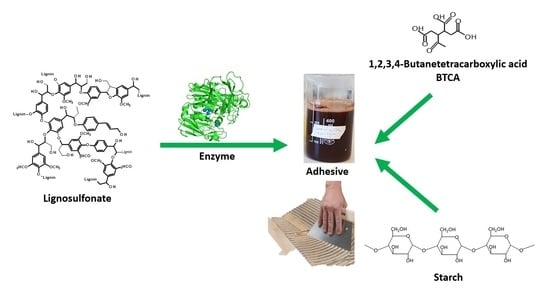
Improving Properties of Starch-Based Adhesives with Carboxylic Acids and Enzymatically Polymerized Lignosulfonates
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Laccase Polymerized Lignosulfonates
2.2. Viscosity
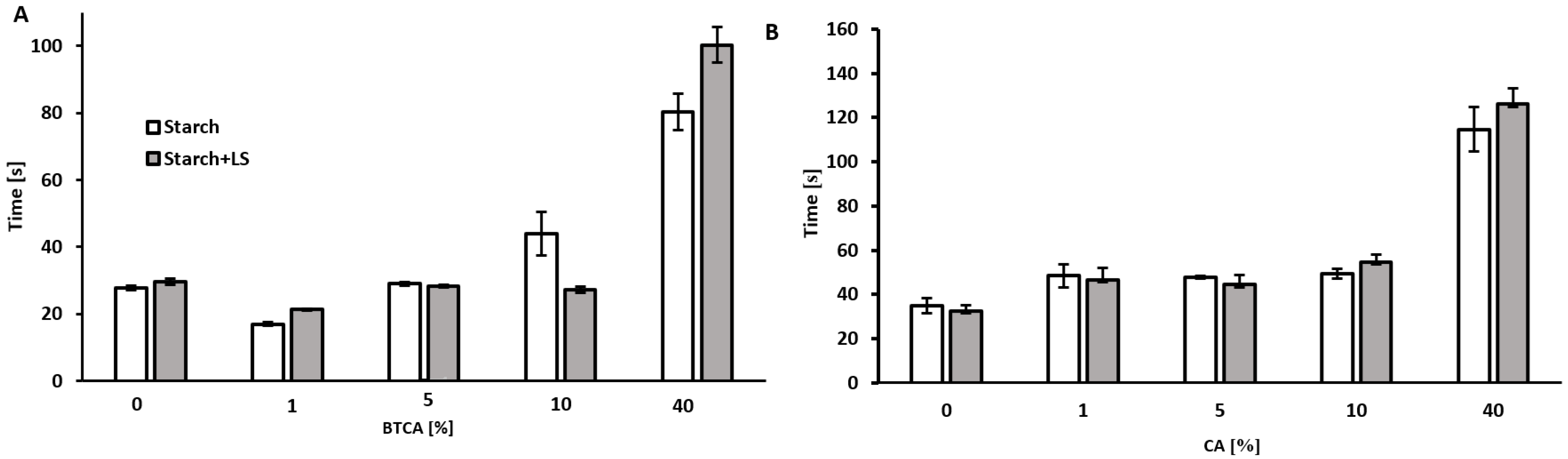
2.3. Bonding Times
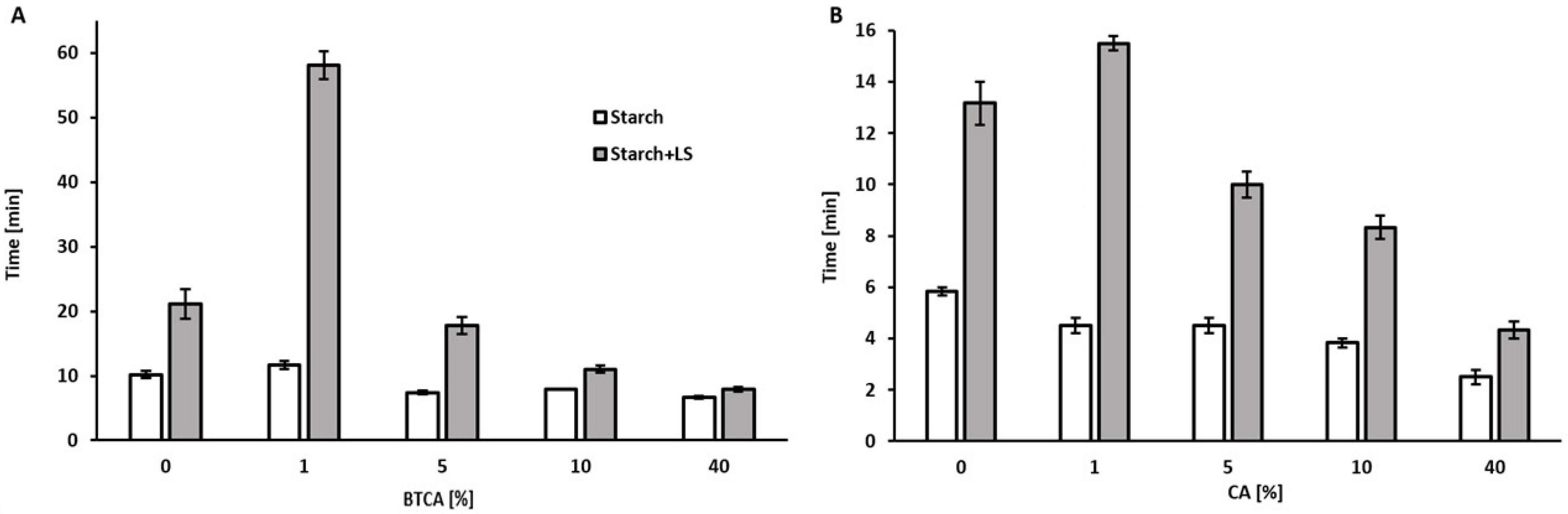
2.4. Wet Resistance
3. Materials and Methods
3.1. Materials and Enzymes
3.2. Determination of Phenol Content
3.3. Enzymatic Polymerization of Lignosulfonates
3.4. Characterization of Laccase Polymerized Lignosulfonates
3.5. Adhesive Synthesis
3.6. Characterization the Adhesive
3.6.1. Viscosity Measurement
3.6.2. Bonding Measurements
3.6.3. Wet Resistance Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nafchi, A.M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic Starches: Properties, Challenges, and Prospects. Starch/Staerke 2013, 65, 61–72. [Google Scholar] [CrossRef]
- Kaur, B.; Ariffin, F.; Bhat, R.; Karim, A.A. Progress in Starch Modification in the Last Decade. Food Hydrocoll. 2012, 26, 398–404. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Dhull, S.B.; Siroha, A.K.; Purewal, S.S.; Kaur, M.; Kidwai, M.K. Oat Starch: Physico-Chemical, Morphological, Rheological Characteristics and Its Applications—A Review. Int. J. Biol. Macromol. 2020, 154, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in Bio-Based Plastics and Plasticizing Modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Starch-Based Adhesives for Wood/Wood Composite Bonding: Review. Open J. Polym. Chem. 2017, 7, 19–32. [Google Scholar] [CrossRef]
- Tako, M.; Tamaki, Y.; Teruya, T.; Takeda, Y. The Principles of Starch Gelatinization and Retrogradation. Food Nutr. Sci. 2014, 05, 280–291. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C.; Liu, Q. Thermoplastic Starch Processing and Characteristics-A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1353–1370. [Google Scholar] [CrossRef]
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.P.; Fernando, N.M.L.; Wanninayaka, D.B.; Dassanayake, R.S.; Rajapaksha, S.M.; Manamperi, A.; Fernando, C.A.N.; Kulatunga, A.K.; et al. Development of Starch-Based Materials Using Current Modification Techniques and Their Applications: A Review. Molecules 2021, 26, 6880. [Google Scholar] [CrossRef]
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.P.; Fernando, N.M.L.; Wanninayaka, D.B.; Manamperi, A.; Dassanayake, R.S.; Rajapaksha, S.M.; Gangoda, M.; Fernando, C.A.N.; et al. Preparation and Characterization of Biodegradable Cassava Starch Thin Films for Potential Food Packaging Applications. Cellulose 2021, 28, 10531–10548. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, L.; Gu, J.; Tan, H.; Zhu, L. Preparation and Properties of a Starch-Based Wood Adhesive with High Bonding Strength and Water Resistance. Carbohydr. Polym. 2015, 115, 32–57. [Google Scholar] [CrossRef]
- Yin, Y.; Li, J.; Liu, Y.; Li, Z. Starch Crosslinked with Poly(Vinyl Alcohol) by Boric Acid. J. Appl. Polym. Sci. 2005, 96, 1394–1397. [Google Scholar] [CrossRef]
- Valodkar, M.; Thakore, S. Isocyanate Crosslinked Reactive Starch Nanoparticles for Thermo-Responsive Conducting Applications. Carbohydr. Res. 2010, 345, 2354–2360. [Google Scholar] [CrossRef] [PubMed]
- Delval, F.; Crini, G.; Bertini, S.; Filiatre, C.; Torri, G. Preparation, Characterization and Sorption Properties of Crosslinked Starch-Based Exchangers. Carbohydr. Polym. 2005, 60, 67–75. [Google Scholar] [CrossRef]
- Gunawardene, O.H.P.; Gunathilake, C.; Amaraweera, S.M.; Fernando, N.M.L.; Wanninayaka, D.B.; Manamperi, A.; Kulatunga, A.K.; Rajapaksha, S.M.; Dassanayake, R.S.; Fernando, C.A.N.; et al. Compatibilization of Starch/Synthetic Biodegradable Polymer Blends for Packaging Applications: A Review. J. Compos. Sci. 2021, 5, 300. [Google Scholar] [CrossRef]
- Soykeabkaew, N.; Supaphol, P.; Rujiravanit, R. Preparation and Characterization of Jute- and Flax-Reinforced Starch-Based Composite Foams. Carbohydr. Polym. 2004, 58, 53–63. [Google Scholar] [CrossRef]
- Kaisangsri, N.; Kerdchoechuen, O.; Laohakunjit, N. Characterization of Cassava Starch Based Foam Blended with Plant Proteins, Kraft Fiber, and Palm Oil. Carbohydr. Polym. 2014, 110, 70–77. [Google Scholar] [CrossRef]
- Shey, J.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Properties of Baked Starch Foam with Natural Rubber Latex. Ind. Crops Prod. 2006, 24, 34–40. [Google Scholar] [CrossRef]
- Cinelli, P.; Chiellini, E.; Lawton, J.W.; Imam, S.H. Foamed Articles Based on Potato Starch, Corn Fibers and Poly(Vinyl Alcohol). Polym. Degrad. Stab. 2006, 91, 1147–1155. [Google Scholar] [CrossRef]
- Singh, R.P.; Pandey, J.K.; Rutot, D.; Degée, P.; Dubois, P. Biodegradation of Poly(ε-Caprolactone)/Starch Blends and Composites in Composting and Culture Environments: The Effect of Compatibilization on the Inherent Biodegradability of the Host Polymer. Carbohydr. Res. 2003, 338, 1759–1769. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Santagata, G.; Gomez d’Ayala, G.; Cerruti, P.; Talens Oliag, P.; Chiralt Boix, M.A.; Malinconico, M. Enhancement of Interfacial Adhesion between Starch and Grafted Poly(ε-Caprolactone). Carbohydr. Polym. 2016, 147, 16–27. [Google Scholar] [CrossRef]
- Chen, L.; Qiu, X.; Deng, M.; Hong, Z.; Luo, R.; Chen, X.; Jing, X. The Starch Grafted Poly(l-Lactide) and the Physical Properties of Its Blending Composites. Polymer 2005, 46, 5723–5729. [Google Scholar] [CrossRef]
- Yin, Q.; Chen, F.; Zhang, H.; Liu, C. Fabrication and Characterisation of Thermoplastic Starch/Poly(Butylene Succinate) Blends with Maleated Poly(Butylene Succinate) as Compatibiliser. Plast. Rubber Compos. 2015, 44, 362–367. [Google Scholar] [CrossRef]
- Bartolome, M.J.; Schwaiger, N.; Flicker, R.; Seidl, B.; Kozich, M.; Nyanhongo, G.S.; Guebitz, G.M. Enzymatic Synthesis of Wet-Resistant Lignosulfonate-Starch Adhesives. N. Biotechnol. 2022, 69, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.; Ortner, A.; Daxbacher, A.; Nyanhongo, G.S.; Bauer, W.; Guebitz, G.M. Influence of Oxygen and Mediators on Laccase-Catalyzed Polymerization of Lignosulfonate. ACS Sustain. Chem. Eng. 2016, 4, 5303–5310. [Google Scholar] [CrossRef]
- Ortner, A.; Huber, D.; Haske-Cornelius, O.; Weber, H.K.; Hofer, K.; Bauer, W.; Nyanhongo, G.S.; Guebitz, G.M. Laccase Mediated Oxidation of Industrial Lignins: Is Oxygen Limiting? Process Biochem. 2015, 50, 1277–1283. [Google Scholar] [CrossRef]
- Ortner, A.; Hofer, K.; Bauer, W.; Nyanhongo, G.S.; Guebitz, G.M. Laccase Modified Lignosulfonates as Novel Binder in Pigment Based Paper Coating Formulations. React. Funct. Polym. 2018, 123, 20–25. [Google Scholar] [CrossRef]
- Jimenez Bartolome, M.; Bischof, S.; Pellis, A.; Konnerth, J.; Wimmer, R.; Weber, H.; Schwaiger, N.; Guebitz, G.M.; Nyanhongo, G.S. Enzymatic Synthesis and Tailoring Lignin Properties: A Systematic Study on the Effects of Plasticizers. Polymer 2020, 202, 122725. [Google Scholar] [CrossRef]
- Monroy, Y.; Rivero, S.; García, M.A. Sustainable Panels Design Based on Modified Cassava Starch Bioadhesives and Wood Processing Byproducts. Ind. Crops Prod. 2019, 137, 171–179. [Google Scholar] [CrossRef]
- Monroy, Y.; Seré, P.; Rivero, S.; García, M.A. Sustainable Panels Based on Starch Bioadhesives: An Insight into Structural and Tribological Performance. Int. J. Biol. Macromol. 2020, 148, 898–907. [Google Scholar] [CrossRef]
- Zain, A.H.M.; Ab Wahab, M.K.; Ismail, H. Biodegradation Behaviour of Thermoplastic Starch: The Roles of Carboxylic Acids on Cassava Starch. J. Polym. Environ. 2018, 26, 691–700. [Google Scholar] [CrossRef]
- Shen, L.; Xu, H.; Kong, L.; Yang, Y. Non-Toxic Crosslinking of Starch Using Polycarboxylic Acids: Kinetic Study and Quantitative Correlation of Mechanical Properties and Crosslinking Degrees. J. Polym. Environ. 2015, 23, 588–594. [Google Scholar] [CrossRef]
- Ghosh Dastidar, T.; Netravali, A.N. “Green” Crosslinking of Native Starches with Malonic Acid and Their Properties. Carbohydr. Polym. 2012, 90, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Seidel, C.; Kulicke, W.M.; Heß, C.; Hartmann, B.; Lechner, M.D.; Lazik, W. Influence of the Cross-Linking Agent on the Gel Structure of Starch Derivatives. Starch/Staerke 2001, 53, 305–310. [Google Scholar] [CrossRef]
- Yang, C.Q. Infrared Spectroscopy Studies of the Cyclic Anhydride as the Intermediate for the Ester Crosslinking of Cotton Cellulose by Polycarboxylic Acids. I. Identification of the Cyclic Anhydride Intermediate. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 1187–1193. [Google Scholar] [CrossRef]
- Yang, C.Q.; Wang, D. Evaluating Ester Crosslinking of Cotton Fabric by a Polycarboxylic Acid Using Acid-Base Titration. Text. Res. J. 2000, 70, 615–620. [Google Scholar] [CrossRef]
- Gharehkhani, S.; Gao, W.; Fatehi, P. In-Situ Rheological Studies of Cationic Lignin Polymerization in an Acidic Aqueous System. Polymers 2020, 12, 2982. [Google Scholar] [CrossRef]
- Nyanhongo, G.S.; Nugroho Prasetyo, E.; Herrero Acero, E.; Guebitz, G.M. Engineering Strategies for Successful Development of Functional Polymers Using Oxidative Enzymes. Chem. Eng. Technol. 2012, 35, 1359–1372. [Google Scholar] [CrossRef]
- Nugroho Prasetyo, E.; Kudanga, T.; Østergaard, L.; Rencoret, J.; Gutiérrez, A.; del Río, J.C.; Ignacio Santos, J.; Nieto, L.; Jiménez-Barbero, J.; Martínez, A.T.; et al. Polymerization of Lignosulfonates by the Laccase-HBT (1-Hydroxybenzotriazole) System Improves Dispersibility. Bioresour. Technol. 2010, 101, 5054–5062. [Google Scholar] [CrossRef]
- Huo, Y.; Zhang, B.; Niu, M.; Jia, C.; Zhao, S.; Huang, Q.; Du, H. An Insight into the Multi-Scale Structures and Pasting Behaviors of Starch Following Citric Acid Treatment. Int. J. Biol. Macromol. 2018, 116, 793–800. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, Z.; Liu, Q.; Han, Y.; Zhang, L.; Chen, D.; Tian, W. Characterization of Citric Acid/Glycerol Co-Plasticized Thermoplastic Starch Prepared by Melt Blending. Carbohydr. Polym. 2007, 69, 748–755. [Google Scholar] [CrossRef]
- Zain, A.H.M.; Kahar, A.W.M.; Noriman, N.Z. Chemical-Mechanical Hydrolysis Technique of Modified Thermoplastic Starch for Better Mechanical Performance. Procedia Chem. 2016, 19, 638–645. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. Hydrogen Bonding in Lignin: A Fourier Transform Infrared Model Compound Study. Biomacromolecules 2005, 6, 2815–2821. [Google Scholar] [CrossRef]
- Yan, M.; Huang, W.; Li, Z. Chitosan Cross-Linked Graphene Oxide/Lignosulfonate Composite Aerogel for Enhanced Adsorption of Methylene Blue in Water. Int. J. Biol. Macromol. 2019, 136, 927–935. [Google Scholar] [CrossRef]
- Gosh Dastidar, T.; Netravali, A. Cross-Linked Waxy Maize Starch-Based “Green” Composites. ACS Sustain. Chem. Eng. 2013, 1, 1537–1544. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Christopher, L.P.; Yao, B.; Ji, Y. Lignin Biodegradation with Laccase-Mediator Systems. Front. Energy Res. 2014, 2, 12. [Google Scholar] [CrossRef]

| Time [min] | Viscosity [mPa·s] | Mw [kDa] |
|---|---|---|
| 0 | 5.859 | 53.74 |
| 20 | 13.77 | 221.1 |
| 50 | 27.84 | 351.5 |
| 90 | 646.4 | 750.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez Bartolome, M.; Padhi, S.S.P.; Fichtberger, O.G.; Schwaiger, N.; Seidl, B.; Kozich, M.; Nyanhongo, G.S.; Guebitz, G.M. Improving Properties of Starch-Based Adhesives with Carboxylic Acids and Enzymatically Polymerized Lignosulfonates. Int. J. Mol. Sci. 2022, 23, 13547. https://doi.org/10.3390/ijms232113547
Jimenez Bartolome M, Padhi SSP, Fichtberger OG, Schwaiger N, Seidl B, Kozich M, Nyanhongo GS, Guebitz GM. Improving Properties of Starch-Based Adhesives with Carboxylic Acids and Enzymatically Polymerized Lignosulfonates. International Journal of Molecular Sciences. 2022; 23(21):13547. https://doi.org/10.3390/ijms232113547
Chicago/Turabian StyleJimenez Bartolome, Miguel, Sidhant Satya Prakash Padhi, Oliver Gabriel Fichtberger, Nikolaus Schwaiger, Bernhard Seidl, Martin Kozich, Gibson S. Nyanhongo, and Georg M. Guebitz. 2022. "Improving Properties of Starch-Based Adhesives with Carboxylic Acids and Enzymatically Polymerized Lignosulfonates" International Journal of Molecular Sciences 23, no. 21: 13547. https://doi.org/10.3390/ijms232113547
APA StyleJimenez Bartolome, M., Padhi, S. S. P., Fichtberger, O. G., Schwaiger, N., Seidl, B., Kozich, M., Nyanhongo, G. S., & Guebitz, G. M. (2022). Improving Properties of Starch-Based Adhesives with Carboxylic Acids and Enzymatically Polymerized Lignosulfonates. International Journal of Molecular Sciences, 23(21), 13547. https://doi.org/10.3390/ijms232113547