Exploring the RNA Editing Events and Their Potential Regulatory Roles in Tea Plant (Camellia sinensis L.)
Abstract
1. Introduction
2. Results
2.1. Identification of RNA Editing Sites in the Chloroplast and Mitochondrial Genomes of Tea Plants
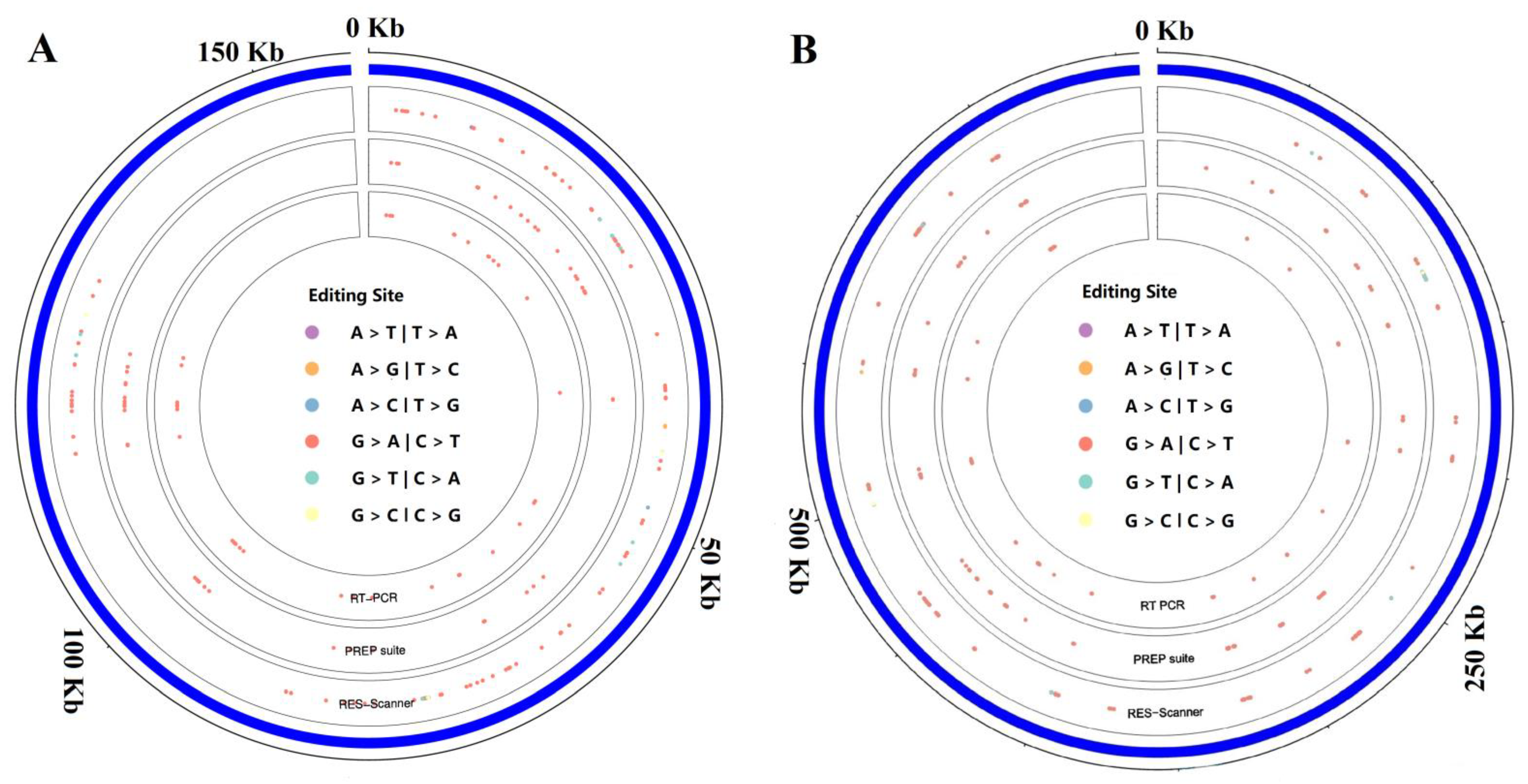
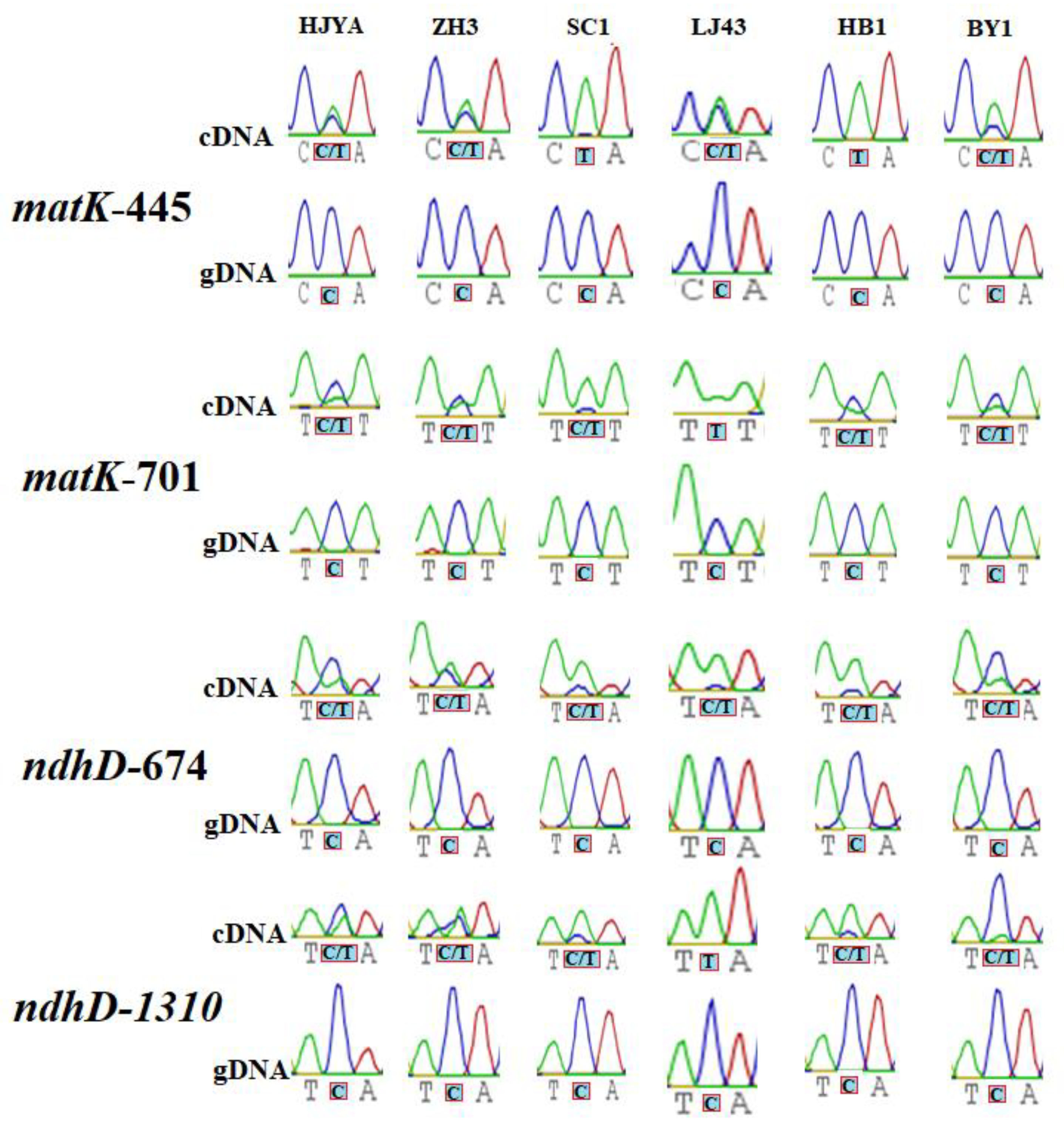
2.2. Confirmation of RNA Editing Sites in Tea Chloroplasts and Mitochondria
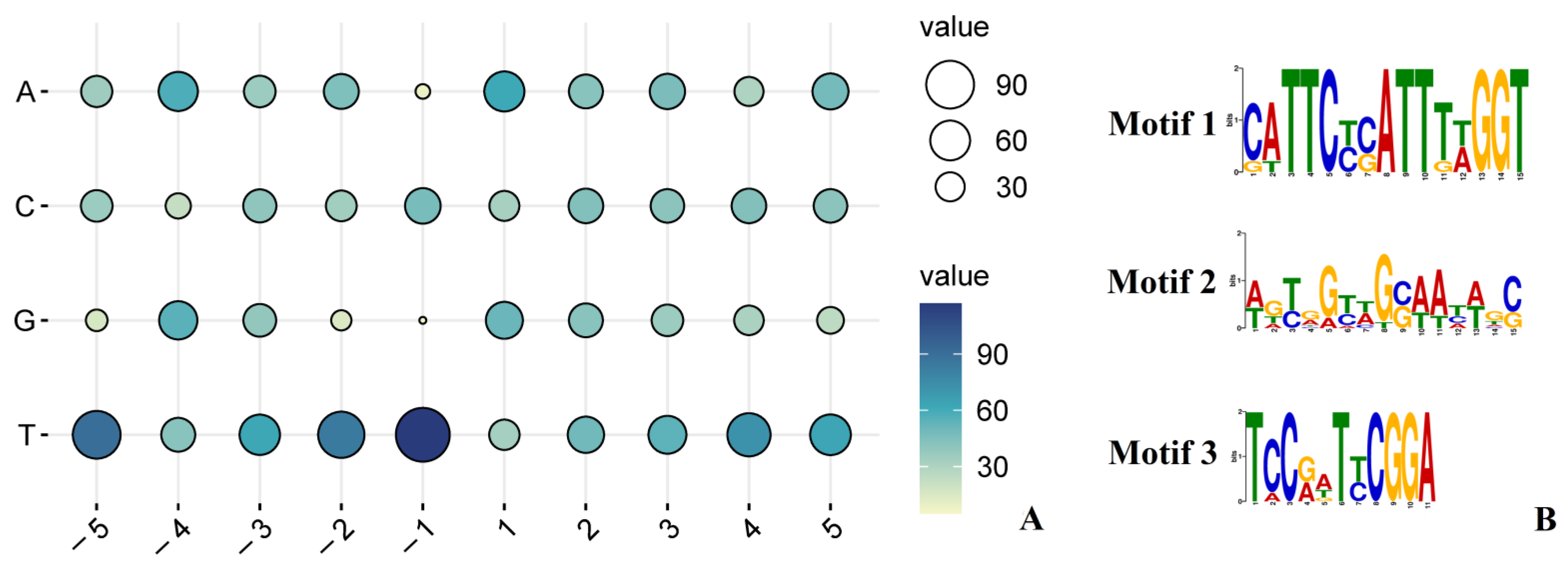
2.3. Sequence Features around RNA Editing Sites
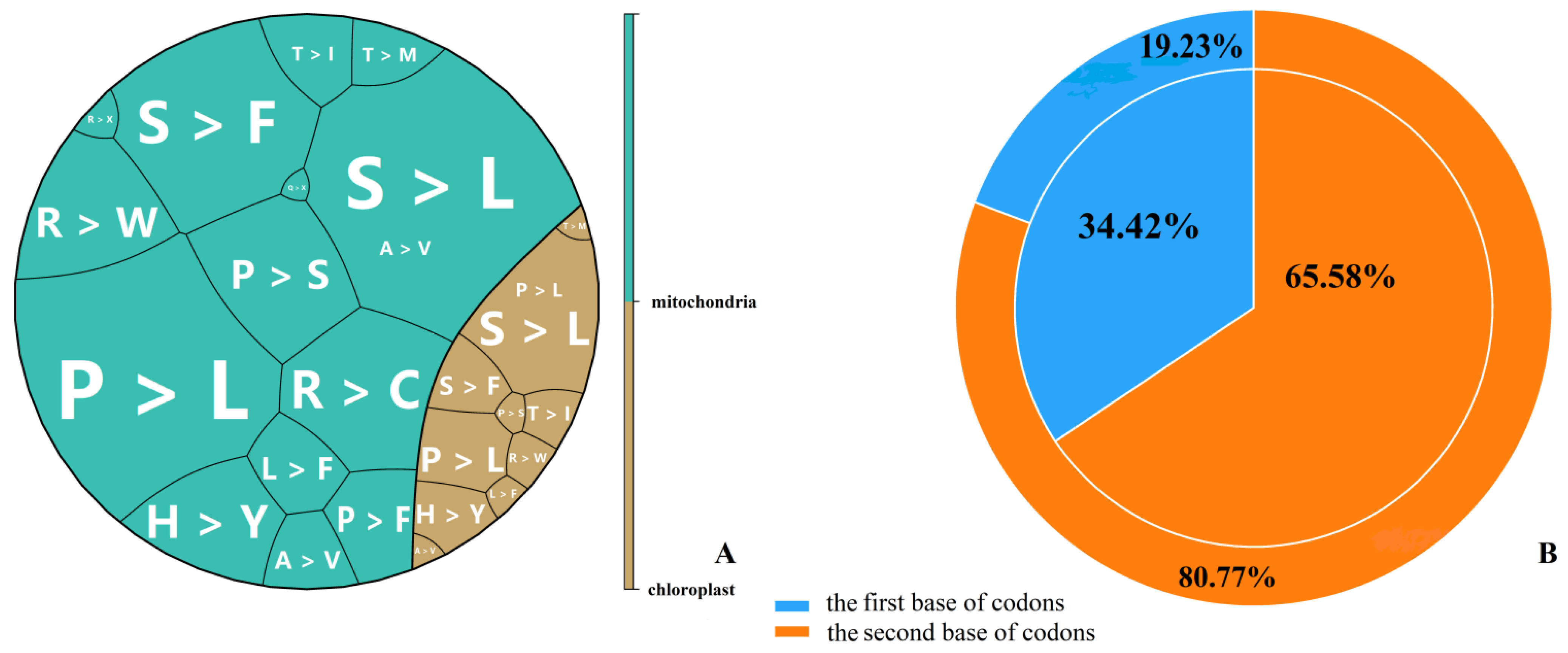
2.4. Impact of RNA Editing on the Subsequent Interpretation of Genetic Information
2.5. Relationship between RNA Editing and Etiolation and Albino Tea Plants
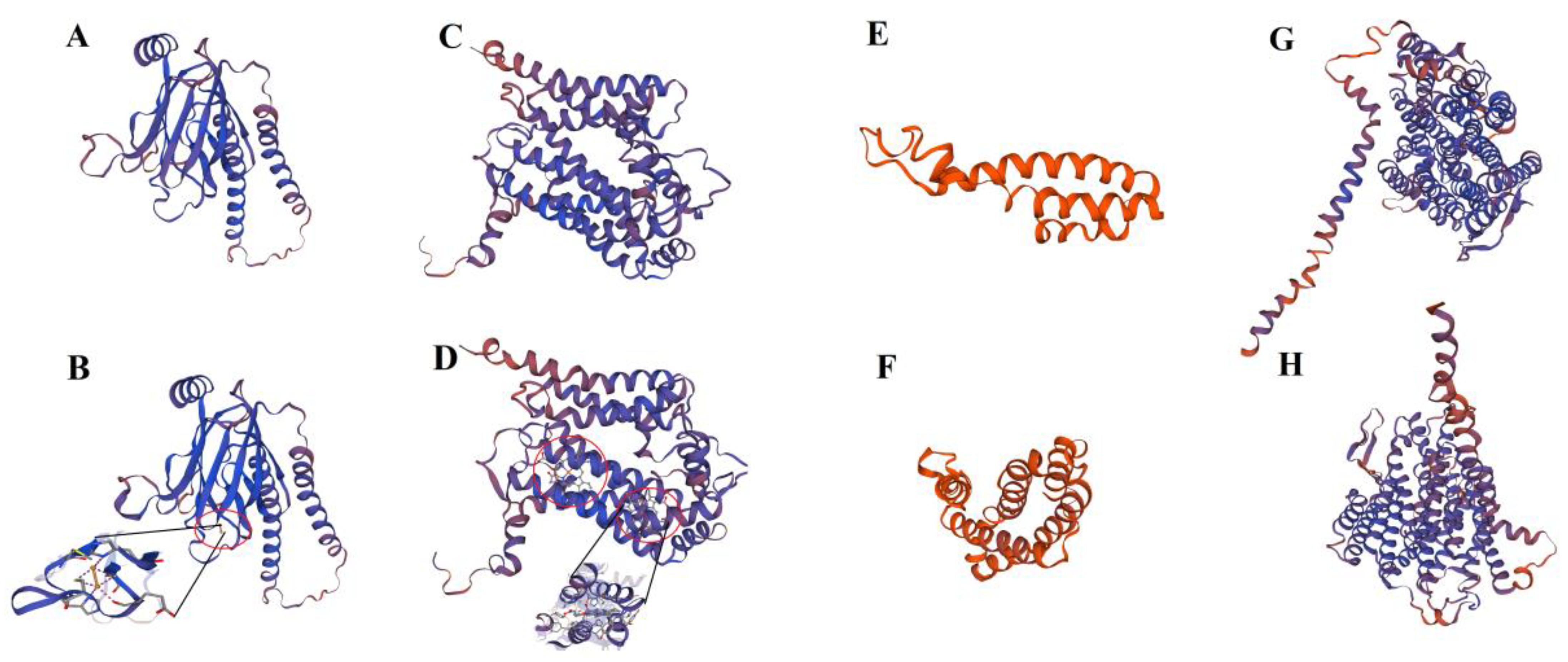
2.6. Interaction Prediction of PLS-CsPPR and the Target Sequences of RNA Editing Sites
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Data Collection
4.3. Identification of RNA Editing Sites in Tea Chloroplasts and Mitochondria
4.4. Validation of Predicted RNA Editing Sites
4.5. Contextual and Potential Motifs Analysis around RNA Editing Sites
4.6. Structural Analysis of Proteins and RNAs
4.7. Interaction Analysis between PLS-CsPPR Proteins and Target Sequence Containing RNA Editing Sites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y. RNA editing of plastid-encoded genes. Photosynthetica 2018, 56, 48–61. [Google Scholar] [CrossRef]
- Small, I.D.; Schallenberg-Rüdinger, M.; Takenaka, M.; Mireau, H.; Ostersetzer-Biran, O. Plant organellar RNA editing: What 30 years of research has revealed. Plant J. 2020, 101, 1040–1056. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, Q.; Yin, P. RNA editing machinery in plant organelles. Sci. China Life Sci. 2018, 61, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Knie, N.; Grewe, F.; Fischer, S.; Knoop, V. Reverse U-to-C editing exceeds C-to-U RNA editing in some ferns—A monilophyte-wide comparison of chloroplast and mitochondrial RNA editing suggests independent evolution of the two processes in both organelles. BMC Evol. Biol. 2016, 16, 134. [Google Scholar] [CrossRef]
- Nishikura, K. Functions and regulation of RNA editing by ADAR deaminases. Annu. Rev. Biochem. 2010, 79, 321–349. [Google Scholar] [CrossRef]
- Göringer, H.U. ‘Gestalt,’ composition and function of the Trypanosoma brucei editosome. Annu. Rev. Microbiol. 2012, 66, 65–82. [Google Scholar] [CrossRef]
- Covello, P.S.; Gray, M.W. RNA editing in plant mitochondria. Nature 1989, 341, 662–666. [Google Scholar] [CrossRef]
- Gualberto, J.; Lamattina, L.; Bonnard, G.; Weil, J.; Grienenberger, J. RNA editing in wheat mitochondria results in the conservation of protein sequences. Nature 1989, 341, 660–662. [Google Scholar] [CrossRef] [PubMed]
- Hiesel, R.; Wissinger, B.; Schuster, W.; Brennicke, A. RNA editing in plant mitochondria. Science 1989, 246, 1632–1634. [Google Scholar] [CrossRef]
- Hoch, B.; Maier, R.M.; Appel, K.; Igloi, G.L.; Kössel, H. Editing of a chloroplast mRNA by creation of an initiation codon. Nature 1991, 353, 178–180. [Google Scholar] [CrossRef]
- Takenaka, M.; Zehrmann, A.; Verbitskiy, D.; Härtel, B.; Brennicke, A. RNA editing in plants and its evolution. Annu. Rev. Genet. 2013, 47, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, D.; Li, Z.A.; Molloy, D.P.; Luo, Z.F.; Su, Y.; Li, H.O.; Liu, Q.; Wang, R.Z.; Xiao, L.T. The PPR protein RARE1-mediated editing of chloroplast accD transcripts is required for fatty acid biosynthesis and heat tolerance in Arabidopsis. Plant Commun. 2022, 100461. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, W.; Wen, K.; Chen, G.; Sun, J.; Tian, Y.; Tang, W.; Yu, J.; An, H.; Wu, T.; et al. OsPPR6, a pentatricopeptide repeat protein involved in editing and splicing chloroplast RNA, is required for chloroplast biogenesis in rice. Plant Mol. Biol. 2017, 95, 345–357. [Google Scholar] [CrossRef]
- He, P.; Wu, S.; Jiang, Y.; Zhang, L.; Tang, M.; Xiao, G.; Yu, J. GhYGL1d, a pentatricopeptide repeat protein, is required for chloroplast development in cotton. BMC Plant Biol. 2019, 19, 350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, W.; Zhang, S.; Lu, J.; Chen, R.; Fu, J.; Gu, R.; Wang, G.; Wang, J.; Cui, Y. Dek504 encodes a mitochondrion-targeted E+-type pentatricopeptide repeat protein essential for RNA editing and seed development in Maize. Int. J. Mol. Sci. 2022, 23, 2513. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Cao, S.K.; Yang, H.; Liu, R.; Sun, F.; Wang, L.; Wang, M.; Tan, B.C. DEK48 is required for RNA editing at multiple mitochondrial sites and seed development in Maize. Int. J. Mol. Sci. 2022, 23, 3064. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, M.; Liu, S.; Teng, Q.; Li, S.; Jiang, Y. Functions of PPR proteins in plant growth and development. Int. J. Mol. Sci. 2021, 22, 11274. [Google Scholar] [CrossRef]
- Chu, D.; Wei, L. Systematic analysis reveals cis and trans determinants affecting C-to-U RNA editing in Arabidopsis thaliana. BMC Genet. 2020, 21, 98. [Google Scholar] [CrossRef]
- Kumbhar, F.; Nie, X.; Xing, G.; Zhao, X.; Lin, Y.; Wang, S.; Weining, S. Identification and characterisation of RNA editing sites in chloroplast transcripts of einkorn wheat (Triticum monococcum): RNA editing sites in einkorn wheat plastome. Ann. Appl. Biol. 2018, 172, 197–207. [Google Scholar] [CrossRef]
- Yang, J.; Cui, Y.; Zhang, X.; Yang, Z.; Lai, J.; Song, W.; Liang, J.; Li, X. Maize PPR278 functions in mitochondrial RNA splicing and editing. Int. J. Mol. Sci. 2022, 23, 3035. [Google Scholar] [CrossRef]
- Wu, B.; Chen, H.; Shao, J.; Zhang, H.; Wu, K.; Liu, C. Identification of symmetrical RNA editing events in the mitochondria of Salvia miltiorrhiza by strand-specific RNA sequencing. Sci. Rep. 2017, 7, 42250. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Takenaka, S.; Wang, T.; Frink, B.; Shikanai, T.; Takenaka, M. DYW deaminase domain has a distinct preference for neighboring nucleotides of the target RNA editing sites. Plant J. 2022, 111, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Barkan, A.; Small, I. Pentatricopeptide repeat proteins in plants. Annu. Rev. Plant Biol. 2014, 65, 415–442. [Google Scholar] [CrossRef]
- Takenaka, M.; Zehrmann, A.; Verbitskiy, D.; Kugelmann, M.; Härtel, B.; Brennicke, A. Multiple organellar RNA editing factor (MORF) family proteins are required for RNA editing in mitochondria and plastids of plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5104–5109. [Google Scholar] [CrossRef] [PubMed]
- Bentolila, S.; Heller, W.P.; Sun, T.; Babina, A.M.; Friso, G.; van Wijk, K.J.; Hanson, M.R. RIP1, a member of an Arabidopsis protein family, interacts with the protein RARE1 and broadly affects RNA editing. Proc. Natl. Acad. Sci. USA 2012, 109, E1453–E1461. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Germain, A.; Giloteaux, L.; Hammani, K.; Barkan, A.; Hanson, M.R.; Bentolila, S. An RNA recognition motif-containing protein is required for plastid RNA editing in Arabidopsis and Maize. Proc. Natl. Acad. Sci. USA 2013, 110, E1169–E1178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Tang, W.; Hedtke, B.; Zhong, L.; Liu, L.; Peng, L.; Lu, C.; Grimm, B.; Lin, R. Tetrapyrrole biosynthetic enzyme protoporphyrinogen IX oxidase 1 is required for plastid RNA editing. Proc. Natl. Acad. Sci. USA 2014, 111, 2023–2028. [Google Scholar] [CrossRef]
- Boussardon, C.; Salone, V.; Avon, A.; Berthomé, R.; Hammani, K.; Okuda, K.; Shikanai, T.; Small, I.; Lurin, C. Two interacting proteins are necessary for the editing of the NdhD-1 site in Arabidopsis plastids. Plant Cell 2012, 24, 3684–3694. [Google Scholar] [CrossRef]
- Sun, T.; Shi, X.; Friso, G.; Van Wijk, K.; Bentolila, S.; Hanson, M.R. A zinc finger motif-containing protein is essential for chloroplast RNA editing. PLoS Genet. 2015, 11, e1005028. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Wu, J.; Han, X.; Gu, X.; Lu, T.; Zhang, Z. The RNA editing factor DUA1 is crucial to chloroplast development at low temperature in rice. New Phytol. 2019, 221, 834–849. [Google Scholar] [CrossRef]
- Sosso, D.; Mbelo, S.; Vernoud, V.; Gendrot, G.; Dedieu, A.; Chambrier, P.; Dauzat, M.; Heurtevin, L.; Guyon, V.; Takenaka, M.; et al. PPR2263, a DYW-Subgroup Pentatricopeptide repeat protein, is required for mitochondrial nad5 and cob transcript editing, mitochondrion biogenesis, and maize growth. Plant Cell 2012, 24, 676–691. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.H.; Zheng, X.Q.; Liang, Y.R. High-light-induced degradation of photosystem II subunits’ involvement in the albino phenotype in tea plants. Int. J. Mol. Sci. 2022, 23, 8522. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Kong, X.; Zhang, Y.; Wang, S.; Zhou, H.; Fang, D.; Yue, W.; Chen, C. Metabolomic profiling in combination with data association analysis provide insights about potential metabolic regulation networks among non-volatile and volatile metabolites in Camellia sinensis cv Baijiguan. Plants 2022, 11, 2557. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; An, C.; Zhao, Y.; Xiao, Y.; Bao, L.; Gong, C.; Gao, Y. Genome-wide identification and characterization of the CsFHY3/FAR1 gene family and expression analysis under biotic and abiotic stresses in tea plants (Camellia sinensis). Plants 2021, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Chen, J.D.; Wang, S.L.; Chen, L.; Ma, C.L.; Yao, M.Z. Repressed gene expression of photosynthetic antenna proteins associated with yellow leaf variation as revealed by bulked segregant RNA-seq in tea plant Camellia sinensis. J. Agric. Food Chem. 2020, 68, 8068–8079. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Y.; Meng, Y.; Xiao, Y.; Zhao, J.; Xiao, B.; An, C.; Gao, Y. PPR proteins in the tea plant (Camellia sinensis) and their potential roles in the leaf color changes. Sci. Hortic-Amsterdam 2022, 293, 110745. [Google Scholar] [CrossRef]
- Mower, J.P. The PREP suite: Predictive RNA editors for plant mitochondrial genes, chloroplast genes and user-defined alignments. Nucleic Acids Res. 2009, 37, W253–W259. [Google Scholar] [CrossRef]
- Wang, Z.; Lian, J.; Li, Q.; Zhang, P.; Zhou, Y.; Zhan, X.; Zhang, G. RES-Scanner: A software package for genome-wide identification of RNA-editing sites. Gigascience 2016, 5, 37. [Google Scholar] [CrossRef]
- Bailey, M.; Ivanauskaite, A.; Grimmer, J.; Akintewe, O.; Payne, A.C.; Osborne, R.; Labandera, A.M.; Etherington, R.D.; Rantala, M.; Baginsky, S.; et al. The Arabidopsis NOT4A E3 ligase promotes PGR3 expression and regulates chloroplast translation. Nat. Commun. 2021, 12, 251. [Google Scholar] [CrossRef]
- Gutmann, B.; Millman, M.; Vincis Pereira Sanglard, L.; Small, I.; Colas des Francs-Small, C. The pentatricopeptide repeat protein MEF100 is required for the editing of four mitochondrial editing sites in Arabidopsis. Cells 2021, 10, 468. [Google Scholar] [CrossRef]
- Oldenkott, B.; Burger, M.; Hein, A.C.; Jörg, A.; Senkler, J.; Braun, H.P.; Knoop, V.; Takenaka, M.; Schallenberg-Rüdinger, M. One C-to-U RNA editing site and two independently evolved editing factors: Testing reciprocal complementation with DYW-Type PPR proteins from the Moss Physcomitrium (Physcomitrella) patens and the flowering plants Macadamia integrifolia and Arabidopsis. Plant Cell 2020, 32, 2997–3018. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cui, L.; Feng, K.; Deng, P.; Du, X.; Wan, F.; Song, W.; Nie, X. Comparative analysis of Asteraceae chloroplast genomes: Structural organization, RNA editing and evolution. Plant Mol. Biol. Rep. 2015, 33, 1526–1538. [Google Scholar] [CrossRef]
- Oldenkott, B.; Yamaguchi, K.; Tsuji-Tsukinoki, S.; Knie, N.; Knoop, V. Chloroplast RNA editing going extreme: More than 3400 events of C-to-U editing in the chloroplast transcriptome of the lycophyte Selaginella uncinata. RNA 2014, 20, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Edera, A.A.; Gandini, C.L.; Sanchez-Puerta, M.V. Towards a comprehensive picture of C-to-U RNA editing sites in angiosperm mitochondria. Plant Mol. Biol. 2018, 97, 215–231. [Google Scholar]
- Ramadan, A.M.; Alnufaei, A.A.; Khan, T.K.; Ali, H.M.; Eissa, H.F.; Hassan, S.M. The first report of RNA U to C or G editing in the mitochondrial NADH dehydrogenase subunit 5 (Nad5) transcript of wild barley. Mol. Biol. Rep. 2021, 48, 6057–6064. [Google Scholar] [CrossRef]
- Liu, R.; Li, W.; Lu, D.; Li, J.; Qu, Y.; Jin, W.; Dong, X. ariation in RNA-editing sites of chloroplast protein-coding genes in early-maturity mutant induced by carbon-ion beam in Sweet Sorghum. Plant Breed. 2020, 139, 762–778. [Google Scholar] [CrossRef]
- Shikanai, T. RNA editing in plants: Machinery and flexibility of site recognition. BBA-Bioenerg. 2015, 1847, 779–785. [Google Scholar] [CrossRef]
- Yagi, Y.; Hayashi, S.; Kobayashi, K.; Hirayama, T.; Nakamura, T. Elucidation of the RNA recognition code for pentatricopeptide repeat proteins involved in organelle RNA editing in plants. PLoS ONE 2013, 8, e57286. [Google Scholar]
- Shen, C.; Wang, X.; Liu, Y.; Li, Q.; Yang, Z.; Yan, N.; Zou, T.; Yin, P. Specific RNA recognition by designer pentatricopeptide repeat protein. Mol. Plant 2015, 8, 667–670. [Google Scholar] [CrossRef]
- Shen, C.; Zhang, D.; Guan, Z.; Liu, Y.; Yang, Z.; Yang, Y.; Wang, X.; Wang, Q.; Zhang, Q.; Fan, S.; et al. Structural basis for specific single-stranded RNA recognition by designer pentatricopeptide repeat proteins. Nat. Commun. 2016, 7, 11285. [Google Scholar] [CrossRef]
- Kobayashi, T.; Yagi, Y.; Nakamura, T. Comprehensive prediction of target RNA editing sites for PLS-Class PPR proteins in Arabidopsis thaliana. Plant Cell Physiol. 2019, 60, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Loudya, N.; Okunola, T.; He, J.; Jarvis, P.; López-Juez, E. Retrograde signalling in a virescent mutant triggers an anterograde delay of chloroplast biogenesis that requires GUN1 and is essential for survival. Philos. Trans. R. Soc. B 2020, 375, 20190400. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, J.A.; Sun, T.; Lin, L.; Hanson, M.R. Cytidine deaminase motifs within the DYW domain of two pentatricopeptide repeat-containing proteins are required for site-specific chloroplast RNA editing. J. Biol. Chem. 2015, 290, 2957–2968. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huang, J.; Chory, J. GUN1 interacts with MORF2 to regulate plastid RNA editing during retrograde signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 10162–10167. [Google Scholar] [CrossRef]
- Wang, X.; Feng, H.; Chang, Y.; Ma, C.; Wang, L.; Hao, X.; Li, A.; Cheng, H.; Wang, L.; Cui, P.; et al. Population sequencing enhances understanding of tea plant evolution. Nat. Commun. 2020, 11, 4447. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. In Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology; AAAI Press: Menlo Park, CA, USA, 1994. [Google Scholar]
- Hofacker, I.L. Vienna RNA secondary structure server. Nucleic Acids Res. 2003, 31, 3429–3431. [Google Scholar] [CrossRef]
- Möller, S.; Croning, M.D.; Apweiler, R. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 2001, 17, 646–653. [Google Scholar] [CrossRef]
- McGuffin, L.J.; Bryson, K.; Jones, D.T. The PSIPRED protein structure prediction server. Bioinformatics 2000, 16, 404–405. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.E.; Bailey, T.L.; Noble, W.S. FIMO: Scanning for occurrences of a given motif. Bioinformatics 2011, 27, 1017–1018. [Google Scholar] [CrossRef] [PubMed]
| Gene | Genome Position | Edited Nucleotide | Amino Acid Changes | Editing Type | Position in Codon |
|---|---|---|---|---|---|
| matK | 2313 | C1234 | H412→Y412 | C→T | 1 |
| matK | 2846 | C701 | S234→F234 | C→T | 2 |
| matK | 3102 | C445 | H149→Y149 | C→T | 1 |
| atpA | 11,605 | C914 | S305→L305 | C→T | 2 |
| atpA | 11,728 | C791 | P264→L264 | C→T | 2 |
| atpF | 13,757 | C92 | P31→H31 | C→T | 2 |
| rps2 | 16,983 | C248 | S83→L83 | C→T | 2 |
| rps2 | 17,097 | C134 | T45→I45 | C→T | 2 |
| rpoC2 | 17,886 | C3728 | S1243→L1243 | C→T | 2 |
| rpoC2 | 18,765 | C2849 | S950→F950 | C→T | 2 |
| rpoC1 | 24,531 | C62 | S21→L21 | C→T | 2 |
| psbZ | 37,856 | C50 | S17→L17 | C→T | 2 |
| ndhK | 52,675 | C81 | F27→F27 | C→T | 3 |
| ndhC | 52,958 | C40 | L14→L14 | C→T | 1 |
| atpB | 56,330 | C23 | S8→F8 | C→T | 2 |
| psaI | 61,448 | C80 | S27→F27 | C→T | 2 |
| psbF | 66,719 | C77 | S26→F26 | C→T | 2 |
| psbE | 66,843 | C214 | P72→S72 | C→T | 1 |
| rps18 | 70,747 | C221 | S74→L74 | C→T | 2 |
| petB | 78,764 | C611 | P204→L204 | C→T | 2 |
| rpoA | 81,234 | C200 | S67→F67 | C→T | 2 |
| rps8 | 82,841 | C182 | P61→L61 | C→T | 2 |
| ndhB | 97,156 | C1481 | P494→L494 | C→T | 2 |
| ndhB | 97,807 | C830 | S277→L277 | C→T | 2 |
| ndhB | 98,570 | C746 | S249→F249 | C→T | 2 |
| ndhB | 98,579 | C737 | P246→L246 | C→T | 2 |
| ndhB | 98,705 | C611 | S204→L204 | C→T | 2 |
| ndhB | 98,729 | C586 | H196→Y196 | C→T | 1 |
| ndhB | 98,848 | C467 | P156→L156 | C→T | 2 |
| ndhB | 99,167 | C149 | S50→L50 | C→T | 2 |
| ndhF | 114,736 | C290 | S97→L97 | C→T | 2 |
| ndhD | 118,369 | C1310 | S437→L437 | C→T | 2 |
| ndhD | 118,792 | C887 | P296→L296 | C→T | 2 |
| ndhD | 118,801 | C878 | S293→L293 | C→T | 2 |
| ndhD | 119,005 | C674 | S225→L225 | C→T | 2 |
| ndhD | 119,296 | C383 | S128→L128 | C→T | 2 |
| ndhA | 124,204 | C341 | S114→L114 | C→T | 2 |
| ndhH | 125,201 | C505 | H169→Y169 | C→T | 1 |
| Gene | Genome Position | Edited Nucleotide | Amino Acid Changes | Editing Type | Position in Codon |
|---|---|---|---|---|---|
| atp9 | 54,812 | C20 | S7→L7 | C→T | 2 |
| atp9 | 54,874 | C82 | L28→F28 | C→T | 1 |
| atp9 | 54,884 | C92 | S31→L31 | C→T | 2 |
| nad6 | 85,722 | C446 | S152→F152 | C→T | 2 |
| cox2 | 138,085 | C742 | R248→W248 | C→T | 1 |
| cox2 | 138,129 | C698 | T233→M233 | C→T | 2 |
| cox2 | 138,195 | C632 | S211→L211 | C→T | 2 |
| cox2 | 138,246 | C581 | S194→L194 | C→T | 2 |
| cox2 | 138,270 | C557 | P186→L186 | C→T | 2 |
| cox2 | 138,283 | C544 | P182→S182 | C→T | 1 |
| cox2 | 138,351 | C476 | S159→L159 | C→T | 2 |
| cox2 | 138,366 | C461 | P154→L154 | C→T | 2 |
| cox2 | 138,384 | C443 | T148→M148 | C→T | 2 |
| cox2 | 138,448 | C379 | R127→W127 | C→T | 1 |
| cox2 | 138,549 | C278 | P93→L93 | C→T | 2 |
| cox2 | 138,574 | C253 | R85→W85 | C→T | 1 |
| cox2 | 138,664 | C163 | R55→W55 | C→T | 1 |
| cox2 | 138,666 | C161 | S54→L54 | C→T | 2 |
| cox2 | 138,756 | C71 | S24→F24 | C→T | 2 |
| rps12 | 196,313 | C284 | S95→F95 | C→T | 2 |
| rps12 | 196,401 | C196 | H66→Y66 | C→T | 1 |
| rps12 | 196,497 | C100 | R34→C34 | C→T | 1 |
| rps12 | 196,526 | C71 | S24→L24 | C→T | 2 |
| nad3 | 196,959 | C43 | P15→S15 | C→T | 1 |
| rps1 | 239,984 | C107 | S36→F36 | C→T | 2 |
| rps1 | 240,035 | C56 | P19→L19 | C→T | 2 |
| matR | 272,897 | C1775 | P592→L592 | C→T | 2 |
| matR | 272,950 | C1722 | Y574→Y574 | C→T | 3 |
| matR | 272,984 | C1688 | P563→L563 | C→T | 2 |
| matR | 273,005 | C1667 | S556→F556 | C→T | 2 |
| ccmFn | 297,223 | C1423 | R475→W475 | C→T | 1 |
| ccmFn | 297,286 | C1486 | L496→F496 | C→T | 1 |
| ccmFn | 297,355 | C1555 | P519→S519 | C→T | 1 |
| atp4 | 323,099 | C416 | T139→I139 | C→T | 2 |
| atp4 | 323,109 | C406 | P136→S136 | C→T | 1 |
| atp4 | 323,120 | C395 | S132→L132 | C→T | 2 |
| atp4 | 323,264 | C251 | P84→L84 | C→T | 2 |
| atp4 | 323,267 | C248 | P83→L83 | C→T | 2 |
| atp4 | 323,288 | C227 | P76→L76 | C→T | 2 |
| atp4 | 323,300 | C215 | S72→L72 | C→T | 2 |
| atp4 | 323,397 | C118 | R40→C40 | C→T | 1 |
| atp4 | 323,426 | C89 | S30→L30 | C→T | 2 |
| atp4 | 323,444 | C71 | S24→L24 | C→T | 2 |
| atp4 | 323,456 | C59 | S20→F20 | C→T | 2 |
| nad41 | 323,824 | C149 | S50→L50 | C→T | 2 |
| nad41 | 323,872 | C101 | S34→L34 | C→T | 2 |
| atp1 | 396,108 | C1039 | P347→S347 | C→T | 1 |
| atp1 | 396,133 | C1064 | S355→L355 | C→T | 2 |
| atp1 | 396,247 | C1178 | S393→L393 | C→T | 2 |
| atp1 | 396,285 | C1216 | L406→F406 | C→T | 1 |
| atp1 | 396,361 | C1292 | P431→L431 | C→T | 2 |
| atp1 | 396,484 | C1415 | P472→L472 | C→T | 2 |
| rps7 | 421,118 | C332 | S111→L111 | C→T | 2 |
| rpl10 | 431,602 | C83 | S28→L28 | C→T | 2 |
| rpl10 | 431,620 | C101 | S34→L34 | C→T | 2 |
| rpl10 | 431,758 | C239 | S80→L80 | C→T | 2 |
| rpl10 | 431,833 | C314 | S105→L105 | C→T | 2 |
| ccmB | 432,360 | C554 | S185→L185 | C→T | 2 |
| ccmB | 432,363 | C551 | S184→L184 | C→T | 2 |
| ccmB | 432,400 | C514 | R172→C172 | C→T | 1 |
| ccmB | 432,402 | C512 | S171→F171 | C→T | 2 |
| ccmB | 432,411 | C503 | P168→L168 | C→T | 2 |
| ccmB | 432,420 | C494 | S165→L165 | C→T | 2 |
| ccmB | 432,429 | C485 | S162→L162 | C→T | 2 |
| ccmB | 432,438 | C476 | P159→L159 | C→T | 2 |
| ccmB | 432,439 | C475 | P159→L159 | C→T | 1 |
| ccmB | 432,477 | C467 | S156→L156 | C→T | 2 |
| ccmB | 432,486 | C428 | S143→L143 | C→T | 2 |
| ccmB | 432,490 | C424 | R142→C142 | C→T | 1 |
| ccmB | 432,535 | C379 | L127→L127 | C→T | 1 |
| ccmB | 432,547 | C367 | R123→W123 | C→T | 1 |
| ccmB | 432,576 | C338 | P113→L113 | C→T | 2 |
| ccmB | 432,601 | C313 | R105→W105 | C→T | 1 |
| ccmB | 432,610 | C304 | R102→C102 | C→T | 1 |
| ccmB | 432,628 | C286 | R96→W96 | C→T | 1 |
| ccmB | 432,720 | C194 | P65→F65 | C→T | 2 |
| ccmB | 432,721 | C193 | P65→F65 | C→T | 1 |
| ccmB | 432,735 | C179 | P60→L60 | C→T | 2 |
| ccmB | 432,742 | C172 | P58→S58 | C→T | 1 |
| ccmB | 432,750 | C164 | P55→L55 | C→T | 2 |
| ccmB | 432,754 | C160 | P54→S54 | C→T | 1 |
| ccmB | 432,760 | C154 | R52→W52 | C→T | 1 |
| ccmB | 432,765 | C149 | P50→L50 | C→T | 2 |
| ccmB | 432,766 | C148 | P50→L50 | C→T | 1 |
| ccmB | 432,777 | C137 | S46→F46 | C→T | 2 |
| ccmB | 432,786 | C128 | S43→L43 | C→T | 2 |
| ccmB | 432,827 | C87 | I29→I29 | C→T | 3 |
| ccmB | 432,834 | C80 | S27→L27 | C→T | 2 |
| ccmB | 432,843 | C71 | P24→L24 | C→T | 2 |
| ccmB | 432,871 | C43 | P15→S15 | C→T | 1 |
| ccmB | 432,886 | C28 | S10→L10 | C→T | 1 |
| atp6 | 450,313 | C548 | S183→F183 | C→T | 2 |
| atp6 | 450,607 | C254 | S85→L85 | C→T | 2 |
| atp6 | 450,824 | C37 | P12→S12 | C→T | 1 |
| nad5 | 504,064 | C155 | P52→L52 | C→T | 2 |
| nad5 | 505,005 | C245 | P82→L82 | C→T | 2 |
| nad5 | 505,035 | C275 | S92→F92 | C→T | 2 |
| nad5 | 505,121 | C361 | P121→F121 | C→T | 1 |
| nad5 | 505,122 | C362 | P121→F121 | C→T | 2 |
| nad5 | 505,137 | C377 | P126→L126 | C→T | 2 |
| nad5 | 505,161 | C401 | S134→F134 | C→T | 2 |
| nad5 | 505,269 | C509 | P170→L170 | C→T | 2 |
| nad5 | 505,302 | C542 | P181→L181 | C→T | 2 |
| nad5 | 505,311 | C551 | S184→L184 | C→T | 2 |
| nad5 | 505,371 | C611 | A204→V204 | C→T | 2 |
| nad5 | 505,392 | C632 | S211→F211 | C→T | 2 |
| nad5 | 505,394 | C634 | R212→C212 | C→T | 1 |
| nad5 | 505,439 | C679 | L227→F227 | C→T | 1 |
| nad5 | 505,476 | C716 | S239→L239 | C→T | 2 |
| nad5 | 505,488 | C728 | S243→L243 | C→T | 2 |
| nad5 | 505,598 | C838 | P280→S280 | C→T | 1 |
| orf115b | 506,667 | C77 | S50→L50 | C→T | 2 |
| ccmFc | 553,661 | C1133 | P378→L378 | C→T | 2 |
| ccmFc | 553,682 | C1154 | S385→L385 | C→T | 2 |
| ccmFc | 553,790 | C1262 | S421→L421 | C→T | 2 |
| sdh3 | 576,179 | C67 | P23→S23 | C→T | 1 |
| sdh3 | 576,186 | C74 | S25→F25 | C→T | 2 |
| rpl5 | 646,335 | C92 | S31→L31 | C→T | 2 |
| rpl5 | 646,412 | C169 | P57→S57 | C→T | 1 |
| rpl5 | 646,415 | C172 | R58→C58 | C→T | 1 |
| rpl5 | 646,693 | C450 | I150→I150 | C→T | 3 |
| rpl5 | 646,761 | C518 | P173→L173 | C→T | 2 |
| rpl5 | 646,764 | C521 | P174→L174 | C→T | 2 |
| rps14 | 647,079 | C271 | P91→S91 | C→T | 1 |
| cob | 648,551 | C118 | P40→S40 | C→T | 1 |
| cob | 648,611 | C178 | H60→Y60 | C→T | 1 |
| cob | 648,719 | C286 | L96→F96 | C→T | 1 |
| cob | 648,731 | C298 | H100→Y100 | C→T | 1 |
| cob | 648,758 | C325 | H109→Y109 | C→T | 1 |
| cob | 648,791 | C358 | R120→W120 | C→T | 1 |
| cob | 648,852 | C419 | P140→L140 | C→T | 2 |
| cob | 649,001 | C568 | H150→Y150 | C→T | 1 |
| cob | 649,113 | C680 | S227→F227 | C→T | 2 |
| cob | 649,241 | C808 | P270→S270 | C→T | 1 |
| cob | 649,286 | C853 | H285→Y285 | C→T | 1 |
| cob | 649,341 | C908 | P303→L303 | C→T | 2 |
| cob | 649,347 | C914 | S305→F305 | C→T | 2 |
| cob | 649,415 | C982 | H327→Y327 | C→T | 1 |
| cob | 649,448 | C1015 | R339→C339 | C→T | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Li, Z.; Wang, Z.; Xiao, Y.; Bao, L.; Wang, M.; An, C.; Gao, Y. Exploring the RNA Editing Events and Their Potential Regulatory Roles in Tea Plant (Camellia sinensis L.). Int. J. Mol. Sci. 2022, 23, 13640. https://doi.org/10.3390/ijms232113640
Zhang M, Li Z, Wang Z, Xiao Y, Bao L, Wang M, An C, Gao Y. Exploring the RNA Editing Events and Their Potential Regulatory Roles in Tea Plant (Camellia sinensis L.). International Journal of Molecular Sciences. 2022; 23(21):13640. https://doi.org/10.3390/ijms232113640
Chicago/Turabian StyleZhang, Mengyuan, Zhuo Li, Zijian Wang, Yao Xiao, Lu Bao, Min Wang, Chuanjing An, and Yuefang Gao. 2022. "Exploring the RNA Editing Events and Their Potential Regulatory Roles in Tea Plant (Camellia sinensis L.)" International Journal of Molecular Sciences 23, no. 21: 13640. https://doi.org/10.3390/ijms232113640
APA StyleZhang, M., Li, Z., Wang, Z., Xiao, Y., Bao, L., Wang, M., An, C., & Gao, Y. (2022). Exploring the RNA Editing Events and Their Potential Regulatory Roles in Tea Plant (Camellia sinensis L.). International Journal of Molecular Sciences, 23(21), 13640. https://doi.org/10.3390/ijms232113640







