Potential Focal Adhesion Kinase Inhibitors in Management of Cancer: Therapeutic Opportunities from Herbal Medicine
Abstract
1. Introduction
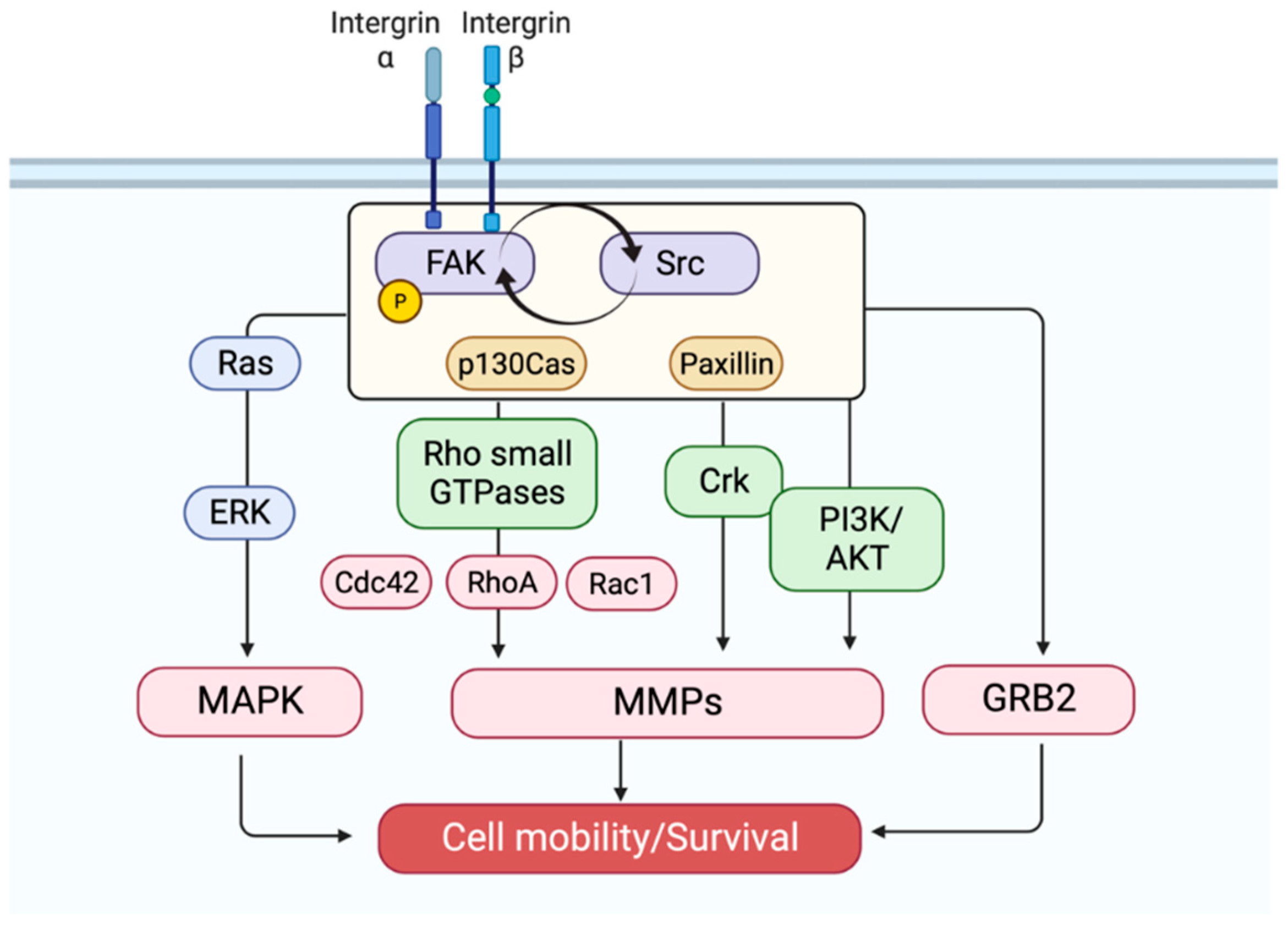
2. Fak Activation
3. Fak Signaling: A Partnership with Src
4. Fak: An Oncogenic Driver
5. Fak Inhibitors from Natural Sources
6. Fak Kinase-Dependent Inhibitors: Subversion of Fak Phosphorylation
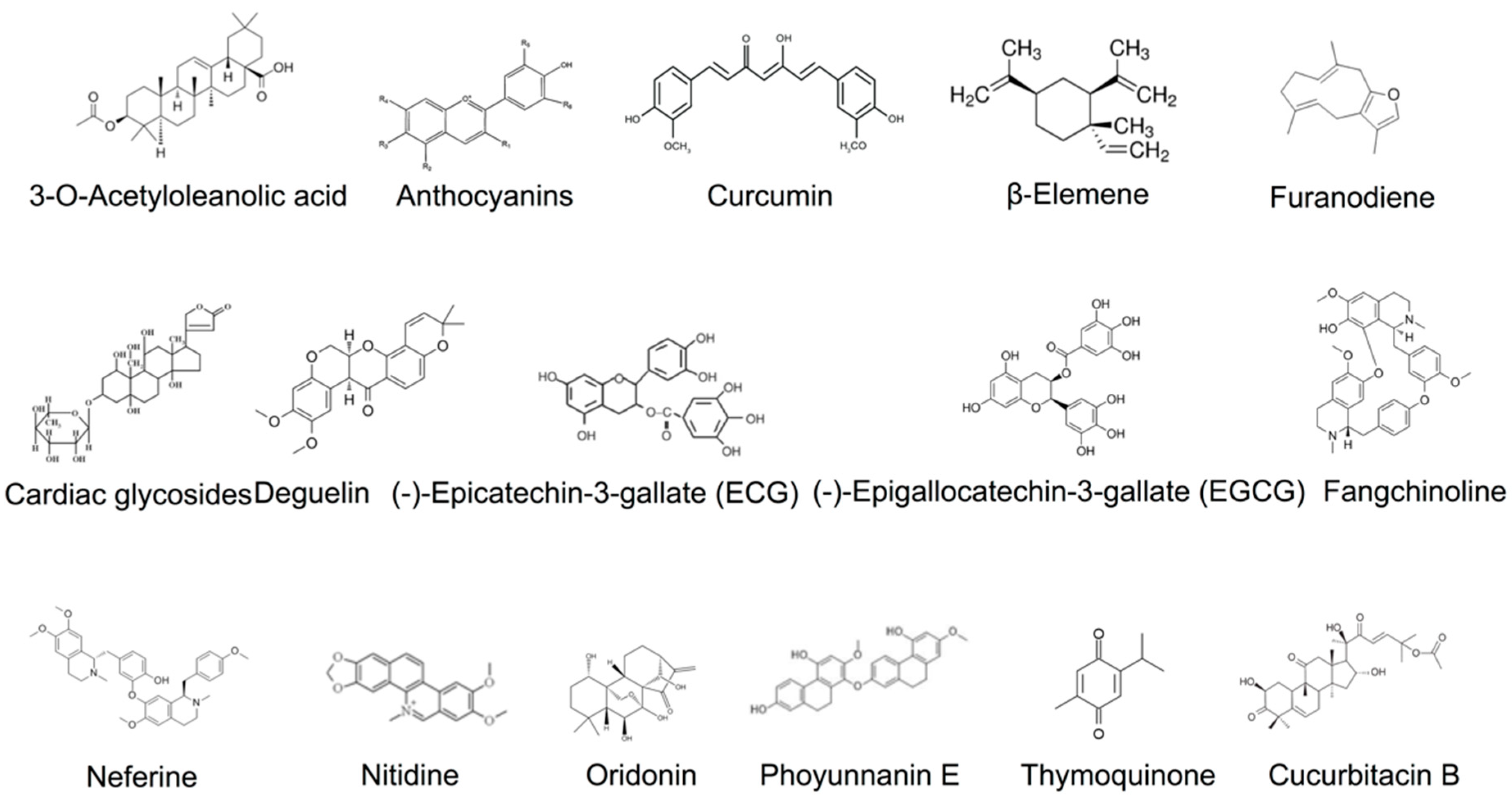
6.1. 3-O-Acetyloleanolic Acid
6.2. Black Rice Anthocyanins
6.3. Curcumin
6.4. β-Elemene
6.5. Furanodiene
6.6. Cardiac Glycosides
6.7. Deguelin
6.8. Epicatechin-3-Gallate
6.9. EGCG
6.10. Fangchinoline
6.11. Neferine
6.12. Nitidine
6.13. Oridonin
6.14. Phoyunnanin E
6.15. Thymoquinone
7. Fak Kinase-Independent Inhibitors: Remodeling of the Actin Cytoskeleton
7.1. Cucurbitacin B
7.2. Thymoquinone
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Tapial Martinez, P.; Lopez Navajas, P.; Lietha, D. FAK Structure and Regulation by Membrane Interactions and Force in Focal Adhesions. Biomolecules 2020, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Guan, J.L. Focal adhesion kinase: From in vitro studies to functional analyses in vivo. Curr. Protein Pept. Sci. 2011, 12, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.T. Focal adhesion kinase: The first ten years. J. Cell Sci. 2003, 116, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Dehart, J.P.; Murphy, J.M.; Lim, S.T. Understanding the roles of FAK in cancer: Inhibitors, genetic models, and new insights. J. Histochem. Cytochem. 2015, 63, 114–128. [Google Scholar] [CrossRef]
- Sulzmaier, F.J.; Jean, C.; Schlaepfer, D.D. FAK in cancer: Mechanistic findings and clinical applications. Nat. Rev. Cancer 2014, 14, 598–610. [Google Scholar] [CrossRef]
- Tai, Y.L.; Chen, L.C.; Shen, T.L. Emerging roles of focal adhesion kinase in cancer. BioMed Res. Int. 2015, 2015, 690690. [Google Scholar] [CrossRef]
- Chauhan, A.; Khan, T. Focal adhesion kinase-An emerging viable target in cancer and development of focal adhesion kinase inhibitors. Chem. Biol. Drug Des. 2021, 97, 774–794. [Google Scholar] [CrossRef]
- Hong, M.; Li, S.; Wang, N.; Tan, H.Y.; Cheung, F.; Feng, Y. A Biomedical Investigation of the Hepatoprotective Effect of Radix salviae miltiorrhizae and Network Pharmacology-Based Prediction of the Active Compounds and Molecular Targets. Int. J. Mol. Sci. 2017, 18, 620. [Google Scholar] [CrossRef]
- Hong, M.; Tan, H.Y.; Li, S.; Cheung, F.; Wang, N.; Nagamatsu, T.; Feng, Y. Cancer Stem Cells: The Potential Targets of Chinese Medicines and Their Active Compounds. Int. J. Mol. Sci. 2016, 17, 893. [Google Scholar] [CrossRef]
- Wang, N.; Tan, H.Y.; Li, L.; Yuen, M.F.; Feng, Y. Berberine and Coptidis Rhizoma as potential anticancer agents: Recent updates and future perspectives. J. Ethnopharmacol. 2015, 176, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ji, J.; Di, L.; Li, J.; Hu, L.; Qiao, H.; Wang, L.; Feng, Y. Resource, chemical structure and activity of natural polysaccharides against alcoholic liver damages. Carbohydr. Polym. 2020, 241, 116355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, N.; Xu, Y.; Tan, H.Y.; Li, S.; Feng, Y. Molecular Mechanisms Involved in Oxidative Stress-Associated Liver Injury Induced by Chinese Herbal Medicine: An Experimental Evidence-Based Literature Review and Network Pharmacology Study. Int. J. Mol. Sci. 2018, 19, 2756. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Cheung, F.; Tan, H.Y.; Wang, N.; Yuen, M.F.; Feng, Y. Hepatoprotective Effects of Chinese Medicinal Herbs: A Focus on Anti-Inflammatory and Anti-Oxidative Activities. Int. J. Mol. Sci. 2016, 17, 465. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhong, Z.; Tan, H.Y.; Guo, W.; Zhang, C.; Tan, C.W.; Li, S.; Wang, N.; Feng, Y. Uncovering the Anticancer Mechanisms of Chinese Herbal Medicine Formulas: Therapeutic Alternatives for Liver Cancer. Front. Pharmacol. 2020, 11, 293. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C.; Zhang, Z.; Feng, Y. Panax notoginseng saponins modulate the gut microbiota to promote thermogenesis and beige adipocyte reconstruction via leptin-mediated AMPKalpha/STAT3 signaling in diet-induced obesity. Theranostics 2020, 10, 11302–11323. [Google Scholar] [CrossRef]
- Huang, J.; Chen, F.; Zhong, Z.; Tan, H.Y.; Wang, N.; Liu, Y.; Fang, X.; Yang, T.; Feng, Y. Interpreting the Pharmacological Mechanisms of Huachansu Capsules on Hepatocellular Carcinoma Through Combining Network Pharmacology and Experimental Evaluation. Front. Pharmacol. 2020, 11, 414. [Google Scholar] [CrossRef]
- Li, D.; Zhang, T.; Lu, J.; Peng, C.; Lin, L. Natural constituents from food sources as therapeutic agents for obesity and metabolic diseases targeting adipose tissue inflammation. Crit. Rev. Food Sci. Nutr. 2020, 1–19. [Google Scholar] [CrossRef]
- Li, D.; Liu, Q.; Lu, X.; Li, Z.; Wang, C.; Leung, C.H.; Wang, Y.; Peng, C.; Lin, L. α-Mangostin remodels visceral adipose tissue inflammation to ameliorate age-related metabolic disorders in mice. Aging 2019, 11, 11084–11110. [Google Scholar] [CrossRef]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol 2005, 6, 56–68. [Google Scholar] [CrossRef]
- Gladkikh, A.; Kovaleva, A.; Tvorogova, A.; Vorobjev, I.A. Heterogeneity of Focal Adhesions and Focal Contacts in Motile Fibroblasts. Methods Mol. Biol. 2018, 1745, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hochwald, S.N. The role of FAK in tumor metabolism and therapy. Pharmacol. Ther. 2014, 142, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.D. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim. Biophys. Acta 2001, 1540, 1–21. [Google Scholar] [CrossRef]
- Schlaepfer, D.D.; Mitra, S.K.; Ilic, D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim. Biophys. Acta 2004, 1692, 77–102. [Google Scholar] [CrossRef]
- Hanks, S.K.; Ryzhova, L.; Shin, N.Y.; Brabek, J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front. Biosci. 2003, 8, d982–d996. [Google Scholar] [CrossRef]
- Calalb, M.B.; Polte, T.R.; Hanks, S.K. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: A role for Src family kinases. Mol. Cell Biol. 1995, 15, 954–963. [Google Scholar] [CrossRef]
- Schlaepfer, D.D.; Mitra, S.K. Multiple connections link FAK to cell motility and invasion. Curr. Opin. Genet. Dev. 2004, 14, 92–101. [Google Scholar] [CrossRef]
- Brabek, J.; Constancio, S.S.; Shin, N.Y.; Pozzi, A.; Weaver, A.M.; Hanks, S.K. CAS promotes invasiveness of Src-transformed cells. Oncogene 2004, 23, 7406–7415. [Google Scholar] [CrossRef]
- Tachibana, K.; Sato, T.; D’Avirro, N.; Morimoto, C. Direct association of pp125FAK with paxillin, the focal adhesion-targeting mechanism of pp125FAK. J. Exp. Med. 1995, 182, 1089–1099. [Google Scholar] [CrossRef]
- Turner, C.E. Paxillin and focal adhesion signalling. Nat. Cell Biol. 2000, 2, E231–E236. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.D. Paxillin: A focal adhesion-associated adaptor protein. Oncogene 2001, 20, 6459–6472. [Google Scholar] [CrossRef] [PubMed]
- Fife, C.M.; McCarroll, J.A.; Kavallaris, M. Movers and shakers: Cell cytoskeleton in cancer metastasis. Brit. J. Pharmacol. 2014, 171, 5507–5523. [Google Scholar] [CrossRef]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane extracellular matrix-cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Raftopoulou, M.; Hall, A. Cell migration: Rho GTPases lead the way. Dev. Biol. 2004, 265, 23–32. [Google Scholar] [CrossRef]
- Wang, H.B.; Dembo, M.; Hanks, S.K.; Wang, Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc. Natl. Acad. Sci. USA 2001, 98, 11295–11300. [Google Scholar] [CrossRef]
- Tilghman, R.W.; Slack-Davis, J.K.; Sergina, N.; Martin, K.H.; Iwanicki, M.; Hershey, E.D.; Beggs, H.E.; Relchardt, L.F.; Parsons, J.T. Focal adhesion kinase is required for the spatial organization of the leading edge in migrating cells. J. Cell Sci. 2005, 118, 2613–2623. [Google Scholar] [CrossRef]
- Lark, A.L.; Livasy, C.A.; Dressler, L.; Moore, D.T.; Millikan, R.C.; Geradts, J.; Iacocca, M.; Cowan, D.; Little, D.; Craven, R.J.; et al. High focal adhesion kinase expression in invasive breast carcinomas is associated with an aggressive phenotype. Mod. Pathol. 2005, 18, 1289–1294. [Google Scholar] [CrossRef]
- Cance, W.G.; Harris, J.E.; Iacocca, M.V.; Roche, E.; Yang, X.; Chang, J.; Simkins, S.; Xu, L. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: Correlation with preinvasive and invasive phenotypes. Clin. Cancer Res. 2000, 6, 2417–2423. [Google Scholar]
- Golubovskaya, V.M.; Kweh, F.A.; Cance, W.G. Focal adhesion kinase and cancer. Histol. Histopathol. 2009, 24, 503–510. [Google Scholar] [CrossRef]
- Ocak, S.; Chen, H.; Callison, C.; Gonzalez, A.L.; Massion, P.P. Expression of focal adhesion kinase in small-cell lung carcinoma. Cancer 2012, 118, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Maeda, T.; Shimada, M.; Aishima, S.; Shirabe, K.; Tanaka, S.; Maehara, Y. Role of expression of focal adhesion kinase in progression of hepatocellular carcinoma. Clin. Cancer Res. 2004, 10, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.B.; Kurago, Z.; Zaharias, R.; Gruman, L.M.; Schaller, M.D.; Hendrix, M.J. Elevated focal adhesion kinase expression facilitates oral tumor cell invasion. Cancer 2002, 95, 2508–2515. [Google Scholar] [CrossRef]
- Aprikian, A.G.; Tremblay, L.; Han, K.; Chevalier, S. Bombesin stimulates the motility of human prostate-carcinoma cells through tyrosine phosphorylation of focal adhesion kinase and of integrin-associated proteins. Int. J. Cancer 1997, 72, 498–504. [Google Scholar] [CrossRef]
- Lai, I.R.; Chu, P.Y.; Lin, H.S.; Liou, J.Y.; Jan, Y.J.; Lee, J.C.; Shen, T.L. Phosphorylation of focal adhesion kinase at Tyr397 in gastric carcinomas and its clinical significance. Am. J. Pathol. 2010, 177, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Beierle, E.A.; Massoll, N.A.; Hartwich, J.; Kurenova, E.V.; Golubovskaya, V.M.; Cance, W.G.; McGrady, P.; London, W.B. Focal adhesion kinase expression in human neuroblastoma: Immunohistochemical and real-time PCR analyses. Clin. Cancer Res. 2008, 14, 3299–3305. [Google Scholar] [CrossRef]
- Korb, T.; Schluter, K.; Enns, A.; Spiegel, H.U.; Senninger, N.; Nicolson, G.L.; Haier, J. Integrity of actin fibers and microtubules influences metastatic tumor cell adhesion. Exp. Cell Res. 2004, 299, 236–247. [Google Scholar] [CrossRef]
- Pawlak, G.; Helfman, D.M. Cytoskeletal changes in cell transformation and tumorigenesis. Curr. Opin. Genet. Dev. 2001, 11, 41–47. [Google Scholar] [CrossRef]
- Wei, W.C.; Lin, H.H.; Shen, M.R.; Tang, M.J. Mechanosensing machinery for cells under low substratum rigidity. Am. J. Physiol. Cell Physiol. 2008, 295, C1579–C1589. [Google Scholar] [CrossRef]
- Jones, G.; Machado, J.; Tolnay, M.; Merlo, A. PTEN-independent induction of caspase-mediated cell death and reduced invasion by the focal adhesion targeting domain (FAT) in human astrocytic brain tumors which highly express focal adhesion kinase (FAK). Cancer Res. 2001, 61, 5688–5691. [Google Scholar]
- Natarajan, M.; Hecker, T.P.; Gladson, C.L. FAK signaling in anaplastic astrocytoma and glioblastoma tumors. Cancer J. 2003, 9, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Pang, D.; Fu, S.B.; Jin, Y.; Yao, L.; Qi, J.P.; Bai, J. Overexpression of focal adhesion kinase correlates with increased lymph node metastasis and poor prognosis in non-small-cell lung cancer. J. Cancer Res. Clin. 2013, 139, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Huang, X.H.; Wang, Q.; Huang, J.Q.; Zhang, L.J.; Chen, X.L.; Lei, J.; Cheng, Z.X. Sonic hedgehog signaling pathway induces cell migration and invasion through focal adhesion kinase/AKT signaling-mediated activation of matrix metalloproteinase (MMP)-2 and MMP-9 in liver cancer. Carcinogenesis 2013, 34, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Huang, X.H.; Wang, Q.; Chen, X.L.; Fu, X.H.; Tan, H.X.; Zhang, L.J.; Li, W.; Bi, J. FAK is involved in invasion and metastasis of hepatocellular carcinoma. Clin. Exp. Metastas. 2010, 27, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Frisch, S.M.; Schaller, M.; Cieply, B. Mechanisms that link the oncogenic epithelial-mesenchymal transition to suppression of anoikis. J. Cell Sci. 2013, 126, 21–29. [Google Scholar] [CrossRef]
- Shibue, T.; Brooks, M.W.; Inan, M.F.; Reinhardt, F.; Weinberg, R.A. The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov. 2012, 2, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Cance, W.G.; Golubovskaya, V.M. Focal adhesion kinase versus p53: Apoptosis or survival? Sci. Signal. 2008, 1, pe22. [Google Scholar] [CrossRef]
- Lim, S.T.; Chen, X.L.; Lim, Y.; Hanson, D.A.; Vo, T.T.; Howerton, K.; Larocque, N.; Fisher, S.J.; Schlaepfer, D.D.; Ilic, D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol. Cell 2008, 29, 9–22. [Google Scholar] [CrossRef]
- Luo, M.; Zhao, X.; Chen, S.; Liu, S.; Wicha, M.S.; Guan, J.L. Distinct FAK activities determine progenitor and mammary stem cell characteristics. Cancer Res. 2013, 73, 5591–5602. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Y. Elaborating the role of natural products-induced autophagy in cancer treatment: Achievements and artifacts in the state of the art. BioMed Res. Int. 2015, 2015, 934207. [Google Scholar] [CrossRef]
- Tan, H.Y.; Wang, N.; Takahashi, M.; Feng, Y.; Li, H.; Feng, Y. New Natural Pigment Fraction Isolated from Saw Palmetto: Potential for Adjuvant Therapy of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2016, 17, 1277. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhong, Z.; Tan, H.Y.; Guo, W.; Zhang, C.; Cheng, C.S.; Wang, N.; Ren, J.; Feng, Y. Suppression of lncRNA MALAT1 by betulinic acid inhibits hepatocellular carcinoma progression by targeting IAPs via miR-22-3p. Clin. Transl. Med. 2020, 10, e190. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, H.Y.; Li, S.; Feng, Y. Atg9b Deficiency Suppresses Autophagy and Potentiates Endoplasmic Reticulum Stress-Associated Hepatocyte Apoptosis in Hepatocarcinogenesis. Theranostics 2017, 7, 2325–2338. [Google Scholar] [CrossRef]
- Guo, W.; Tan, H.Y.; Li, S.; Wang, N.; Feng, Y. Glutamic-Pyruvic Transaminase 1 Facilitates Alternative Fuels for Hepatocellular Carcinoma Growth-A Small Molecule Inhibitor, Berberine. Cancers 2020, 12, 1854. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, N.; Tan, H.Y.; Guo, W.; Chen, F.; Zhong, Z.; Man, K.; Tsao, S.W.; Lao, L.; Feng, Y. Direct inhibition of the TLR4/MyD88 pathway by geniposide suppresses HIF-1alpha-independent VEGF expression and angiogenesis in hepatocellular carcinoma. Br. J. Pharmacol. 2020, 177, 3240–3257. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Leiderman, R.S.E.D.B. Cannabis for Medical Use: FDA and DEA Regulation in the Hall of Mirrors. Food Drug Law J. 2019, 74, 246–279. [Google Scholar]
- Hwang-Bo, J.; Bae, M.G.; Park, J.H.; Chung, I.S. 3-O-Acetyloleanolic acid inhibits VEGF-A-induced lymphangiogenesis and lymph node metastasis in an oral cancer sentinel lymph node animal model. BMC Cancer 2018, 18, 714. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, Y.F.; Chen, X.Y.; Han, B.; Li, F.; Chen, J.Y.; Peng, X.L.; Luo, L.P.; Chen, W.; Yu, X.P. Black rice-derived anthocyanins inhibit HER-2-positive breast cancer epithelial-mesenchymal transition-mediated metastasis in vitro by suppressing FAK signaling. Int. J. Mol. Med. 2017, 40, 1649–1656. [Google Scholar] [CrossRef]
- Chen, C.C.; Sureshbabul, M.; Chen, H.W.; Lin, Y.S.; Lee, J.Y.; Hong, Q.S.; Yang, Y.C.; Yu, S.L. Curcumin Suppresses Metastasis via Sp-1, FAK Inhibition, and E-Cadherin Upregulation in Colorectal Cancer. Evid.-Based Compl. Altern. Med. 2013, 2013, 541695. [Google Scholar] [CrossRef]
- Mani, J.; Fleger, J.; Rutz, J.; Maxeiner, S.; Bernd, A.; Kippenberger, S.; Zoller, N.; Chun, F.K.H.; Relja, B.; Juengel, E.; et al. Curcumin combined with exposure to visible light blocks bladder cancer cell adhesion and migration by an integrin dependent mechanism. Eur. Rev. Med. Pharm. 2019, 23, 10564–10574. [Google Scholar]
- Deng, M.; Zhang, Y.; Liu, B.; Chen, Y.; Song, H.; Yu, R.; Che, X.; Qu, X.; Liu, Y.; Hu, X.; et al. beta-Elemene inhibits peritoneal metastasis of gastric cancer cells by modulating FAK/Claudin-1 signaling. Phytother. Res. 2019, 33, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Tan, W.; Chen, X.; Wang, Y. Furanodiene, a natural small molecule suppresses metastatic breast cancer cell migration and invasion in vitro. Eur. J. Pharmacol. 2014, 737, 1–10. [Google Scholar] [CrossRef]
- Zhong, Z.F.; Tan, W.; Tian, K.; Yu, H.; Qiang, W.A.; Wang, Y.T. Combined effects of furanodiene and doxorubicin on the migration and invasion of MDA-MB-231 breast cancer cells in vitro. Oncol. Rep. 2017, 37, 2016–2024. [Google Scholar] [CrossRef]
- Schneider, N.F.; Geller, F.C.; Persich, L.; Marostica, L.L.; Padua, R.M.; Kreis, W.; Braga, F.C.; Simoes, C.M. Inhibition of cell proliferation, invasion and migration by the cardenolides digitoxigenin monodigitoxoside and convallatoxin in human lung cancer cell line. Nat. Prod. Res. 2016, 30, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, X.; Ma, X.; Xie, L.; Xia, Z.; Liu, L.; Yu, X.; Wang, J.; Zhou, H.; Zhou, X.; et al. Deguelin attenuates non-small cell lung cancer cell metastasis through inhibiting the CtsZ/FAK signaling pathway. Cell Signal. 2018, 50, 131–141. [Google Scholar] [CrossRef]
- Huang, S.F.; Horng, C.T.; Hsieh, Y.S.; Hsieh, Y.H.; Chu, S.C.; Chen, P.N. Epicatechin-3-gallate reverses TGF-beta1-induced epithelial-to-mesenchymal transition and inhibits cell invasion and protease activities in human lung cancer cells. Food Chem. Toxicol. 2016, 94, 1–10. [Google Scholar] [CrossRef]
- Sen, T.; Dutta, A.; Chatterjee, A. Epigallocatechin-3-gallate (EGCG) downregulates gelatinase-B (MMP-9) by involvement of FAK/ERK/NFkappaB and AP-1 in the human breast cancer cell line MDA-MB-231. Anticancer Drugs 2010, 21, 632–644. [Google Scholar] [CrossRef]
- Sen, T.; Chatterjee, A. Epigallocatechin-3-gallate (EGCG) downregulates EGF-induced MMP-9 in breast cancer cells: Involvement of integrin receptor alpha5beta1 in the process. Eur. J. Nutr. 2011, 50, 465–478. [Google Scholar] [CrossRef]
- Sen, T.; Moulik, S.; Dutta, A.; Choudhury, P.R.; Banerji, A.; Das, S.; Roy, M.; Chatterjee, A. Multifunctional effect of epigallocatechin-3-gallate (EGCG) in downregulation of gelatinase-A (MMP-2) in human breast cancer cell line MCF-7. Life Sci. 2009, 84, 194–204. [Google Scholar] [CrossRef]
- Chen, P.N.; Chu, S.C.; Kuo, W.H.; Chou, M.Y.; Lin, J.K.; Hsieh, Y.S. Epigallocatechin-3 gallate inhibits invasion, epithelial-mesenchymal transition, and tumor growth in oral cancer cells. J. Agric. Food Chem. 2011, 59, 3836–3844. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.S.; Park, K.K.; Chung, W.Y. Epigallocatechin-3 gallate inhibits cancer invasion by repressing functional invadopodia formation in oral squamous cell carcinoma. Eur. J. Pharmacol. 2013, 715, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.D.; Chen, S.H.; Lin, C.L.; Tsai, S.H.; Liang, Y.C. Inhibition of melanoma growth and metastasis by combination with (-)-epigallocatechin-3-gallate and dacarbazine in mice. J. Cell Biochem. 2001, 83, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Guo, B.; Hui, Q.; Chang, P.; Tao, K. Fangchinoline suppresses growth and metastasis of melanoma cells by inhibiting the phosphorylation of FAK. Oncol. Rep. 2017, 38, 63–70. [Google Scholar] [CrossRef]
- Guo, B.; Su, J.; Zhang, T.; Wang, K.; Li, X. Fangchinoline as a kinase inhibitor targets FAK and suppresses FAK-mediated signaling pathway in A549. J. Drug Target 2015, 23, 266–274. [Google Scholar] [CrossRef]
- Thiyagarajan, V.; Lin, S.H.; Chang, Y.C.; Weng, C.F. Identification of novel FAK and S6K1 dual inhibitors from natural compounds via ADMET screening and molecular docking. Biomed. Pharmacother. 2016, 80, 52–62. [Google Scholar] [CrossRef]
- Pan, X.; Han, H.; Wang, L.; Yang, L.; Li, R.; Li, Z.; Liu, J.; Zhao, Q.; Qian, M.; Liu, M.; et al. Nitidine Chloride inhibits breast cancer cells migration and invasion by suppressing c-Src/FAK associated signaling pathway. Cancer Lett. 2011, 313, 181–191. [Google Scholar] [CrossRef]
- Wang, S.; Zhong, Z.; Wan, J.; Tan, W.; Wu, G.; Chen, M.; Wang, Y. Oridonin induces apoptosis, inhibits migration and invasion on highly-metastatic human breast cancer cells. Am. J. Chin. Med. 2013, 41, 177–196. [Google Scholar] [CrossRef]
- Yao, H.; Xie, S.; Ma, X.; Liu, J.; Wu, H.; Lin, A.; Yao, H.; Li, D.; Xu, S.; Yang, D.H.; et al. Identification of a Potent Oridonin Analogue for Treatment of Triple-Negative Breast Cancer. J. Med. Chem. 2020, 63, 8157–8178. [Google Scholar] [CrossRef]
- Li, D.; Wang, H.; Ding, Y.; Zhang, Z.; Zheng, Z.; Dong, J.; Kim, H.; Meng, X.; Zhou, Q.; Zhou, J.; et al. Targeting the NRF-2/RHOA/ROCK signaling pathway with a novel aziridonin, YD0514, to suppress breast cancer progression and lung metastasis. Cancer Lett. 2018, 424, 97–108. [Google Scholar] [CrossRef]
- Petpiroon, N.; Sritularak, B.; Chanvorachote, P. Phoyunnanin E inhibits migration of non-small cell lung cancer cells via suppression of epithelial-to-mesenchymal transition and integrin alphav and integrin beta3. BMC Complement. Altern. Med. 2017, 17, 553. [Google Scholar] [CrossRef] [PubMed]
- Kolli-Bouhafs, K.; Boukhari, A.; Abusnina, A.; Velot, E.; Gies, J.P.; Lugnier, C.; Ronde, P. Thymoquinone reduces migration and invasion of human glioblastoma cells associated with FAK, MMP-2 and MMP-9 down-regulation. Investig. New Drugs 2012, 30, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, X.L.; Yuan, J.W.; Zhang, H.R.; Liu, D.; Hao, J.; Ji, W.; Wu, X.Z.; Chen, D. Cucurbitacin B inhibits the migration and invasion of breast cancer cells by altering the biomechanical properties of cells. Phytother. Res. PTR 2019, 33, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Cui, E.J.; Hwang-Bo, J.; Park, J.H.; Baek, N.I.; Kim, J.; Hong, S.G.; Chung, I.S. 3-O-Acetyloleanolic acid exhibits anti-angiogenic effects and induces apoptosis in human umbilical vein endothelial cells. Biotechnol. Lett. 2013, 35, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.H.; Park, J.H.; Cui, E.J.; Kim, K.I.; Kim, J.Y.; Kim, J.; Hong, S.G.; Baek, N.I.; Chung, I.S. 3-O-acetyloleanolic acid induces apoptosis in human colon carcinoma HCT-116 cells. Phytother. Res. 2012, 26, 1541–1546. [Google Scholar] [CrossRef]
- Yoo, K.H.; Park, J.H.; Lee, D.Y.; Hwang-Bo, J.; Baek, N.I.; Chung, I.S. Corosolic Acid Exhibits Anti-angiogenic and Anti-lymphangiogenic Effects on In Vitro Endothelial Cells and on an In Vivo CT-26 Colon Carcinoma Animal Model. Phytother. Res. 2015, 29, 714–723. [Google Scholar] [CrossRef]
- Kong, S.; Kim, D.J.; Oh, S.K.; Choi, I.S.; Jeong, H.S.; Lee, J. Black rice bran as an ingredient in noodles: Chemical and functional evaluation. J. Food Sci. 2012, 77, C303–C307. [Google Scholar] [CrossRef]
- Sehitoglu, M.H.; Farooqi, A.A.; Qureshi, M.Z.; Butt, G.; Aras, A. Anthocyanins: Targeting of signaling networks in cancer cells. Asian Pac. J. Cancer Prev. 2014, 15, 2379–2381. [Google Scholar] [CrossRef]
- Yang, F.Q.; Wang, Y.T.; Li, S.P. Simultaneous determination of 11 characteristic components in three species of Curcuma rhizomes using pressurized liquid extraction and high-performance liquid chromatography. J. Chromatogr. A 2006, 1134, 226–231. [Google Scholar] [CrossRef]
- Ashraf, K.; Mujeeb, M.; Ahmad, A.; Ahmad, N.; Amir, M. Determination of Curcuminoids in Curcuma longa Linn. by UPLC/Q-TOF-MS: An Application in Turmeric Cultivation. J. Chromatogr. Sci. 2015, 53, 1346–1352. [Google Scholar] [CrossRef]
- Leu, T.H.; Su, S.L.; Chuang, Y.C.; Maa, M.C. Direct inhibitory effect of curcumin on Src and focal adhesion kinase activity. Biochem. Pharmacol. 2003, 66, 2323–2331. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P.; Thiele, W.; Cremers, N.; Muppala, S.; Krachulec, J.; Diefenbacher, M.; Kassel, O.; Mudduluru, G.; Allgayer, H.; Frame, M.; et al. CD24 interacts with and promotes the activity of c-src within lipid rafts in breast cancer cells, thereby increasing integrin-dependent adhesion. Cell Mol. Life Sci. 2012, 69, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Laubach, V.; Kaufmann, R.; Bernd, A.; Kippenberger, S.; Zoller, N. Extrinsic or Intrinsic Apoptosis by Curcumin and Light: Still a Mystery. Int. J. Mol. Sci. 2019, 20, 905. [Google Scholar] [CrossRef]
- Bernd, A. Visible light and/or UVA offer a strong amplification of the anti-tumor effect of curcumin. Phytochem. Rev. 2014, 13, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R.; Liu, B.; Zhou, L.; Huang, Y.X. MicroRNA-124-3p suppresses cell migration and invasion by targeting ITGA3 signaling in bladder cancer. Cancer Biomark. 2019, 24, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Zhang, N.; Han, X.; Li, Q.; Zhang, M.; Chen, X.; Li, G.; Zhang, R.; Chen, P.; Wang, W.; et al. Molecular targets of beta-elemene, a herbal extract used in traditional Chinese medicine, and its potential role in cancer therapy: A review. Biomed. Pharmacother. 2019, 114, 108812. [Google Scholar] [CrossRef]
- Gunzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Cherradi, S.; Ayrolles-Torro, A.; Vezzo-Vie, N.; Gueguinou, N.; Denis, V.; Combes, E.; Boissiere, F.; Busson, M.; Canterel-Thouennon, L.; Mollevi, C.; et al. Antibody targeting of claudin-1 as a potential colorectal cancer therapy. J. Exp. Clin. Cancer Res. 2017, 36, 89. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Fukasawa, M.; Kuniyasu, H.; Yagi, K.; Kondoh, M. Claudin-targeted drug development using anti-claudin monoclonal antibodies to treat hepatitis and cancer. Ann. N. Y. Acad. Sci. 2017, 1397, 5–16. [Google Scholar] [CrossRef]
- Stebbing, J.; Filipovic, A.; Giamas, G. Claudin-1 as a promoter of EMT in hepatocellular carcinoma. Oncogene 2013, 32, 4871–4872. [Google Scholar] [CrossRef]
- do Carmo, G.M.; Doleski, P.H.; de Sa, M.F.; Grando, T.H.; Bottari, N.B.; Leal, D.B.R.; Gressler, L.T.; Mendes, R.E.; Stefani, L.M.; Monteiro, S.G.; et al. Purinergic ecto-enzymes participate in the thromboregulation in acute in mice infection by Trypanosoma cruzi. Mol. Cell Biochem. 2017, 432, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Guo, D.W.; Lao, Q.C.; Xu, Y.Q.; Meng, Z.K.; Xia, B.; Yang, H.; Li, C.Q.; Li, P. Sensitization and synergistic anti-cancer effects of Furanodiene identified in zebrafish models. Sci. Rep. 2019, 9, 4541. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Ma, Y.; Yu, J.; Chen, X. Predicted molecular targets and pathways for germacrone, curdione, and furanodiene in the treatment of breast cancer using a bioinformatics approach. Sci. Rep. 2017, 7, 15543. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Amalraj, A.; Jacob, J.; Kunnumakkara, A.B.; Gopi, S. Non-Curcuminoids from Turmeric and Their Potential in Cancer Therapy and Anticancer Drug Delivery Formulations. Biomolecules 2019, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.F.; Yu, H.B.; Wang, C.M.; Qiang, W.A.; Wang, S.P.; Zhang, J.M.; Yu, H.; Cui, L.; Wu, T.; Li, D.Q.; et al. Corrigendum: Furanodiene Induces Extrinsic and Intrinsic Apoptosis in Doxorubicin-Resistant MCF-7 Breast Cancer Cells via NF-kappaB-Independent Mechanism. Front. Pharmacol. 2017, 8, 934. [Google Scholar] [CrossRef]
- Cohen, L.A.; Guan, J.L. Mechanisms of focal adhesion kinase regulation. Curr. Cancer Drug Targets 2005, 5, 629–643. [Google Scholar] [CrossRef]
- Parsons, J.T.; Martin, K.H.; Slack, J.K.; Taylor, J.M.; Weed, S.A. Focal adhesion kinase: A regulator of focal adhesion dynamics and cell movement. Oncogene 2000, 19, 5606–5613. [Google Scholar] [CrossRef]
- Janku, F.; Yap, T.A.; Meric-Bernstam, F. Targeting the PI3K pathway in cancer: Are we making headway? Nat. Rev. Clin. Oncol. 2018, 15, 273–291. [Google Scholar] [CrossRef]
- Rahimtoola, S.H.; Tak, T. The use of digitalis in heart failure. Curr. Probl. Cardiol. 1996, 21, 781–853. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, Q.; Shen, J.J.; Li, D.D.; Jiang, X.J.; Si, S.Y.; Shao, R.G.; Wang, Z. Cardiac glycosides induce autophagy in human non-small cell lung cancer cells through regulation of dual signaling pathways. Int. J. Biochem. Cell Biol. 2012, 44, 1813–1824. [Google Scholar] [CrossRef]
- Cerella, C.; Dicato, M.; Diederich, M. Assembling the puzzle of anti-cancer mechanisms triggered by cardiac glycosides. Mitochondrion 2013, 13, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Pongrakhananon, V.; Chunhacha, P.; Chanvorachote, P. Ouabain suppresses the migratory behavior of lung cancer cells. PLoS ONE 2013, 8, e68623. [Google Scholar] [CrossRef]
- Liu, N.; Li, Y.; Su, S.; Wang, N.; Wang, H.; Li, J. Inhibition of cell migration by ouabain in the A549 human lung cancer cell line. Oncol. Lett. 2013, 6, 475–479. [Google Scholar] [CrossRef]
- Murillo, G.; Salti, G.I.; Kosmeder, J.W., 2nd; Pezzuto, J.M.; Mehta, R.G. Deguelin inhibits the growth of colon cancer cells through the induction of apoptosis and cell cycle arrest. Eur. J. Cancer 2002, 38, 2446–2454. [Google Scholar] [CrossRef]
- Lee, H.Y.; Oh, S.H.; Woo, J.K.; Kim, W.Y.; Van Pelt, C.S.; Price, R.E.; Cody, D.; Tran, H.; Pezzuto, J.M.; Moriarty, R.M.; et al. Chemopreventive effects of deguelin, a novel Akt inhibitor, on tobacco-induced lung tumorigenesis. J. Natl. Cancer Inst. 2005, 97, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, J.H.; Jung, K.H.; Hong, S.S. Deguelin promotes apoptosis and inhibits angiogenesis of gastric cancer. Oncol. Rep. 2010, 24, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Boreddy, S.R.; Srivastava, S.K. Deguelin suppresses pancreatic tumor growth and metastasis by inhibiting epithelial-to-mesenchymal transition in an orthotopic model. Oncogene 2013, 32, 3980–3991. [Google Scholar] [CrossRef]
- Zhao, D.; Han, W.; Liu, X.; Cui, D.; Chen, Y. Deguelin inhibits epithelial-to-mesenchymal transition and metastasis of human non-small cell lung cancer cells by regulating NIMA-related kinase 2. Thorac. Cancer 2017, 8, 320–327. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, S.; Cai, H.; Kang, M.; Qin, W.; Li, C.; Wu, Y. Deguelin inhibits proliferation and migration of human pancreatic cancer cells in vitro targeting hedgehog pathway. Oncol. Lett. 2016, 12, 2761–2765. [Google Scholar] [CrossRef]
- Zhao, H.; Jiao, Y.; Zhang, Z. Deguelin inhibits the migration and invasion of lung cancer A549 and H460 cells via regulating actin cytoskeleton rearrangement. Int. J. Clin. Exp. Pathol. 2015, 8, 15582–15590. [Google Scholar]
- Akkari, L.; Gocheva, V.; Kester, J.C.; Hunter, K.E.; Quick, M.L.; Sevenich, L.; Wang, H.W.; Peters, C.; Tang, L.H.; Klimstra, D.S.; et al. Distinct functions of macrophage-derived and cancer cell-derived cathepsin Z combine to promote tumor malignancy via interactions with the extracellular matrix. Genes Dev. 2014, 28, 2134–2150. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Jevnikar, Z.; Obermajer, N. The role of cathepsin X in cell signaling. Cell Adh. Migr. 2009, 3, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Lechner, A.M.; Assfalg-Machleidt, I.; Zahler, S.; Stoeckelhuber, M.; Machleidt, W.; Jochum, M.; Nagler, D.K. RGD-dependent binding of procathepsin X to integrin alphavbeta3 mediates cell-adhesive properties. J. Biol. Chem. 2006, 281, 39588–39597. [Google Scholar] [CrossRef]
- Chowdhury, A.; Sarkar, J.; Chakraborti, T.; Pramanik, P.K.; Chakraborti, S. Protective role of epigallocatechin-3-gallate in health and disease: A perspective. Biomed. Pharmacother. 2016, 78, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yang, C.; Jiang, Z.; Wang, Y.; Zhu, F.; Li, T.; Wan, X.; Xu, Y.; Xie, Z.; Li, D.; et al. Epicatechin-3-Gallate Signaling and Protection against Cardiac Ischemia/Reperfusion Injury. J. Pharmacol. Exp. Ther. 2019, 371, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Garbisa, S.; Sartor, L.; Biggin, S.; Salvato, B.; Benelli, R.; Albini, A. Tumor gelatinases and invasion inhibited by the green tea flavanol epigallocatechin-3-gallate. Cancer 2001, 91, 822–832. [Google Scholar] [CrossRef]
- Jung, Y.D.; Ellis, L.M. Inhibition of tumour invasion and angiogenesis by epigallocatechin gallate (EGCG), a major component of green tea. Int. J. Exp. Pathol. 2001, 82, 309–316. [Google Scholar] [CrossRef]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109 (Suppl. S1), 69–75. [Google Scholar] [CrossRef]
- Merarchi, M.; Sethi, G.; Fan, L.; Mishra, S.; Arfuso, F.; Ahn, K.S. Molecular Targets Modulated by Fangchinoline in Tumor Cells and Preclinical Models. Molecules 2018, 23, 2538. [Google Scholar] [CrossRef]
- Wang, C.D.; Yuan, C.F.; Bu, Y.Q.; Wu, X.M.; Wan, J.Y.; Zhang, L.; Hu, N.; Liu, X.J.; Zu, Y.; Liu, G.L.; et al. Fangchinoline inhibits cell proliferation via Akt/GSK-3beta/cyclin D1 signaling and induces apoptosis in MDA-MB-231 breast cancer cells. Asian Pac. J. Cancer Prev. 2014, 15, 769–773. [Google Scholar] [CrossRef]
- Wang, C.D.; Huang, J.G.; Gao, X.; Li, Y.; Zhou, S.Y.; Yan, X.; Zou, A.; Chang, J.L.; Wang, Y.S.; Yang, G.X.; et al. Fangchinoline induced G1/S arrest by modulating expression of p27, PCNA, and cyclin D in human prostate carcinoma cancer PC3 cells and tumor xenograft. Biosci. Biotechnol. Biochem. 2010, 74, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Peng, J.M.; Su, L.D.; Wang, D.Y.; Yu, Y.J. Fangchinoline inhibits the proliferation of SPC-A-1 lung cancer cells by blocking cell cycle progression. Exp. Ther. Med. 2016, 11, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lu, Y.; Sun, P.; Feng, L.X.; Liu, M.; Hu, L.H.; Wu, W.Y.; Jiang, B.H.; Yang, M.; Qu, X.B.; et al. Inhibition on Proteasome beta1 Subunit Might Contribute to the Anti-Cancer Effects of Fangchinoline in Human Prostate Cancer Cells. PLoS ONE 2015, 10, e0141681. [Google Scholar] [CrossRef]
- Lee, H.S.; Safe, S.; Lee, S.O. Inactivation of the orphan nuclear receptor NR4A1 contributes to apoptosis induction by fangchinoline in pancreatic cancer cells. Toxicol. Appl. Pharmacol. 2017, 332, 32–39. [Google Scholar] [CrossRef]
- Wang, N.; Pan, W.; Zhu, M.; Zhang, M.; Hao, X.; Liang, G.; Feng, Y. Fangchinoline induces autophagic cell death via p53/sestrin2/AMPK signalling in human hepatocellular carcinoma cells. Br. J. Pharmacol. 2011, 164, 731–742. [Google Scholar] [CrossRef]
- Deramaudt, T.B.; Dujardin, D.; Noulet, F.; Martin, S.; Vauchelles, R.; Takeda, K.; Ronde, P. Altering FAK-paxillin interactions reduces adhesion, migration and invasion processes. PLoS ONE 2014, 9, e92059. [Google Scholar] [CrossRef]
- Siesser, P.M.; Hanks, S.K. The signaling and biological implications of FAK overexpression in cancer. Clin. Cancer Res. 2006, 12, 3233–3237. [Google Scholar] [CrossRef]
- Pham, D.C.; Chang, Y.C.; Lin, S.R.; Fuh, Y.M.; Tsai, M.J.; Weng, C.F. FAK and S6K1 Inhibitor, Neferine, Dually Induces Autophagy and Apoptosis in Human Neuroblastoma Cells. Molecules 2018, 23, 3110. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, W.; Zhang, Z.; Qian, M.; Du, B. Nitidine chloride inhibits LPS-induced inflammatory cytokines production via MAPK and NF-kappaB pathway in RAW 264.7 cells. J. Ethnopharmacol. 2012, 144, 145–150. [Google Scholar] [CrossRef]
- Bouquet, J.; Rivaud, M.; Chevalley, S.; Deharo, E.; Jullian, V.; Valentin, A. Biological activities of nitidine, a potential anti-malarial lead compound. Malar. J. 2012, 11, 67. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Lin, L.; He, L.; Wu, Y.; Zhang, L.; Yi, Z.; Chen, Y.; Pang, X.; Liu, M. Inhibition of STAT3 signaling pathway by nitidine chloride suppressed the angiogenesis and growth of human gastric cancer. Mol. Cancer Ther. 2012, 11, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Tang, Y.; Jiao, W.; Xing, Z.; Guo, Z.; Wang, W.; Shi, B.; Xu, Z.; Liu, Z. Nitidine chloride inhibits renal cancer cell metastasis via suppressing AKT signaling pathway. Food Chem. Toxicol. 2013, 60, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xu, T.; Zheng, J.X.; Lin, J.M.; Cai, Q.Y.; Yu, D.B.; Peng, J. Nitidine chloride inhibits hepatocellular carcinoma cell growth in vivo through the suppression of the JAK1/STAT3 signaling pathway. Int. J. Mol. Med. 2013, 32, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Tang, Y.; Jiao, W.; Xing, Z.; Guo, Z.; Wang, W.; Xu, Z.; Liu, Z. Nitidine chloride induces apoptosis and inhibits tumor cell proliferation via suppressing ERK signaling pathway in renal cancer. Food Chem. Toxicol. 2014, 66, 210–216. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, N.; Wang, X.; Cai, C.; Cun, J.; Li, Y.; Lv, S.; Yang, Q. Nitidine chloride induces apoptosis, cell cycle arrest, and synergistic cytotoxicity with doxorubicin in breast cancer cells. Tumour. Biol. 2014, 35, 10201–10212. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wang, S.; Gao, Y.; Zhang, X.; Lu, C. Oridonin phosphate-induced autophagy effectively enhances cell apoptosis of human breast cancer cells. Med. Oncol. 2015, 32, 365. [Google Scholar] [CrossRef]
- Luo, D.; Yi, Y.; Peng, K.; Liu, T.; Yang, J.; Liu, S.; Zhao, W.; Qu, X.; Yu, W.; Gu, Y.; et al. Oridonin derivatives as potential anticancer drug candidates triggering apoptosis through mitochondrial pathway in the liver cancer cells. Eur. J. Med. Chem. 2019, 178, 365–379. [Google Scholar] [CrossRef]
- Ke, Y.; Wang, W.; Zhao, L.F.; Liang, J.J.; Liu, Y.; Zhang, X.; Feng, K.; Liu, H.M. Design, synthesis and biological mechanisms research on 1,2,3-triazole derivatives of Jiyuan Oridonin A. Bioorg. Med. Chem. 2018, 26, 4761–4773. [Google Scholar] [CrossRef]
- Shen, Q.K.; Deng, H.; Wang, S.B.; Tian, Y.S.; Quan, Z.S. Synthesis, and evaluation of in vitro and in vivo anticancer activity of 14-substituted oridonin analogs: A novel and potent cell cycle arrest and apoptosis inducer through the p53-MDM2 pathway. Eur. J. Med. Chem. 2019, 173, 15–31. [Google Scholar] [CrossRef]
- Hu, X.; Bai, Z.; Qiao, J.; Li, H.; Xu, S.; Wang, X.; Xu, Y.; Xu, J.; Hua, H.; Li, D. Effective enmein-type mimics of clinical candidate HAO472: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2019, 171, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Liang, J.J.; Hou, R.J.; Li, M.M.; Zhao, L.F.; Wang, W.; Liu, Y.; Xie, H.; Yang, R.H.; Hu, T.X.; et al. Synthesis and biological evaluation of novel Jiyuan Oridonin A-1,2,3-triazole-azole derivatives as antiproliferative agents. Eur. J. Med. Chem. 2018, 157, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Sukphan, P.; Sritularak, B.; Mekboonsonglarp, W.; Lipipun, V.; Likhitwitayawuid, K. Chemical constituents of Dendrobium venustum and their antimalarial and anti-herpetic properties. Nat. Prod. Commun. 2014, 9, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.; Ocker, M.; Kuester, D.; Krueger, S.; El-Hajj, Z.; Diestel, A.; Evert, M.; El-Najjar, N.; Peters, B.; Jurjus, A.; et al. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J. Cell Mol. Med. 2008, 12, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Jafri, S.H.; Glass, J.; Shi, R.; Zhang, S.; Prince, M.; Kleiner-Hancock, H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J. Exp. Clin. Cancer Res. 2010, 29, 87. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Cho, S.G.; Yi, Z.; Pang, X.; Rodriguez, M.; Wang, Y.; Sethi, G.; Aggarwal, B.B.; Liu, M. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol. Cancer Ther. 2008, 7, 1789–1796. [Google Scholar] [CrossRef]
- El-Najjar, N.; Chatila, M.; Moukadem, H.; Vuorela, H.; Ocker, M.; Gandesiri, M.; Schneider-Stock, R.; Gali-Muhtasib, H. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis 2010, 15, 183–195. [Google Scholar] [CrossRef]
- Mon, N.N.; Hasegawa, H.; Thant, A.A.; Huang, P.; Tanimura, Y.; Senga, T.; Hamaguchi, M. A role for focal adhesion kinase signaling in tumor necrosis factor-alpha-dependent matrix metalloproteinase-9 production in a cholangiocarcinoma cell line, CCKS1. Cancer Res. 2006, 66, 6778–6784. [Google Scholar] [CrossRef]
- Xu, H.Y.; Qian, A.R.; Shang, P.; Xu, J.; Kong, L.M.; Bian, H.J.; Chen, Z.N. siRNA targeted against HAb18G/CD147 inhibits MMP-2 secretion, actin and FAK expression in hepatocellular carcinoma cell line via ERK1/2 pathway. Cancer Lett. 2007, 247, 336–344. [Google Scholar] [CrossRef]
- Kumar, S.; Weaver, V.M. Mechanics, malignancy, and metastasis: The force journey of a tumor cell. Cancer Metastasis Rev. 2009, 28, 113–127. [Google Scholar] [CrossRef]
- De Pascalis, C.; Etienne-Manneville, S. Single and collective cell migration: The mechanics of adhesions. Mol. Biol. Cell 2017, 28, 1833–1846. [Google Scholar] [CrossRef]
- Weaver, A.M. Invadopodia: Specialized cell structures for cancer invasion. Clin. Exp. Metastasis 2006, 23, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Guck, J.; Schinkinger, S.; Lincoln, B.; Wottawah, F.; Ebert, S.; Romeyke, M.; Lenz, D.; Erickson, H.M.; Ananthakrishnan, R.; Mitchell, D.; et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys. J. 2005, 88, 3689–3698. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, U.; Aeri, V.; Mir, S.R. Cucurbitacins—An insight into medicinal leads from nature. Pharmacogn. Rev. 2015, 9, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zi Jiang, Y.; Shi, H.; Mi, C.; Li, J.; Xing Nan, J.; Wu, X.; Joon Lee, J.; Jin, X. Cucurbitacin B inhibits the translational expression of hypoxia-inducible factor-1alpha. Eur. J. Pharmacol. 2014, 723, 46–54. [Google Scholar] [CrossRef]
- Garg, S.; Kaul, S.C.; Wadhwa, R. Cucurbitacin B and cancer intervention: Chemistry, biology and mechanisms (Review). Int. J. Oncol. 2018, 52, 19–37. [Google Scholar] [CrossRef]
- Chen, J.C.; Chiu, M.H.; Nie, R.L.; Cordell, G.A.; Qiu, S.X. Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat. Prod. Rep. 2005, 22, 386–399. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Gao, M.X.; Yang, K. Cucurbitacin B inhibits cell proliferation and induces apoptosis in human osteosarcoma cells via modulation of the JAK2/STAT3 and MAPK pathways. Exp. Ther. Med. 2017, 14, 805–812. [Google Scholar] [CrossRef]
- Shukla, S.; Khan, S.; Kumar, S.; Sinha, S.; Farhan, M.; Bora, H.K.; Maurya, R.; Meeran, S.M. Cucurbitacin B Alters the Expression of Tumor-Related Genes by Epigenetic Modifications in NSCLC and Inhibits NNK-Induced Lung Tumorigenesis. Cancer Prev. Res. 2015, 8, 552–562. [Google Scholar] [CrossRef]
- Promkan, M.; Dakeng, S.; Chakrabarty, S.; Bogler, O.; Patmasiriwat, P. The effectiveness of cucurbitacin B in BRCA1 defective breast cancer cells. PLoS ONE 2013, 8, e55732. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, X.; Tanaka, M.; Peixoto, H.S.; Wink, M. Cucurbitacins: Elucidation of their interactions with the cytoskeleton. PeerJ 2017, 5, e3357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Xu, L.H.; Lu, Q.; Liu, K.P.; Liu, P.Y.; Ji, F.; Liu, X.M.; Ouyang, D.Y.; He, X.H. VASP activation via the Galpha13/RhoA/PKA pathway mediates cucurbitacin-B-induced actin aggregation and cofilin-actin rod formation. PLoS ONE 2014, 9, e93547. [Google Scholar] [CrossRef]
- Marostica, L.L.; Silva, I.T.; Kratz, J.M.; Persich, L.; Geller, F.C.; Lang, K.L.; Caro, M.S.; Duran, F.J.; Schenkel, E.P.; Simoes, C.M. Synergistic Antiproliferative Effects of a New Cucurbitacin B Derivative and Chemotherapy Drugs on Lung Cancer Cell Line A549. Chem. Res. Toxicol. 2015, 28, 1949–1960. [Google Scholar] [CrossRef]
- Liu, C.Y.; Lin, H.H.; Tang, M.J.; Wang, Y.K. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget 2015, 6, 15966–15983. [Google Scholar] [CrossRef] [PubMed]
- Bergert, M.; Chandradoss, S.D.; Desai, R.A.; Paluch, E. Cell mechanics control rapid transitions between blebs and lamellipodia during migration. Proc. Natl. Acad. Sci. USA 2012, 109, 14434–14439. [Google Scholar] [CrossRef]
- Canel, M.; Serrels, A.; Frame, M.C.; Brunton, V.G. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J. Cell Sci. 2013, 126, 393–401. [Google Scholar] [CrossRef]
- Ridley, A.J. Rho GTPase signalling in cell migration. Curr. Opin. Cell Biol. 2015, 36, 103–112. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Morris, L.G.; Chan, T.A. Therapeutic targeting of tumor suppressor genes. Cancer 2015, 121, 1357–1368. [Google Scholar] [CrossRef]
- Rahman, H.S. Natural Products for Cancer Therapy. Dual Diagn. 2016, 1, 1–3. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Tabaczar, S.; Koceva-Chyla, A.; Matczak, K.; Gwozdzinski, K. Molecular mechanisms of antitumor activity of taxanes. I. Interaction of docetaxel with microtubules. Postepy Hig. Med. Dosw. 2010, 64, 568–581. [Google Scholar]
- Mekhail, T.M.; Markman, M. Paclitaxel in cancer therapy. Expert Opin. Pharmacother. 2002, 3, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234–12248. [Google Scholar] [CrossRef]

| Natural Compound | Herbal Source | Tumor Type | Key Findings | Reference |
|---|---|---|---|---|
| FAK kinase-dependent inhibitors | ||||
| 3AOA | V. sinensis K. | Oral cancer | 3AOA suppresses tumor growth, tumor-triggered lymphangiogenesis, and sentinel lymph node metastasis by suppressing the phosphorylation of AKT, FAK, PI3K, and ERK1/2. | [68] |
| Anthocyanins | Black rice | HER-2–positive breast cancer | BRACs suppress the metastasis of HER-2–positive breast cancer in vitro via the Src/FAK/p130Cas pathway. | [69] |
| Curcumin | Curcuma C. longa | Colon cancer | Downregulation of CD24 by curcumin inhibits the interaction of CD24 with FAK and then prevents the proliferation and invasion of colon cancer cells. | [70] |
| Bladder cancer | Treatment with curcumin and light blocks bladder cancer cell adhesion and migration through inhibition of integrin/pFAK signaling. | [71] | ||
| β-elemene | Curcuma C. longa | Gastric cancer | β-elemene inhibits gastric cancer cell metastasis via modulation of the FAK/claudin-1 signaling pathway. | [72] |
| Furanodiene | Curcuma C. longa | Breast cancer | The integrin/FAK and PI3K/AKT pathways jointly contribute to the metastasis-inhibiting effect of furanodiene in breast cancer cases. | [73] |
| Highly metastatic breast cancer | Furanodiene has the potential to improve the anticancer efficacy of doxorubicin by downregulating the phosphorylation of FAK, Src, paxillin, p85, and AKT. | [74] | ||
| Cardiac glycosides (digitoxigenin monodigitoxoside and convallatoxin) | Digitalis lanata Ehrh. (digitoxigenin monodigitoxoside), Convallaria majalis L. (convallatoxin) | Lung cancer | Cardenolides decrease the expression of pFAK, MMP-9, and MMP-2 to inhibit cancer cell migratory behavior. | [75] |
| Deguelin | D. trifoliata Lour. and M. sericea | Lung cancer | Deguelin exerts antimigratory and anti-invasive effects partly by disrupting the physical interaction of cathepsin Z with integrin β3 and attenuating activation of the FAK/Src/paxillin-signaling cascade. | [76] |
| ECG | Green tea | Lung cancer | Invasion of A549 lung cancer cells is inhibited by ECG partly through inhibition of the FAK-signaling pathway. | [77] |
| EGCG | Green tea | Breast cancer | EGCG-induced FAK/ERK inhibition disrupts the binding activities of nuclear factor-κB and activator protein 1, leading to dysregulation of MMP-9 gene transcription. | [78,79] |
| Breast cancer | Downregulation of FAK is induced by EGCG in MCF-7 breast cancer cells, which results in blockade of MMP-2 activation and expression. | [80] | ||
| Oral squamous cell carcinoma | EGCG decreases the levels of pFAK, pSrc, snail-1, vimentin, and MMP-9 in vivo and in vitro, demonstrating the antimetastatic. effect of EGCG on oral squamous cell carcinoma. | [81] | ||
| Oral squamous cell carcinoma | EGCG inhibits functional invadopodia formation by inhibiting the activation of RhoA, cortactin, FAK, and Src in oral squamous cell carcinomas. | [82] | ||
| Melanoma | EGCG is correlated with inhibition of cell invasion along with downregulation of MMP-9 and FAK in melanoma cells. | [83] | ||
| Fangchinoline | S. tetrandra | Melanoma | The inhibitory effect of fangchinoline on melanoma may result from suppressing phosphorylation of FAK and its downstream FAK/paxillin-signaling pathway. | [84] |
| Lung cancer | Fangchinoline effectively represses cell invasion and metastasis in A549 lung cancer cells by inhibiting the FAK-paxillin/MMP-2/MMP-9 pathway. | [85] | ||
| Neferine | N. nucifera | Glioma | Neferine A and B are proposed to be novel inhibitors of tumor growth via dual FAK and S6K1 docking. | [86] |
| Nitidine | Z. nitidum | Breast cancer | At low concentrations, NC inhibits breast cancer cell metastasis by blocking the c-Src/FAK signaling pathway. | [87] |
| Oridonin | R. rubescens | Breast cancer | The migration and invasion of MDA-MB-231 cells by oridonin may be attributed to blockade of the integrin β1/FAK pathway. | [88] |
| Oridonin analogs | Metastatic breast cancer | Oridonin analogs may retain the antimetastatic property of oridonin and exert anticancer effects via inhibition of the integrin/FAK pathway. | [89,90] | |
| Phoyunnanin E | D. venustum | Lung cancer | Phoyunnanin E promotes EMT suppression in and inhibits the migration of lung cancer cells via the integrin/FAK/AKT cascade. | [91] |
| Thymoquinone | N. sativa | Glioblastoma | Thymoquinone exerts antimigratory and anti-invasive effects via modulation of the FAK/ERK pathway in glioblastoma cells. | [92] |
| FAK kinase-independent inhibitors | ||||
| CuB | T. kirilowii Maximowicz and M. charantia L. | Breast cancer | CuB mediates the reorganization of cytoskeletal proteins in breast cancer cells via the RAC1/CDC42/RhoA signaling pathway. | [93] |
| Thymoquinone | N. sativa | Glioblastoma | Thymoquinone-induced morphological changes in glioblastoma cells are attributed, to a certain extent, to disruption of focal contacts and actin cytoskeletal organization. | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Zhong, Z.; Zhang, C.; Lu, Y.; Chan, Y.-T.; Wang, N.; Zhao, D.; Feng, Y. Potential Focal Adhesion Kinase Inhibitors in Management of Cancer: Therapeutic Opportunities from Herbal Medicine. Int. J. Mol. Sci. 2022, 23, 13334. https://doi.org/10.3390/ijms232113334
Chen F, Zhong Z, Zhang C, Lu Y, Chan Y-T, Wang N, Zhao D, Feng Y. Potential Focal Adhesion Kinase Inhibitors in Management of Cancer: Therapeutic Opportunities from Herbal Medicine. International Journal of Molecular Sciences. 2022; 23(21):13334. https://doi.org/10.3390/ijms232113334
Chicago/Turabian StyleChen, Feiyu, Zhangfeng Zhong, Cheng Zhang, Yuanjun Lu, Yau-Tuen Chan, Ning Wang, Di Zhao, and Yibin Feng. 2022. "Potential Focal Adhesion Kinase Inhibitors in Management of Cancer: Therapeutic Opportunities from Herbal Medicine" International Journal of Molecular Sciences 23, no. 21: 13334. https://doi.org/10.3390/ijms232113334
APA StyleChen, F., Zhong, Z., Zhang, C., Lu, Y., Chan, Y.-T., Wang, N., Zhao, D., & Feng, Y. (2022). Potential Focal Adhesion Kinase Inhibitors in Management of Cancer: Therapeutic Opportunities from Herbal Medicine. International Journal of Molecular Sciences, 23(21), 13334. https://doi.org/10.3390/ijms232113334











