Spatial and Quantitative Analysis of Tumor-Associated Macrophages: Intratumoral CD163-/PD-L1+ TAMs as a Marker of Favorable Clinical Outcomes in Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Results
2.1. Clinicopathological Features
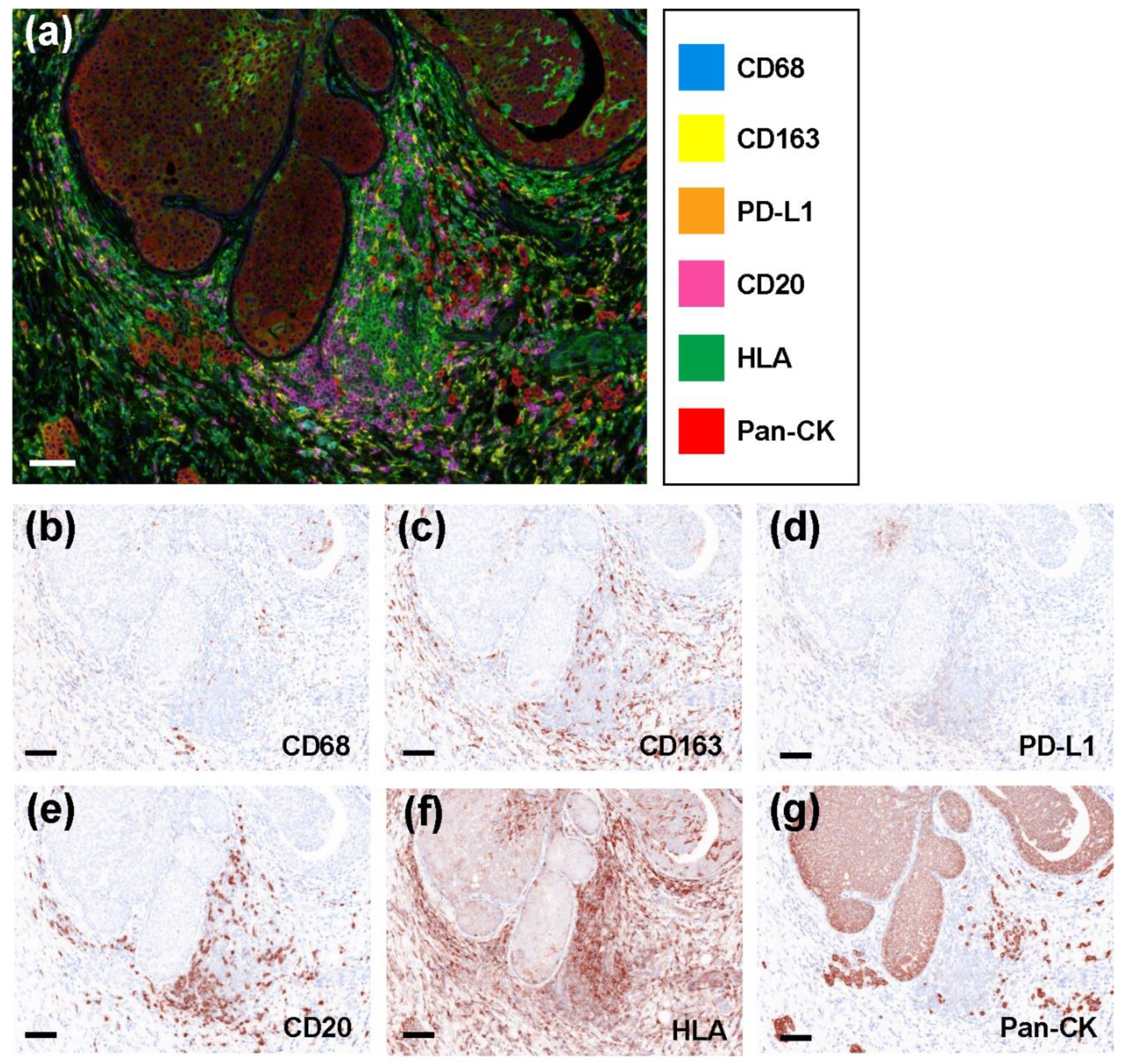
2.2. Representative Multicolored Immunostaining Images
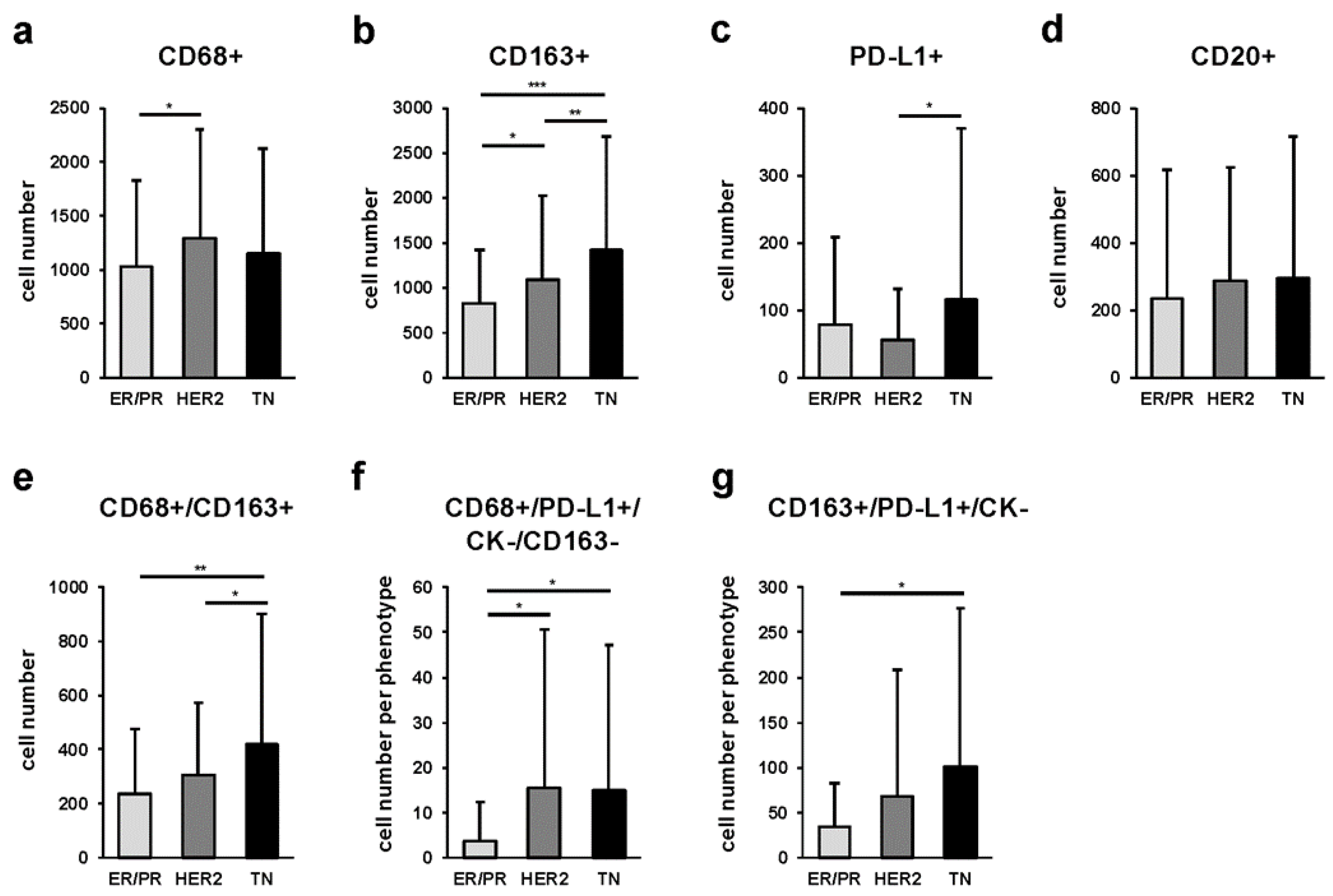
2.3. Quantitative Comparison of Stromal Tumor-Associated Macrophages (sTAMs), Immune Modification, and B-Cell-Marker-Positive Cells among Breast Cancer Subtypes
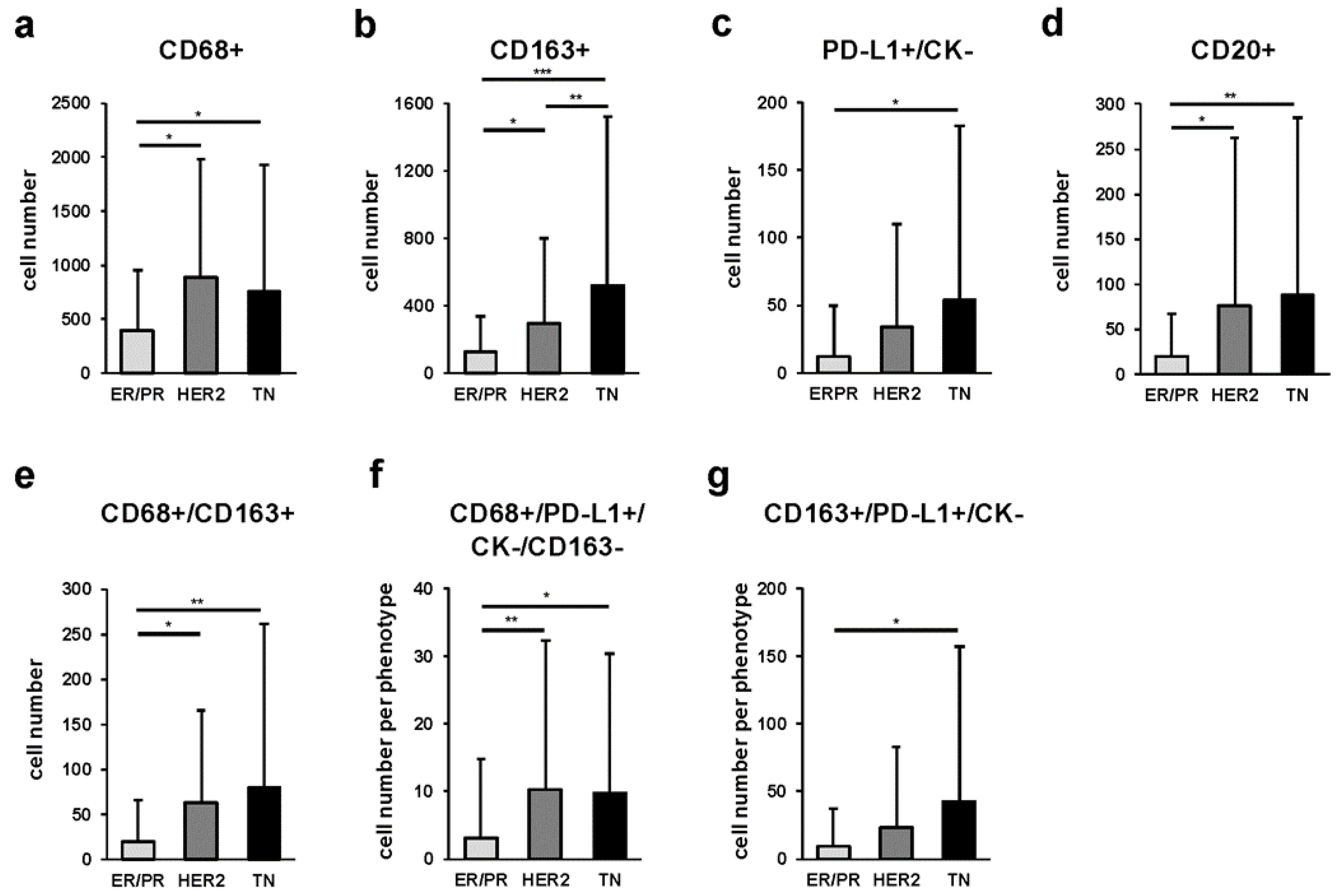
2.4. Quantitative Comparison of Intratumoral Tumor-Associated Macrophages (iTAMs), Immune Modification, and B-Cell-Marker-Positive Cells among Breast Cancer Subtypes
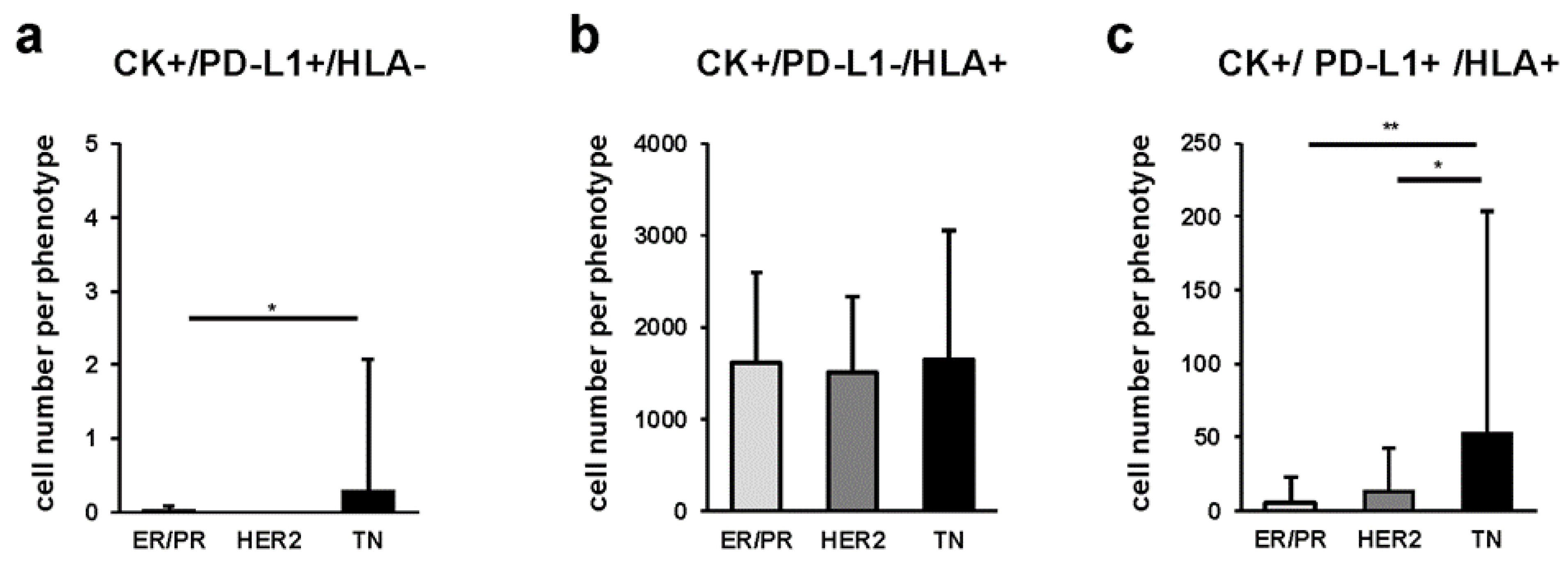
2.5. Quantitative Comparison of Cancer Cells among Breast Cancer Subtypes
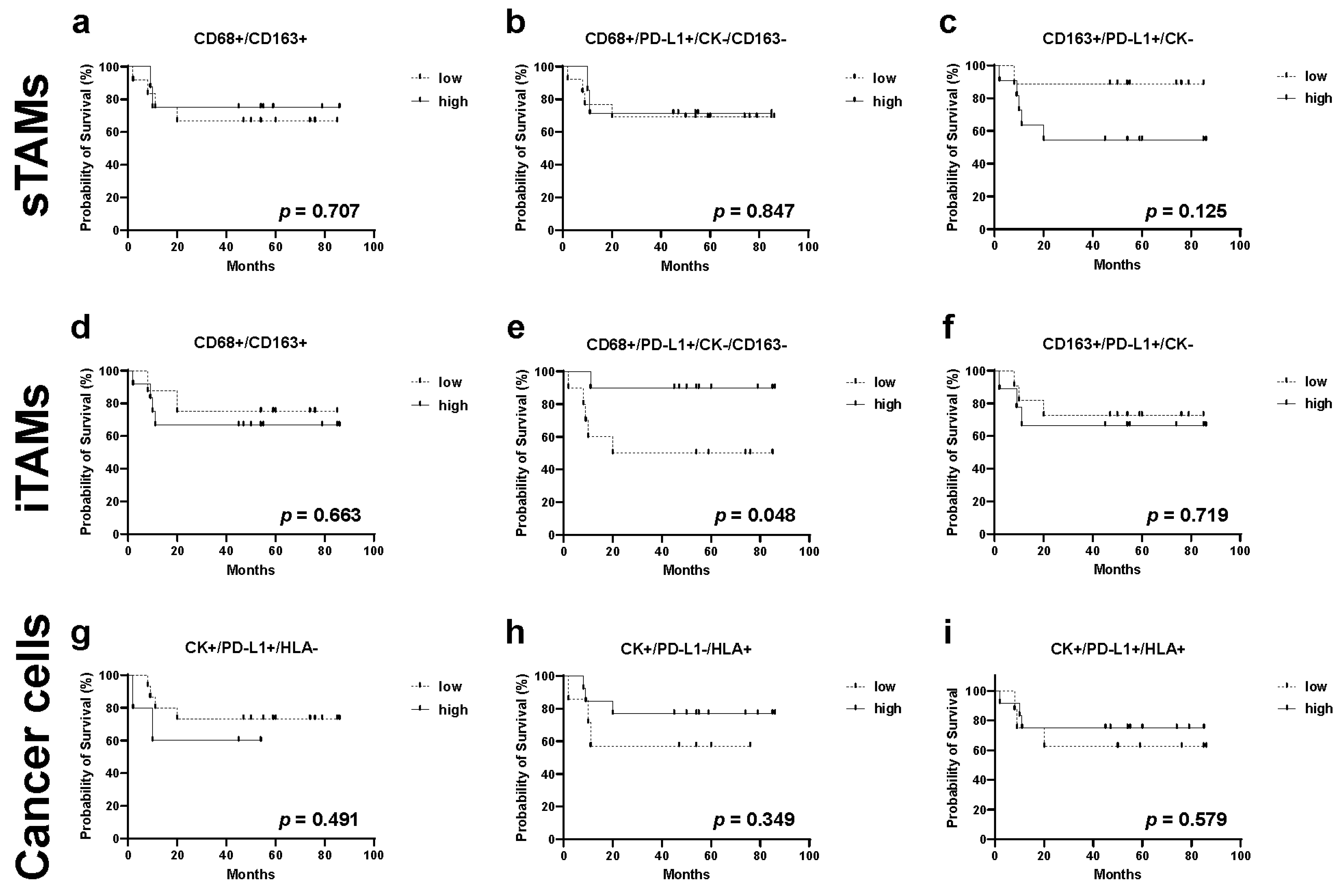
2.6. Relationship between TAMs, Immune Modification, B-Cell-Marker-Positive Cells, and Recurrence in TN Subtype
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Multiplex Immunohistochemistry
4.3. Quantitative Image Analysis
4.4. Data Analysis and Statistical Procedures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lakhani, S.R.; Ellis, I.O.; Schnitt, S.J.; Tan, P.H.; van de Vijver, M.J. WHO Classification of Tumours of the Breast, 4th ed.; WHO: Geneva, Switzerland; Volume 4.
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Pardoll, D.M. Blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The future of cancer treatment includes immunomodulation, CARs, and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273–290. [Google Scholar] [CrossRef]
- Okazaki, T.; Chikuma, S.; Iwai, Y.; Fagarasan, S.; Honjo, T. A rheostat for immune responses: The unique properties of PD-1 and its advantages for clinical application. Nat. Immunol. 2013, 14, 1212–1218. [Google Scholar] [CrossRef]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Park, I.J.; An, S.; Kim, S.Y.; Lim, H.M.; Hong, S.M.; Kim, M.J.; Kim, Y.J.; Yu, C.S. Prediction of radio-responsiveness with immune-profiling in patients with rectal cancer. Oncotarget 2017, 8, 79793–79802. [Google Scholar] [CrossRef][Green Version]
- Kaiyue, W.; Kangjia, L.; Xiaoyan, L.; Xiangliang, Y.; Peiqing, X.; Peihua, N.; Dakang, X. Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front. Immunol. 2020, 4, 1731. [Google Scholar] [CrossRef]
- Pupa, S.M.; Bufalino, R.; Invernizzi, A.M.; Andreola, S.; Rilke, F.; Lombardi, L.; Colnaghi, M.I.; Ménard, S. Macrophage infiltrate and prognosis in c-erbB-2-overexpressing breast carcinomas. J. Clin. Oncol. 1996, 14, 85–94. [Google Scholar] [CrossRef]
- Lewis, C.E.; Leek, R.; Harris, A.; McGee, J.O. Cytokine regulation of angiogenesis in breast cancer: The role of tumor-associated macrophages. J. Leukoc. Biol. 1995, 57, 747–751. [Google Scholar] [CrossRef]
- Sica, A.; Schioppa, T.; Mantovani, A.; Allavena, P. Tumor-associated macrophages are a distinct M2 polarized population that promotes tumor progression and is a potential target of anti-cancer therapy. Eur. J. Cancer 2006, 42, 717–727. [Google Scholar] [CrossRef]
- Ambarus, C.A.; Krausz, S.; van Eijk, M.; Hamann, J.; Radstake, T.R.; Reedquist KATak, P.P.; Baeten, D.L. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J. Immunol. Methods 2012, 375, 196–206. [Google Scholar] [CrossRef]
- Lau, S.K.; Chu, P.G.; Weiss, L.M. CD163: A specific marker of macrophages in paraffin-embedded tissue samples. Am. J. Clin. Pathol. 2004, 122, 794–801. [Google Scholar] [CrossRef]
- Ding, J.; Jin, W.; Chen, C.; Shao, Z.; Wu, J. Tumor-associated macrophage × cancer cell hybrids may acquire cancer stem cell properties in breast cancer. PLoS ONE 2012, 7, e41942. [Google Scholar] [CrossRef]
- Tang, X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Nat. Cancer Lett. 2013, 332, 3–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, S.; Zhang, M.; Zhen, L.; Pang, D.; Zhang, Q.; Li, Z. High-infiltration of tumor-associated macrophages predicts unfavorable clinical outcomes for node-negative breast cancer. PLoS ONE 2013, 8, e76147. [Google Scholar] [CrossRef]
- Ch’ng, E.S.; Tuan Sharif, S.E.; Jaafar, H.H. In human invasive breast ductal carcinoma, tumor stromal macrophages and tumor nest macrophages have distinct relationships with clinicopathological parameters and angiogenesis. Virchows Arch. 2013, 462, 257–267. [Google Scholar] [CrossRef]
- Gómez, V.; Eykyn, T.R.; Mustapha, R.; Flores-Borja, F.; Male, V.; Barber, P.R.; Patsialou, A.; Green, R.; Panagaki, F.; Li, C.W.; et al. Breast cancer-associated macrophages promote tumorigenesis by suppressing succinate dehydrogenase in tumor cells. Sci. Signal. 2020, 13, eaax4585. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Y.; Wang, L.; Lou, W. Tumor-regulated macrophage type 2 differentiation promotes immunosuppression in laryngeal squamous cell carcinoma. Life Sci. 2021, 267, 118798. [Google Scholar] [CrossRef]
- Eisinger, S.; Sarhan, D.; Boura, V.F.; Ibarlucea-Benitez, I.; Tyystjärvi, S.; Oliynyk, G.; Arsenian-Henriksson, M.; Lane, D.; Wikström, S.L.; Kiessling, R.; et al. Targeting scavenger receptors on tumor-associated macrophages activates tumor cell killing by natural killer cells. Proc. Natl. Acad. Sci. USA 2020, 117, 32005–32016. [Google Scholar] [CrossRef]
- Xia, Q.; Jia, J.; Hu, C.; Lu, J.; Li, J.; Xu, H.; Fang, J.; Feng, D.; Wang, L.; Chen, Y. Tumor-associated macrophages promote PD-L1 expression in tumor cells by regulating PKM2 nuclear translocation in pancreatic ductal adenocarcinoma. Oncogene 2022, 41, 865–877. [Google Scholar] [CrossRef]
- Cavalcante, R.S.; Ishikawa, U.; Silva, E.S.; Silva-Júnior, A.A.; Araújo, A.A.; Cruz, L.J.; Chan, A.B.; de Araújo, R.F., Jr. STAT3/NF-κB signalling disruption in M2 tumor-associated macrophages is a major target of PLGA nanocarriers/PD-L1 antibody immunomodulatory therapy in breast cancer. Br. J. Pharmacol. 2021, 178, 2284–2304. [Google Scholar] [CrossRef]
- Shan, T.; Chen, S.; Chen, X.; Wu, T.; Yang, Y.; Li, S.; Ma, J.; Zhao, J.; Lin, W.; Li, W.; et al. M2-TAM subsets altered by lactic acid promote T-cell apoptosis through the PD-L1/PD-1 pathway. Oncol. Rep. 2020, 44, 1885–1894. [Google Scholar] [CrossRef]
- Park, D.J.; Sung, P.S.; Lee, G.W.; Cho, S.; Kim, S.M.; Kang, B.Y.; Hur, W.; Yang, H.; Lee, S.K.; Lee, S.H.; et al. Preferential expression of programmed death ligand 1 in tumor-associated macrophages and its potential role in immunotherapy for hepatocellular carcinoma. Int. J. Mol. Sci. 2021, 22, 4710. [Google Scholar] [CrossRef]
- Vasaturo, A.; Galon, J. Multiplexed immunohistochemistry for immune cell phenotyping, quantification, and spatial distribution in situ. Methods Enzymol. 2020, 635, 51–66. [Google Scholar]
- Hayashi, K.; Nogawa, D.; Kobayashi, M.; Ohata, Y.; Kitagawa, S.; Kubota, K.; Takahashi, H.; Yamada, M.; Oda, G.; Nakagawa, T.; et al. Quantitative high-throughput analysis of tumor infiltration lymphocytes in breast cancer. Front. Oncol. 2022, 12, 901591. [Google Scholar] [CrossRef]
- Li, L.; Sun, R.; Miao, Y.; Tran, T.; Adams, L.; Roscoe, N.; Xu, B.; Manyam, G.C.; Tan, X.; Zhang, H.; et al. PD-1/PD-L1 expression and interaction by automated quantitative immunofluorescent analysis show adverse prognostic impact in patients with diffuse large B-cell lymphoma with T-cell infiltration: A study from the International DLBCL Consortium Program. Mod. Pathol. 2019, 32, 741–754. [Google Scholar] [CrossRef]
- Carvajal-Hausdorf, D.; Altan, M.; Velcheti, V.; Gettinger, S.N.; Herbst, R.S.; Rimm, D.L.; Schalper, K.A. Expression and clinical significance of PD-L1, B7-H3, B7-H4, and TILs in human small cell lung cancer (SCLC). J. Immunother. Cancer 2019, 7, 65. [Google Scholar] [CrossRef]
- Halse, H.; Colebatch, A.J.; Petrone, P.; Henderson, M.A.; Mills, J.K.; Snow, H.; Westwood, J.A.; Sandhu, S.; Raleigh, J.M.; Behren, A.; et al. Multiplex immunohistochemistry accurately defines the immune context of metastatic melanoma. Sci. Rep. 2018, 8, 11158. [Google Scholar] [CrossRef]
- Huang, Y.K.; Wang, M.; Sun YDi, C.N.; Mitchell, C.; Achuthan, A.; Hamilton, J.A.; Busuttil, R.A.; Boussioutas, A. Macrophage spatial heterogeneity in gastric cancer defined by multiplex immunohistochemistry. Nat. Commun. 2019, 10, 3928. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, Y.R.; Ji, P.; Hu, X.; Shao, Z.M. Impact of molecular subtypes on metastatic breast cancer patients: A SEER population-based study. Sci. Rep. 2017, 7, 45411. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Esposito, A.; Curigliano, G. Tumor-stroma crosstalk: Targeting stroma in breast cancer. Curr. Opin. Oncol. 2014, 26, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qu, J.; Sun, Y.; Wang, J.; Liu, X.; Wang, F.; Zhang, H.; Wang, W.; Ma, X.; Gao, X.; et al. Prognostic significance of tumor-associated macrophages in breast cancer: A meta-analysis of the literature. Oncotarget 2017, 8, 30576–30586. [Google Scholar] [CrossRef] [PubMed]
- Murri, A.M.A.; Hilmy, M.; Bell, J.; Wilson, C.; McNicol, A.M.; Lannigan, A.; Doughty, J.C.; McMillan, D.C. The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic and macrophage infiltration, microvessel density and survival in patients with primary operable breast cancer. Br. J. Cancer 2008, 99, 1013–1019. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Luo, R.Z.; Peng, R.J.; Wang, S.S.; Xue, C. High infiltration of tumor-associated macrophages in triple-negative breast cancer is associated with a higher risk of distant metastasis. OncoTargets Ther. 2014, 7, 1475–1480. [Google Scholar] [CrossRef]
- Cai, L.; Michelakos, T.; Deshpande, V.; Arora, K.S.; Yamada, T.; Ting, D.T.; Taylor, M.S.; Castillo, C.F.; Warshaw, A.L.; Lillemoe, K.D.; et al. Role of tumor-associated macrophages in the clinical course of pancreatic neuroendocrine tumors (PanNETs). Clin. Cancer Res. 2019, 25, 2644–2655. [Google Scholar] [CrossRef]
- Yagi, T.; Baba, Y.; Okadome, K.; Kiyozumi, Y.; Hiyoshi, Y.; Ishimoto, T.; Iwatsuki, M.; Miyamoto, Y.; Yoshida, N.; Watanabe, M.; et al. Tumour-associated macrophages are associated with poor prognosis and programmed death ligand 1 expression in oesophageal cancer. Eur. J. Cancer 2019, 111, 38–49. [Google Scholar] [CrossRef]
- Klingen, T.A.; Chen, Y.; Aas, H.; Wik, E.; Akslen, L.A. Tumor associated macrophages are strongly related to vascular invasion, non-luminal subtypes and interval breast cancer. Hum. Pathol. 2017, 69, 72–80. [Google Scholar] [CrossRef]
- Medrek, C.; Pontén, F.; Jirström, K.; Leandersson, K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 2012, 12, 306. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International Tils Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Khoury, T.; Nagrale, V.; Opyrchal, M.; Peng, X.; Wang, D.; Yao, S. Prognostic significance of stromal versus intratumoral infiltrating lymphocytes in different subtypes of breast cancer treated with cytotoxic neoadjuvant chemotherapy. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Catacchio, I.; Silvestris, N.; Scarpi, E.; Schirosi, L.; Scattone, A.; Mangia, A. Intratumoral, rather than stromal, CD8+ T cells could be a potential negative prognostic marker in invasive breast cancer patients. Transl. Oncol. 2019, 12, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Li, X.; Wetherilt, C.S.; Krishnamurti, U.; Yang, J.; Ma, Y.; Styblo, T.M.; Meisel, J.L.; Peng, L.; Siddiqui, M.T.; Cohen, C.; et al. Stromal PD-L1 expression is associated with better disease-free survival in triple-negative breast cancer. Am. J. Clin. Pathol. 2016, 146, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Kubo, M.; Yamaguchi, R.; Nishimura, R.; Osako, T.; Arima, N.; Okumura, Y.; Okido, M.; Yamada, M.; Kai, M.; et al. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget 2017, 8, 15584–15592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, H.; Zhao, S.; Wang, Y.; Pu, H.; Wang, Y.; Zhang, Q. Expression of PD-L1 and prognosis in breast cancer: A meta-analysis. Oncotarget 2017, 8, 31347–31354. [Google Scholar] [CrossRef] [PubMed]
- Hartley, G.P.; Chow, L.; Ammons, D.T.; Wheat, W.H.; Dow, S.W. Programmed cell death ligand 1 (PD-L1) signaling regulates macrophage proliferation and activation. Cancer Immunol. Res. 2018, 6, 1260–1273. [Google Scholar] [CrossRef]
- Sumitomo, R.; Hirai, T.; Fujita, M.; Murakami, H.; Otake, Y.; Huang, C.L. PD-L1 expression on tumor-infiltrating immune cells is highly associated with M2 TAM and aggressive malignant potential in patients with resected non-small cell lung cancer. Lung Cancer 2019, 136, 136–144. [Google Scholar] [CrossRef]
- Shima, T.; Shimoda, M.; Shigenobu, T.; Ohtsuka, T.; Nishimura, T.; Emoto, K.; Hayashi, Y.; Iwasaki, T.; Abe, T.; Asamura, H.; et al. Infiltration of tumor-associated macrophages is involved in tumor programmed death-ligand 1 expression in early lung adenocarcinoma. Cancer Sci. 2020, 111, 727–738. [Google Scholar] [CrossRef]
- Madonna, G.; Ballesteros-Merino, C.; Feng, Z.; Bifulco, C.; Capone, M.; Giannarelli, D.; Mallardo, D.; Simeone, E.; Grimaldi, A.M.; Caracò, C.; et al. PD-L1 expression with immune-infiltrate evaluation and outcome prediction in melanoma patients treated with ipilimumab. Oncoimmunology 2018, 7, e1405206. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Park, H.E.; Kim, J.H.; Wen, X.; Jeong, S.; Cho, N.Y.; Gwon, H.G.; Kim, K.; Lee, H.S.; Jeong, S.Y.; et al. Whole-slide image analysis reveals quantitative landscape of tumor-immune microenvironment in colorectal cancers. Clin. Cancer Res. 2020, 26, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Müller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.C.; Bamboat, Z.M.; Maker, A.V.; Shia, J.; Pillarisetty, V.G.; Yopp, A.C.; Hedvat, C.V.; Gonen, M.; Jarnagin, W.R.; Fong, Y.; et al. Regulatory T cell infiltration predicts outcome following resection of colorectal cancer liver metastases. Ann. Surg. Oncol. 2013, 20, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.M.; Paish, E.C.; Powe, D.G.; Macmillan, R.D.; Grainge, M.J.; Lee, A.H.; Ellis, I.O.; Green, A.R. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J. Clin. Oncol. 2011, 29, 1949–1955. [Google Scholar] [CrossRef]
- Ono, T.; Azuma, K.; Kawahara, A.; Sasada, T.; Hattori, S.; Sato, F.; Shin, B.; Chitose, S.I.; Akiba, J.; Hirohito, U. Association between PD-L1 expression combined with tumor-infiltrating lymphocytes and the prognosis of patients with advanced hypopharyngeal squamous cell carcinoma. Oncotarget 2017, 8, 92699–92714. [Google Scholar] [CrossRef][Green Version]
- Hou, Y.; Nitta, H.; Wei, L.; Banks, P.M.; Parwani, A.V.; Li, Z. Evaluation of immune reaction and PD-L1 expression using multiplex immunohistochemistry in HER2-positive breast cancer: The association with response to anti-HER2 neoadjuvant therapy. Clin. Breast Cancer 2018, 18, e237–e244. [Google Scholar] [CrossRef]

| Clinicopathological Features | ER/PR (n = 43) | HER2 (n = 18) | TN (n = 20) | |
|---|---|---|---|---|
| Age | Median (Range) | 59 (35–86) | 54 (33–71) | 63 (36–88) |
| pT classification | T1 | 22 | 11 | 8 |
| T2 | 16 | 7 | 8 | |
| T3 | 4 | 0 | 3 | |
| T4 | 1 | 0 | 1 | |
| Neoadjuvant chemotherapy | + | 6 | 2 | 7 |
| − | 37 | 16 | 13 | |
| Lymph node metastasis | Positive | 13 | 6 | 4 |
| Negative | 28 | 10 | 12 | |
| NX | 2 | 2 | 4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinohara, H.; Kobayashi, M.; Hayashi, K.; Nogawa, D.; Asakawa, A.; Ohata, Y.; Kubota, K.; Takahashi, H.; Yamada, M.; Tokunaga, M.; et al. Spatial and Quantitative Analysis of Tumor-Associated Macrophages: Intratumoral CD163-/PD-L1+ TAMs as a Marker of Favorable Clinical Outcomes in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2022, 23, 13235. https://doi.org/10.3390/ijms232113235
Shinohara H, Kobayashi M, Hayashi K, Nogawa D, Asakawa A, Ohata Y, Kubota K, Takahashi H, Yamada M, Tokunaga M, et al. Spatial and Quantitative Analysis of Tumor-Associated Macrophages: Intratumoral CD163-/PD-L1+ TAMs as a Marker of Favorable Clinical Outcomes in Triple-Negative Breast Cancer. International Journal of Molecular Sciences. 2022; 23(21):13235. https://doi.org/10.3390/ijms232113235
Chicago/Turabian StyleShinohara, Hajime, Maki Kobayashi, Kumiko Hayashi, Daichi Nogawa, Ayaka Asakawa, Yae Ohata, Kazuishi Kubota, Hisashi Takahashi, Miyuki Yamada, Masanori Tokunaga, and et al. 2022. "Spatial and Quantitative Analysis of Tumor-Associated Macrophages: Intratumoral CD163-/PD-L1+ TAMs as a Marker of Favorable Clinical Outcomes in Triple-Negative Breast Cancer" International Journal of Molecular Sciences 23, no. 21: 13235. https://doi.org/10.3390/ijms232113235
APA StyleShinohara, H., Kobayashi, M., Hayashi, K., Nogawa, D., Asakawa, A., Ohata, Y., Kubota, K., Takahashi, H., Yamada, M., Tokunaga, M., Kinugasa, Y., Oda, G., Nakagawa, T., Onishi, I., Kinowaki, Y., Kurata, M., Ohashi, K., Kitagawa, M., & Yamamoto, K. (2022). Spatial and Quantitative Analysis of Tumor-Associated Macrophages: Intratumoral CD163-/PD-L1+ TAMs as a Marker of Favorable Clinical Outcomes in Triple-Negative Breast Cancer. International Journal of Molecular Sciences, 23(21), 13235. https://doi.org/10.3390/ijms232113235






