Coordination of AMPK and YAP by Spatholobi Caulis and Procyanidin B2 Provides Antioxidant Effects In Vitro and In Vivo
Abstract
1. Introduction
2. Results
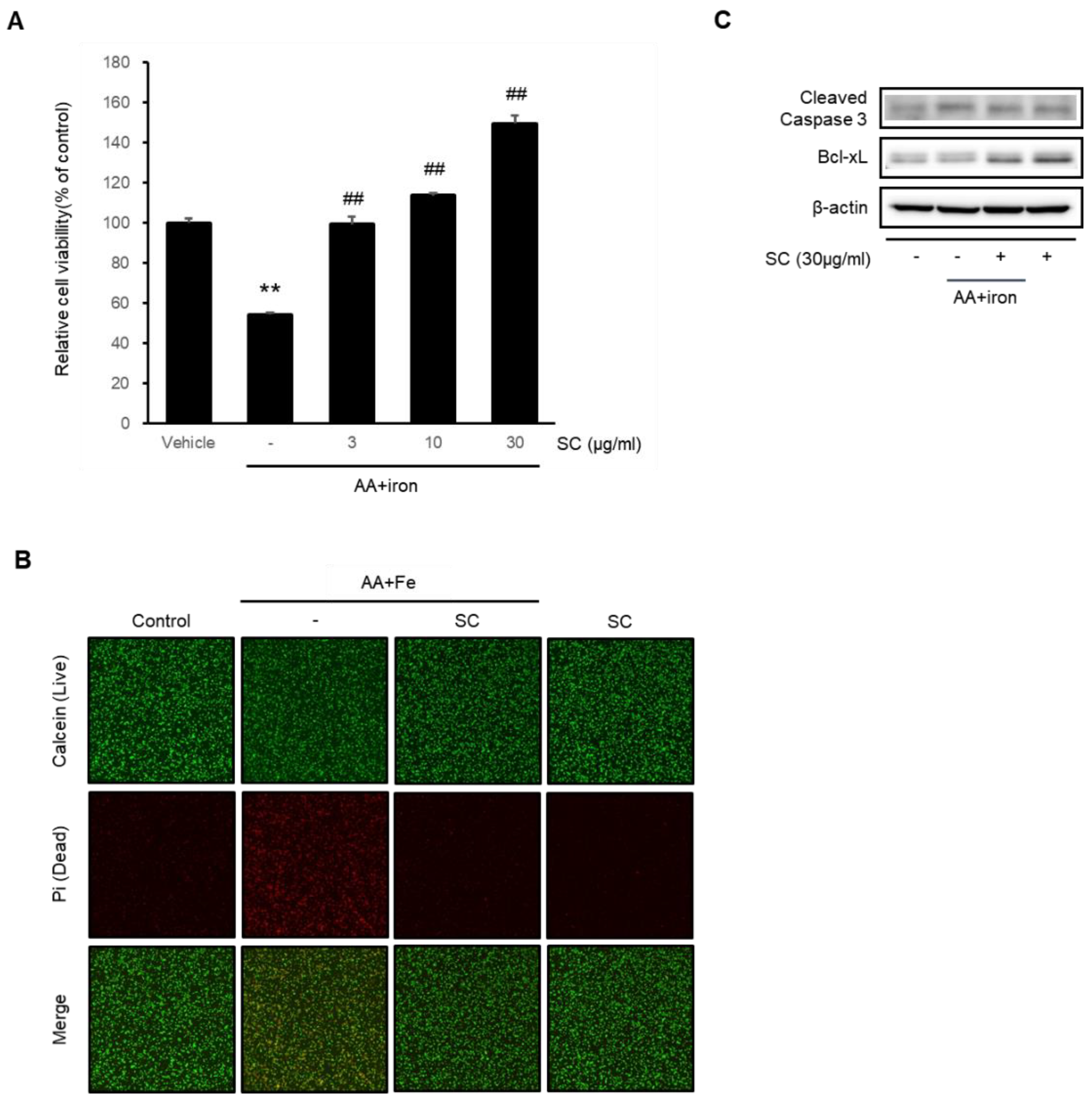
2.1. SC Protects Hepatocytes against AA + Iron-Induced Cytotoxicity
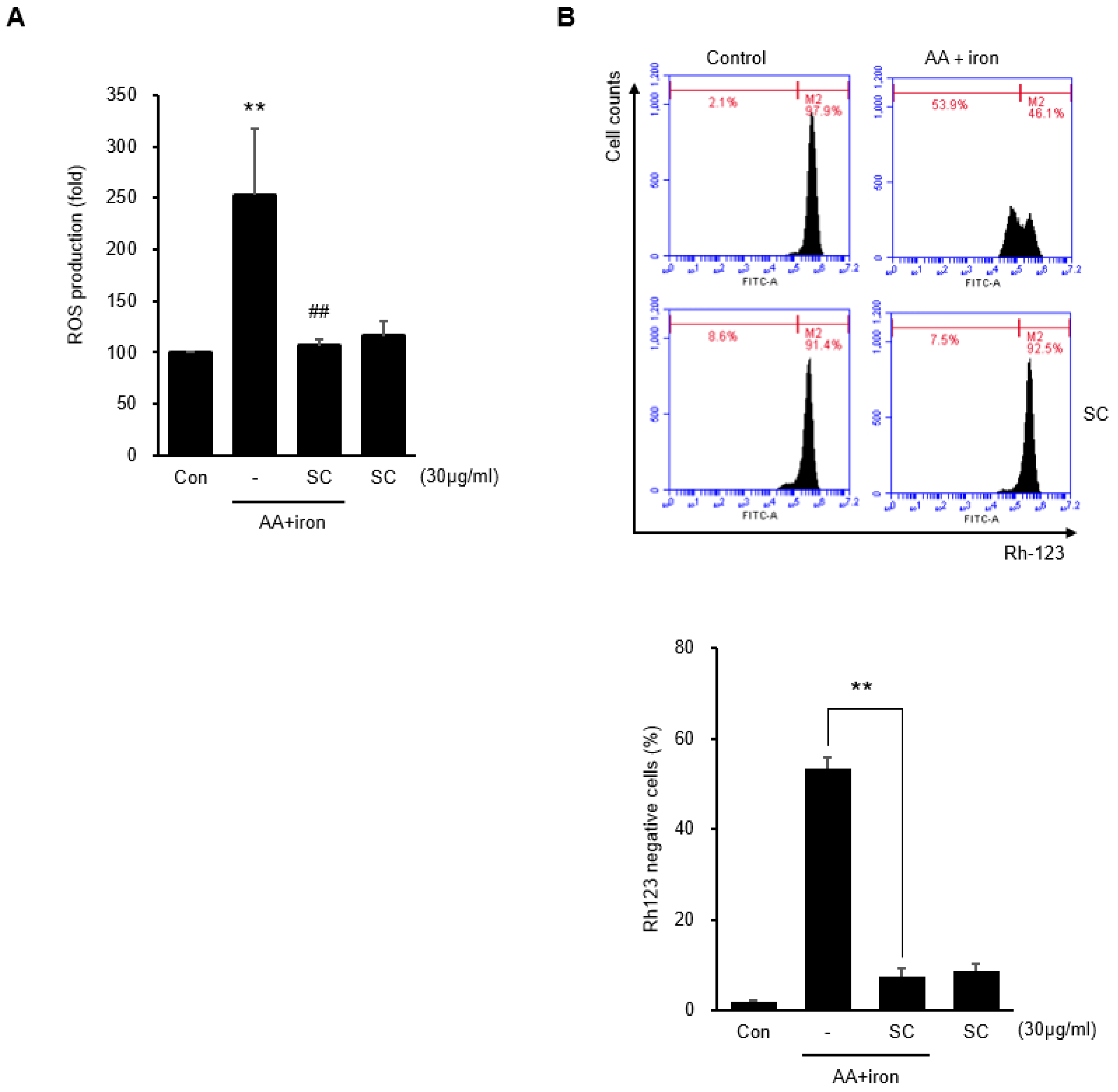
2.2. SC Inhibits AA + Iron-Induced ROS Generation and Mitochondrial Dysfunction
2.3. SC Activates AMPKα
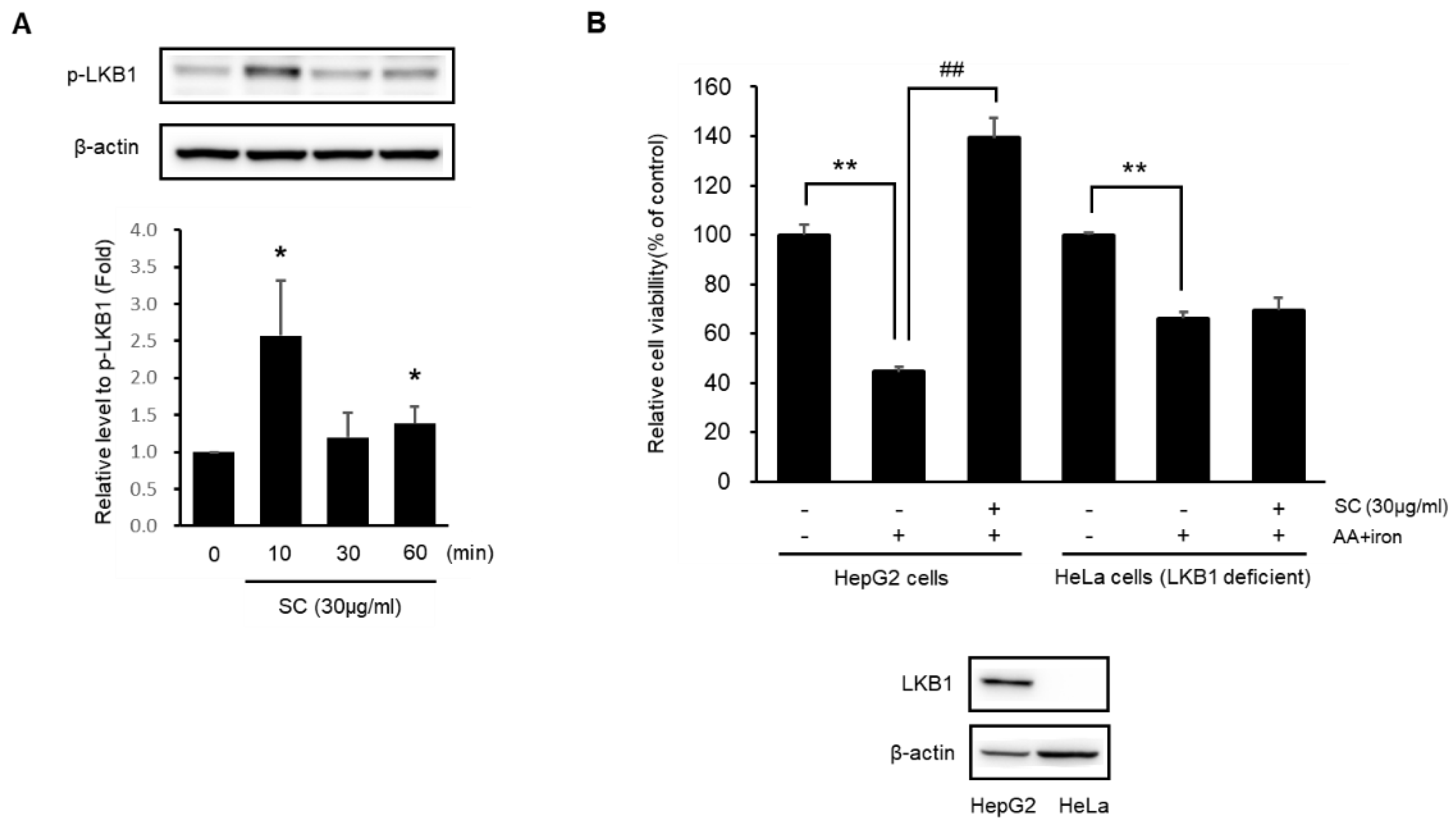
2.4. SC Protects Hepatocytes via Activation of the LKB1-AMPK Pathway
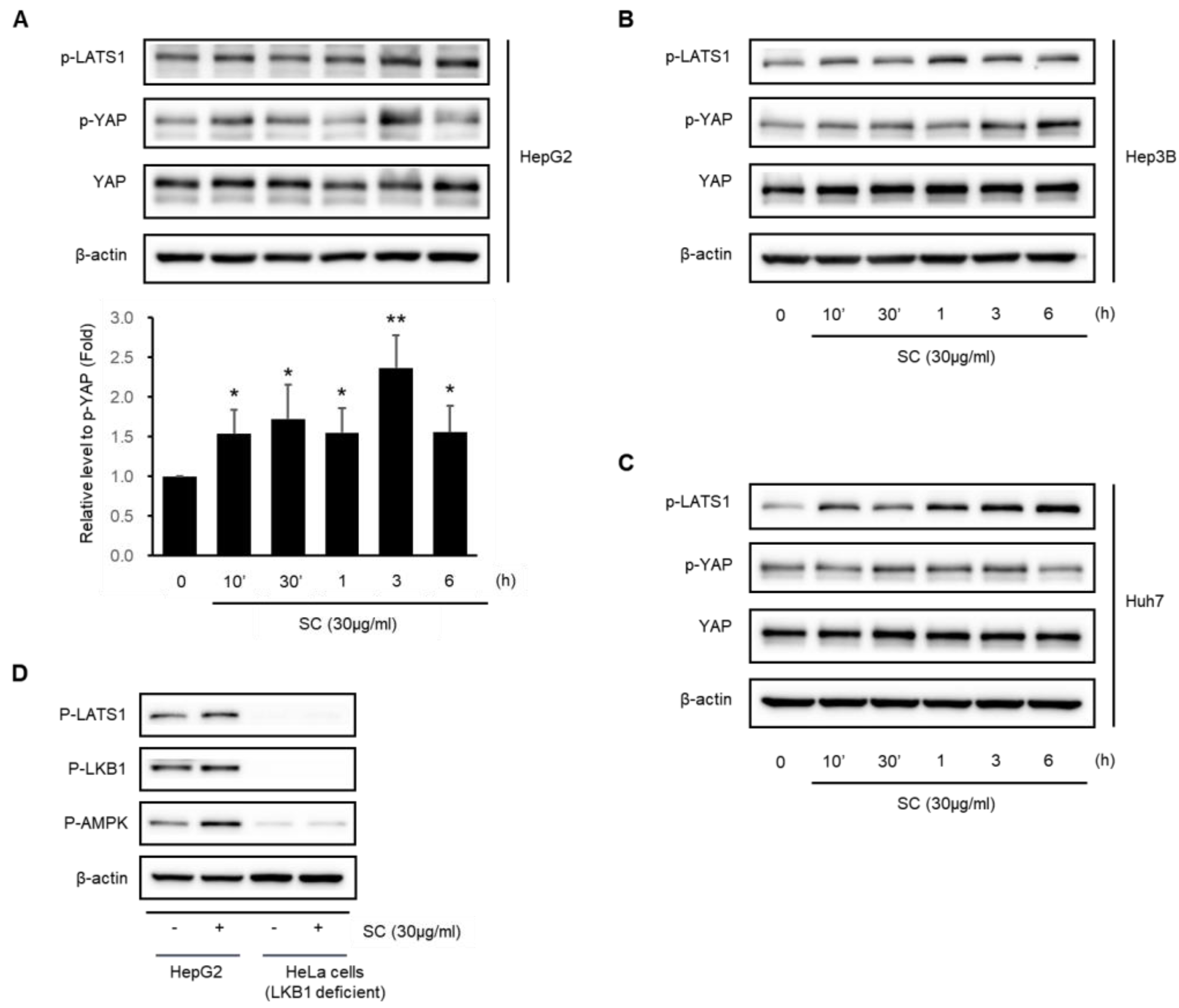
2.5. SC Activates the Hippo–YAP Pathway
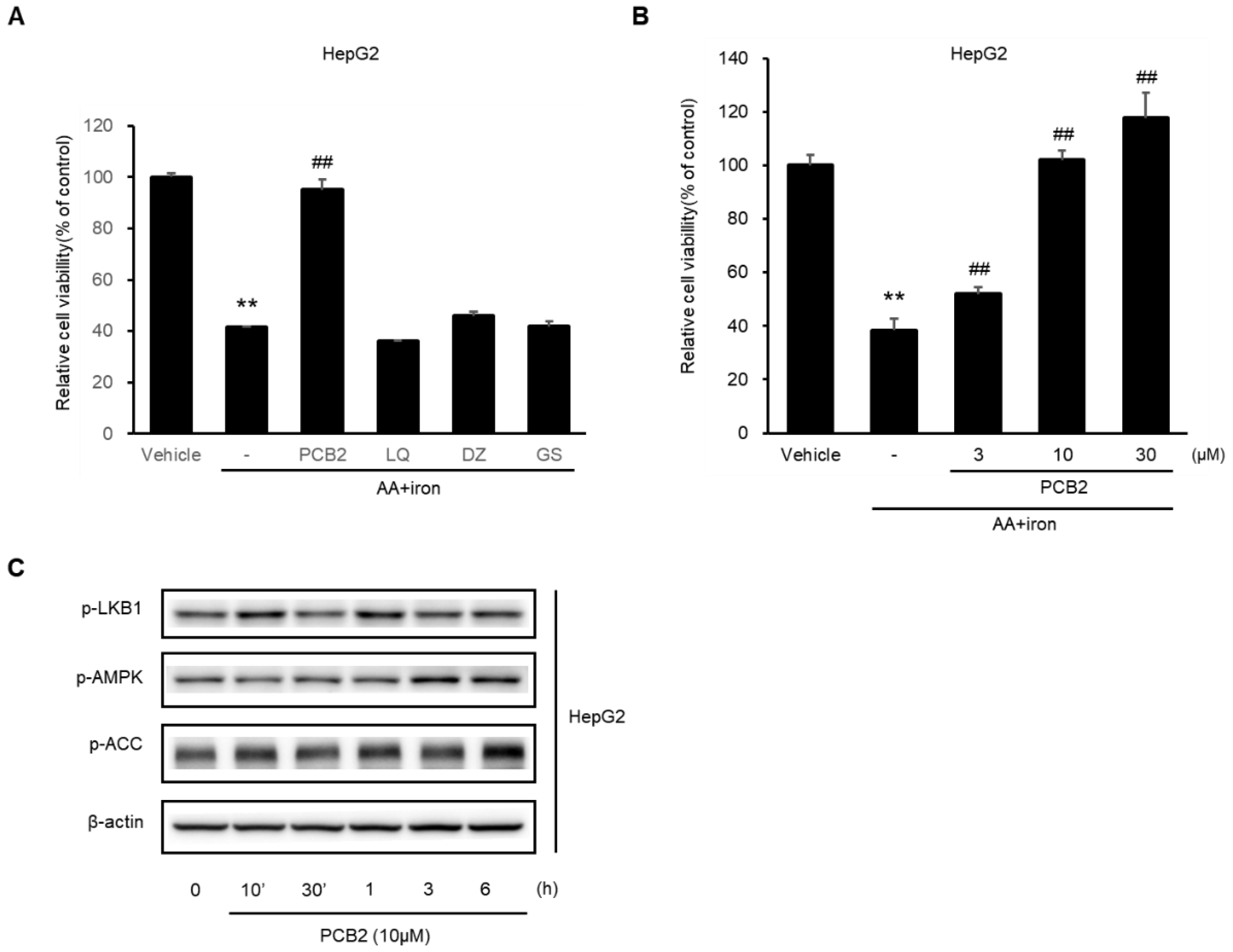
2.6. Procyanidin B2 in SC Protects Hepatocytes
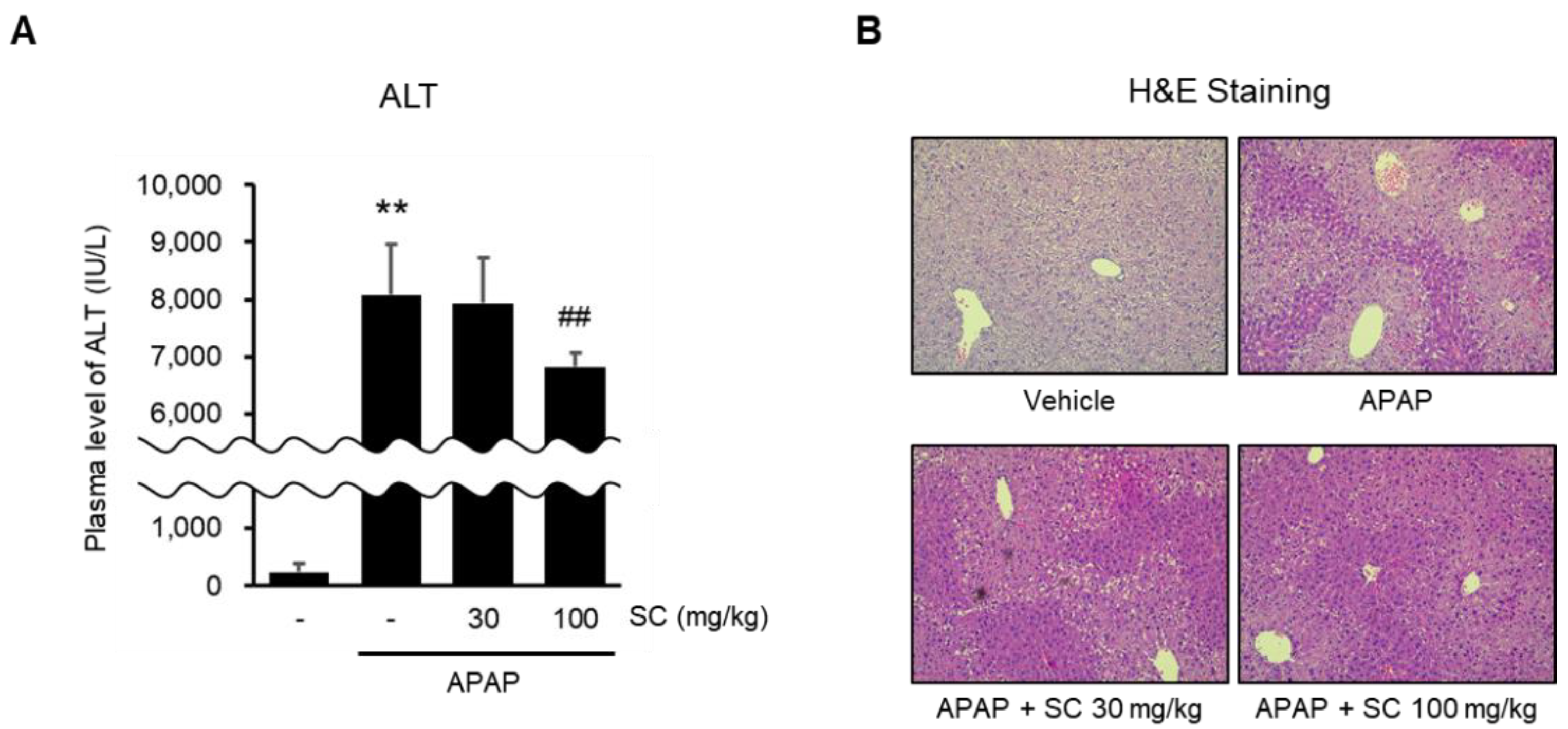
2.7. SC Ameliorates APAP-Induced Acute Liver Injury
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Lines and Cell Culture
4.3. Cell Viability Assay
4.4. Measurement of Reactive Oxygen Species (ROS) Production
4.5. Measurement of Mitochondrial Membrane Potential
4.6. Immunoblot Analysis
4.7. Animals and Treatment
4.8. Blood Chemistry
4.9. Histopathology
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.L.; Shin, H.J.; Feitelson, M.A.; Yu, D.Y. Oxidative stress and antioxidants in hepatic pathogenesis. World J. Gastroenterol. 2010, 16, 6035–6043. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Shadel, G.S.; Ghosh, S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011, 11, 389–402. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Hardie, D.G. AMPK: Positive and negative regulation, and its role in whole-body energy homeostasis. Curr. Opin. Cell Biol. 2015, 33, 1–7. [Google Scholar] [CrossRef]
- Cool, B.; Zinker, B.; Chiou, W.; Kifle, L.; Cao, N.; Perham, M.; Dickinson, R.; Adler, A.; Gagne, G.; Iyengar, R.; et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006, 3, 403–416. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An Emerging Drug Target for Diabetes and the Metabolic Syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Kwon, D.Y.; Park, O.J.; Kim, M.S. Resveratrol protects ROS-induced cell death by activating AMPK in H9c2 cardiac muscle cells. Genes Nutr. 2008, 2, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.B.; Ruan, J.W.; Liu, W.; Zhu, L.Q.; Zhu, X.H.; Yi, H.; Cui, S.Y.; Zhao, J.N.; Cui, Z.M. miR-135b expression downregulates Ppm1e to activate AMPK signaling and protect osteoblastic cells from dexamethasone. Oncotarget 2016, 7, 70613–70622. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.P.; Guo, X.H.; Geng, D.; Weng, L.J. d-Limonene protects PC12 cells against corticosterone-induced neurotoxicity by activating the AMPK pathway. Environ. Toxicol. Pharmacol. 2019, 70, 103192. [Google Scholar] [CrossRef] [PubMed]
- Pietrzyk, N.; Zakłos-Szyda, M.; Koziołkiewicz, M.; Podsędek, A. Viburnum opulus L. fruit phenolic compounds protect against FFA-induced steatosis of HepG2 cells via AMPK pathway. J. Funct. Foods 2021, 80, 104437. [Google Scholar] [CrossRef]
- Plouffe, S.W.; Hong, A.W.; Guan, K.L. Disease implications of the Hippo/YAP pathway. Trends Mol. Med. 2015, 21, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Baek, S.Y.; Park, J.Y.; Kim, Y.W. Emodin in Rheum undulatum inhibits oxidative stress in the liver via AMPK with Hippo/Yap signalling pathway. Pharm Biol. 2020, 58, 333–341. [Google Scholar] [CrossRef]
- Wu, H.; Xiao, Y.; Zhang, S.; Ji, S.; Wei, L.; Fan, F.; Geng, J.; Tian, J.; Sun, X.; Qin, F.; et al. The Ets Transcription Factor GABP Is a Component of the Hippo Pathway Essential for Growth and Antioxidant Defense. Cell Rep. 2013, 3, 1663–1677. [Google Scholar] [CrossRef]
- Song, S.R.; Jang, B.; Lee, S.M.; Bae, S.J.; Bak, S.B.; Kim, Y.W. Angelica gigas NAKAI and Its Active Compound, Decursin, Inhibit Cellular Injury as an Antioxidant by the Regulation of AMP-Activated Protein Kinase and YAP Signaling. Molecules 2022, 27, 1858. [Google Scholar] [CrossRef]
- Park, H.R.; Lee, H.; Lee, J.J.; Yim, N.H.; Gu, M.J.; Ma, J.Y. Protective Effects of Spatholobi Caulis Extract on Neuronal Damage and Focal Ischemic Stroke/Reperfusion Injury. Mol. Neurobiol. 2018, 55, 4650–4666. [Google Scholar] [CrossRef]
- Kang, I.-C.; Kim, S.-A.; Song, G.Y.; Baek, N.-I.; Park, Y.-D.; Ryu, S.Y.; Saiki, I.; Kim, S.-H. Effects of the ethyl acetate fraction of Spatholobi caulis on tumour cell aggregation and migration. Phytother. Res. 2003, 17, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Y.; Huan, L.Y.; Zhao, W.R.; Tang, N.; Jin, Y.; Tang, J.Y. Spatholobi Caulis extracts promote angiogenesis in HUVECs in vitro and in zebrafish embryos in vivo via up-regulation of VEGFRs. J. Ethnopharmacol. 2017, 200, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.R.; Wang, A.Q.; Lin, L.G.; Qiu, H.C.; Wang, Y.T.; Wang, Y. In Vitro Study on Anti-Hepatitis C Virus Activity of Spatholobus suberectus Dunn. Molecules 2016, 21, 1367. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Liu, H.; Lin, B.; Yang, W.; Chen, W.; Lu, Z.; Li, P.; Gui, S.; Zhan, Y.; Lin, B. Spatholobi Caulis dispensing granule reduces deep vein thrombus burden through antiinflammation via SIRT1 and Nrf2. Phytomedicine 2020, 77, 153285. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; He, X.; Sheng, Y.; Yang, C.; Li, H.; Xu, J.; Xu, W.; Huang, K. Caulis Spatholobi Ameliorates Obesity through Activating Brown Adipose Tissue and Modulating the Composition of Gut Microbiota. Int. J. Mol. Sci. 2019, 20, 5150. [Google Scholar] [CrossRef]
- Yang, Y.M.; Han, C.Y.; Kim, Y.J.; Kim, S.G. AMPK-associated signaling to bridge the gap between fuel metabolism and hepatocyte viability. World J. Gastroenterol. 2010, 16, 3731–3742. [Google Scholar] [CrossRef]
- Lee, E.H.; Baek, S.Y.; Park, J.Y.; Kim, Y.W. Rifampicin activates AMPK and alleviates oxidative stress in the liver as mediated with Nrf2 signaling. Chem.-Biol. Interact. 2020, 315, 108889. [Google Scholar] [CrossRef]
- Chen, C.; Kassan, A.; Castaneda, D.; Gabani, M.; Choi, S.K.; Kassan, M. Metformin prevents vascular damage in hypertension through the AMPK/ER stress pathway. Hypertens. Res. 2019, 42, 960–969. [Google Scholar] [CrossRef]
- Xu, Z.; Feng, W.; Shen, Q.; Yu, N.; Yu, K.; Wang, S.; Chen, Z.; Shioda, S.; Guo, Y. Rhizoma Coptidis and Berberine as a Natural Drug to Combat Aging and Aging-Related Diseases via Anti-Oxidation and AMPK Activation. Aging Dis. 2017, 8, 760–777. [Google Scholar] [CrossRef]
- Seo, H.L.; Baek, S.Y.; Lee, E.H.; Lee, J.-H.; Lee, S.-G.; Kim, K.-Y.; Jang, M.H.; Park, M.-H.; Kim, J.-H.; Kim, K.-J.; et al. Liqustri lucidi Fructus inhibits hepatic injury and functions as an antioxidant by activation of AMP-activated protein kinase in vivo and in vitro. Chem.-Biol. Interact. 2017, 262, 57–68. [Google Scholar] [CrossRef]
- Zhao, B.; Li, L.; Lei, Q.; Guan, K.L. The Hippo-YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev. 2010, 24, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Moya, I.M.; Halder, G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 2019, 20, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.S.; Meng, Z.; Kim, Y.C.; Park, H.W.; Hansen, C.G.; Kim, S.; Lim, D.S.; Guan, K.L. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat. Cell Biol. 2015, 17, 500–510. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, Z.D.; Li, X.; Aziz, K.E.; Gan, B.; Johnson, R.L.; Chen, J. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat. Cell Biol. 2015, 17, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Byrne, C.D. Omega-3 Fatty Acids, Hepatic Lipid Metabolism, and Nonalcoholic Fatty Liver Disease. Annu. Rev. Nutr. 2013, 33, 231–248. [Google Scholar] [CrossRef]
- Ghazali, R.; Mehta, K.J.; Bligh, S.W.A.; Tewfik, I.; Clemens, D.; Patel, V.B. High omega arachidonic acid/docosahexaenoic acid ratio induces mitochondrial dysfunction and altered lipid metabolism in human hepatoma cells. World J. Hepatol. 2020, 12, 84–98. [Google Scholar] [CrossRef]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. J. Physiol. Pharmacol. 2013, 64, 409–421. [Google Scholar]
- Colquhoun, A.; Schumacher, R.I. γ-Linolenic acid and eicosapentaenoic acid induce modifications in mitochondrial metabolism, reactive oxygen species generation, lipid peroxidation and apoptosis in Walker 256 rat carcinosarcoma cells. BBA-Mol. Cell Biol. Lipids 2001, 1533, 207–219. [Google Scholar] [CrossRef]
- Hasenjaeger, A.; Gillissen, B.; Mueller, A.; Normand, G.; Hemmati, P.G.; Schuler, M.; Doerken, B.; Daniel, P.T. Smac induces cytochrome c release and apoptosis independently from Bax/Bcl-xL in a strictly caspase-3-dependent manner in human carcinoma cells. Oncogene 2004, 23, 4523–4535. [Google Scholar] [CrossRef]
- Murie, P. Role of free radicals in liver diseases. Hepatol. Int. 2009, 3, 526–536. [Google Scholar] [CrossRef]
- Price, L.; Kowdley, K.V. The Role of Iron in the Pathophysiology and Treatment of Chronic Hepatitis C. Can. J. Gastroenterol. 2009, 23, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Mattera, R.; Stone, G.P.; Bahhur, N.; Kuryshev, Y.A. Increased Release of Arachidonic Acid and Eicosanoids in Iron-Overloaded Cardiomyocytes. Circulation 2001, 103, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.M.; Kim, S.G. Inhibition of arachidonic acid and iron-induced mitochondrial dysfunction and apoptosis by oltipraz and novel 1, 2-dithiole-3-thione congeners. Mol. Pharmacol. 2009, 75, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Caro, A.A.; Cederbaum, A.I. Synergistic toxicity of iron and arachidonic acid in HepG2 cells overexpressing CYP2E1. Mol. Pharmacol. 2001, 60, 742–752. [Google Scholar] [PubMed]
- Dong, G.Z.; Lee, J.H.; Ki, S.H.; Yang, J.H.; Cho, I.J.; Kang, S.H.; Zhao, R.J.; Kim, S.C.; Kim, Y.W. AMPK activation by isorhamnetin protects hepatocytes against oxidative stress and mitochondrial dysfunction. Eur. J. Pharmacol. 2014, 740, 634–640. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, Y.W.; Kim, S.G. AMPK-mediated GSK3beta inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress. Biochem. Pharmacol. 2010, 79, 1352–1362. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Duan, L.; Dong, X.; Zhou, P.; Liu, E.H.; Li, P. Simultaneous determination of 16 phenolic constituents in Spatholobi Caulis by high performance liquid chromatography/electrospray ionization triple quadrupole mass spectrometry. J. Pharm. Biomed. Anal. 2015, 102, 110–118. [Google Scholar] [CrossRef]
- Yang, H.; Xiao, L.; Yuan, Y.; Luo, X.; Jiang, M.; Ni, J.; Wang, N. Procyanidin B2 inhibits NLRP3 inflammasome activation in human vascular endothelial cells. Biochem. Pharmacol. 2014, 92, 599–606. [Google Scholar] [CrossRef]
- Wu, S.; Yue, Y.; Li, J.; Li, Z.; Li, X.; Niu, Y.; Xiang, J.; Ding, H. Procyanidin B2 attenuates neurological deficits and blood-brain barrier disruption in a rat model of cerebral ischemia. Mol. Nutr. Food Res. 2015, 59, 1930–1941. [Google Scholar] [CrossRef]
- Wang, W.; Chen, R.; Wang, J. Procyanidin B2 ameliorates carrageenan-induced chronic nonbacterial prostatitis in rats via anti-inflammatory and activation of the Nrf2 pathway. Biochem. Biophys. Res. Commun. 2017, 493, 794–799. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, X.; Yang, M.; Yu, Y.; Zhou, Y.; Gao, Y.; Zhang, L.; Li, Z.; Xiao, Y.; Moses, R.E.; et al. Procyanidin B2 Promotes Intestinal Injury Repair and Attenuates Colitis-Associated Tumorigenesis via Suppression of Oxidative Stress in Mice. Antioxid. Redox Signal. 2021, 35, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.W.; Lei, G.T.; Wu, Q.H.; Jiang, Y.; Huang, M.X. Procyanidin B2 protects against diet-induced obesity and non-alcoholic fatty liver disease via the modulation of the gut microbiota in rabbits. World J. Gastroenterol. 2019, 25, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Zhang, X.Y.; Guan, S.W.; Hua, Z.C. Protective Effect of Procyanidin B2 against CCl4-Induced Acute Liver Injury in Mice. Molecules 2015, 20, 12250–12265. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, Z.C.; Battello, N.; Rothley, M.; Piorońska, W. Liver cancer cell lines distinctly mimic the metabolic gene expression pattern of the corresponding human tumours. J. Exp. Clin. Cancer Res. 2018, 37, 211. [Google Scholar] [CrossRef]
- Shi, J.; Wang, X.; Lyu, L.; Jiang, H.; Zhu, H.J. Comparison of protein expression between human livers and the hepatic cell lines HepG2, Hep3B, and Huh7 using SWATH and MRM-HR proteomics: Focusing on drug-metabolizing enzymes. Drug Metab. Pharmacokinet. 2018, 33, 133–140. [Google Scholar] [CrossRef]
- Qiu, G.H.; Xie, X.; Xu, F.; Shi, X.; Wang, Y.; Deng, L. Distinctive pharmacological differences between liver cancer cell lines HepG2 and Hep3B. Cytotechnology 2015, 67, 1–12. [Google Scholar] [CrossRef]
- Punzalan, C.S.; Barry, C.T. Acute Liver Failure: Diagnosis and Management. J. Intensive Care Med. 2016, 31, 642–653. [Google Scholar] [CrossRef]
- Lefkowitch, J.H. The Pathology of Acute Liver Failure. Adv. Anat. Pathol. 2016, 23, 144–158. [Google Scholar] [CrossRef]
- Stravitz, R.T.; Lee, W.M. Acute liver failure. Lancet 2019, 394, 869–881. [Google Scholar] [CrossRef]
- Ramachandran, A.; Jaeschke, H. Acetaminophen Hepatotoxicity. Semin. Liver Dis. 2019, 39, 221–234. [Google Scholar] [CrossRef]
- Truong, V.L.; Ko, S.Y.; Jun, M.; Jeong, W.S. Quercitrin from Toona sinensis (Juss.) M.Roem. Attenuates Acetaminophen-Induced Acute Liver Toxicity in HepG2 Cells and Mice through Induction of Antioxidant Machinery and Inhibition of Inflammation. Nutrients 2016, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, M.A.; Jang, S.; Banerjee, A.; Hardwick, J.P.; Song, B.J. Robust protein nitration contributes to acetaminophen-induced mitochondrial dysfunction and acute liver injury. Free Radic. Biol. Med. 2013, 60, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, J.Y.; Jang, E.J.; Jegal, K.H.; Moon, S.Y.; Ku, S.K.; Kang, S.H.; Cho, I.J.; Park, S.J.; Lee, J.R.; et al. Combination of honokiol and magnolol inhibits hepatic steatosis through AMPK-SREBP-1c pathway. Exp. Biol. Med. 2014, 240, 508–518. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, S.-J.; Bak, S.B.; Kim, Y.W. Coordination of AMPK and YAP by Spatholobi Caulis and Procyanidin B2 Provides Antioxidant Effects In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 13730. https://doi.org/10.3390/ijms232213730
Bae S-J, Bak SB, Kim YW. Coordination of AMPK and YAP by Spatholobi Caulis and Procyanidin B2 Provides Antioxidant Effects In Vitro and In Vivo. International Journal of Molecular Sciences. 2022; 23(22):13730. https://doi.org/10.3390/ijms232213730
Chicago/Turabian StyleBae, Su-Jin, Seon Been Bak, and Young Woo Kim. 2022. "Coordination of AMPK and YAP by Spatholobi Caulis and Procyanidin B2 Provides Antioxidant Effects In Vitro and In Vivo" International Journal of Molecular Sciences 23, no. 22: 13730. https://doi.org/10.3390/ijms232213730
APA StyleBae, S.-J., Bak, S. B., & Kim, Y. W. (2022). Coordination of AMPK and YAP by Spatholobi Caulis and Procyanidin B2 Provides Antioxidant Effects In Vitro and In Vivo. International Journal of Molecular Sciences, 23(22), 13730. https://doi.org/10.3390/ijms232213730





