1. Introduction
Arrhythmogenic cardiomyopathy (ACM) is an inherited heart disease associated with progressive muscle deterioration, lethal arrhythmias, exercise-induced disease penetrance, inflammation, and fibrofatty replacement of the myocardium [
1]. Known as a “disease of the cardiac desmosome,” the majority of clinical reports are linked to inherited pathogenic variants (primarily, autosomal dominant) in desmosomal genes, such as
PKP2 (
Plakophilin-2) and
DSG2 (
Desmoglein-2) [
1]. Despite experimental and clinical evidence on the efficacy of current pharmacological treatment options, such as antiarrhythmics and angiotensin receptor blockers (ARBs), little is known regarding ARBs’ role in the origin of ventricular arrhythmias and increased myocardial lipolysis via the peroxisome proliferator-activated receptor (PPAR) pathway. Kim, C. et. al. demonstrated in induced pluripotent stem-cell-derived cardiomyocytes (iPSC-CMs) from a patient harboring a pathogenic
PKP2 variant that introducing exogenous PPARγ caused a shift in metabolic respiration, increased lipolysis and apoptosis, decreased β-catenin activity, and abnormal calcium handling [
2]. In the present study, we used an animal model of ACM-homozygous
Desmoglein-2 mutant mice (
Dsg2mut/mut) [
3,
4,
5]. This is a robust animal model of ACM that recapitulates the disease time course seen in patients with ACM. Specifically,
Dsg2mut/mut mice are asymptomatic at birth and show subclinical phenotypes (e.g., ECG depolarization/repolarization abnormalities) until 8 weeks of age [
3,
4,
5] whereby, at 8 weeks of age, echocardiographic analyses demonstrate mild dysfunction [
3,
4,
5]. From 8 to 16 weeks of age, large-scale myocyte cell death and both innate and extrinsic inflammation lead to pathological progression [
3,
4,
5], whereby, at 16 weeks of age,
Dsg2mut/mut mice demonstrate numerous PVCs, severe cardiac dysfunction, and biventricular fibrosis (
Supplementary Figure S1) [
3,
4,
5].
Thus, Dsg2mut/mut mice were utilized in this study to provide further insight into the efficacy and safety of two specific ARBs with discordant effects on PPAR signaling, Valsartan and Telmisartan, as a pharmacological therapeutic in ACM. Moreover, we treated mice with Rosiglitazone, a selective PPARγ agonist with no PPARα-binding affinity to test whether drugs with PPARγ agonism (i.e., Telmisartan or Rosiglitazone) worsen ACM phenotypes.
Recent studies demonstrated the deleterious effects of PPARγ signaling in the pathophysiology of ACM progression [
2,
6]. However, these prior studies lack in vivo testing of thiazolidinediones (i.e., PPARγ agonists) in animal models of ACM. Our study reveals the detrimental impact of PPARγ agonism in ACM mice, as a 100% mortality rate occurred in Rosiglitazone-treated
Dsg2mut/mut mice before the study endpoint. Yet, even at the study midpoint, Rosiglitazone-treated
Dsg2mut/mut mice had significant cardiac dysfunction. Caution is warranted when prescribing ARBs to ACM patients, such as those with comorbidities (e.g., diabetes mellitus type-2). Even so, thiazolidinediones have been known to cause fluid retention and thus may complicate treatment with ACM patients developing or with developed heart failure. Alternatively, while Valsartan-treated
Dsg2mut/mut mice did not delay the progression of cardiac dysfunction, it normalized QRS duration, reduced ventricular ectopy, and prevented further fibrotic progression. Interestingly, Valsartan-treated
Dsg2mut/mut mice showed increased nuclear PPARα levels and increased active β-catenin with elevated Wnt/β-catenin transcripts, the latter of which is down-regulated in ACM [
7].
In summary, this article provides evidence that Valsartan is a safe and effective ARB to reduce ventricular ectopy and ECG repolarization and depolarization abnormalities, prevent fibrofatty scar formation, and increase Wnt-mediated gene transcription. More studies should be conducted regarding drug titration levels of Valsartan to acquire the optimal dose for the preservation of cardiac function without adverse outcomes. Although Telmisartan-treated Dsg2mut/mut mice did show the preservation of cardiac function, caution should be implemented for providers in prescribing Telmisartan, especially considering our results indicate worsening of arrhythmic burden, a variety of cardiac wall motion abnormalities, and increased fibrotic remodeling in Telmisartan-treated Dsg2mut/mut mice.
2. Results
To test the efficacy and safety of angiotensin receptor blockers (ARBs) in ameliorating or delaying ACM phenotypes, wild-type (WT) and
Dsg2mut/mut mice were provided either Valsartan (Val), Telmisartan (Tel), or Vehicle (Veh) via drinking water for 13 weeks starting at 3 weeks of age. At the study endpoint (i.e., 16 weeks of age, [16 W]), 100% of Val-treated
Dsg2mut/mut mice survived, whereas, at week 15, n = 1 Veh-treated
Dsg2mut/mut mouse died (overall cohort survival = 85.7%;
Figure 1A). Interestingly, n = 1 Tel-treated
Dsg2mut/mut mouse died 3 weeks prior (overall cohort survival = 85.7%;
Figure 1A) to the Veh-treated
Dsg2mut/mut mouse that died. No deaths occurred in Veh-treated WT mice (
Figure 1A).
The damaging effects of PPARγ agonism in ACM have been previously demonstrated [
2,
6], yet to our knowledge, this has yet to be tested in vivo. Therefore, we treated
Dsg2mut/mut mice with Rosiglitazone (Ros), a thiazolidinedione that specifically acts as a PPARγ agonist. Of monumental importance, Ros-treated
Dsg2mut/mut mice exhibited a 100% mortality rate by 9 weeks of age (
Figure 1A). Considering the rapid mortality rate in Ros-treated
Dsg2mut/mut mice, we collected Echos from the remaining n = 3 mice at 7W in the event that more deaths occurred in this cohort. We observed a stark decrease in cardiac function and aberrant cardiac remodeling in Ros-treated
Dsg2mut/mut mice vs. Veh-treated
Dsg2mut/mut, Veh-treated WT, and Ros-treated WT cohorts at 7 weeks of age (
Supplementary Figure S2A–D).
At the study midpoint (8W) and endpoint (16W) (
Supplementary Table S1 and
Table 1, respectively), echocardiographic, ECG, and blood pressure plethysmography (BPP) data were collected.
Dsg2mut/mut mice treated with either Veh or Val presented with reduced cardiac function (%EF) at 16W compared to Veh-treated WT mice (
Figure 1B;
Table 1). That said, Val-treated
Dsg2mut/mut mice still showed improved cardiac function compared to Veh-treated
Dsg2mut/mut mice (
Figure 1B,
Table 1). Conversely, Tel-treated
Dsg2mut/mut mice showed the preservation of cardiac function compared to Veh- and Val-treated
Dsg2mut/mut counterparts (
Figure 1B;
Table 1). Despite this finding, Echo analyses in Tel-treated
Dsg2mut/mut mice demonstrated that this cohort presented with hypokinesia, dyskinesia, and aneurysms of the left ventricular anterior and posterior free walls (
Figure 1C, D;
Table 1). Next, signal-average ECGs (SAECGs) showed that Val- and Tel-treated
Dsg2mut/mut mice displayed shortening of the RR-Interval (RR-I) and increased heart rate (HR) compared to Veh-
Dsg2mut/mut mice (
Figure 1E,F;
Table 1). Although Veh-
Dsg2mut/mut mice showed a trend towards prolonged QRS-duration (QRSd), only Tel-treated
Dsg2mut/mut mice showed increased QRSd compared to WT mice (
Figure 1F;
Table 1). Additionally, Tel-treated
Dsg2mut/mut mice demonstrated augmented premature ventricular contractions (PVCs;
Figure 1G) and an increased Q-amplitude (
Figure 1E;
Table 1); thus, Telmisartan appears to cause ventricular depolarization abnormalities in ACM mice. As a vasodilator, Telmisartan exhibited a nearly 30% reduction in systolic, diastolic, and mean blood pressure compared to Veh- and Val-treated
Dsg2mut/mut mice (
Table 1). A similar reduction in blood pressure was observed in Tel-treated WT mice, yet Valsartan appeared to have no effect in WT mice.
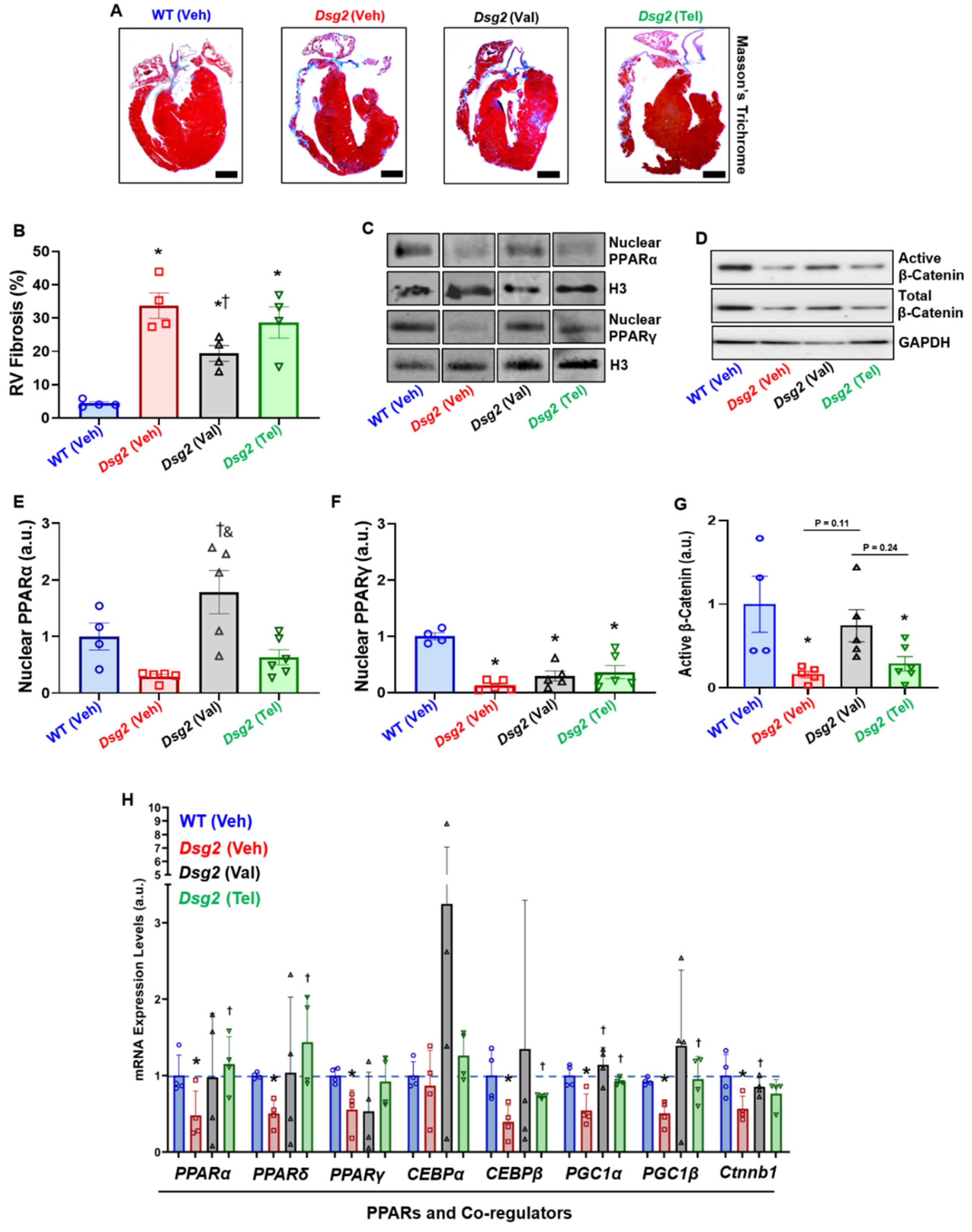
Next, the cardiac histopathological analysis showed a significant increase in right ventricular fibrosis (RVF) in Veh-treated
Dsg2mut/mut mice compared to Veh-treated WT mice (
Figure 2A,B). Although RVF was increased in Val-treated
Dsg2mut/mut mice compared to Veh-treated WT mice, this cohort displayed a reduction in RVF compared to Veh-treated
Dsg2mut/mut mice (
Figure 2B). Meanwhile, Tel-treated
Dsg2mut/mut mice showed a null-to-minimal reduction in RVF (
Figure 2A,B). To assess the influence of ARBs on PPARα/γ activation and PPAR-mediated transcription, immunoblots were performed in nuclear lysates and isolated mRNA, respectively. Veh-treated
Dsg2mut/mut mice displayed a trend towards reduced nuclear PPARα levels compared to Veh-treated WT mice (
Figure 2C,E). Interestingly, nuclear PPARα levels were elevated in Val-treated
Dsg2mut/mut mice compared to Veh- and Tel-treated
Dsg2mut/mut mice (
Figure 2C,E). Regardless of which ARB treatment,
Dsg2mut/mut mice displayed a reduction in nuclear PPARγ levels (
Figure 2C,F) compared to WT mice, counter to our original hypothesis. Conversely, we examined the levels of β-catenin, considering PPARγ and Wnt act in opposition to one another. As previously reported in ACM, [
2]
Dsg2mut/mut mice harbored a significant reduction in the level of active (non-phosphorylated) β-catenin (
Figure 2D,G).
Lastly, ARB-induced gene transcription was analyzed for changes in PPARs, PPAR co-regulators, and metabolism and lipid transcripts (
Figure 2H and
Supplementary Figure S2E,F). The most interesting results uncovered were that both
Pparα,
Pparδ, and
PPARγ co-activator-1α (
Pgc1α) and -
1β (
Pgc1β) expression was elevated in Tel-treated
Dsg2mut/mut mice (
Figure 2H). This was particularly interesting considering PPARγ nuclear protein levels were reduced in these same mice. Of note,
pyruvate dehydrogenase (
Pdh) and
Pdh kinase isoform-2 (
Pdk2) expressions were up-regulated in Val-treated
Dsg2mut/mut mice compared to Veh-treated
Dsg2mut/mut mice (
Supplementary Figure S2E). Intriguingly, the top transcription factor binding sites for
Pdh and
Pdk2 gene promoters are
c-Myc and
Nfat1-4, whereby the former is a primordial Wnt/β-catenin transcript and the latter increases the transcription of the Frizzled—A Wnt co-receptor [
8]. These findings are certainly intriguing given that Val-treated
Dsg2mut/mut mice showed elevated levels of β-catenin (
Ctnnb1;
Figure 2H). There was a stark decline in
Pdk4 expression, an endogenous inhibitor of
Pdh, in Val-treated
Dsg2mut/mut mice compared to Veh-treated
Dsg2mut/mut mice (
Supplementary Figure S2E). Although the expression of
adiponectin (
Adipo) was elevated in nearly all
Dsg2-cohorts (
Supplementary Figure S2F), only Val-treated
Dsg2mut/mut mice showed reduced levels of the adipokine,
fatty acid binding protein-4 (
Fabp4;
Supplementary Figure S2F). In conclusion, it appears Valsartan increases gene transcripts to catabolize fat into energy (i.e., ATP) through the TCA cycle, in addition to increasing Wnt/β-catenin transcription.
3. Discussion
Considering that fibrofatty replacement of the myocardium is a pathological hallmark of ACM, we were interested in the effects of thiazolidinediones (i.e., Rosiglitazone) and/or partial-thiazolidinediones (i.e., Telmisartan), on cardiac function, lipolytic gene expression, and fibrofatty infiltration. The ARB, Telmisartan, was of particular interest given recent work that demonstrated it harbors partial-PPARγ agonism [
9]. This is crucial in ACM given that the suppression of the Wnt/β-catenin signaling pathway is implicated in ACM pathogenesis [
7] and PPARγ and Wnt share an inverse relationship with one another. Specifically, PPARs work in complete opposition to Wnt, such that if PPARs are up-regulated then Wnt is down-regulated, and vice versa [
10].
As an ARB, Telmisartan exceeded the blood pressure reduction compared to Valsartan at both 8 and 16 weeks of age. Additionally, Telmisartan preserved left ventricular function to nearly WT levels. However, Telmisartan-treated
Dsg2mut/mut mice showed increased wall motion abnormalities, myocardial fibrosis, PVCs, and depolarization abnormalities—all Task Force Criteria phenotypes for ACM. Alternatively, while Valsartan preserved cardiac function, it was nearly devoid of conduction abnormalities, arrhythmias, and reduced RVF, and myocardial samples showed increased PPARα nuclear levels, active β-catenin, and Wnt-mediated mRNA transcription. Prior work demonstrated PPARα activation is necessary to combat ROS-mediated cellular damage and inflammation via the suppression of NFκB [
11]. Exercise-induced ROS generation [
5] and NFκB-mediated myocardial inflammation [
4] are two key pathological triggers we recently demonstrated in ACM.
In addition to either ARBs’ efficacy in preventing cardiac dysfunction and fibrosis, we would be remiss to not address the tantalizing outcomes of
Pdh and
Pdk4 expression in Val-treated
Dsg2mut/mut mice. Pyruvate dehydrogenase catalyzes pyruvate and NAD+ into acetyl-CoA and NADH, the latter of which acts as a reducing agent for the mitochondrial Thioredoxin-2/Peroxiredoxin-3 pathway, a pathway we have shown is severely reduced in ACM myocardial samples [
5]. Thus, it appears Valsartan not only inadvertently increases reducing agents (i.e., NADH) to combat ROS levels but also elevates acetyl-CoA levels and thus energy production through the TCA cycle. This is a cycle that has additionally been shown to be down-regulated in ACM myocytes [
2]. Lastly, PDK4 inhibits PDH via phosphorylation, thereby contributing to the regulation of fatty-acid oxidation (FAO) via the reduction of glucose metabolism. Considering heart failure (HF) is common in ACM patients, it may seem counterintuitive that
Pdk4 expression was significantly down-regulated in Val-treated
Dsg2mut/mut mice. Heart failure is a disease state that utilizes glucose metabolism over FAO, which produces far less ATP. Yet, recent work demonstrated that altered cellular metabolism, from glucose metabolism to the TCA cycle, is mediated by the reduction in PDK4 levels [
12,
13]. That said, Sun Y et al. showed PDK4 binds to the apoptosis-inducing factor (AIF), a mitochondrial protein that we recently showed contributes to cell death in ACM [
5].
Although HF shifts the heart to a less energy-efficient metabolic state, new advancements have indicated that increasing glucose utilization in cardiomyocytes is associated with increased cardiac function [
12,
13]. In conclusion, providers should consider moving alongside antiarrhythmics in ACM, such as the administration of Valsartan as this ARB demonstrates additional metabolic therapeutic effects to increase substrate utilization even in diseased states (i.e., HF) [
12,
13].
4. Materials and Methods
4.1. Study Approval
All experiments conformed to the Guide for the Care and Use of Laboratory Animals from the National Institute of Health (NIH publication no. 85–23, revised 1996). Animal study protocol was approved by the Johns Hopkins University Animal Care and Use Committee (Protocol Code: MO19M94; Date of Approval: 26 March 2019).
4.2. In Vivo Drug Treatment
Two mouse lines were utilized in this study: Wild-type (WT) controls and homozygous
Desmoglein-2 mutant mice (
Dsg2mut/mut). All mouse lines are of a C57BL/6J background and were bred in-house via heterozygous x heterozygous pairing (i.e.,
Dsg2mut/+ x
Dsg2mut/+) in order to ensure all mice were age- and litter-matched, with n≥5 mice/cohort/treatment [
5]. As previously described, [
3] mice with the targeted allele were mated with mice harboring ubiquitous expression of CMV-Cre to remove the fourth and fifth exons of
Dsg2 (
Dsg2mut/mut), which results in the loss of exons 4 and 5 due to a frameshift mutation and premature termination of translation. [
3] This led to germline deletion of exons four and five, thus no longer requiring additional Cre-recombinase.
Litters were genotyped at 3 weeks of age then separated into treatment cages and administered one of the following via drinking water: (a) Valsartan (30 mg/kg/day) [
14] (Cat. No. SML0142, Sigma; Milwaukee, WI, USA); (b) Telmisartan (10 mg/kg/day) [
15] (Cat. No. T8949, Sigma); (c) Rosiglitazone (25 mg/kg/day) [
16] (Cat. No. R2408, Sigma); or (d) Vehicle (deionized [DI] H
20). All drinking water bottles contained 5% Splenda. Genotypes were known prior to the separation into treatment cohorts to ensure n ≥ 5 mice/cohort/treatment. ARBs and Rosiglitazone were obtained as ≥98% HPLC grade powder, dissolved in DMSO, where stock solutions were then added to DI H
20 containing 5% Splenda and filter-sterilized with Express PLUS Stericups (0.22 μm; Cat. No. SCGPCAPRE, Millipore Sigma; Milwaukee, WI, USA). Mice were given access to treatment or vehicle water bottles ad libitum, and water bottles were replaced every 48 h.
4.3. Cardiac Function and Histopathology
Disease functional characterization was performed at 8 and 16 weeks (8 W and 16 W, respectively) of age. Specifically, echocardiography, electrocardiography, and blood pressure plethysmography were obtained at 8 W and 16 W, and histopathology, PPAR protein levels, and qPCR expression were obtained at 16 W.
Echocardiography: A 2100 Vevo Visualsonic was used to assess cardiac function. An ultrahigh-frequency linear array microscan transducer (40 MHz; acquired at a sweep speed of 200 mm/s,) was utilized to obtain short-axis, m-mode and parasternal, long-axis, B-mode images. All images at the level of the papillary muscles were obtained and analyzed following the American Society of Echocardiography guidelines for animals, as previously described [
5]. Each mouse had 3–5 measurements obtained for each echocardiographic parameter and then were averaged for statistical evaluation, as previously described [
3]. Additionally, all mice were evaluated for the presence of hypokinesia, dyskinesia, and aneurysms at the study endpoint; these functional abnormalities and corresponding data are included in
Table 1.
Electrocardiography (ECG)
: Surface ECGs were performed in anesthetized mice (nose cone anesthesia using 2% Isoflurane in 100% vaporized oxygen [O
2]). PowerLab (ADInstruments) instruments for Lead I recordings were utilized, as previously described [
5]. ECG Analysis Add-on Software (LabChart Pro 8, MLS360/8, AD Instruments) was utilized to analyze signal-averaged ECGs (SAECGs;
Supplementary Table S1 and
Table 1 for 8 W and 16 W, respectively). Additionally, 5 min recordings were used to obtain the percent ventricular extrasystoles (%PVCs).
Blood pressure plethysmography (BPP): Non-invasive BPP measurements were obtained via the ADInstruments Tail-cuff BPP System. Specifically, non-anesthetized mice (a) were led into a rodent restrainer (Cat. No. MLA5016); (b) using the slide piston, each mouse was adjusted in order to maximize tail length for placement in the BPP tail cuff holder (Cat. No. MLA5030); (c) a blood pressure cuff (Cat. No. MLT125/m) was then placed around the base of the tail. All BPP measurements were conducted in a 37 °C designated quiet area (JHU Physiology Suite). Prior to BPP measurements, (d) mice were acclimated to the ADInstruments Tail-cuff BPP System for 15mins. Following acclimation, (e) a total of n = 6 BPP measurements were recorded. The first n = 3 measurements were deemed ‘acclimation cycles’ and discarded, whereas the last n = 3 measurements were averaged for systolic (SBP), diastolic (DBP), and mean BP (MBP) recordings.
Histopathology: Following endpoint functional analyses, mice were euthanized via cervical dislocation, their hearts were extracted and placed in 1X PBS, and they were cut via the long axis. One-half of the heart containing the atrium was processed for immunohistochemical analyses via Masson’s Trichrome, as previously described [
5]. The remaining half was flash-frozen in liquid nitrogen for downstream analyses.
4.4. PPAR Pathway Analyses
Subcellular fractionation: Mouse ventricular myocardium was lysed in RIPA buffer containing 1:100 protease and phosphatase inhibitors (Sigma; Cat. No. P1860 and P0044, respectively). Following the removal of cytosolic and membrane-bound lysates, nuclear fractions were extracted as follows: 5000 g for 5 min at 4 °C (Subcellular Fractionation Kit for Tissues, ThermoFisher, Cat. No. 87790). Protein concentrations were then measured using a BCA protein assay kit (Pierce, Cat. No. 23225)
Western immunoblotting: Following subcellular fractionation, extracted nuclear fractions (10 µg) were boiled (95 °C) in Laemmli buffer for 10 min and separated via electrophoresis in NuPAGETM 4–12% Bis-Tris gels at 100 V. Protein-captured gels were then transferred to nitrocellulose membranes using the iBlotTM-2 system at 20 V for 7 min. Membranes were blocked using the iBindTM flex FD solution (ThermoFisher; Cat. No. SLF2020) for 30 min at room temperature. Membranes were placed in the iBindTM flex system and probed with the following primary antibodies: Mouse anti-active β-catenin (Sigma; Cat. No. 05-665; at 1:1000); rabbit anti- β-catenin (Sigma; Cat. No. 06-734; at 1:1000); mouse anti-Histone-3 (H3; Cell Signaling; Cat. No. 14269S; at 1:1000); rabbit polyclonal anti-PPARα (Abcam; Cat. No. 8934; at 1:500); and rabbit polyclonal anti-PPARγ (GeneTex; Cat. No. 50828; at 1:500). To avoid signal overlap, immunoblots for PPARα and PPARγ were performed separately. Secondary antibodies were additionally added as follows: (a) Either added to the iBindTM flex system: Goat anti-rabbit IRDye 680RD (LI-CORTM; Cat. No. 926-6807 at 1:1000); or blots were incubated at room temperature for 1 h with donkey anti-mouse IRDye 800CW (LI-CORTM; Cat. No. 926-32212; at 1:1000 dilution). Membranes were then washed three times (5 min each) with 1X TBS with 0.05% TweenTM-20 and imaged using the OdysseyTM CLx imaging system (LI-CORTM). Protein signal intensities (arbitrary units; [a.u.]) were quantified using the Odyssey Imaging system (Image Studio 4.0 software). Protein signals corresponding to nuclear PPARα/γ were normalized to H3 signal intensities, then cohorts were normalized to Veh-treated WT signals.
4.5. qPCR Expression
RNA (1 µg/reaction) was isolated using the PicoPure RNA Isolation Kit (ThermoFisher, Cat. No. KIT0204) and cDNA (0.5 µg/reaction) generated using the High-capacity cDNA Reverse Transcription Kit (Fisher Scientific, Cat. No. 43-688-14; Foster City, CA, USA). The SYBR Green Select Master Mix (ThermoFisher, Cat. No. 4472919; Gainesville, FL, USA) was utilized for qPCR reactions in the CFX384 Biorad Real-Time PCR System (BioRad; Cat. No. 1855484). All reactions were normalized to
Gapdh and then normalized to Veh-treated WT data. Forward and reverse qPCR primers and protocol information can be found in
Supplementary Table S2.
4.6. Statistical Analyses
Data are presented as mean ± SEM, with a
p < 0.05 deemed significant using Student’s t-test and One-way or Two-way ANOVA with Tukey’s post-hoc analysis. A detailed description of statistical analyses can be found in each figure legend and/or table. The number of animals used (n = 5–6) in this study was determined based on our prior publications [
3,
4,
5] that demonstrated n ≥ 4 mice was sufficient to show substantial cardiovascular and pathological differences between cohorts (i.e., WT vs.
Dsg2mut/mut). Our prior work demonstrated 80–99% Power Analyses and received a statistical probability of
p < 0.05 by utilizing an n ≥ 4 mice/genotype/cohort/parameter.