Met expression, which resulted in the oncogenic prostate transformation. Nevertheless, the presence of METamp and PTEN deletion leads to prostate neoplasia and prostatic adenocarcinomas, inducing an epithelial-mesenchymal transition and an increase of metastasis events [5]. As a result, METamp caused cell transformation; however, this had to take place in cooperation with another alteration in Met signaling pathways or MET parallel signaling co-activation, which could be needed to support the MET activities. All of this was the initial knowledge about MET in cancer; nevertheless, the development of target therapies against MET was not initiated until the discovery of METex14, which is by far the most common MET mutation. In this review, we will discuss the current therapeutic opportunities for MET driver alterations and the current understanding of MET resistance mechanisms.
2. MET in Cancer Initiation and Driver Mutations
2.1. MET Amplification
In 1996, Ichimura et al. revealed that MET protein and its specific ligand, hepatocyte growth factor (HGF), were highly expressed in lung cancer cell lines, as well as in non-small cell lung cancer (NSCLC) biopsies [6,7]. However, the protein levels did not reveal whether these results were associated with a specific MET mutation or with the gene copy number gain (GCN) [8], which could arise from polysomy or amplification. Still, the amplification represents a biologic selection process for MET as an oncogenic driver. As METamp has been recognized as a bad prognosis biomarker in NSCLC, HCC, gastric cancer, and triple-negative breast cancer [9,10,11,12], its identification in precision medicine through the GCN is currently calculated using the copy number variation (CNV) from next-generation sequencing (NGS) or using the standard assay fluorescence in situ hybridization (FISH) [13].
The current METamp frequency in different solid cancers could be estimated thanks to the AACR Project Genomics Evidence Neoplasia Information Exchange (GENIE), which is an international pan-cancer registry [14]. According to GENIE, METamp represents a 2% in non-small cell lung cancer (NSCLC 10,451 patients), a 1.2% in renal (1556 patients), and hepatobiliary (1854 patients) cancers, followed by a 0.4% in colorectal (7370 patients), 0.2% in ovarian (4481 patients), breast cancer (8365 patients), and prostate cancers (3530 patients). NSCLC has the highest METamp and driver mutations frequency (Table 1), as the METex14 is the most common MET driver and actionable alterations [15,16], where, according to cancer genomics, a driver mutation starts the tumor development, and an actionable mutation is a driver alteration that has assigned a target therapy. Now, as much as METamp and METex14 are recognized as actionable because they account for different target therapies replacing the conventional chemotherapies; despite the success in METamp, the therapy response will depend on the GCN degree, as we will later review.

Table 1.
METex14 and METamp frequency in NSCLC, renal, hepatobiliary, colorectal, ovarian, breast, and prostate cancers in the GENIE cohort [13]. Germinal mutations, uncertain significance variants, and patients missing MET analysis were filtered out using available tools at https://genie.cbioportal.org/ (last time accessed on 10 November 2022).
2.2. MET Exon 14 Skipping Alterations
The METex14 was first reported in small cell lung cancer and then in NSCLC patients. Furthermore, when METex14 was expressed in normal mouse NIH3T3 fibroblasts, cells were transformed and then became tumorigenic in vivo, which confirmed METex14 as a driver alteration [17], whereas Paik and colleagues were demonstrating METex14 tumor cells were sensitive to MET tyrosine kinase inhibitors (TKIs), and the clinical benefit for NSCLC patients was demonstrated [18].
Normally, in MET pre-mRNA, the introns flanking the exon 14 are spliced out, resulting in an mRNA containing exon 14, which encodes the juxtamembrane domain (JMD), which is key for MET protein degradation. The METex14 causes the loss of JMD when mutations at the splice donor or acceptor sites result in exon 14 loss, such as base substitutions, insertions, deletions, and intronic noncoding regions immediately adjacent to the splice acceptor site and the whole-exon deletion. When some of these alternatives derived in a truncated MET receptor, it shows a constitutive expression because the loss of the Tyr1003 residue located in the JMD prevents the binding of the E3 ubiquitin ligase Cbl and proteasomal degradation, which have shown overactive MET signaling pathways, triggering an exacerbate cell proliferation and invasion, contributing to the evolution of cancer and bad prognosis [19,20].
METex14 occurs in 3 to 4% of NSCLC patients (Table 1), and it has been identified as a potent therapeutic target encouraging the approval of TKIs [21,22]. However, the actionability of different variants which originate the METex14 alteration have not been totally validated yet. Currently, several MET mutations are recognized as actionable METex14 by the approved test Foundation One (such as splice site 2888-10_2911del34, splice site 2888-37_2888-30delCGTCTTA, splice site 2888-18_2888-5del14, D1010N, splice site 3028+2T>C, splice site 2999_3028+4del34, splice site 3028+1G>A, and splice site 3028_3028+2delGGT), which were searched in the GENIE public database, and, as a result, only D1010N was found, representing 9.6% of 439 MET driver mutations (Figure 1 and Table S1). In addition, other frequent mutations recognized as a driver by GENIE impacting the exon 14 of MET were found, as the X1010_splice (29%), followed by the X963_splice (20.9%) and D1010H (9.7%) (Figure 1).
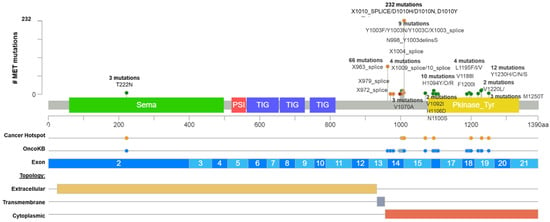
Figure 1.
Common MET driver mutations impact principally the juxtamembrane and kinase domains (exon 14). Lolliplot of MET protein domains (Sema, PSI, TIG, juxtamembrane, and Pkinase) showing common driver mutations identified in solid tumors from Table 1. Additionally, the protein structure shows the cancer hotspot (yellow circle), OncoKB prediction therapies (blue circles), exons numbered (blue and light blue rectangles), and protein topology. Yellow is the extracellular region, red, the cytoplasmic region, and, gray, the transmembrane region.
After that, we interrogate all these variants according to the bioinformatic driver predictors, and OncoKB as the actionability predictor, which is a precision oncology knowledge base developed at Memorial Sloan Kettering Cancer Center that contains biologic and oncogenic effects, prognostic, and predictive significance of somatic alterations [23]. As is shown in Table S1, sixteen alterations in exon 14 (affecting 374 patients) were recognized by different predictors, including OncoKB as oncogenic/likely oncogenic mutations with level 1 of evidence, which means these mutations concur with an FDA-approved drug. According to these results, many driver and actionable MET mutations had not been functionally or clinically validated yet (Table S1). Additionally, there is an extensive list of mutations in the MET hot spot between exons 14 and 19 with drugs predictions, which must be evaluated as actionable alterations (Figure 1 and Table S1).
Still, the validation and approval of additional MET driver alterations should be the next step to offer many potentially METex14 targetable mutations [24,25]. Indeed, a study identified five hundred genetic alterations that lead to METex14, and the analysis revealed that the most frequent regions impacted were the splice donor site (42%), followed by the polypyrimidine tract (15%), the splice acceptor site (~5%), and both the splice acceptor site and the polypyrimidine tract (13%). All these alterations resulted in the elimination of exon 14 with an mRNA containing the exon 13 fused to exon 15 [26].
Thereby, given the diversity of alterations leading to METex14 revealed in mRNA, the diagnosis sensitivity could challenge the identification of them in DNA assays. In contrast, RNA approaches directly identify 13–15 exons fusion in the transcript. For this reason, the amplicon-based approaches may fail to find METex14 alterations because it does not allow the detection of large deletions. However, hybrid capture is more amenable to detecting the alterations leading to METex14. Furthermore, this method generally isolates larger fragments of DNA, including sequences that flank the regions of interest, compared with amplicon-based methods when using DNA as the input material [27]. Additionally, 60% of positive results according to the RNA-based assay were negative using the DNA-based assay [28]. Likewise, the mRNA-based quantitative reverse transcriptase RT-PCR demonstrated 100% sensitivity in detecting METex14, compared to 61.5% sensitivity using conventional DNA-based Sanger sequencing [29], so RNA analysis seems to be best way to identify METex14 to follow with target drugs’ prescriptions.
3. Actionable MET Mutations and MET-Target Therapies
The validation of new driver alterations and their actionability helps improve target drug availability, which contributes to the development of precision medicine [30]. As it is conceivable that some cancers are primarily driven by the MET mutations [31], to date, METamp and METex14, have shown their actionability in NSCLC, such as prognosis, and expanding the therapeutic opportunities for patients without EGFR and ALK as usual alterations [32].
The NSCLC subtype adenocarcinoma has been the most advanced cancer subtype in the implementation of targeted therapies, and at the current date, two categories of MET tyrosine kinase inhibitors (TKI) have been validated (Table 2), such as type I, which included crizotinib, capmatinib, tepotinib, and savolitinib that binds to an active MET conformation, and type II (cabozantinib, merestinib, and glesatinib) that binds to an inactive MET [33]. The TKI crizotinib was the first target drug FDA-approved with multi-kinase activity against MET, ALK, and ROS1 [34,35,36]; however, the best patient response to crizotinib was in ALK-positive NSCLC patients compared to MET and ROS1 positive patients [37].

Table 2.
Classification of current MET target therapies, and clinical trial results, approved by FDA.
3.1. Type I MET-Targeting Drugs Directed to Actionable Mutations
3.1.1. Crizotinib
Crizotinib was validated in 2010 for METamp in NSCLC patients [38], and in 2016, its efficacy and safety on METex14 advance NSCLC (aNSCLC) patients were confirmed [39]. However, concerning METamp, not all GCN levels experience the same therapeutic results, as was demonstrated in the clinical trial PROFILE-1001 in 2021 (NCT00585195), where low levels (≥1.8–≤2.2 copies), medium (>2.2–<4 copies), and high (>5 copies) experienced a progression-free survival (PFS) of 1.8, 1.9, and 6.7 months, respectively [40]. Drilon et al. [41] reported the safety and response rate of 69 patients with METex14 alteration from an expansion cohort of NCT00585195, reporting an objective response rate of 32%, mPFS of 7.3 months, and OS of 20.5 months (95% CI, 14.3–21.8).
3.1.2. Tepotinib
Tepotinib is a kinase inhibitor that targets MET alterations, including METex14. It was FDA approved in 2021 for patients with metastatic NSCLC (mNSCLC). Tepotinib inhibits the hepatocyte growth factor of dependent and independent MET phosphorylation and MET-dependent downstream signaling pathways [42]. In the VISION trial, 152 METex14 patients were treated with Tepotinib (NCT028649920, Table 2), which was associated with an objective response rate of 56%. The median duration of response and mPFS was 11.1 and 8.5 months, respectively. In patients with central nervous metastasis, the observed response rate was 55%, with mPFS of 10.9 months.
3.1.3. Capmatinib
Capmatinib is a selective MET TKIs ATP-competitive MET inhibitor (type Ib) and was screened against >400 kinases, showing high selectivity for MET. In 2020, capmatinib was FDA approved for patients with mNSCLC harboring METex14 showing a significant antitumor activity, particularly in those not previously treated, and this drug has been approved in several countries including UE, the EU, US, Switzerland, and Japan. The overall response rate observed with capmatinib in patients with METex14 skipping mutation was 41% in 69 patients who had received previous treatment and an ORR of 68% in patients naïve to treatment. In terms of time of response, 82% of patients who had been previously treated with other lines of therapy had tumor response at the first CT scan of evaluation. Capmatinib showed high response rates in patients with intracranial disease with 33% of patients achieving complete response. In addition, the response to METamp was higher in tumors with a high GCN than in those with a lower GCN (Table 2). Capmatinib also has been documented in METamp in combination with gefitinib (NCT01610336 and NCT01324479) in NSCLC with METex14 and MET GCN > 6 (high) [43]. Lower efficacy was observed among NSCLC patients with METex14 skipping mutations who previously had received one or two lines of therapies, with an overall response in 41% of the patients but showed limited activity in patients who had METamp with a gene copy number of less than 10 (Table 2).
3.1.4. Savolitinib
Savolitinib is a potent and highly selective oral MET TKI that provides clinical benefits for patients with mNSCLC papillary and clear cell renal cell carcinoma (RCC), gastric cancer, and colorectal cancer [44]. In a phase 2 trial that included patients with pulmonary sarcomatoid carcinoma, a rare and poorly differentiated type of NSCLC and NSCLC adenocarcinoma treated with savolitinib showed a similar objective response rate of 42%. Most of the patients were male and the median age of the 70 patients was 68.7 years. The median time to response was 1.4 months with a high disease control rate (82%). The pulmonary sarcomatoid carcinoma median duration of response was 17.9 months, suggesting that the treatment response to savolitinib was acceptable, durable, and rapid in patients with pulmonary sarcomatoid carcinoma and other NSCLC subtypes positive for METex14 [45]. This drug had been approved China’s National Medical Products Administration (NMPA) (conditional on the results of a phase III trial) to treat mNSCLC with METex14 in patients who have progressed after or who are unable to tolerate platinum-based chemotherapy. Currently, the FDA has not approved savolitinib against METex14 yet. However, it was FDA approved mixing savolitinib with osimertinib therapy because it helps patients with the EGFR-mutant that had developed resistance to prior EGFR-targeted therapies through MET-gene amplification [46].
In relationship with the immunotherapy, MET alterations have gained relevance since NSCLC patients with low MET GCN (METamp GCN ≤ 10) have been treated with immune checkpoint inhibitors (ICI), which showed a median survival of 19 versus 8 months with chemotherapy. Nevertheless, in patients with high amplification levels (GCN ≥10), the median survival was 36 months with ICI compared to 4 months with chemotherapy. However, the patients with METex14 did not present significant differences, and there was a trend toward improved survival with ICI therapy compared to chemotherapy (16.0 months versus 10.0 months) [21].
3.2. MET Alterations and Treatment in the Context of Mestastasic NSCLC
In general, the treatment for mNSCLC, which occurs in one-third of METex14-altered lung cancers at diagnosis [47], currently is based on systemic therapy using cytotoxic and/or molecularly targeted agents and palliative radiotherapy for symptomatic metastases with poor prognosis. Patients with METex14–positive NSCLC and brain metastasis have been through a long journey to find the target drug with good central nervous system activity [48,49]. Since 2020, the FDA has approved three target drugs for mNSCLC patients with METex14 alteration, such as capmatinib, which showed an intracranial response in 7 of 13 patients (54%) [43]. The intracranial responses were also observed in a phase II trial of savolitinib and the VISION phase II study of tepotinib that had 11 patients with brain metastases and a response rate of 55%, with a median duration of response of 9.5 months, and median duration of progression-free survival of 10.9 months [42]. Furthermore, on 10 August 2022, the FDA granted regular approval to capmatinib for adult patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have a mutation leading to METex14.
The METamp shows a vast heterogeneity in GCN; however, the best therapy prognosis has been seen in the high levels (GCN > 10) as depicted in Table 2, where the therapy success decreases proportionally to GCN decrease. Therefore, if the METamp is increased without any known actionable alteration that drives the tumor, the evaluation of immunotherapy additional to MET TKIs is recommended. As such, the MET inhibitors plus immune checkpoint inhibitors (ICIs) strategy has been improving pancreatic cancer immunotherapeutic efficacy [50], evidencing potential crosstalk between c-MET inhibition and immune escape since PD-L1 expression positively correlates with METamp [51]. Furthermore, a clinical trial in progress is studying the safety and efficacy of capmatinib plus pembrolizumab vs. pembrolizumab alone in NSCLC with PD-L1 ≥ 50% (NCT04139317), which might be the confirmation of a new improvement to the only MET-TKI therapies in patients with MET alterations.
Additionally, an mNSCLC case report treated with chemotherapy switched to pembrolizumab (PD-L1 70%) with tepotinib that enabled stable disease following this therapeutic decision for 31 months [52]. Accordingly, the MET alterations and high PD-L1 expression must be considered recurrent with actionable co-alterations in NSCLC with the option to combine MET-TKIs with ICIs, which could also be the best strategy in case of resistance to other target therapies. Nonetheless, the evidence to support clinical recommendations, such as the best sequentially of MET inhibitors and immunotherapy, is still limited. In addition, considering the advance stage patient setting, therapy options are limited by drug toxicities, patient comorbidities, and adverse reactions [42].
4. Resistance Mutations as a Bypass of MET-Target Therapies
Even though targeted therapies have increased the life expectancy of patients with druggable driver alterations [53], their efficacy could be limited when tumor cells acquiree therapy resistance mutations, which, depending on the resistance mechanism used, could impact the same molecular target (On-target), MET parallel tyrosine kinase signaling, or its own signaling pathways with the aim of treatment breakdown [54,55], so, the knowledge about the driver’s signaling pathways alterations will be relevant to predicting the possible drug bypasses at diagnosis [56].
Although the study of signaling pathways has been confined to basic science, it gives insight into relevant information for precision medicine when the driver alterations are spatially localized in their signaling pathways to predict future resistance mechanisms according to a personalized somatic mutational profile analysis, which does not only consider the diagnosis of actionable mutations with the highest mutation frequency, because intratumor low-frequency alterations should also be considered as a possible future resistance mutation. The manual curation of somatic mutations after the NGS bioinformatic analysis is rich in information to envisage possible sensitivity and resistance alterations when considering known variants at low frequency could then be stimulated by the target drugs based on the sub clonal evolution, where the treatments may serve as a selective pressure to increase the frequency of these possible resistance mutations [57].
4.1. Acquisition of On-Target Mutations Resistance to MET TKIs
The scene of resistance mechanisms to MET TKIs has not been well characterized, and its frequency impact is unknown. However, acquiring resistance mutations could have on-target preference after MET-TKIs fail (Figure 2A). One form to identify resistance mutations is through patients’ analysis before and after MET-TKIs therapies.
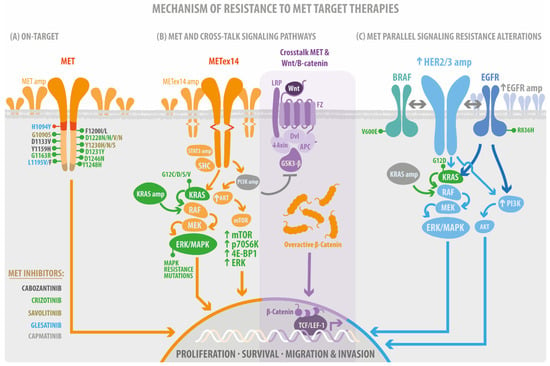
Figure 2.
Mechanism of resistance to MET target therapies. (A) the on-target resistance mutations. These mutations impacted the Juxtamembrane domain (RED) and the Tyrosine kinase domain (orange). The multi-docking site (brown) without evidence of resistance mutations. (B) Canonical MET signaling pathways acquire resistance mutations such as STAT, PI3K, and KRAS amplification (amp), AKT, mTOR signaling overexpression (arrow up), KRAS mutations, and crosstalk’s mediate PI3Kamp and GSK3b inhibition, Axin downregulation (down arrow) followed by overactivation of b-catenin signaling. (C) The acquired resistance mutations impact the MET parallel receptor tyrosine kinase signaling after using MET-TKI. Therefore, the colors of the target drugs (MET inhibitors) are related to the color of mutations.
A study considering 20 plasma and tissues analyzed by NGS from METex14 NSCLC patients demonstrated known and suspected resistance mechanisms in 15 patients. The on-target acquired resistance mechanisms, including MET kinase domain mutations in codons H1094, G1163, L1195, D1228, and Y1230 in seven patients (35%), showed high MET exon 14-mutant allele amplification [33]. Another group of patients with METex14 mutations demonstrated specific resistance mechanisms to crizotinib and type II MET TKI glesatinib. In four cases (33%), acquired MET alterations were identified, including one patient with amplification of the mutated METex14 allele and three cases with MET tyrosine kinase domain secondary site mutations; in two of these cases, more than one MET resistance mutation was present in the same patient. Secondary mutations in MET included H1094Y, G1163R, L1195F, L1195V, D1228N, Y1230H, and Y1230S [58]. In addition, it has been reported that MET D1228 and Y1230 are the hotspots for the secondary resistant mutation for type I MET TKIs in NSCLC carrying METex14 using in vitro models (Figure 2A) [59,60]. After the use of the TKI gefitinib, 12 patients with advanced NSCLC were analyzed using capture-based targeted ultra-deep sequencing, revealing MET Y1248H and D1246N as acquired mutations in two patients, further confirming their resistance against type I MET-TKIs in silico, in vitro, and in vivo, which could be solved using TKIs type II, according to in vitro experiments’ results [61].
4.2. MET Parallel Signaling Resistance Alteration
MET activation is an effective resistance mechanism to targeted therapies against several RTKs, including EGFR, HER2, VEGFR, and against signaling hubs such as BRAF [62]. An example is METamp, which has been shown to be one of the most relevant mechanisms responsible for the acquired resistance against EGFR TKIs for both first and second-generation TKIs. It has also been observed as the most common resistance mechanism affecting 15–20% of ctDNAs in patients with NSCLC (NCT02296125).
The concomitant use of MET inhibitors, such as crizotinib, in combination with osimertinib, can improve the sensitivity of patients with osimertinib-resistant EGFR mutations and with amplified MET, which were detected using ctDNA [63]. Currently, the combination of nazartinib with capmatinib is under study (NCT02335944), and tepotinib has been tested in combination with gefitinib in participants with T790M negative METamp [64] positive in locally advanced or mNSCLC with EGFR mutations, and the results do not show significant improvement in OS compared to standard chemotherapy; one reason for this response is that this combination may have better response rates in high MET overexpression (IHC3+) or METamp (GCN ≥ 5).
METamp due to gefitinib resistance drives the drug bypass through ERBB3 dependent activation of PI3K [65], and METamp cell lines treated with capmatinib have suggested that the activation of EGFR-PIK3CA signaling may mediate the resistance without any EGFR activating mutation or amplification (Figure 2C). Therefore, it may indicate a preferable PI3K signaling pathway activation, independent of MET or EGFR acquiring resistance or primary alteration [66]. Perhaps, the combination of capmatinib with gefitinib is a promising treatment for patients with EGFR-mutated and METamp in NSCLC [67]. Acquired amplification of wild-type EGFR, KRAS, HER3, and BRAF has been reported and was detected in 45% of MET exon 14-mutant NSCLC patients previously treated with crizotinib [33].
4.3. Resistance Mutation on MET Signaling Pathways
As we noticed in the beginning of this review, the MET gene is a critical driver. In addition, the signaling pathways affected by MET alterations are essential to predicting the tumor phenotype, as it is a proliferative or invasive disease. The knowledge of the signaling pathways impacted by the driver mutations is becoming more relevant following the initial studies of the resistance mechanism to target therapies. Still, the design of specific target therapies directed to driver mutation related to specific altered signaling pathways has not been accomplished in oncology. Until now, the MET signaling pathways are affected by resistance mutations after Met-TKIs, and, in some cases, co-alterations in the same signaling pathways have been reported (Figure 2B). So far, the solution has been to test the combination of target therapies.
4.3.1. MET/RAS/MAPK, the Proliferative and Survival Signaling
MET activates RAS through the recruitment of effectors [68], which subsequently recruit Sos that convert inactive RAS into an active conformation (RasGDP RasGTP) [69]. RasGTP successively activates Raf, Mek, MAPKs, ERK, JNK (Jun N-terminal kinase), and p38 (HOG). When MAPKs signaling enters the nucleus, it activates transcription factors such as Elk1, Etsl, [70], and c-Myc [71], promoting cell proliferation and cell cycle progression [72]. MAPK can also promote tumor cell invasion, which is early coordinated by HGF-SC/MET [73,74], as depicted in Figure 2B.
Prominent co-occurring RAS–MAPK pathway gene alterations were detected in aNSCLCs patients with METex14 alterations compared with EGFR, demonstrating an association between decreased MET treatment response and the RAS-MAPK pathway co-occurring alteration [75]. Enrichment analysis of transcriptomic data from patients with METex14 revealed the preferential activation of the KRAS (G12S) pathway in lung cancers as a crizotinib-resistant response [76].
KRAS activation also mediates resistance to targeted therapy in METex14 NSCLC and tumor cell persistence through MAPK signaling overactivation [77]. This similarly happens with the selective Met inhibitor tepotinib, even with its prolonged on-target activity [42]. In addition, Rotow et al. demonstrated a resistance overcome when combining crizotinib and the MEK inhibitor trametinib polytherapy in METex14-mutated preclinical models. However, the early evidence of molecular response to combined crizotinib and trametinib therapies in a patient with acquired KRAS amplification was poorly tolerated in the context of overall clinical decline, with fatigue, fluid retention, and diarrhea [75,76].
4.3.2. MET/PI3K/AKT/mTOR Cellular Motility and Invasion Signaling
This signaling pathway is activated by the HGF/SF receptor and includes the generation of D-3-phosphorylated inositol phospholipids (Figure 2B), which regulate cytoskeletal functions, membrane trafficking, and receptor signaling by recruiting protein complexes to cell- and endosomal-membrane, as demonstrated early in 1991 [78]. PI3K pathway alterations are also common in METex14 NSCLC and confer primary resistance to MET TKIs. Accordingly, by combining a MET TKI with a PI3K inhibitor, the sensitivity to MET TKIs is restored in preclinical models [79]. MET has also been described as a potential therapeutic target for multiple myeloma (MM), where Akt/mTOR is a crucial signaling component through which Met protects multiple myeloma cells from chemotherapy-induced growth inhibition and apoptosis. c-Met/Akt/mTOR pathway is a potential therapeutic target to overcome the chemoresistance of MM [80]. In fact, c-Met depletion significantly enhanced the sensitivity of U266 cells to bortezomib in vitro, indicating that c-Met promotes chemoresistance in MM. Additionally, the knock-down of Met enhanced the cytotoxicity and caspase-mediated apoptosis in human MM U266 cells after bortezomib treatment. These findings raise the possibility that novel combined pharmacologic inhibitors of the c-Met⁄Akt⁄mTOR pathway could enhance the effectiveness of bortezomib in the treatment of myeloma [80].
A study investigated the therapeutic potential of cabozantinib using a large panel of HCC cell lines, and distinct oncogene-driven HCC in mice, where cabozantinib inhibited c-MET and its MAPK signaling activity; however, it was ineffective in inhibiting the MET/Akt/mTOR cascade, but mixing cabozantinib and pan-mTOR inhibitors, has shown synergistic properties in inhibiting HCC growth in vitro and in driving tumor regression in mice [81].
4.3.3. MET/Src/Fak Mechanosensing Signaling
Focal adhesion kinase (FAK) regulates the reorganization of the actin cytoskeleton, cell polarization, cell migration, and adhesion, spreading through MET in medulloblastoma [82]. The combined targeting against MET and FAK could be advantageous for medulloblastoma therapy [82], and the interaction of FAK with MET was required for HGF-induced cell invasion [83], whereas Src-dependent phosphorylation of MET requires cell-matrix adhesion, as well as actin stress fiber assembly. Phosphorylation of FAK by Src is also required for Src-induced MET phosphorylation. Thus, a novel role of the Src/FAK interaction network in the positive regulation of MET activation occurs in an HGF-independent manner in breast epithelial cells and neuronal cells [84].
The inhibition of MET alone has been demonstrated to have limited efficacy in colon cancer; although the Src inhibitor, dasatinib, reduced MET autophosphorylation and decreased MET phosphorylation by stimulating factors, the inhibition is not complete. In addition, the simultaneous inhibition of MET and Src demonstrated that the combination treatment reduced cell viability and increased the apoptosis rate in mutant and wild-type Ras colon cancer cells [85].
In recent years, it has been reported that activation of the MET/FAK signaling axis leads to CDK4/6-independent CDK2 activation, and the blockage of the three targets reduces cell proliferation and enhances tumor growth inhibition in vivo [57]. In addition, verticillin, targeting MET/FAK/Src, inhibited the migration ability through HGF/MET deactivation in gastric and cervical cancer cells [86].
A series of studies confirmed the importance of FAK/Src signaling in resistance to first-(erlotinib), second- (afatinib), and third-generation (osimertinib) EGFR TKIs; thus, with the current diagnosis and mono target therapies against MET, this signaling could be implicated in MET-TKI resistance with an emphasis in metastatic tumors because it activates the signaling pathways such as MET/FAK involved in polymerization and contraction of the actin cytoskeleton to cell migration, followed by intra-extravasation through invasion of basal membrane and metastasis.
Crosstalk among signaling in HCC, such as the MET/β-Catenin axis, resulting in FAK is required for MET/β-Catenin-driven hepatocarcinogenesis. Additionally, in this study, the authors demonstrated that FAK deficiency in hepatocytes largely blocks HCC development induced by MET/β-catenin [34,35].
4.3.4. MET/Wnt/β-Catenin
The crosstalk between HGF/Met and Wnt/β-catenin is strongly implicated in hepatocarcinogenesis [83,87]. It has also been observed as active in glioblastoma, the most lethal and common type of primary brain tumor. The axis MET/Wnt/β-catenin is critical to maintaining cancer stem cells, which possess high levels of MET and Wnt/β-catenin to drive tumor propagation [88]. In the same line, in HCC, targeting Met with tepotinib alone had a minor effect on Met-β-catenin-HCC development, although tepotinib improved overall survival by 1.5–2 weeks. Thus, single therapy with the Met inhibitor will be insufficient for sustained response in Met/β-catenin HCC, requiring assessment of additional combinations [89].
A mechanism of c-Met inhibitor resistance in melanoma was suggested using melanoma in vivo and in vitro models with high levels of phospho Met. After inducing the resistance to MET-TKI (SU11274 and JNJ38877605), cell lines displayed upregulation of phosphorylated Met and overactive β-catenin, suggesting the involvement of the Wnt pathway (Figure 2B). When Met activates Akt, it then inhibits GSK-3 and or directly phosphorylates β-catenin at Ser552, which enhances β-catenin nuclear accumulation [90]. Interestingly, the resistance mechanism was not mediated here by mutations in the Met tyrosine kinase domain (TKD) [91].
Finally, we used all resistance mutations from Figure 2 to investigate co-mutations related to resistance, showing that most patients have only one driver mutation. Still, GENIE samples showed co-mutations in MET and MET with molecules as part of its parallel signaling pathways (Figures S1 and S2). However, information about WNT1 is unavailable because this gene is not part of the GENIE sequencing panel. For this reason, if we consider resistance mutation since the initial diagnosis, it would be relevant to analyze mutations related to MET signaling pathways to predict or avoid all known resistance mutations associated with MET target therapies.
5. Conclusions
A complete understanding of the driver mutations and pathways altered is essential to identify potential therapeutic options and vulnerabilities to the therapies, such as treatment resistance. The relevance of a deep analysis of tumor mutational profile in a study of METamp as a consequence of EGFR TKIs resistance therapies detected clones displaying METamp at a shallow frequency before any treatment with an EGFR TKI, suggesting that these clones were selected under therapeutic pressure [92]. Perhaps, in an initial diagnosis, the prediction of potential resistance mutations will consider analyzing the low-frequency alterations related to driver and/or actionable mutations’ signaling pathways. Nevertheless, to develop treatment resistance, patient access to drugs is a must. Currently, in Chile, none of the drugs approved by the NMPA, FDA and EMA against MET actionable alteration are registered [93], so patients whose tumors harbor actionable variants in the MET gene are facing a significant disparity in the access to target therapies. In Chile, comprehensive tumor profiling is not part of the lung cancer standard of care, or any national health policies. Precision medicine, and target drugs’ implementation is challenging due to the high cost and lack of biomarker testing options. Patients diagnosed with NSCLC should undergo examination for EGFR, ALK, ROS1, and PDL-1 protein expression according to the drugs registered and available in Chile. Therefore, MET mutations are not considered in the evaluation, and there are no registered MET inhibitors in Chile, except for crizotinib, but it is approved only for patients with ALK and ROS1 alterations. If a lung cancer patient in Chile is diagnosed with a MET actionable alteration, off label use of crizotinib or participation in MET specific inhibitor clinical trial are possibilities. Standard of care treatment option would include surgery, chemotherapy, radiotherapy, or palliative care for these patients. As of today, there is not any public indication of any attempt to register MET inhibitors in Chile. Expectations regarding comprehensive tumor profiling and better access to high-cost drugs resurged with the implementation of recently approved legislation (a Chile National Cancer Law).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232213898/s1.
Author Contributions
Conceptualization, S.R. and R.A.; writing—original draft preparation, S.R.; writing—review and editing, S.R., A.M., S.S., E.G.-F. and R.A.; formal analysis and data curation, S.R., E.G.-F. and R.A.; visualization, S.R., and R.A.; supervision, R.A.; funding acquisition, S.R. and R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANID Anillo ACT210079 (R.A.) and FONDECYT post doctorate 3210455 (S.R.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
https://genie.cbioportal.org/ (last time accessed on 10 November 2022).
Acknowledgments
The authors would like to thank Felipe Serrano (contact@illustrative-science.com) for his work in Figure 2.
Conflicts of Interest
R.A. received honoraria for conferences, advisory boards, and educational activities from Roche and Janssen and grants and support for scientific research from Pfizer, Roche & Thermo Fischer Scientific. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.S.; Park, M.; Blair, D.G.; Tainsky, M.A.; Huebner, K.; Croce, C.M.; Vande Woude, G.F. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 1984, 311, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ferrell, L.D.; Faouzi, S.; Maher, J.J.; Bishop, J.M. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J. Cell Biol. 2001, 153, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Tward, A.D.; Jones, K.D.; Yant, S.; Cheung, S.T.; Fan, S.T.; Chen, X.; Kay, M.A.; Wang, R.; Bishop, J.M. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc. Natl. Acad. Sci. USA 2007, 104, 14771–14776. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Hooker, E.; Balog, S.; Zeng, H.; Johnson, D.T.; He, Y.; Yu, E.J.; Wu, H.; Le, V.; Lee, D.H.; et al. Activation of hepatocyte growth factor/MET signaling initiates oncogenic transformation and enhances tumor aggressiveness in the murine prostate. J. Biol. Chem. 2018, 293, 20123–20136. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, E.; Maeshima, A.; Nakajima, T.; Nakamura, T. Expression of c-met/HGF receptor in human non-small cell lung carcinomas in vitro and in vivo and its prognostic significance. Jpn. J. Cancer Res. 1996, 87, 1063–1069. [Google Scholar] [CrossRef]
- Siegfried, J.M.; Weissfeld, L.A.; Luketich, J.D.; Weyant, R.J.; Gubish, C.T.; Landreneau, R.J. The clinical significance of hepatocyte growth factor for non-small cell lung cancer. Ann. Thorac. Surg. 1998, 66, 1915–1918. [Google Scholar] [CrossRef]
- Overbeck, T.R.; Cron, D.A.; Schmitz, K.; Rittmeyer, A.; Korber, W.; Hugo, S.; Schnalke, J.; Lukat, L.; Hugo, T.; Hinterthaner, M.; et al. Top-level MET gene copy number gain defines a subtype of poorly differentiated pulmonary adenocarcinomas with poor prognosis. Transl. Lung Cancer Res. 2020, 9, 603–616. [Google Scholar] [CrossRef]
- Yin, W.; Cheng, J.; Tang, Z.; Toruner, G.; Hu, S.; Guo, M.; Robinson, M.; Medeiros, L.J.; Tang, G. MET Amplification (MET/CEP7 Ratio >/= 1.8) Is an Independent Poor Prognostic Marker in Patients With Treatment-naive Non-Small-cell Lung Cancer. Clin. Lung Cancer 2021, 22, e512–e518. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.S.; Kim, B.J.; Jang, H.J.; Lee, J. Prognostic value of c-Met overexpression in hepatocellular carcinoma: A meta-analysis and review. Oncotarget 2017, 8, 90351–90357. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Dai, C.; Liu, X.; Li, W.; Huang, M.; Zhao, X.; Ji, D.; Li, J.; Guo, W. Amplification and expression of c-MET correlate with poor prognosis of patients with gastric cancer and upregulate the expression of PDL1. Acta Biochim. Biophys. Sin. (Shanghai) 2021, 53, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Zagouri, F.; Bago-Horvath, Z.; Rossler, F.; Brandstetter, A.; Bartsch, R.; Papadimitriou, C.A.; Dimitrakakis, C.; Tsigginou, A.; Papaspyrou, I.; Giannos, A.; et al. High MET expression is an adverse prognostic factor in patients with triple-negative breast cancer. Br. J. Cancer 2013, 108, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Noonan, S.A.; Berry, L.; Lu, X.; Gao, D.; Baron, A.E.; Chesnut, P.; Sheren, J.; Aisner, D.L.; Merrick, D.; Doebele, R.C.; et al. Identifying the Appropriate FISH Criteria for Defining MET Copy Number-Driven Lung Adenocarcinoma through Oncogene Overlap Analysis. J. Thorac. Oncol. 2016, 11, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Consortium, A.P.G. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Frampton, G.M.; Ali, S.M.; Rosenzweig, M.; Chmielecki, J.; Lu, X.; Bauer, T.M.; Akimov, M.; Bufill, J.A.; Lee, C.; Jentz, D.; et al. Activation of MET via Diverse Exon 14 Splicing Alterations Occurs in Multiple Tumor Types and Confers Clinical Sensitivity to MET Inhibitors. Cancer Discov. 2015, 5, 850–859. [Google Scholar] [CrossRef]
- Paik, P.K.; Drilon, A.; Fan, P.D.; Yu, H.; Rekhtman, N.; Ginsberg, M.S.; Borsu, L.; Schultz, N.; Berger, M.F.; Rudin, C.M.; et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov. 2015, 5, 842–849. [Google Scholar] [CrossRef]
- Onozato, R.; Kosaka, T.; Kuwano, H.; Sekido, Y.; Yatabe, Y.; Mitsudomi, T. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancers. J. Thorac. Oncol. 2009, 4, 5–11. [Google Scholar] [CrossRef]
- Kong-Beltran, M.; Seshagiri, S.; Zha, J.; Zhu, W.; Bhawe, K.; Mendoza, N.; Holcomb, T.; Pujara, K.; Stinson, J.; Fu, L.; et al. Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer Res. 2006, 66, 283–289. [Google Scholar] [CrossRef]
- Kron, A.; Scheffler, M.; Heydt, C.; Ruge, L.; Schaepers, C.; Eisert, A.K.; Merkelbach-Bruse, S.; Riedel, R.; Nogova, L.; Fischer, R.N.; et al. Genetic Heterogeneity of MET-Aberrant NSCLC and Its Impact on the Outcome of Immunotherapy. J. Thorac. Oncol. 2021, 16, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Sattler, M.; Salgia, R. MET in the Driver’s Seat: Exon 14 Skipping Mutations as Actionable Targets in Lung Cancer. J. Thorac. Oncol. 2016, 11, 1381–1383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chakravarty, D.; Gao, J.; Phillips, S.M.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis. Oncol. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, J.; Tawfik, O. Detection of MET exon 14 skipping mutations in non-small cell lung cancer: Overview and community perspective. Expert Rev. Anticancer Ther. 2021, 21, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Pennell, N.A.; Davies, K.D. MET Exon 14 Skipping Mutations in Non-Small-Cell Lung Cancer: An Overview of Biology, Clinical Outcomes, and Testing Considerations. JCO Precis. Oncol. 2021, 5. [Google Scholar] [CrossRef]
- Awad, M.M.; Lee, J.K.; Madison, R.; Classon, A.; Kmak, J.; Frampton, G.M.; Alexander, B.M.; Venstrom, J.; Schrock, A.B. Characterization of 1,387 NSCLCs with MET exon 14 (METex14) skipping alterations (SA) and potential acquired resistance (AR) mechanisms. J. Clin. Oncol. 2020, 38, 9511. [Google Scholar] [CrossRef]
- Poirot, B.; Doucet, L.; Benhenda, S.; Champ, J.; Meignin, V.; Lehmann-Che, J. MET Exon 14 Alterations and New Resistance Mutations to Tyrosine Kinase Inhibitors: Risk of Inadequate Detection with Current Amplicon-Based NGS Panels. J. Thorac. Oncol. 2017, 12, 1582–1587. [Google Scholar] [CrossRef]
- Davies, K.D.; Lomboy, A.; Lawrence, C.A.; Yourshaw, M.; Bocsi, G.T.; Camidge, D.R.; Aisner, D.L. DNA-Based versus RNA-Based Detection of MET Exon 14 Skipping Events in Lung Cancer. J. Thorac. Oncol. 2019, 14, 737–741. [Google Scholar] [CrossRef]
- Kim, E.K.; Kim, K.A.; Lee, C.Y.; Kim, S.; Chang, S.; Cho, B.C.; Shim, H.S. Molecular Diagnostic Assays and Clinicopathologic Implications of MET Exon 14 Skipping Mutation in Non-small-cell Lung Cancer. Clin. Lung Cancer 2019, 20, e123–e132. [Google Scholar] [CrossRef]
- Bailey, M.H.; Tokheim, C.; Porta-Pardo, E.; Sengupta, S.; Bertrand, D.; Weerasinghe, A.; Colaprico, A.; Wendl, M.C.; Kim, J.; Reardon, B.; et al. Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell 2018, 173, 371–385 e318. [Google Scholar] [CrossRef]
- Drilon, A.; Cappuzzo, F.; Ou, S.I.; Camidge, D.R. Targeting MET in Lung Cancer: Will Expectations Finally Be MET? J. Thorac. Oncol. 2017, 12, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Le, X. Heterogeneity in MET-Aberrant NSCLC. J. Thorac. Oncol. 2021, 16, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Recondo, G.; Bahcall, M.; Spurr, L.F.; Che, J.; Ricciuti, B.; Leonardi, G.C.; Lo, Y.C.; Li, Y.Y.; Lamberti, G.; Nguyen, T.; et al. Molecular Mechanisms of Acquired Resistance to MET Tyrosine Kinase Inhibitors in Patients with MET Exon 14-Mutant NSCLC. Clin. Cancer Res. 2020, 26, 2615–2625. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.G.; Zou, H.Y.; Arango, M.E.; Li, Q.; Lee, J.H.; McDonnell, S.R.; Yamazaki, S.; Alton, G.R.; Mroczkowski, B.; Los, G. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol. Cancer Ther. 2007, 6, 3314–3322. [Google Scholar] [CrossRef] [PubMed]
- McDermott, U.; Iafrate, A.J.; Gray, N.S.; Shioda, T.; Classon, M.; Maheswaran, S.; Zhou, W.; Choi, H.G.; Smith, S.L.; Dowell, L.; et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 2008, 68, 3389–3395. [Google Scholar] [CrossRef]
- Yasuda, H.; de Figueiredo-Pontes, L.L.; Kobayashi, S.; Costa, D.B. Preclinical rationale for use of the clinically available multitargeted tyrosine kinase inhibitor crizotinib in ROS1-translocated lung cancer. J. Thorac. Oncol. 2012, 7, 1086–1090. [Google Scholar] [CrossRef]
- Solomon, B.J.; Mok, T.; Kim, D.W.; Wu, Y.L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef]
- Rodig, S.J.; Shapiro, G.I. Crizotinib, a small-molecule dual inhibitor of the c-Met and ALK receptor tyrosine kinases. Curr. Opin. Investig. Drugs 2010, 11, 1477–1490. [Google Scholar]
- Drilon, A.E.; Camidge, D.R.; Ou, S.-H.I.; Clark, J.W.; Socinski, M.A.; Weiss, J.; Riely, G.J.; Winter, M.; Wang, S.C.; Monti, K.; et al. Efficacy and safety of crizotinib in patients (pts) with advanced MET exon 14-altered non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2016, 34, 108. [Google Scholar] [CrossRef]
- Camidge, D.R.; Otterson, G.A.; Clark, J.W.; Ignatius Ou, S.H.; Weiss, J.; Ades, S.; Shapiro, G.I.; Socinski, M.A.; Murphy, D.A.; Conte, U.; et al. Crizotinib in Patients With MET-Amplified NSCLC. J. Thorac. Oncol. 2021, 16, 1017–1029. [Google Scholar] [CrossRef]
- Drilon, A.; Clark, J.W.; Weiss, J.; Ou, S.H.I.; Camidge, D.R.; Solomon, B.J.; Otterson, G.A.; Villaruz, L.C.; Riely, G.J.; Heist, R.S. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat. Med. 2020, 26, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Seto, T.; Han, J.Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Gavine, P.R.; Ren, Y.; Han, L.; Lv, J.; Fan, S.; Zhang, W.; Xu, W.; Liu, Y.J.; Zhang, T.; Fu, H.; et al. Volitinib, a potent and highly selective c-Met inhibitor, effectively blocks c-Met signaling and growth in c-MET amplified gastric cancer patient-derived tumor xenograft models. Mol. Oncol. 2015, 9, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Fang, J.; Li, X.; Cao, L.; Zhou, J.; Guo, Q.; Liang, Z.; Cheng, Y.; Jiang, L.; Yang, N.; et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: A multicentre, single-arm, open-label, phase 2 study. Lancet Respir. Med. 2021, 9, 1154–1164. [Google Scholar] [CrossRef]
- Sequist, L.V.; Han, J.Y.; Ahn, M.J.; Cho, B.C.; Yu, H.; Kim, S.W.; Yang, J.C.; Lee, J.S.; Su, W.C.; Kowalski, D.; et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: Interim results from a multicentre, open-label, phase 1b study. Lancet Oncol. 2020, 21, 373–386. [Google Scholar] [CrossRef]
- Tamura, T.; Kurishima, K.; Nakazawa, K.; Kagohashi, K.; Ishikawa, H.; Satoh, H.; Hizawa, N. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol. Clin. Oncol. 2015, 3, 217–221. [Google Scholar] [CrossRef]
- Offin, M.; Luo, J.; Guo, R.; Lyo, J.K.; Falcon, C.; Dienstag, J.; Wilkins, O.; Chang, J.; Rudin, C.M.; Riely, G.; et al. CNS Metastases in Patients With MET Exon 14-Altered Lung Cancers and Outcomes With Crizotinib. JCO Precis. Oncol. 2020, 4, 871–876. [Google Scholar] [CrossRef]
- Klempner, S.J.; Borghei, A.; Hakimian, B.; Ali, S.M.; Ou, S.I. Intracranial Activity of Cabozantinib in MET Exon 14-Positive NSCLC with Brain Metastases. J. Thorac. Oncol. 2017, 12, 152–156. [Google Scholar] [CrossRef]
- Li, E.; Huang, X.; Zhang, G.; Liang, T. Combinational blockade of MET and PD-L1 improves pancreatic cancer immunotherapeutic efficacy. J. Exp. Clin. Cancer Res. 2021, 40, 279. [Google Scholar] [CrossRef]
- Domenech, M.; Munoz Marmol, A.M.; Mate, J.L.; Estival, A.; Moran, T.; Cucurull, M.; Saigi, M.; Hernandez, A.; Sanz, C.; Hernandez-Gallego, A.; et al. Correlation between PD-L1 expression and MET gene amplification in patients with advanced non-small cell lung cancer and no other actionable oncogenic driver. Oncotarget 2021, 12, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.G.; Mambetsariev, I.; Salgia, R. Prolonged survival and response to tepotinib in a non-small-cell lung cancer patient with brain metastases harboring MET exon 14 mutation: A research report. Cold Spring Harb. Mol. Case Stud. 2020, 6, a005785. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Vander Velde, R.; Yoon, N.; Marusyk, V.; Durmaz, A.; Dhawan, A.; Miroshnychenko, D.; Lozano-Peral, D.; Desai, B.; Balynska, O.; Poleszhuk, J.; et al. Resistance to targeted therapies as a multifactorial, gradual adaptation to inhibitor specific selective pressures. Nat. Commun. 2020, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Meador, C.B.; Hata, A.N. Acquired resistance to targeted therapies in NSCLC: Updates and evolving insights. Pharmacol. Ther. 2020, 210, 107522. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.C. Mapping the Pathways of Resistance to Targeted Therapies. Cancer Res. 2015, 75, 4247–4251. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, S.; Swanton, C.; Taylor, B.S.; Costello, J.F. Treatment-Induced Mutagenesis and Selective Pressures Sculpt Cancer Evolution. Cold Spring Harb. Perspect. Med. 2017, 7, a026617. [Google Scholar] [CrossRef]
- Awad, M.M.; Bahcall, M.; Sholl, L.M.; Wilson, F.H.; Paweletz, C.; Capelletti, M.; Leonardi, G.C.; Watanabe, M.; Baba, H.; Chambers, E.S.; et al. Mechanisms of acquired resistance to MET tyrosine kinase inhibitors (TKIs) in MET exon 14 (METex14) mutant non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2018, 36, 9069. [Google Scholar] [CrossRef]
- Fujino, T.; Kobayashi, Y.; Suda, K.; Koga, T.; Nishino, M.; Ohara, S.; Chiba, M.; Shimoji, M.; Tomizawa, K.; Takemoto, T.; et al. Sensitivity and Resistance of MET Exon 14 Mutations in Lung Cancer to Eight MET Tyrosine Kinase Inhibitors In Vitro. J. Thorac. Oncol. 2019, 14, 1753–1765. [Google Scholar] [CrossRef]
- Ou, S.I.; Young, L.; Schrock, A.B.; Johnson, A.; Klempner, S.J.; Zhu, V.W.; Miller, V.A.; Ali, S.M. Emergence of Preexisting MET Y1230C Mutation as a Resistance Mechanism to Crizotinib in NSCLC with MET Exon 14 Skipping. J. Thorac. Oncol. 2017, 12, 137–140. [Google Scholar] [CrossRef]
- Li, A.; Yang, J.J.; Zhang, X.C.; Zhang, Z.; Su, J.; Gou, L.Y.; Bai, Y.; Zhou, Q.; Yang, Z.; Han-Zhang, H.; et al. Acquired MET Y1248H and D1246N Mutations Mediate Resistance to MET Inhibitors in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2017, 23, 4929–4937. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Jamme, P.; Cortot, A.B.; Kherrouche, Z.; Tulasne, D. When the MET receptor kicks in to resist targeted therapies. Oncogene 2021, 40, 4061–4078. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Kiedrowski, L.A.; Ravera, E.; Cheng, H.; Halmos, B. Response to Dual Crizotinib and Osimertinib Treatment in a Lung Cancer Patient with MET Amplification Detected by Liquid Biopsy Who Acquired Secondary Resistance to EGFR Tyrosine Kinase Inhibition. J. Thorac. Oncol. 2018, 13, e169–e172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, S.; Wang, K.; Sun, S.Y. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. J. Hematol. Oncol. 2019, 12, 63. [Google Scholar] [CrossRef]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.; Hyland, C.; Park, J.O.; Lindeman, N.; Gale, C.M.; Zhao, X.; Christensen, J.; et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007, 316, 1039–1043. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.M.; Kim, D.W.; Kim, S.; Kim, M.; Ahn, Y.O.; Keam, B.; Heo, D.S. Acquired Resistance of MET-Amplified Non-small Cell Lung Cancer Cells to the MET Inhibitor Capmatinib. Cancer Res. Treat. 2019, 51, 951–962. [Google Scholar] [CrossRef]
- Wu, Y.L.; Zhang, L.; Kim, D.W.; Liu, X.; Lee, D.H.; Yang, J.C.; Ahn, M.J.; Vansteenkiste, J.F.; Su, W.C.; Felip, E.; et al. Phase Ib/II Study of Capmatinib (INC280) Plus Gefitinib After Failure of Epidermal Growth Factor Receptor (EGFR) Inhibitor Therapy in Patients With EGFR-Mutated, MET Factor-Dysregulated Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 3101–3109. [Google Scholar] [CrossRef]
- Vogel, W.; Ullrich, A. Multiple in vivo phosphorylated tyrosine phosphatase SHP-2 engages binding to Grb2 via tyrosine 584. Cell Growth Differ. 1996, 7, 1589–1597. [Google Scholar]
- Graziani, A.; Gramaglia, D.; dalla Zonca, P.; Comoglio, P.M. Hepatocyte growth factor/scatter factor stimulates the Ras-guanine nucleotide exchanger. J. Biol. Chem. 1993, 268, 9165–9168. [Google Scholar] [CrossRef]
- Paumelle, R.; Tulasne, D.; Kherrouche, Z.; Plaza, S.; Leroy, C.; Reveneau, S.; Vandenbunder, B.; Fafeur, V. Hepatocyte growth factor/scatter factor activates the ETS1 transcription factor by a RAS-RAF-MEK-ERK signaling pathway. Oncogene 2002, 21, 2309–2319. [Google Scholar] [CrossRef]
- Li, Y.; Guessous, F.; Johnson, E.B.; Eberhart, C.G.; Li, X.N.; Shu, Q.; Fan, S.; Lal, B.; Laterra, J.; Schiff, D.; et al. Functional and molecular interactions between the HGF/c-Met pathway and c-Myc in large-cell medulloblastoma. Lab. Invest. 2008, 88, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Fujita-Sato, S.; Galeas, J.; Truitt, M.; Pitt, C.; Urisman, A.; Bandyopadhyay, S.; Ruggero, D.; McCormick, F. Enhanced MET Translation and Signaling Sustains K-Ras-Driven Proliferation under Anchorage-Independent Growth Conditions. Cancer Res. 2015, 75, 2851–2862. [Google Scholar] [CrossRef] [PubMed]
- Scorilas, A.; Karameris, A.; Arnogiannaki, N.; Ardavanis, A.; Bassilopoulos, P.; Trangas, T.; Talieri, M. Overexpression of matrix-metalloproteinase-9 in human breast cancer: A potential favourable indicator in node-negative patients. Br. J. Cancer 2001, 84, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.B.; Nabha, S.M.; Atanaskova, N. Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev. 2003, 22, 395–403. [Google Scholar] [CrossRef]
- Rotow, J.K.; Gui, P.; Wu, W.; Raymond, V.M.; Lanman, R.B.; Kaye, F.J.; Peled, N.; Fece de la Cruz, F.; Nadres, B.; Corcoran, R.B.; et al. Co-occurring Alterations in the RAS-MAPK Pathway Limit Response to MET Inhibitor Treatment in MET Exon 14 Skipping Mutation-Positive Lung Cancer. Clin. Cancer Res. 2020, 26, 439–449. [Google Scholar] [CrossRef]
- Suzawa, K.; Offin, M.; Lu, D.; Kurzatkowski, C.; Vojnic, M.; Smith, R.S.; Sabari, J.K.; Tai, H.; Mattar, M.; Khodos, I.; et al. Activation of KRAS Mediates Resistance to Targeted Therapy in MET Exon 14-mutant Non-small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 1248–1260. [Google Scholar] [CrossRef]
- Bahcall, M.; Awad, M.M.; Sholl, L.M.; Wilson, F.H.; Xu, M.; Wang, S.; Palakurthi, S.; Choi, J.; Ivanova, E.V.; Leonardi, G.C.; et al. Amplification of Wild-type KRAS Imparts Resistance to Crizotinib in MET Exon 14 Mutant Non-Small Cell Lung Cancer. Clin. Cancer Res. 2018, 24, 5963–5976. [Google Scholar] [CrossRef]
- Graziani, A.; Gramaglia, D.; Cantley, L.C.; Comoglio, P.M. The tyrosine-phosphorylated hepatocyte growth factor/scatter factor receptor associates with phosphatidylinositol 3-kinase. J. Biol. Chem. 1991, 266, 22087–22090. [Google Scholar] [CrossRef]
- Jamme, P.; Fernandes, M.; Copin, M.C.; Descarpentries, C.; Escande, F.; Morabito, A.; Gregoire, V.; Jamme, M.; Baldacci, S.; Tulasne, D.; et al. Alterations in the PI3K Pathway Drive Resistance to MET Inhibitors in NSCLC Harboring MET Exon 14 Skipping Mutations. J. Thorac. Oncol. 2020, 15, 741–751. [Google Scholar] [CrossRef]
- Que, W.; Chen, J.; Chuang, M.; Jiang, D. Knockdown of c-Met enhances sensitivity to bortezomib in human multiple myeloma U266 cells via inhibiting Akt/mTOR activity. APMIS 2012, 120, 195–203. [Google Scholar] [CrossRef]
- Shang, R.; Song, X.; Wang, P.; Zhou, Y.; Lu, X.; Wang, J.; Xu, M.; Chen, X.; Utpatel, K.; Che, L.; et al. Cabozantinib-based combination therapy for the treatment of hepatocellular carcinoma. Gut 2021, 70, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Guessous, F.; Yang, Y.; Johnson, E.; Marcinkiewicz, L.; Smith, M.; Zhang, Y.; Kofman, A.; Schiff, D.; Christensen, J.; Abounader, R. Cooperation between c-Met and focal adhesion kinase family members in medulloblastoma and implications for therapy. Mol. Cancer Ther. 2012, 11, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Chen, H.C. Direct interaction of focal adhesion kinase (FAK) with Met is required for FAK to promote hepatocyte growth factor-induced cell invasion. Mol. Cell. Biol. 2006, 26, 5155–5167. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.Y.; Meens, J.A.; Schick, C.; Organ, S.L.; Qiao, H.; Tremblay, E.A.; Schaeffer, E.; Uniyal, S.; Chan, B.M.; Elliott, B.E. Src and FAK mediate cell-matrix adhesion-dependent activation of Met during transformation of breast epithelial cells. J. Cell. Biochem. 2009, 107, 1168–1181. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Qu, X.; Liu, S.; Zhang, S.; Liu, J.; Qu, J.; Zheng, H.; Liu, Y.; Che, X. Dual inhibition of MET and SRC kinase activity as a combined targeting strategy for colon cancer. Exp. Ther. Med. 2017, 14, 1357–1366. [Google Scholar] [CrossRef][Green Version]
- Lu, J.; Li, X.; Tu, K.; Guan, Y.; Fung, K.P.; Liu, F. Verticillin A suppresses HGF-induced migration and invasion via repression of the c-Met/FAK/Src pathway in human gastric and cervical cancer cells. OncoTargets Ther. 2019, 12, 5823–5833. [Google Scholar] [CrossRef]
- Kaposi-Novak, P.; Lee, J.S.; Gomez-Quiroz, L.; Coulouarn, C.; Factor, V.M.; Thorgeirsson, S.S. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J. Clin. Investig. 2006, 116, 1582–1595. [Google Scholar] [CrossRef]
- Kim, K.H.; Seol, H.J.; Kim, E.H.; Rheey, J.; Jin, H.J.; Lee, Y.; Joo, K.M.; Lee, J.; Nam, D.H. Wnt/beta-catenin signaling is a key downstream mediator of MET signaling in glioblastoma stem cells. NeuroOncology 2013, 15, 161–171. [Google Scholar] [CrossRef]
- Zhan, N.; Michael, A.A.; Wu, K.; Zeng, G.; Bell, A.; Tao, J.; Monga, S.P. The Effect of Selective c-MET Inhibitor on Hepatocellular Carcinoma in the MET-Active, beta-Catenin-Mutated Mouse Model. Gene Expr. 2018, 18, 135–147. [Google Scholar] [CrossRef]
- Fang, D.; Hawke, D.; Zheng, Y.; Xia, Y.; Meisenhelder, J.; Nika, H.; Mills, G.B.; Kobayashi, R.; Hunter, T.; Lu, Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J. Biol. Chem. 2007, 282, 11221–11229. [Google Scholar] [CrossRef]
- Etnyre, D.; Stone, A.L.; Fong, J.T.; Jacobs, R.J.; Uppada, S.B.; Botting, G.M.; Rajanna, S.; Moravec, D.N.; Shambannagari, M.R.; Crees, Z.; et al. Targeting c-Met in melanoma: Mechanism of resistance and efficacy of novel combinatorial inhibitor therapy. Cancer Biol. Ther. 2014, 15, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Davies, K.D. MET Copy Number as a Secondary Driver of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Resistance in EGFR-Mutant Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2019, 37, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Rivas, S.; Armisén, R. El cáncer de pulmón de células no pequeñas en la era de la medicina de precisión (Non-small cell lung cancer in the era of precision medicine). Rev. Médica Clínica Condes 2022, 33, 25–35. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).