Abstract
Genetic alterations of DNA repair genes, particularly BRCA2 in patients with prostate cancer, are associated with aggressive behavior of the disease. It has reached consensus that somatic and germline tests are necessary when treating advanced prostate cancer patients. Yet, it is unclear whether the mutations are associated with any presenting clinical features. We assessed the incidences and characteristics of BRCA2 mutated cancers by targeted sequencing in 126 sets of advanced prostate cancer tissue sequencing data. At the time of diagnosis, cT3/4, N1 and M1 stages were 107 (85%), 54 (43%) and 35 (28%) samples, respectively. BRCA2 alterations of clinical significance by AMP/ASCO/CAP criteria were found in 19 of 126 samples (15.1%). The BRCA2 mutated cancer did not differ in the distributions of TNM stage, Gleason grade group or histological subtype compared to BRCA2 wild-type cancers. Yet, they had higher tumor mutation burden, and higher frequency of ATM and BRCA1 mutations (44% vs. 10%, p = 0.002 and 21% vs. 4%, p = 0.018, respectively). Of the metastatic subgroup (M1, n = 34), mean PSA was significantly lower in BRCA2 mutated cancers than wild-type (p = 0.018). In the non-metastatic subgroup (M0, n = 64), PSA was not significantly different (p = 0.425). A similar trend was noted in multiple metastatic prostate cancer public datasets. We conclude that BRCA2 mutated metastatic prostate cancers may present in an advanced stage with relatively low PSA.
1. Introduction
Germline mutations of DNA homologous recombination repair (HRR) genes, particularly BRCA2, are frequently found in advanced prostate cancers (PCs) [1,2], and predict poor outcomes for conventional therapies [3,4,5,6]. Therefore, PCa screening using serum prostate-specific antigen (PSA) is recommended in BRCA1/2-carrying males [7]. Also, somatic alterations of BRCA1/2 are found in 5–10% of de novo metastatic cancers and 10–15% of metastatic castration-resistant prostate cancers (mCRPCs) [8,9]. Furthermore, BRCA2 mutation in mCRPC is associated with poor response to docetaxel chemotherapies and new hormonal agents [5,10,11]. In contrast, recently, polyadenosine diphosphate-ribose polymerase (PARP) inhibitors olaparib and rucaparib have demonstrated improved overall survival in BRCA2-altered metastatic PCs, combined with or followed by conventional chemo-hormonal therapies [12,13,14]. This suggests that BRCA2-altered PCs are genetically and biologically distinguished from the rest, which is linked to drug sensitivity.
It has been known that some PCs present with low serum PSA but high-grade histology, which often rapidly progresses to lethal disease [15,16]. Reportedly, the PSA-low tumors are characterized by aberrant histology such as neuroendocrine, ductal/intraductal, or sarcomatoid features [15,17,18]. In particular, intraductal and cribriform histology are frequently found in BRCA2 and other HRR gene-mutated PCs [19,20,21].
We hypothesized that BRCA2 mutation—either somatic or germline—is a special subgroup of PC and may be clinically or pathologically distinguished from those wild-type tumors. In this paper, we reviewed a consecutive series of targeted gene panel sequencing data of high-risk PCs—Gleason grade group (GGG) 4–5, cT3/4 stage, N1 or M1 stage at diagnosis. We identified those with BRCA2 mutations, and compared their clinical, pathological and genomics characteristics with the wild-type tumors.
2. Results
We evaluated 126 sets of consecutive prostate cancer tissue NGS data (from June 2019 and April 2021). There were 26 patients with 44 BRCA2 genetic alterations (42 mutations, 1 amplification and 1 heterozygous loss) (Table S1). Among the 26 patients, we identified 19 (15.1%) patients with BRCA2 alterations that were classified as “Pathogenic” or “Likely Pathogenic” by ACMG classification and “Tier I” or “Tier II” by AMP/ASCO/CAP classification (Table S2). Of note, the 1 hetloss was considered as an alteration whereas the 1 amplification was considered as wild-type, since BRCA2 is a tumor-suppressor gene.
2.1. Clinical Characteristics of BRCA2 Mutated Prostate Cancers
We compared the clinical characteristics of the 19 cases of BRCA2 oncogenic alterations with the remaining 107 wild-type cases. Patient age or TNM stage distribution were not significantly different between BRCA2 mutated (BRCA2mut) and wild-type (BRCA2WT) tumors (Table 1). Interestingly, mean PSA at diagnosis of BRCA2mut group tended to be lower than that of the BRCA2WT group (94.7 ng/mL vs. 242.7 ng/mL, p = 0.101, Table 1 and Table S3).

Table 1.
Clinical and Pathologic Characteristics of BRCA2 mutated cancers.
2.2. Pathologic Characteristics of BRCA2 Mutated Prostate Cancers
Gleason grade group (GGG) 5 tumors were more frequent in BRCA2mut group than the BRCA2WT group, yet not statistically significant (68% vs. 50%, p = 0.140, Table 1). Neuroendocrine/small histology was more frequent in BRCA2mut group, yet not statistically significant either (11% vs. 1%, p = 0.06, Table 1). Lastly, ductal adenocarcinoma was less significant in BRCA2mut group (5% vs. 15%, p = 0.06, Table 1).
2.3. PSA at Presentation by Initial Stage and by BRCA2 Mutation
We separated the cohort into metastasis at presentation (M1, n = 34) and no metastasis at presentation (M0, n = 92) subgroups. The mean serum PSAs at presentation were: 102 ng/mL (BRCA2mut M1, n = 8), 869 ng/mL (BRCA2WT M1, n = 26), 89 ng/mL (BRCA2mut M0, n = 11) and 39 ng/mL (BRCA2WT M0, n = 80), respectively. In the M1 subgroup, mean PSA at presentation was significantly lower in the BRCA2mut group than the BRCA2WT group (102 ng/mL vs. 869 ng/mL, p = 0.018, t-test with Welch’s correction, Table S3). In the non-metastatic subgroup, contrastingly, serum PSA was not significantly different between the BRCA2mut group and the BRCA2WT group (89 ng/mL vs. 39 ng/mL, p = 0.425, t-test with Welch’s correction).
2.4. Genomic Characteristics of BRCA2 Mutated Prostate Cancers
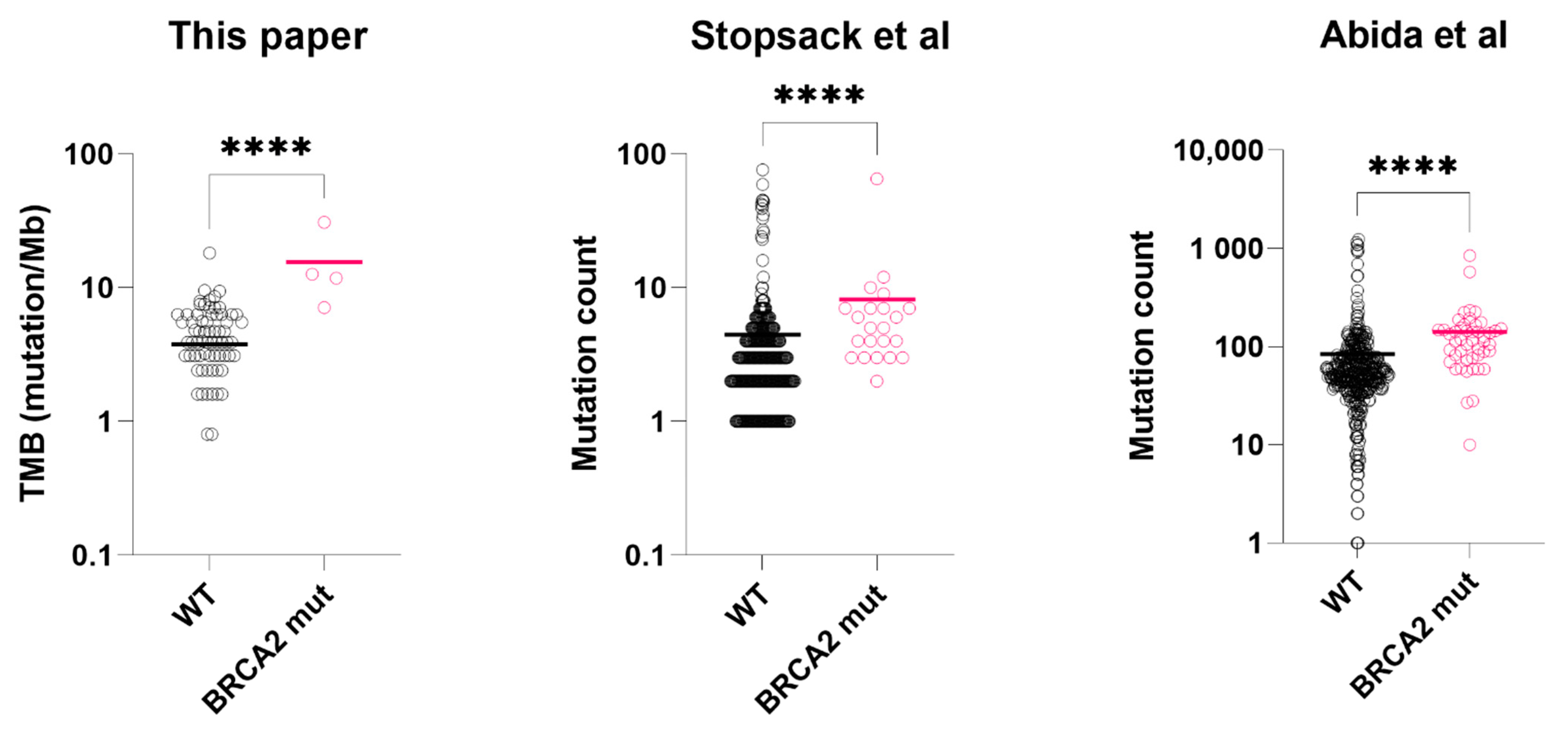
Common genetic alterations in prostate cancer, such as TMPRSS2-ERG fusion, TP53, AR, MYC, or CDK12 were not significantly associated with BRCA2 mutations. Enriched gene mutations (including variants of unknown significance) in BRCA2mut group included ATM, APC and BRCA1 (Table 2, sample data available in Table S1). Enriched gene mutations in wild-type tumors include SPOP (7.5% vs. 0.0%, p = 0.606, Fisher’s exact test) (Table S1). Median tumor mutation burden (TMB) was calculable in 87 (69%) of the 126 sequenced samples, and microsatellite instability (MSI) was calculable in 89 (71%) of the samples. TMB of BRCA2mut group was significantly higher than BRCA2WT group (12.2 vs. 3.9 mutation/Mb, p < 0.001, Mann-Whitney test, Table S3). In contrast, median microsatellite instability (MSI) was not significantly different between the two groups (2.90% vs. 3.23%, p = 0.895, Mann-Whitney test).

Table 2.
Genomic Characteristics of BRCA2 mutated cancers.
2.5. Serum PSA Level Validation in Public Datasets
To validate our findings, we assessed four large public datasets of metastatic castration-resistant (n = 444, 123) [6,22], metastatic castration-sensitive (n = 424) [23], and localized prostate cancers (n = 187) with serum PSA data available [24]. The prevalence of BRCA2 alterations in the studies are summarized in Table 3.

Table 3.
BRCA2 mutation incidences in prostate cancer datasets with PSA level data available.
2.5.1. Localized BRCA2 Mutated Prostate Cancer
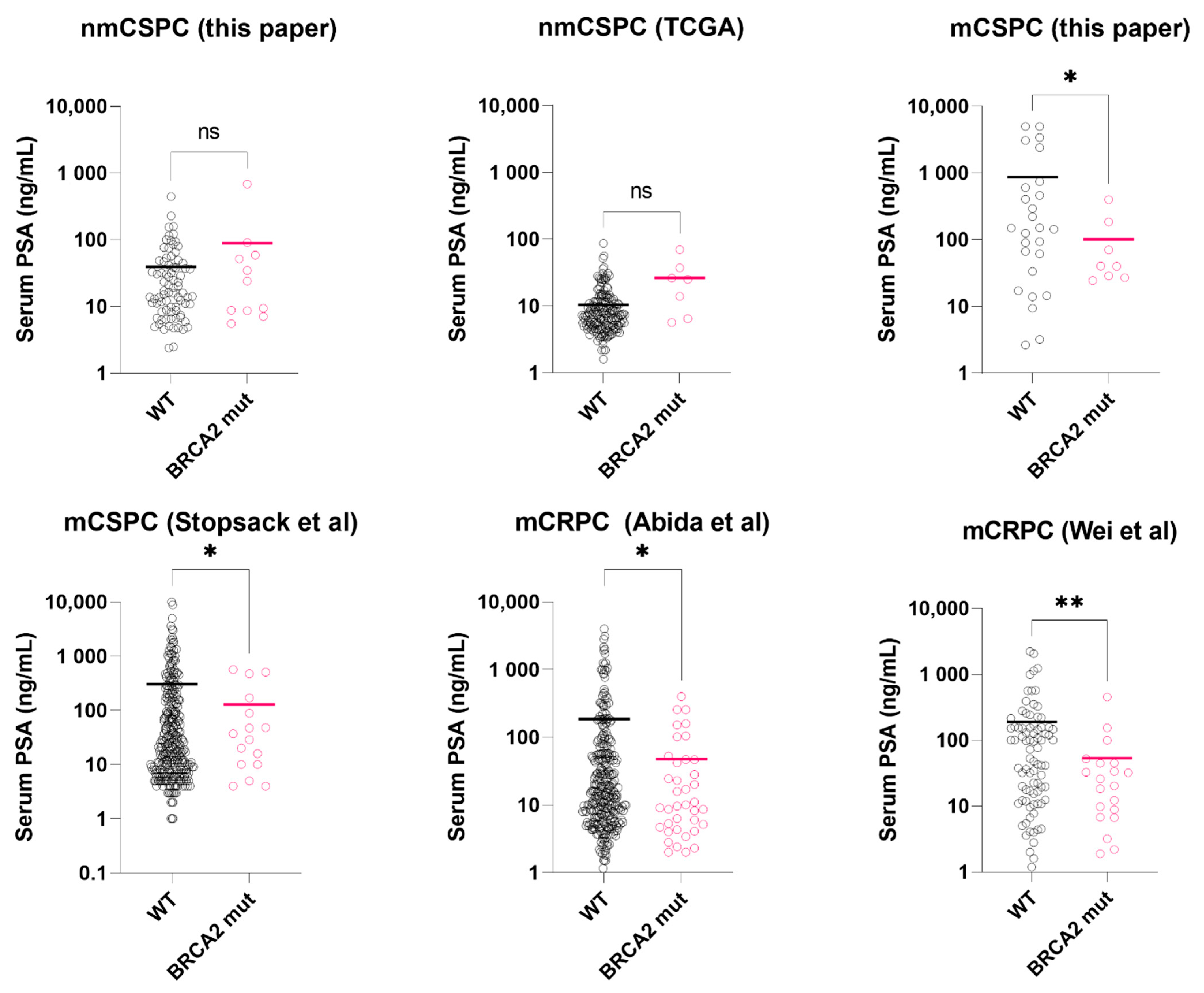
In the TCGA-localized prostate cancers, BRCA2 genetic alterations (mutations or deep deletion, somatic/germline) were found in 7 (4%) patients. In this group, Serum PSA of BRCA2-mutated tumors was not significantly different from the wild-types (26.4 ng/mL vs. 10.4 ng/mL, p = 0.107, t-test with Welch’s correction, Figure 1), consistent with our data. Median mutation count was higher in the BRCA2-mutated group, yet did not reach statistical significance (30 vs. 21, p = 0.067, Mann-Whitney test).
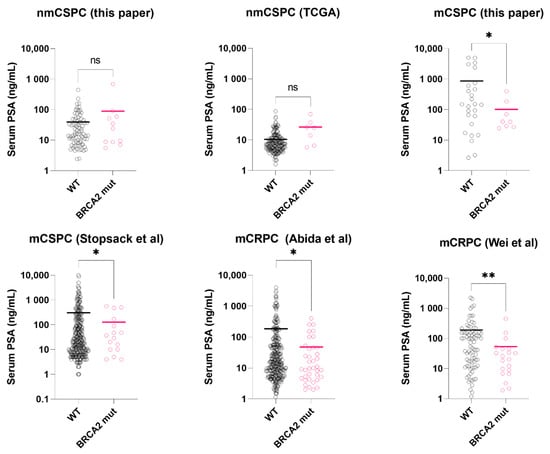
Figure 1.
Serum PSA level at diagnosis in BRCA2-mutated vs. wild-type prostate cancer, stratified by metastatic stage and castration-sensitivity. Asterisks: * p < 0.05; ** p < 0.01. black circle: BRCA2 wild-type sample (WT); pink circle: BRCA2 mutated sample (mut). Colored bar = median. Datasets: This paper, TCGA. [24], Stopsack et al. [23], Abida et al. [22], Wei et al. [6].
2.5.2. Metastatic Castration-Sensitive BRCA2 Mutated Prostate Cancer
In the mCSPC dataset (Stopsack et al.), we excluded those received androgen deprivation therapy (n = 104) for PSA level comparison. In the hormone-naïve subgroup (n = 297), Serum PSA of BRCA2-mutated tumors was significantly lower than the wild-types (127 ng/mL vs. 306 ng/mL, p = 0.031, t-test with Welch’s correction, Figure 1). For mutation count comparison, we used the full dataset. BRCA2-mutated tumors (n = 23) had higher mutation count than the wild-types (n = 401) (p < 0.0001, Mann-Whitney test, Figure 2), consistent with our results.
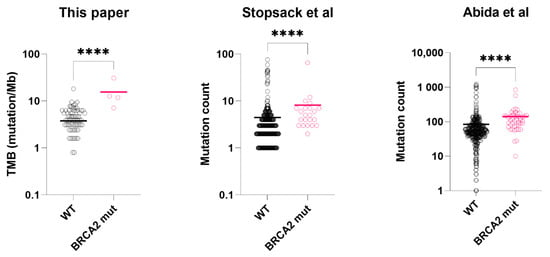
Figure 2.
Tumor mutation burden or mutation count of BRCA2-mutated prostate cancer vs. wild-type prostate cancer tissues. From left to right: tumor mutation burden (mutation/Mb), data from this paper; tumor mutation count (total), data from Stopsack et al.; tumor mutation count (total), data from Abida et al. Asterisks: **** p < 0.0001. black circle: BRCA2 wild-type sample (WT); pink circle: BRCA2 mutated sample (mut). Colored bar = median. Datasets: This paper, TCGA. [24], Stopsack et al. [23], Abida et al. [22], Wei et al. [6].
2.5.3. Metastatic Castration-Resistant BRCA2 Mutated Prostate Cancer
In the first mCRPC dataset (Abida et al.), clinically significant BRCA2 alterations were found in 47 samples (10%). Compared to those with wild-type BRCA2 (n = 374), BRCA2-mutated samples had significantly lower serum PSA at presentation (48 ng/mL vs. 186 ng/mL, p = 0.017, t-test with Welch’s correction, Figure 1) and higher mutation count (p < 0.0001, Mann-Whitney test, Figure 2). In the second mCRPC dataset (Wei et al.), BRCA2 alterations (somatic and germline) were found in 20 samples (19%). Similarly, their serum PSA at presentation was significantly lower than the wild-type tumors (53.4 ng/mL vs. 190 ng/mL, p = 0.006, t-test with Welch’s correction, Figure 1).
2.6. Genomic Characteristics Validation in Public Dataset
Using the cBioPortal, we validated the co-occurrence or mutual exclusivity of gene mutations with BRCA2 genetic alterations in prostate cancer public datasets (6875 patients or 7151 samples from 22 studies). BRCA2 genetic alterations were found in 383 (6%) of queried patients. Tumor mutation burden was significantly higher in the BRCA2-altered samples. We confirmed enriched genomic alterations of ATM (11% vs. 5.1%, p = 1.442 × 10−5) and APC (16% vs. 5.8%, p < 1 × 10−10) (Fisher’s exact test), consistent with our data. SPOP mutation was more frequent in BRCA2-altered group (16% vs. 9.9%, p = 6.079 × 10−4), opposite to our data.
3. Discussion
We found that BRCA2-mutated metastatic PCs present with relatively low serum PSA. In localized tumors, however, serum PSA of BRCA2 mutated PCs was not lower than the wild-type PCs. Also, we validated our findings with multiple publicly available mCSPC and mCRPC datasets. To our knowledge, this is first report that suggests BRCA2-mutated metastatic tumors differ from the wild-type tumor by PSA level at diagnosis. Velho et al. reported that germline DNA-repair gene mutation-positive prostate cancer patients (52% BRCA1 or BRCA2) had lower median PSA levels at diagnosis than mutation-negative patients. The patient cohort analyzed by Velho et al. consisted of 53–62% of T3/4 and 14–23% M1 disease at diagnosis [25]. Based on our analysis, we speculate that the PSA-low advanced stage tumors are enriched by BRCA2 mutation carriers. For metastatic PCs, it is now recommended by the NCCN, AUA or EAU guidelines to run both.
Serum PSA is a highly sensitive biomarker for prostate cancer diagnosis as well as monitoring for recurrence and drug response. Yet, its specificity is not high enough for metastatic disease monitoring, leaving cases with clinical or radiographic progression without PSA elevation [26]. Our findings speculate that BRCA2-altered prostate cancer progresses rapidly to metastatic and castration-resistant disease not necessarily accompanied by increased serum PSA level. Molecular studies suggest that BRCA2 mutation combined with RB1 alteration, which is located closely on chromosome 13q14, can generate an aggressive PC model with castration resistance [27].
Our study shows that BRCA2 mutation is associated with lower PSA at the metastatic state, but not in the localized setting. We suggest some explanations, but these are not yet definitive. First, the major source of serum PSA in patients with metastatic prostate cancer is the cancer cells themselves. However, in a localized setting, theoretically, PSA released from adjacent normal cells may contribute more significantly than in a metastatic setting. Secondly, genetic characteristics of BRCA2 wild-type localized cancer may be different from those of BRCA2 wild-type metastatic cancer. As BRCA2 mutation is enriched in metastatic prostate cancer, TP53 mutation or RB1 deletions are also enriched in metastatic disease, whereas SPOP mutation is more frequent in localized disease [6,21,22,28,29]. Lastly, the metastatic microenvironment may pressure cancer cells to express PSA more or less than the primary site. In other words, a BRCA2-mutated cancer cell at the primary site (prostate) may express high PSA while a cell with same genetic background but located in bone marrow may express less PSA.
Among the various DNA-repair genes of HRR or mismatch repair, we focused on BRCA2 alteration because it is the most frequently mutated HRR gene in advanced PCs [1], and represents a subset of patients that are likely to respond to PARP inhibitors [12,13]. While earlier studies combined ATM mutation in this subgroup [4], recent evidence shows that PARP inhibitor alone is not enough to treat the ATM mutated PCs [30]. Indeed, we found that ATM mutation frequently accompanies BRCA2, which may explain the earlier-documented benefit of PARP inhibitors in ATM-mutated cases. ATM germline mutation increases the likelihood of PC development [31], and ATM mutation or protein loss are enriched in high-grade cancer [32]. In response to DNA double strand break, the ATM protein modulates diverse aspects of the cell’s responses such as cell cycle checkpoint arrest, apoptosis—not limited to DNA repair itself [33,34,35]. This may lead to varying responses to DNA-damaging agents such as PARP inhibitors or radiations in patients with ATM somatic or germline alterations unlike BRCA1 or BRCA2 [36,37,38].
We point to some limitations in this study. Interpretation of our data is limited due to the study’s retrospective design. Serum PSA data was available in only a subset of the datasets analyzed in this study, which may increase selection bias. We did not perform survival analysis as the consecutive treatments after sequencing analysis were rather heterogenous. Particularly, some of the patients with HRR mutations underwent clinical trials involving PARP inhibitors which would deviate survival outcome. Lastly, we tried setting some arbitrary cut-off points (100 ng/mL, 10 ng/mL) of PSA threshold or values that could possibly be considered suspicious and warrant genetic testing in metastatic PC at presentation, which were not successful. We argue that somatic and germline genetic testing is recommended in patients with metastatic PC no matter what their PSA level is.
It has been known that there is a clinically distinctive aggressive variant prostate cancer (AVPC), presenting with low-serum PSA, neuroendocrine small cell or ductal histology, which frequently metastasizes to visceral organs and rapidly develops castration resistance. Yet there is substantial genomic and transcriptomic heterogeneity that exists within these tumors [39]. Our paper addresses the contention that BRCA2-mutated metastatic PC fits in the criteria of AVPC, providing further understanding on this disease entity.
4. Materials and Methods
Patient data: The YUHS Big-Data team identified cases of prostate cancer patients who had performed next generation sequencing on their achieved prostate cancer tissues (prostatectomy, biopsy or transurethral resection specimen) between January 2019 and May 2021. Associated clinical and pathological information was retrieved from electrical medical records. The study design was approved by Severance Hospital Institutional Review Board (IRB #4-2020-0812). Patient consent was waived due to the retrospective nature of the study. All data underwent deidentification process.
Panel Sequencing of Prostate Cancer Tissues: Targeted DNA and RNA sequencing were performed using TruSight Tumor 170 or 500 (Illumina, San Diego, CA, USA). Briefly, 40 ng of formalin-fixed paraffin-embedded (FFPE) tissue-derived DNA and RNA were extracted using QIAGEN AllPrep DNA/RNA FFPE Kit (Qiagen, Hilden, Germany). After hybridization capture-based target enrichment, paired-end sequencing (2 × 150 bp) was performed using a NextSeq sequencer (Illumina) according to the manufacturer’s instructions. Variants with a total depth of at least 100× were included for analysis. Variant interpretation was based on recommendations from the Association for Molecular Pathology (AMP), American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP) [40]. AMP/ASCO/CAP Tier 1 variant included level 1 and level 2 genetic alterations that are FDA-approved biomarkers and standard of care. Tier 2 variant included alterations with compelling clinical or preclinical evidence to drug response. Variant interpretations followed the “Standards and guidelines for the interpretation of sequence variants” published by the American College of Medical Genetics and genomics (ACMG) [41]. Each variant was initially queried using Varsome (Varsome.com) which annotates variants by combining data resources including AACT Clinical Trials from clinicaltrials.gov, Pharmacogenomics Knowledge Base (PharmGKB), COSMIC by the Sanger Institute, Polyphen-2 scores by Harvard University, CADD scores by the University of Washington, Clinical Pharmacogenetics Implementation Consortium (CPIC), the Drug-Gene Interaction Database (DGIdb) and OMIM [42]. Output variant annotation was manually reviewed in OncoKB website (http://www.OncoKB.org) (last accessed on 30 September 2022).
Public Dataset Analysis: We downloaded annotated clinical and genomic data from the cBioportal—“Prostate Adenocarcinoma (TGCA, Cell 2015)”, “Metastatic castration-sensitive prostate cancer (MSK, Clin Cancer Res 2020)”, and “Metastatic Prostate Adenocarcinoma (SU2C/PCF Dream Team, PNAS 2019)”. Additionally, we downloaded annotated clinical and genomic data of metastatic castration-resistant prostate cancer from the publication of Wei et al. [6].
Statistical Methods: Cases were grouped by their germline or somatic alteration status of BRCA2 (mutation, deletion). Serum PSA at diagnosis and tumor mutation count were compared between BRCA2-altered group and wild-type from each study. Baseline characteristics, including Gleason grade group, TNM stage and other gene mutations were compared between the two groups using Pearson’s chi-squared test or Fisher’s exact test. Continuous variables of age at diagnosis and PSA at diagnosis were compared using t test with Welch’s correction. Tumor mutation burden or total mutation count were compared using Mann-Whitney test. All p values were two-sided and p < 0.05 was defined as statistically significant. Statistical analysis was performed using SPSS 26.0 and GraphPad Prism 9.2.0.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232113426/s1.
Author Contributions
Conceptualization, H.H. and K.S.C.; methodology, H.H. and C.K.P.; software, H.H.; validation, H.H.; investigation, N.H.C., K.S.C. and J.L.; resources, W.S.J., W.S.H. and Y.D.C.; data curation, H.H.; writing—original draft preparation, H.H.; writing—review and editing, H.H., C.K.P. and K.S.C.; visualization, H.H.; supervision, Y.D.C. and K.S.C.; project administration, H.H.; funding acquisition, H.H. and K.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Korea Health Industry Development Institute (KHIDI) grant for research under the Biomedical Global Talent Nurturing Program of KHIDI (HI19C0723) (PI: H.H.) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2022R1A2C3005586) (PI: H.H. and J.S.L).
Institutional Review Board Statement
The study design was approved by Severance Hospital Institutional Review Board (IRB #4-2020- 0812).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study. All data underwent deidentification process.
Data Availability Statement
All processed genomic, pathologic and clinical data are presented in Supplementary Tables. Original genomic data will be provided upon request. The link and location of public data used in this paper is described in method section.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef]
- Castro, E.; Goh, C.; Olmos, D.; Saunders, E.; Leongamornlert, D.; Tymrakiewicz, M.; Mahmud, N.; Dadaev, T.; Govindasami, K.; Guy, M.; et al. Germline brca mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J. Clin. Oncol. 2013, 31, 1748–1757. [Google Scholar] [CrossRef]
- Gallagher, D.J.; Gaudet, M.M.; Pal, P.; Kirchhoff, T.; Balistreri, L.; Vora, K.; Bhatia, J.; Stadler, Z.; Fine, S.W.; Reuter, V.; et al. Germline brca mutations denote a clinicopathologic subset of prostate cancer. Clin. Cancer Res. 2010, 16, 2115–2121. [Google Scholar] [CrossRef]
- Na, R.; Zheng, S.L.; Han, M.; Yu, H.J.; Jiang, D.K.; Shah, S.; Ewing, C.M.; Zhang, L.T.; Novakovic, K.; Petkewicz, J.; et al. Germline mutations in atm and brca1/2 distinguish risk for lethal and indolent prostate cancer and are associated with early age at death. Eur. Urol. 2017, 71, 740–747. [Google Scholar] [CrossRef]
- Castro, E.; Romero-Laorden, N.; del Pozo, A.; Lozano, R.; Medina, A.; Puente, J.; Piulats, J.M.; Lorente, D.; Saez, M.I.; Morales-Barrera, R.; et al. Prorepair-b: A prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J. Oflinical Oncol. 2019, 37, 490–503. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, J.; Gu, W.; Wang, J.; Lin, G.; Qin, X.; Dai, B.; Gan, H.; Ye, D.; Zhu, Y. Prognostic value of germline DNA repair gene mutations in de novo metastatic and castration-sensitive prostate cancer. Oncologist 2020, 25, e1042–e1050. [Google Scholar] [CrossRef]
- Page, E.C.; Bancroft, E.K.; Brook, M.N.; Assel, M.; Hassan Al Battat, M.; Thomas, S.; Taylor, N.; Chamberlain, A.; Pope, J.; Raghallaigh, H.N.; et al. Interim results from the impact study: Evidence for prostate-specific antigen screening in brca2 mutation carriers. Eur. Urol. 2019, 76, 831–842. [Google Scholar] [CrossRef]
- Fan, L.; Fei, X.; Zhu, Y.; Pan, J.; Sha, J.; Chi, C.; Gong, Y.; Du, X.; Zhou, L.; Dong, B.; et al. Comparative analysis of genomic alterations across castration sensitive and castration resistant prostate cancer via circulating tumor DNA sequencing. J. Urol. 2021, 205, 461–469. [Google Scholar] [CrossRef]
- Beltran, H.; Yelensky, R.; Frampton, G.M.; Park, K.; Downing, S.R.; MacDonald, T.Y.; Jarosz, M.; Lipson, D.; Tagawa, S.T.; Nanus, D.M.; et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur. Urol. 2013, 63, 920–926. [Google Scholar] [CrossRef]
- Nientiedt, C.; Heller, M.; Endris, V.; Volckmar, A.L.; Zschabitz, S.; Tapia-Laliena, M.A.; Duensing, A.; Jager, D.; Schirmacher, P.; Sultmann, H.; et al. Mutations in brca2 and taxane resistance in prostate cancer. Sci. Rep. 2017, 7, 4574. [Google Scholar] [CrossRef]
- Annala, M.; Vandekerkhove, G.; Khalaf, D.; Taavitsainen, S.; Beja, K.; Warner, E.W.; Sunderland, K.; Kollmannsberger, C.; Eigl, B.J.; Finch, D.; et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 2018, 8, 444–457. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Abida, W.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; Sautois, B.; Vogelzang, N.J.; Bambury, R.M.; Voog, E.; et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a brca1 or brca2 gene alteration. J. Clin. Oncol. 2020, 38, 3763–3772. [Google Scholar] [CrossRef]
- Stopsack, K.H. Efficacy of parp inhibition in metastatic castration-resistant prostate cancer is very different with non-brca DNA repair alterations: Reconstructing prespecified endpoints for cohort b from the phase 3 profound trial of olaparib. Eur. Urol. 2021, 79, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Mahal, B.A.; Yang, D.D.; Wang, N.Q.; Alshalalfa, M.; Davicioni, E.; Choeurng, V.; Schaeffer, E.M.; Ross, A.E.; Spratt, D.E.; Den, R.B.; et al. Clinical and genomic characterization of low-prostate-specific antigen, high-grade prostate cancer. Eur. Urol. 2018, 74, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Beltran, H.; Tomlins, S.; Aparicio, A.; Arora, V.; Rickman, D.; Ayala, G.; Huang, J.; True, L.; Gleave, M.E.; Soule, H.; et al. Aggressive variants of castration-resistant prostate cancer. Clin. Cancer Res. 2014, 20, 2846–2850. [Google Scholar] [CrossRef]
- Morgan, T.M.; Welty, C.J.; Vakar-Lopez, F.; Lin, D.W.; Wright, J.L. Ductal adenocarcinoma of the prostate: Increased mortality risk and decreased psa secretion. J. Urol. 2010, 183, E95–E96. [Google Scholar] [CrossRef][Green Version]
- Markowski, M.C.; Eisenberger, M.A.; Zahurak, M.; Epstein, J.I.; Paller, C.J. Sarcomatoid carcinoma of the prostate: Retrospective review of a case series from the johns hopkins hospital. Urology 2015, 86, 539–543. [Google Scholar] [CrossRef]
- Risbridger, G.P.; Taylor, R.A.; Clouston, D.; Sliwinski, A.; Thorne, H.; Hunter, S.; Li, J.; Mitchell, G.; Murphy, D.; Frydenberg, M.; et al. Patient-derived xenografts reveal that intraductal carcinoma of the prostate is a prominent pathology in brca2 mutation carriers with prostate cancer and correlates with poor prognosis. Eur. Urol. 2015, 67, 496–503. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Antonarakis, E.S.; Bismar, T.A.; Guedes, L.B.; Cheng, H.H.; Tretiakova, M.S.; Vakar-Lopez, F.; Klemfuss, N.; Konnick, E.Q.; Mostaghel, E.A.; et al. Genomic characterization of prostatic ductal adenocarcinoma identifies a high prevalence of DNA repair gene mutations. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Lozano, R.; Salles, D.C.; Sandhu, S.; Aragon, I.M.; Thorne, H.; Lopez-Campos, F.; Rubio-Briones, J.; Gutierrez-Pecharroman, A.M.; Maldonado, L.; di Domenico, T.; et al. Association between brca2 alterations and intraductal and cribriform histologies in prostate cancer. Eur. J. Cancer 2021, 147, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Cyrta, J.; Heller, G.; Prandi, D.; Armenia, J.; Coleman, I.; Cieslik, M.; Benelli, M.; Robinson, D.; Van Allen, E.M.; et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 11428–11436. [Google Scholar] [CrossRef]
- Stopsack, K.H.; Nandakumar, S.; Wibmer, A.G.; Haywood, S.; Weg, E.S.; Barnett, E.S.; Kim, C.J.; Carbone, E.A.; Vasselman, S.E.; Nguyen, B.; et al. Oncogenic genomic alterations, clinical phenotypes, and outcomes in metastatic castration-sensitive prostate cancer. Clin. Cancer Res. 2020, 26, 3230–3238. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An integrated tcga pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell 2018, 173, 400–416.e11. [Google Scholar] [CrossRef]
- Velho, P.I.; Silberstein, J.L.; Markowski, M.C.; Luo, J.; Lotan, T.L.; Isaacs, W.B.; Antonarakis, E.S. Intraductal/ductal histology and lymphovascular invasion are associated with germline DNA-repair gene mutations in prostate cancer. Prostate 2018, 78, 401–407. [Google Scholar] [CrossRef]
- Bryce, A.H.; Alumkal, J.J.; Armstrong, A.; Higano, C.S.; Iversen, P.; Sternberg, C.N.; Rathkopf, D.; Loriot, Y.; de Bono, J.; Tombal, B.; et al. Radiographic progression with nonrising psa in metastatic castration-resistant prostate cancer: Post hoc analysis of prevail. Prostate Cancer Prostatic Dis. 2017, 20, 221–227. [Google Scholar] [CrossRef]
- Chakraborty, G.; Armenia, J.; Mazzu, Y.Z.; Nandakumar, S.; Stopsack, K.H.; Atiq, M.O.; Komura, K.; Jehane, L.; Hirani, R.; Chadalavada, K.; et al. Significance of brca2 and rb1 co-loss in aggressive prostate cancer progression. Clin. Cancer Res. 2020, 26, 2047–2064. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Erho, N.; Chan, J.M.; Feng, F.Y.; Fishbane, N.; Zhao, S.G.; Simko, J.P.; Cowan, J.E.; Lehrer, J.; Alshalalfa, M.; et al. The diverse genomic landscape of clinically low-risk prostate cancer. Eur. Urol. 2018, 74, 444–452. [Google Scholar] [CrossRef]
- Marshall, C.H.; Sokolova, A.O.; McNatty, A.L.; Cheng, H.H.; Eisenberger, M.A.; Bryce, A.H.; Schweizer, M.T.; Antonarakis, E.S. Differential response to olaparib treatment among men with metastatic castration-resistant prostate cancer harboring brca1 or brca2 versus atm mutations. Eur. Urol. 2019, 76, 452–458. [Google Scholar] [CrossRef]
- Karlsson, Q.; Brook, M.N.; Dadaev, T.; Wakerell, S.; Saunders, E.J.; Muir, K.; Neal, D.E.; Giles, G.G.; MacInnis, R.J.; Thibodeau, S.N.; et al. Rare germline variants in atm predispose to prostate cancer: A practical consortium study. Eur. Urol. Oncol. 2021, 4, 570–579. [Google Scholar] [CrossRef]
- Kaur, H.; Salles, D.C.; Murali, S.; Hicks, J.L.; Nguyen, M.; Pritchard, C.C.; De Marzo, A.M.; Lanchbury, J.S.; Trock, B.J.; Isaacs, W.B.; et al. Genomic and clinicopathologic characterization of atm-deficient prostate cancer. Clin. Cancer Res. 2020, 26, 4869–4881. [Google Scholar] [CrossRef] [PubMed]
- Gatei, M.; Scott, S.P.; Filippovitch, I.; Soronika, N.; Lavin, M.F.; Weber, B.; Khanna, K.K. Role for atm in DNA damage-induced phosphorylation of brca1. Cancer Res. 2000, 60, 3299–3304. [Google Scholar] [PubMed]
- Khoronenkova, S.V.; Dianov, G.L. Regulation of usp7/hausp in response to DNA damage: Yet another role for atm. Cell Cycle 2012, 11, 2409–2410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shibata, A.; Jeggo, P.A. Atm’s role in the repair of DNA double-strand breaks. Genes 2021, 12, 1370. [Google Scholar] [CrossRef]
- Xiong, H.; Liao, Z.; Liu, Z.; Xu, T.; Wang, Q.; Liu, H.; Komaki, R.; Gomez, D.; Wang, L.E.; Wei, Q. Atm polymorphisms predict severe radiation pneumonitis in patients with non-small cell lung cancer treated with definitive radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1066–1073. [Google Scholar] [CrossRef]
- Ishikawa, A.; Suga, T.; Shoji, Y.; Kato, S.; Ohno, T.; Ishikawa, H.; Yoshinaga, S.; Ohara, K.; Ariga, H.; Nomura, K.; et al. Genetic variants of npat-atm and aurka are associated with an early adverse reaction in the gastrointestinal tract of patients with cervical cancer treated with pelvic radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1144–1152. [Google Scholar] [CrossRef]
- Andreassen, C.N.; Rosenstein, B.S.; Kerns, S.L.; Ostrer, H.; De Ruysscher, D.; Cesaretti, J.A.; Barnett, G.C.; Dunning, A.M.; Dorling, L.; West, C.M.L.; et al. Individual patient data meta-analysis shows a significant association between the atm rs1801516 snp and toxicity after radiotherapy in 5456 breast and prostate cancer patients. Radiother. Oncol. 2016, 121, 431–439. [Google Scholar] [CrossRef]
- Han, H.; Lee, H.H.; Choi, K.; Moon, Y.J.; Heo, J.E.; Ham, W.S.; Jang, W.S.; Rha, K.H.; Cho, N.H.; Giancotti, F.G.; et al. Prostate epithelial genes define therapy-relevant prostate cancer molecular subtype. Prostate Cancer 2021, 24, 1080–1092. [Google Scholar] [CrossRef]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: A joint consensus recommendation of the association for molecular pathology, american society of clinical oncology, and college of american pathologists. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the american college of medical genetics and genomics and the association for molecular pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Albarca Aguilera, M.; Meyer, R.; Massouras, A. Varsome: The human genomic variant search engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).