The Association between Cyclin Dependent Kinase 2 Associated Protein 1 (CDK2AP1) and Molecular Subtypes of Lethal Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Tissue Microarray Construction
2.2. Immunohistochemistry
2.3. Pathological Analysis
2.4. Cell line and Western Blotting
2.5. Protein–Protein Interaction Using Co-Immunoprecipitation and Molecular Docking
2.6. CDK2AP1 mRNA Expression in TCGA PRAD and Associated Gene Set Enrichment Analysis
2.7. Statistical Analysis
3. Results
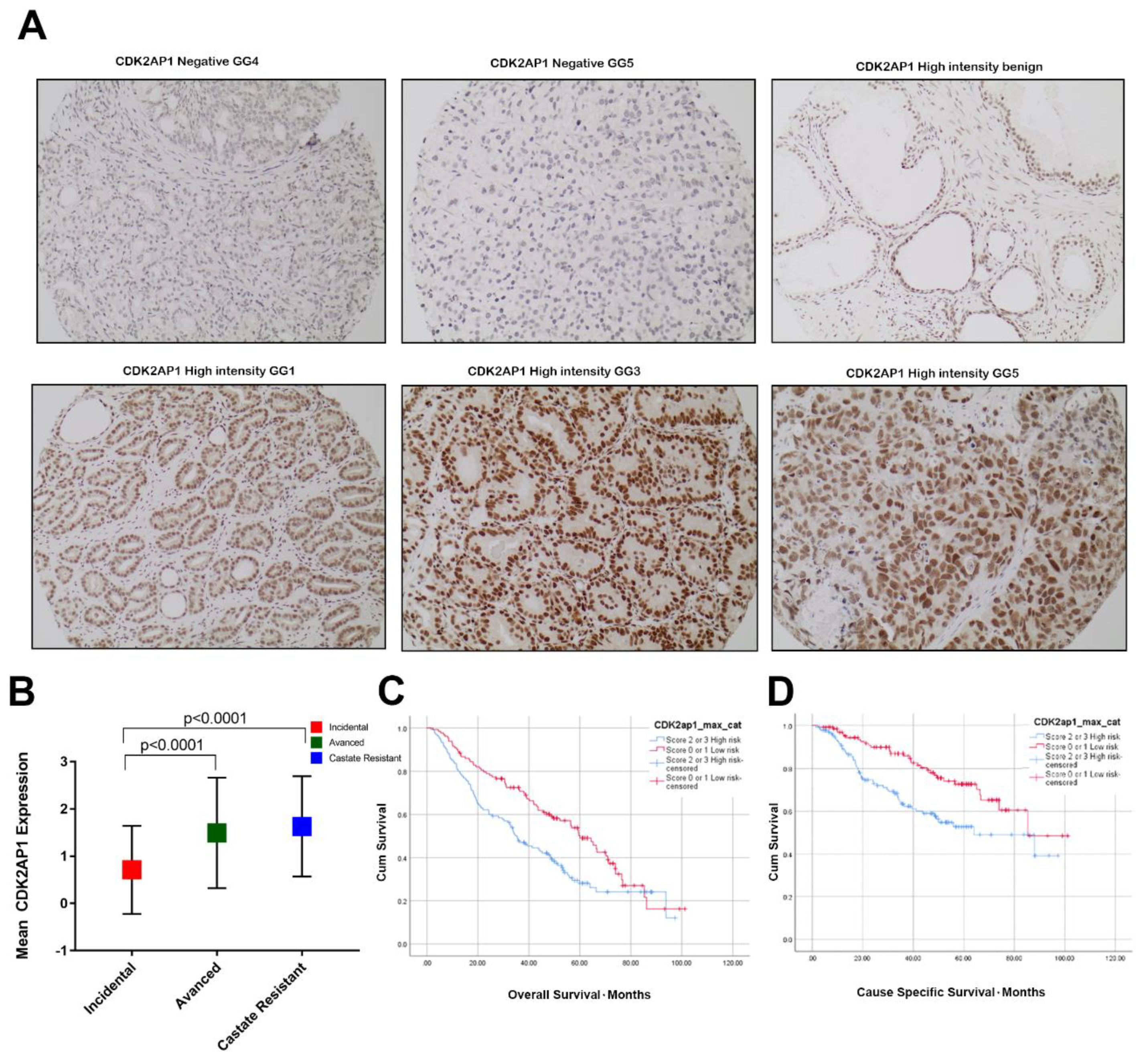
3.1. CDK2AP1 Expression in PCa
3.2. High CDK2AP1 Expression Is Associated with Higher Gleason Group (GG) and Metastatic Disease
3.3. High CDK2AP1 Expression Is Associated with Metastatic Prostate Cancer
3.4. High CDK2AP1 Expression Is Associated with Poor Overall and Cause Specific Survival in PCa
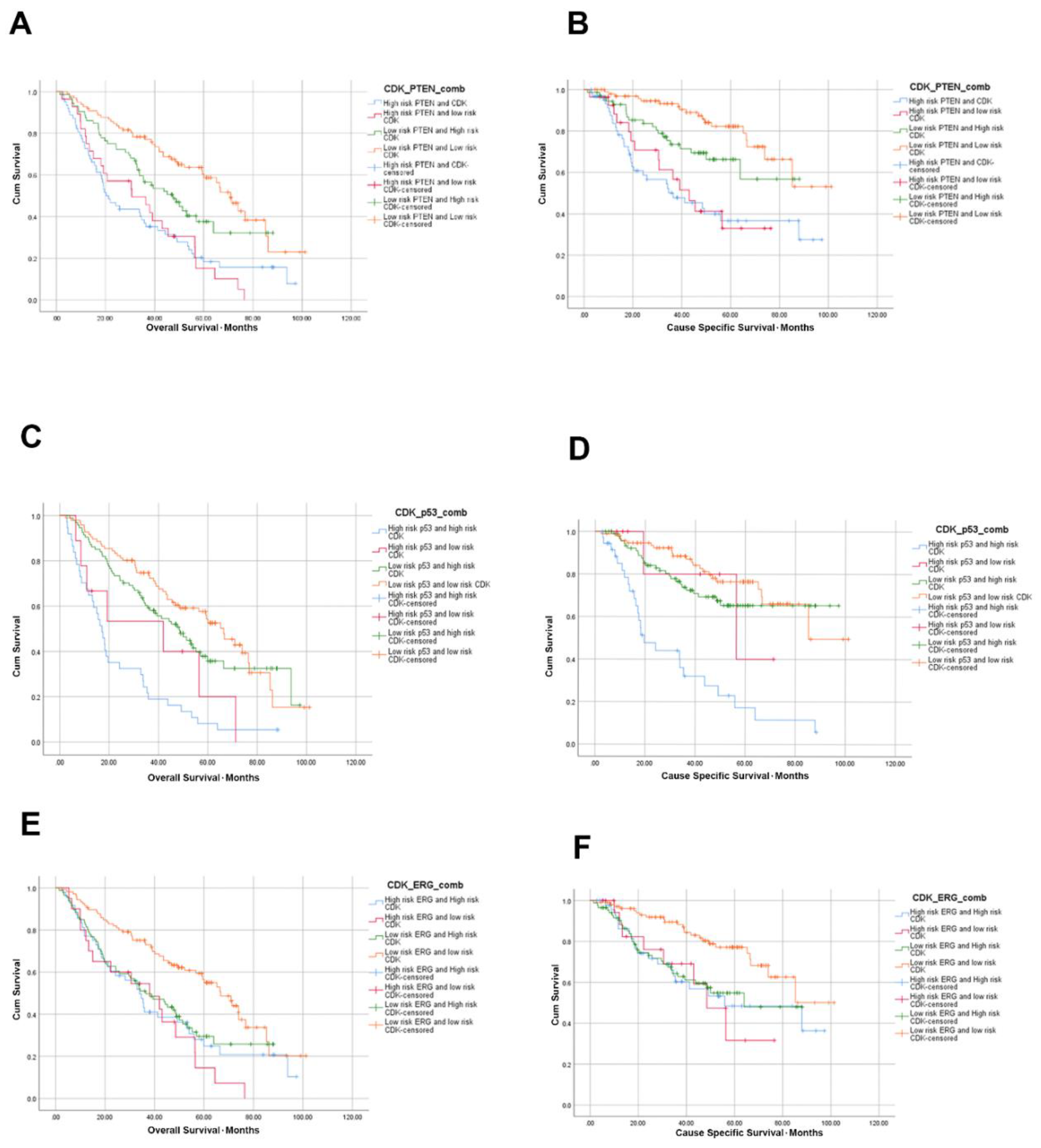
3.5. High CDK2AP1 Expression in Combination with ERG, PTEN, p53 or AR Signify Poorer Overall and Cause Specific Survival
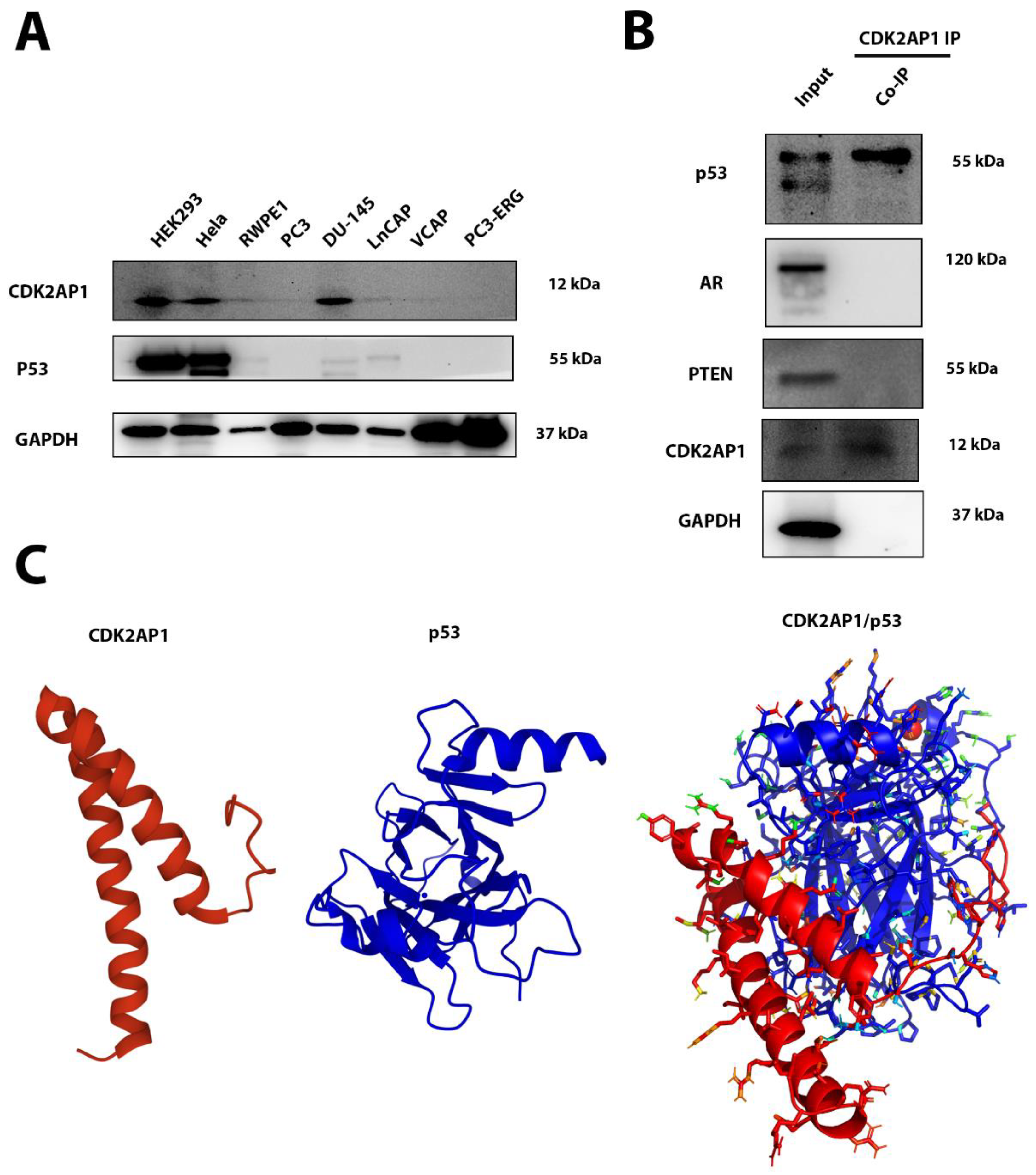
3.6. CDK2AP1 Interacts with p53
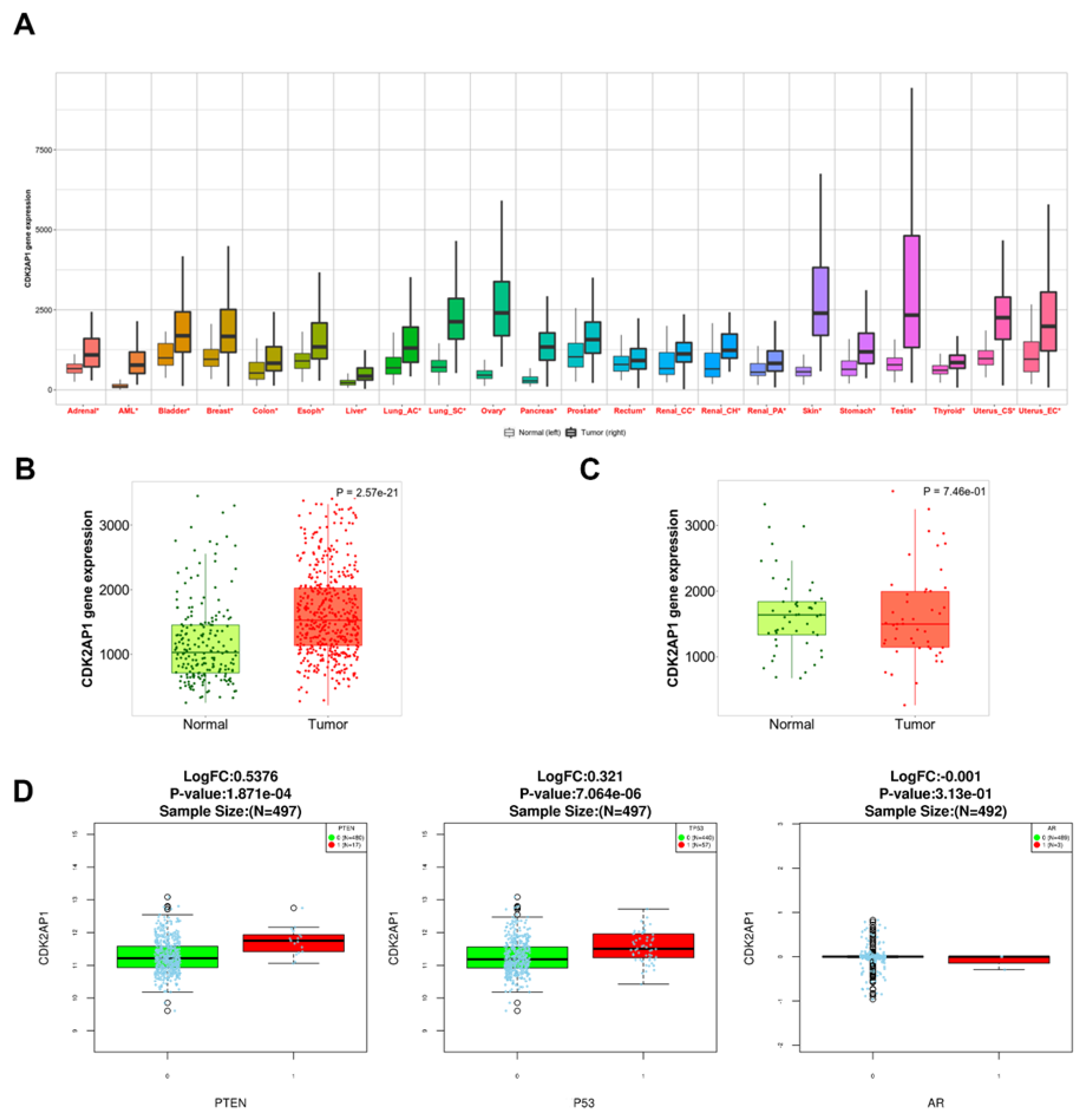
3.7. CDK2AP1 mRNA Expression in Pan-Cancer Data
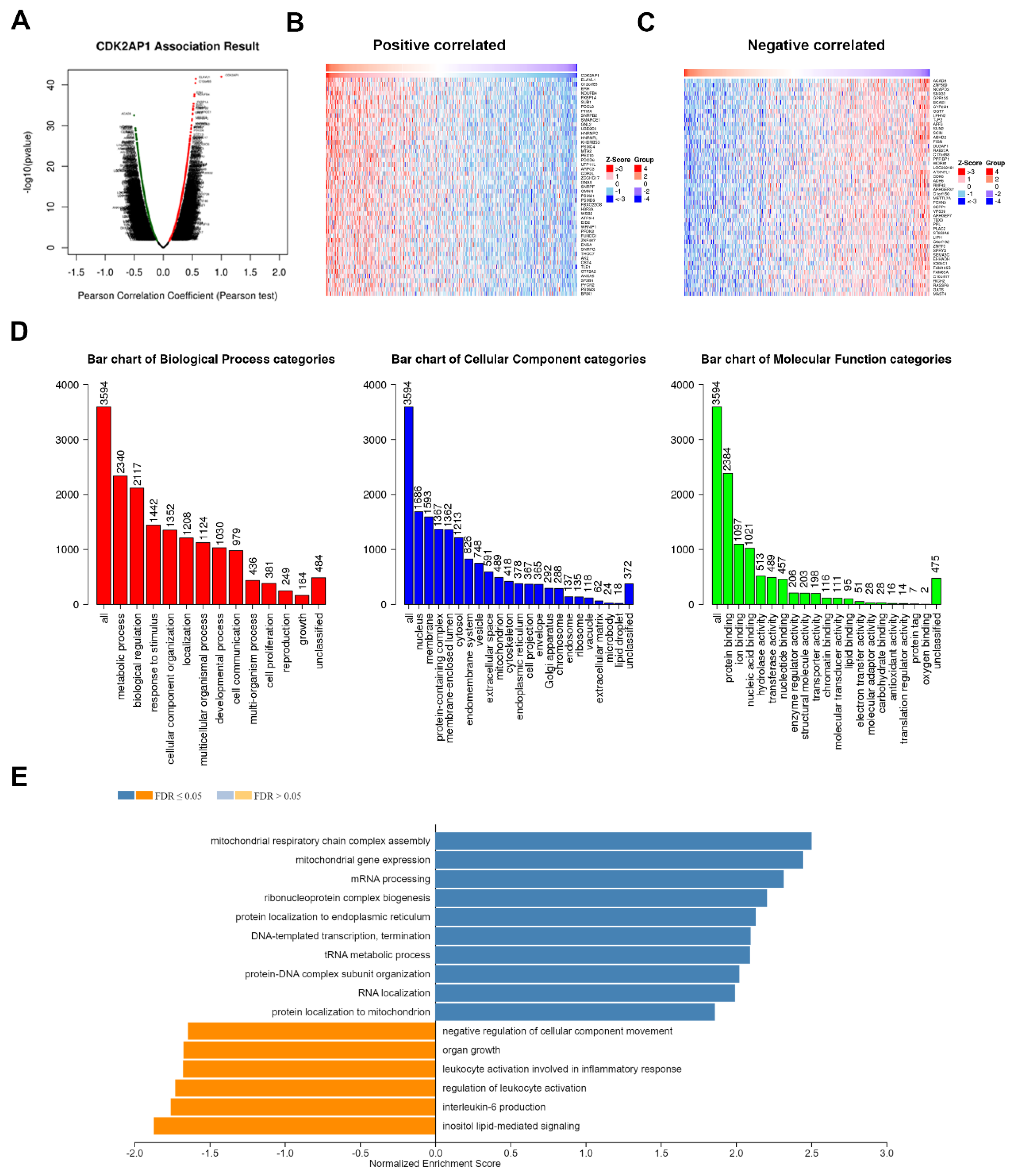
3.8. CDK2AP1 Gene Set Enrichment Analysis in PCa TCGA PRAD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDK2AP1 | Cyclin Dependent Kinase 2 Associated Protein 1 |
| PCa | Prostate cancer |
| TURP | Transurethral resection of the prostate |
| CSS | Cause-specific survival |
| OS | Overall survival |
| GG | Gleason Grade Group |
| Co-IP | Co-Immunoprecipitation |
| ERG | ETS-related gene |
| PTEN | Phosphatase tensin homologue |
| AR | Androgen receptor |
| CRPC | Castration-resistant prostate cancer |
| ADT | Androgen deprivation therapy |
| TMAs | Tissue microarrays |
| IHC | Immunohistochemistry |
| FISH | Fluorescence in situ hybridization |
| FCC | Fraction of Common Contacts |
| RSA | Relative solvent accessibility |
| KM | Kaplan–Meier |
| CI | Confidence intervals |
| HR | Hazard ratios |
| TAD | Transactivation’s domains |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Arora, K.; Barbieri, C.E. Molecular Subtypes of Prostate Cancer. Curr. Oncol. Rep. 2018, 20, 58. [Google Scholar] [CrossRef]
- Imada, E.L.; Sanchez, D.F.; Dinalankara, W.; Vidotto, T.; Ebot, E.M.; Tyekucheva, S.; Franco, G.R.; Mucci, L.A.; Loda, M.; Schaeffer, E.M.; et al. Transcriptional Landscape of PTEN Loss in Primary Prostate Cancer. BMC Cancer 2021, 21, 856. [Google Scholar] [CrossRef]
- Bismar, T.A.; Hegazy, S.; Feng, Z.; Yu, D.; Donnelly, B.; Palanisamy, N.; Trock, B.J. Clinical Utility of Assessing PTEN and ERG Protein Expression in Prostate Cancer Patients: A Proposed Method for Risk Stratification. J. Cancer Res. Clin. Oncol. 2018, 144, 2117–2125. [Google Scholar] [CrossRef]
- Khosh Kish, E.; Choudhry, M.; Gamallat, Y.; Buharideen, S.M.; Dhananjaya, D.; Bismar, T.A. The Expression of Proto-Oncogene ETS-Related Gene (ERG) Plays a Central Role in the Oncogenic Mechanism Involved in the Development and Progression of Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 4772. [Google Scholar] [CrossRef]
- Abou-Ouf, H.; Assem, H.; Ghosh, S.; Karnes, R.J.; Stoletov, K.; Palanisamy, N.; Lewis, J.D.; Bismar, T.A. High Serine-arginine Protein Kinase 1 Expression with PTEN Loss Defines Aggressive Phenotype of Prostate Cancer Associated with Lethal Outcome and Decreased Overall Survival. Eur. Urol. Open Sci. 2021, 23, 1–8. [Google Scholar] [CrossRef]
- Wasmuth, E.V.; Hoover, E.A.; Antar, A.; Klinge, S.; Chen, Y.; Sawyers, C.L. Modulation of Androgen Receptor Dna Binding Activity through Direct Interaction with the ETS Transcription Factor ERG. Proc. Natl. Acad. Sci. USA 2020, 117, 8584–8592. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, H.; Zhu, Y.; Xia, Y.; Cui, J.; Shi, K.; Fan, Y.; Shi, B.; Chen, S. TP53 Alterations of Hormone-Naïve Prostate Cancer in the Chinese Population. Prostate Cancer Prostatic Dis. 2021, 24, 482–491. [Google Scholar] [CrossRef]
- Stern, N.; Ly, T.L.; Welk, B.; Chin, J.; Ballucci, D.; Haan, M.; Power, N. Association of Race and Ethnicity with Prostate Cancer–Specific Mortality in Canada. JAMA Netw. Open 2021, 4, e2136364. [Google Scholar] [CrossRef]
- Lotan, T.L.; Tomlins, S.A.; Bismar, T.A.; Van Der Kwast, T.H.; Grignon, D.; Egevad, L.; Kristiansen, G.; Pritchard, C.C.; Rubin, M.; Bubendorf, L. Report from the International Society of Urological Pathology (ISUP) Consultation Conference on Molecular Pathology of Urogenital Cancers. I. Molecular Biomarkers in Prostate Cancer. Am. J. Surg. Pathol. 2020, 44, e15–e29. [Google Scholar] [CrossRef]
- Todd, R.; McBride, J.; Tsujl, T.; Donoff, R.B.; Nagai, M.; Chou, M.Y.; Chiang, T.; Wong, D.T.W. Deleted in Oral Cancer-1 (doc-l), a Novel Oral Tumor Suppressor Gene. FASEB J. 1995, 9, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Daigo, Y.; Suzuki, K.; Maruyama, O.; Miyoshi, Y.; Yasuda, T.; Kabuto, T.; Imaoka, S.; Fujiwara, T.; Takahashi, E.; Fujino, M.A.; et al. Isolation, Mapping and Mutation Analysis of a Human cDNA Homologous to the doc-1 Gene of the Chinese Hamster, a Candidate Tumor Suppressor for Oral Cancer. Genes Chromosom. Cancer 1997, 20, 204–207. [Google Scholar] [CrossRef]
- Wong, D.T.; Kim, J.J.; Khalid, O.; Sun, H.-H.; Kim, Y. Double Edge: CDK2AP1 in Cell-Cycle Regulation and Epigenetic Regulation. J. Dent. Res. 2012, 91, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Spruijt, C.G.; Bartels, S.J.J.; Brinkman, A.B.; Tjeertes, J.V.; Poser, I.; Stunnenberg, H.G.; Vermeulen, M. CDK2AP1/DOC-1 is a Bona Fide Subunit of the Mi-2/NuRD Complex. Mol. Biosyst. 2010, 6, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Gera, R.; Mokbel, L.; Jiang, W.G.; Mokbel, K. mRNA Expression of CDK2AP1 in Human Breast Cancer: Correlation with Clinical and Pathological Parameters. Cancer Genom. Proteom. 2018, 15, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Shintani, S.; Ohyama, H.; Zhang, X.; McBride, J.; Matsuo, K.; Tsuji, T.; Hu, M.G.; Hu, G.; Kohno, Y.; Lerman, M.; et al. p12DOC-1 Is a Novel Cyclin-Dependent Kinase 2-Associated Protein. Mol. Cell. Biol. 2000, 20, 6300–6307. [Google Scholar] [CrossRef]
- Sun, M.; Jiang, R.; Wang, G.; Zhang, C.; Li, J.; Jin, C.; Zhang, X. Cyclin-Dependent Kinase 2-Associated Protein 1 Suppresses Growth and Tumorigenesis of Lung Cancer. Int. J. Oncol. 2013, 42, 1376–1382. [Google Scholar] [CrossRef][Green Version]
- Xu, Y.; Wang, J.; Fu, S.; Wang, Z. Knockdown of CDK2AP1 by RNA Interference Inhibits Cell Growth and Tumorigenesis of Human Glioma. Neurol. Res. 2014, 36, 659–665. [Google Scholar] [CrossRef]
- Huang, K.-C.; Bégin, L.R.; Palanisamy, N.; Donnelly, B.; Bismar, T.A. SPINK1 Expression in Relation to PTEN and ERG in Matched Primary and Lymph Node Metastatic Prostate Cancer: Implications for Biomarker Development. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 235.e1–235.e10. [Google Scholar] [CrossRef]
- Bismar, T.A.; Yoshimoto, M.; Duan, Q.; Liu, S.; Sircar, K.; Squire, J.A. Interactions and Relationships of PTEN, ERG, SPINK1 and AR in Castration-Resistant Prostate Cancer. Histopathology 2012, 60, 645–652. [Google Scholar] [CrossRef]
- Yemelyanova, A.; Vang, R.; Kshirsagar, M.P.; Lu, D.; Marks, M.A.; Shih, I.M.; Kurman, R.J. Immunohistochemical Staining Patterns of p53 Can Serve as a Surrogate Marker for TP53 Mutations in Ovarian Carcinoma: An Immunohistochemical and Nucleotide Sequencing Analysis. Mod. Pathol. 2011, 24, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Guedes, L.B.; Almutairi, F.; Haffner, M.C.; Rajoria, G.; Liu, Z.; Klimek, S.; Zoino, R.; Yousefi, K.; Sharma, R.; De Marzo, A.M.; et al. Analytic, Preanalytic, and Clinical Validation of p53 IHC for Detection of TP53 Missense Mutation in Prostate Cancer. Clin. Cancer Res. 2017, 23, 4693–4703. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Brenner, J.C.; Sabolch, A.; Jackson, W.; Speers, C.; Wilder-Romans, K.; Knudsen, K.E.; Lawrence, T.S.; Chinnaiyan, A.M.; Feng, F.Y. Targeted Radiosensitization of ETS Fusion-Positive Prostate Cancer through PARP1 Inhibition. Neoplasia 2013, 15, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rosengarth, A.; Luecke, H. Structure of the Human p53 Core Domain in the Absence of DNA. Acta Crystallogr. D Biol. Crystallogr. 2007, 63 Pt. 3, 276–281. [Google Scholar] [CrossRef]
- Ertekin, A.; Aramini, J.M.; Rossi, P.; Leonard, P.G.; Janjua, H.; Xiao, R.; Maglaqui, M.; Lee, H.-W.; Prestegard, J.H.; Montelione, G.T. Human Cyclin-dependent Kinase 2-associated Protein 1 (CDK2AP1) Is Dimeric in Its Disulfide-reduced State, with Natively Disordered N-terminal Region. J. Biol. Chem. 2012, 287, 16541–16549. [Google Scholar] [CrossRef]
- Honorato, R.V.; Koukos, P.I.; Jiménez-García, B.; Tsaregorodtsev, A.; Verlato, M.; Giachetti, A.; Rosato, A.; Bonvin, A.M.J.J. Structural Biology in the Clouds: The WeNMR-EOSC Ecosystem. Front. Mol. Biosci. 2021, 8, 729513. [Google Scholar] [CrossRef]
- Bartha, Á.; Győrffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: Analyzing Multi-Omics Data within and across 32 Cancer Types. Nucleic Acids Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene Set Analysis Toolkit with Revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef]
- Jamaspishvili, T.; Berman, D.M.; Ross, A.E.; Scher, H.I.; De Marzo, A.M.; Squire, J.A.; Lotan, T.L. Clinical Implications of PTEN Loss in Prostate Cancer. Nat. Rev. Urol. 2018, 15, 222–234. [Google Scholar] [CrossRef]
- Garcia-Marques, F.; Liu, S.; Totten, S.M.; Bermudez, A.; Bs, C.T.; Hsu, E.; Ba, R.N.; Hembree, A.; Stoyanova, T.; Brooks, J.D.; et al. Protein Signatures to Distinguish Aggressive from Indolent Prostate Cancer. Prostate 2022, 82, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Gesztes, W.; Schafer, C.; Young, D.; Fox, J.; Jiang, J.; Chen, Y.; Kuo, H.-C.; Mwamukonda, K.B.; Dobi, A.; Burke, A.P.; et al. Focal p53 Protein Expression and Lymphovascular Invasion in Primary Prostate Tumors Predict Metastatic Progression. Sci. Rep. 2022, 12, 5404. [Google Scholar] [CrossRef] [PubMed]
- Alsayegh, K.N.; Gadepalli, V.S.; Iyer, S.; Rao, R.R. Knockdown of CDK2AP1 in Primary Human Fibroblasts Induces p53 Dependent Senescence. PLoS ONE 2015, 10, e0120782. [Google Scholar] [CrossRef] [PubMed]
- Hafner, A.; Bulyk, M.L.; Jambhekar, A.; Lahav, G. The Multiple Mechanisms that Regulate p53 Activity and Cell Fate. Nat. Rev. Mol. Cell Biol. 2019, 20, 199–210. [Google Scholar] [CrossRef]
- Katarkar, A.; Patel, L.; Mukherjee, S.; Ray, J.G.; Haldar, P.K.; Chaudhuri, K. Association of Oral Tumor Suppressor Gene Deleted in Oral Cancer-1 (DOC-1) in Progression of Oral Precancer to Cancer. Oral Sci. Int. 2015, 12, 15–21. [Google Scholar] [CrossRef]
- Xiao, J.; Cohen, P.; Stern, M.C.; Odedina, F.; Carpten, J.; Reams, R. Mitochondrial Biology and Prostate Cancer Ethnic Disparity. Carcinogenesis 2018, 39, 1311–1319. [Google Scholar] [CrossRef]
- Cihan, Y.B.; Arslan, A.; Ergul, M.A. Subtypes of White Blood Cells in Patients with Prostate Cancer or Benign Prostatic Hyperplasia and Healthy Individuals. Asian Pac. J. Cancer Prev. 2013, 14, 4779–4783. [Google Scholar] [CrossRef]
- Tsui, K.-H.; Feng, T.-H.; Hsieh, W.-C.; Chang, P.-L.; Juang, H.-H. Expression of interleukin-6 is Downregulated by 17-(allylamino)-17-demethoxygeldanamycin in Human Prostatic Carcinoma Cells. Acta Pharmacol. Sin. 2008, 29, 1334–1341. [Google Scholar] [CrossRef][Green Version]
| Variables | Total (n = 275) (%) |
|---|---|
| Gleason Score | |
| Grade group 1 | 82 (29.8) |
| Grade group 2 (3 + 4) | 29 (10.5) |
| Grade group 3 (4 + 3) | 17 (6.2) |
| Grade group 4 (8) | 20 (7.3) |
| Grade group 5 (9/10) | 121 (44.0) |
| Missing | 6 (2.2) |
| Deceased | |
| Yes | 165 (60.0) |
| No | 108 (39.3) |
| Missing | 2 (0.7) |
| Prostate cancer specific mortality | |
| Yes | 85 (30.9) |
| No | 188 (68.4) |
| Missing | 2 (0.7) |
| Cancer Subgroup | |
| Incidental | 94 (34.2) |
| Advanced | 109 (39.6) |
| Castrate Resistant | 72 (26.2) |
| CDK2AP1 Score (Score by Cancer subgroup) | |
| Score 2 or 3 | 141 (51.3%) |
| Incidental Score | 29 (10.5) |
| Advanced Score | 66 (24.0) |
| Castrate Resistant Score | 46 (16.7) |
| Score 0 or 1 | 134 (48.7) |
| Incidental Score | 65 (23.6) |
| Advanced Score | 43 (15.6) |
| Castrate Resistant Score | 26 (9.5) |
| PTEN and CDK2AP1 combined | |
| PTEN negative and CDK2AP1 Score 2, 3 | 66 (24.0) |
| PTEN negative and CDK2AP1 Score 0, 1 | 28 (10.2) |
| PTEN positive and CDK2AP1 score 2, 3 | 73 (26.5) |
| PTEN positive and CDK2AP1 Score 0, 1 | 102 (37.1) |
| Missing | 6 (2.2) |
| ERG and CDK2AP1 Combined | |
| ERG positive and CDK2AP1 Score 2, 3 | 50 (18.2) |
| ERG positive and CDK2AP1 Score 0, 1 | 20 (7.3) |
| ERG negative and CDK2AP1 Score 2, 3 | 89 (32.4) |
| ERG negative and CDK2AP1 Score 0, 1 | 110 (40.0) |
| Missing | 6 (2.2) |
| AR and CDK2AP1 Combined | |
| AR Score 0, 1 and CDK2AP1 Score 2, 3 | 10 (3.6) |
| AR Score 0, 1 and CDK2AP1 Score 0, 1 | 12 (4.4) |
| AR Score 2, 3 and CDK2AP1 Score 2, 3 | 126 (45.8) |
| AR Score 2, 3 and CDK2AP1 Score 0, 1 | 115 (41.8) |
| Missing | 12 (4.4) |
| p53 and CDK2AP1 Combined | |
| p53 Score 0, 2, 3 and CDK2AP1 Score 2, 3 | 39 (14.2) |
| p53 Score 0, 2, 3 and CDK2AP1 Score 0, 1 | 10 (3.6) |
| p53 Score 1 and CDK2AP1 Score 2, 3 | 98 (35.6) |
| p53 Score 1 and CDK2AP1 Score 0, 1 | 99 (36.0) |
| Missing | 29 (10.5) |
| Variables | Overall Survival | Prostate Cancer Specific Survival | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| CDK2AP1 (Score 0, 1) (n = 129) | ||||
| Score 2, 3 (n = 135) | 1.62 (1.19–2.21) | 0.002 | 2.01 (1.29–3.13) | 0.002 |
| PTEN (Positive) (n = 170) | ||||
| PTEN negative (n = 89) | 2.44 (1.79–3.33) | <0.0001 | 3.26 (2.12–5.01) | <0.0001 |
| CDK2AP1 and PTEN (PTEN positive and CDK Score 0, 1) (n = 98) | ||||
| PTEN negative and CDK score 2, 3 (n = 62) | 2.83 (1.89–4.24) | <0.0001 | 4.43 (2.28–7.93) | <0.0001 |
| PTEN negative and CDK score 0, 1 (n = 28) | 3.07 (1.87–5.01) | <0.0001 | 4.18 (2.07–8.42) | <0.0001 |
| PTEN positive and CDK score 2, 3 (n = 72) | 1.64 (1.08–2.51) | 0.021 | 1.97 (1.05–3.71) | <0.0001 |
| CDK2AP1 and ERG (ERG negative and CDK score 0, 1) (n = 106) | ||||
| ERG positive and CDK score 2, 3 (n = 48) | 2.00 (1.31–3.06) | 0.001 | 2.42 (1.33–4.40) | 0.004 |
| ERG positive and CDK score 0, 1 (n = 20) | 2.62 (1.51–4.53) | 0.001 | 2.63 (1.18–5.88) | 0.018 |
| ERG negative and CDK score 2, 3 (n = 86) | 1.85 (1.27–2.69) | 0.001 | 2.28 (1.34–3.87) | 0.002 |
| CDK2AP1 and AR (AR score 2, 3 and CDK score 0, 1) (n = 110) | ||||
| AR score 0, 1 and CDK score 2, 3 (n = 10) | 3.53 (1.73–7.17) | 0.001 | 5.41 (2.19–13.37) | <0.0001 |
| AR score 0, 1 and CDK score 0, 1 (n = 12) | 2.09 (1.06–4.12) | 0.033 | 2.81 (1.14–6.92) | 0.025 |
| AR score 2, 3 and CDK score 2, 3 (n = 120) | 1.74 (1.24–2.44) | 0.001 | 2.20 (1.34–3.62) | 0.002 |
| CDK2AP1 and p53 (p53 score 1 and CDK score 0, 1) (n = 96) | ||||
| p53 score 0, 2, 3 and CDK score 2, 3 (n = 37) | 3.81 (2.46–5.90) | <0.0001 | 6.01 (3.36–10.76) | <0.0001 |
| p53 score 0, 2, 3 and CDK score 0, 1 (n = 9) | 2.37 (1.08–5.24) | 0.033 | 1.49 (0.35–6.35) | 0.589 |
| p53 score 1 and CDK score 2, 3 (n = 94) | 1.34 (0.92–1.97) | 0.130 | 1.44 (0.82–2.54) | 0.203 |
| Variables | Overall Survival | Prostate Cancer Specific Mortality | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| CDK2AP1 and PTEN (PTEN positive and CDK2AP1 Score 0, 1) (n = 91) | ||||
| PTEN negative and CDK2AP1 score 2, 3 (n = 60) | 1.74 (1.12–2.71) | 0.014 | 1.84 (0.99–3.42) | 0.054 |
| PTEN negative and CDK2AP1 score 0, 1 (n = 26) | 2.36 (1.41–3.93) | 0.001 | 2.39 (1.14–5.00) | 0.021 |
| PTEN positive and CDK2AP1 score 2, 3 (n = 68) | 1.44 (0.93 –2.22) | 0.102 | 1.54 (0.80–2.94) | 0.193 |
| ERG and CDK2AP1 (ERG negative and CDK2AP1 score 0, 1) (n = 98) | ||||
| ERG positive and CDK2AP1 score 2, 3 (n = 46) | 1.22 (0.77–1.93) | 0.390 | 1.06 (0.56–2.01) | 0.861 |
| ERG positive and CDK2AP1 score 0, 1 (n = 19) | 2.30 (1.32–4.01) | 0.003 | 2.08 (0.92–4.71) | 0.078 |
| ERG negative and CDK2AP1 score 2, 3 (n = 82) | 1.58 (1.07–2.33) | 0.021 | 1.79 (1.04–3.10) | 0.037 |
| AR and CDK2AP1 (AR score 2, 3 and CDK2AP1 score 0, 1) (n = 104) | ||||
| AR score 0, 1 and CDK2AP1 score 2, 3 (n = 10) | 2.07 (1.00–4.29) | 0.050 | 2.17 (0.87–5.42) | 0.098 |
| AR score 0, 1 and CDK2AP1 score 0, 1 (n = 11) | 1.40 (0.68–2.87) | 0.361 | 1.27 (0.48–3.40) | 0.629 |
| AR score 2, 3 and CDK2AP1 score 2, 3 (n = 115) | 1.32 (0.92–1.89) | 0.128 | 1.32 (0.79–2.22) | 0.294 |
| P53 and CDK2AP1 (p53 score 1 and CDK2AP1 score 0, 1) (n = 91) | ||||
| p53 score 0, 2, 3 and CDK2AP1 score 2, 3 (n = 37) | 2.29 (1.42–3.70) | 0.001 | 2.36 (1.28–4.34) | 0.006 |
| p53 score 0, 2, 3 and CDK2AP1 score 0, 1 (n = 9) | 1.51 (0.67–3.40) | 0.325 | 0.63 (0.15–2.72) | 0.631 |
| p53 score 1 and CDK2AP1 score 2, 3 (n = 88) | 1.07 (0.71–1.60) | 0.757 | 0.89 (0.49–1.61) | 0.697 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamallat, Y.; Bakker, A.; Khosh Kish, E.; Choudhry, M.; Walker, S.; Aldakheel, S.; Seyedi, S.; Huang, K.-C.; Ghosh, S.; Gotto, G.; et al. The Association between Cyclin Dependent Kinase 2 Associated Protein 1 (CDK2AP1) and Molecular Subtypes of Lethal Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 13326. https://doi.org/10.3390/ijms232113326
Gamallat Y, Bakker A, Khosh Kish E, Choudhry M, Walker S, Aldakheel S, Seyedi S, Huang K-C, Ghosh S, Gotto G, et al. The Association between Cyclin Dependent Kinase 2 Associated Protein 1 (CDK2AP1) and Molecular Subtypes of Lethal Prostate Cancer. International Journal of Molecular Sciences. 2022; 23(21):13326. https://doi.org/10.3390/ijms232113326
Chicago/Turabian StyleGamallat, Yaser, Andrea Bakker, Ealia Khosh Kish, Muhammad Choudhry, Simon Walker, Saood Aldakheel, Sima Seyedi, Kuo-Cheng Huang, Sunita Ghosh, Geoffrey Gotto, and et al. 2022. "The Association between Cyclin Dependent Kinase 2 Associated Protein 1 (CDK2AP1) and Molecular Subtypes of Lethal Prostate Cancer" International Journal of Molecular Sciences 23, no. 21: 13326. https://doi.org/10.3390/ijms232113326
APA StyleGamallat, Y., Bakker, A., Khosh Kish, E., Choudhry, M., Walker, S., Aldakheel, S., Seyedi, S., Huang, K.-C., Ghosh, S., Gotto, G., & Bismar, T. A. (2022). The Association between Cyclin Dependent Kinase 2 Associated Protein 1 (CDK2AP1) and Molecular Subtypes of Lethal Prostate Cancer. International Journal of Molecular Sciences, 23(21), 13326. https://doi.org/10.3390/ijms232113326






