In Situ Growth of Nanosilver on Fabric for Flexible Stretchable Electrodes
Abstract
1. Introduction
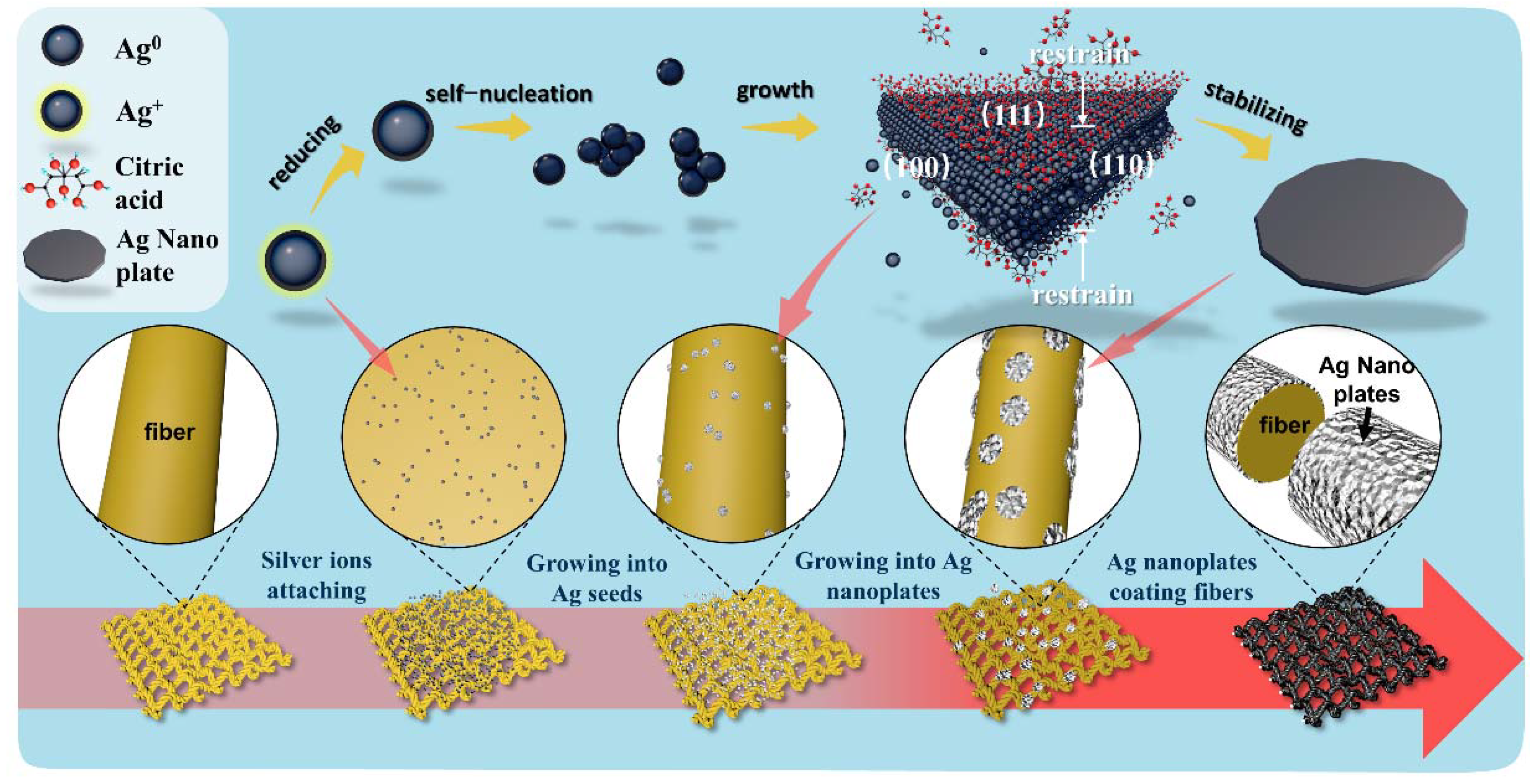
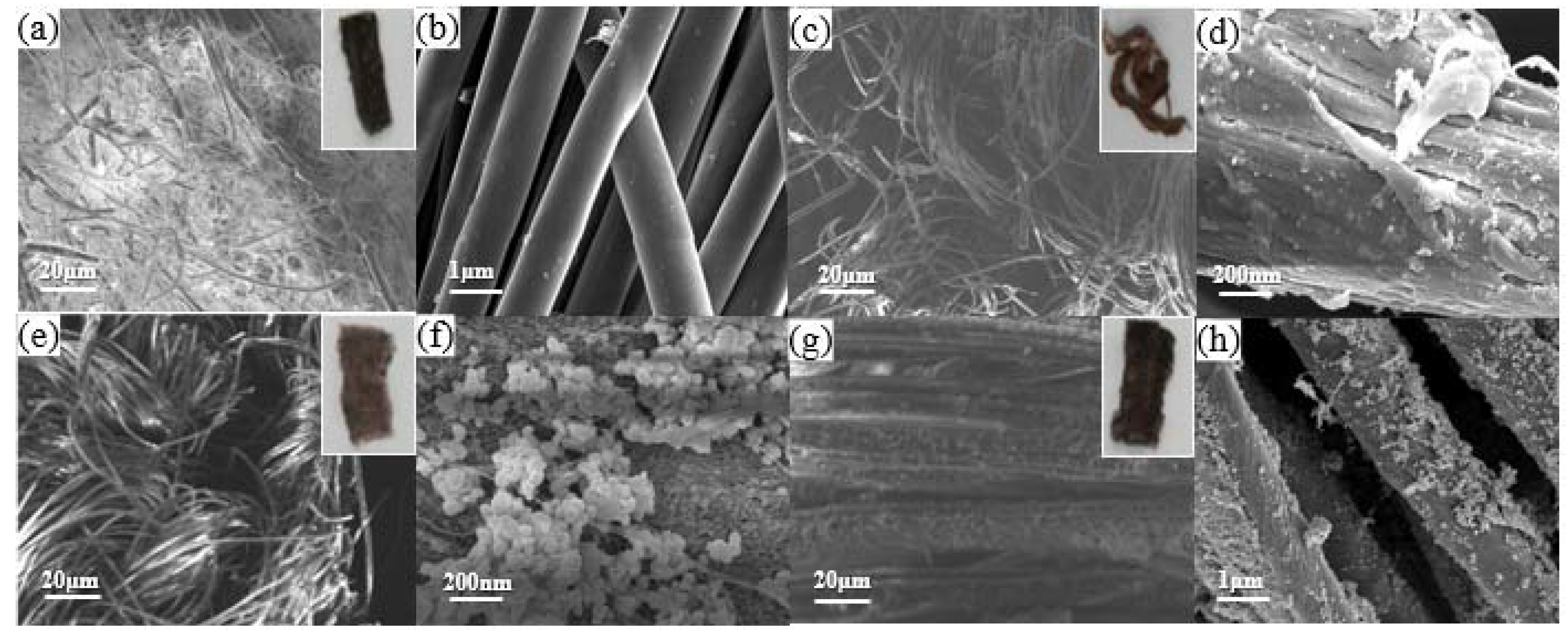
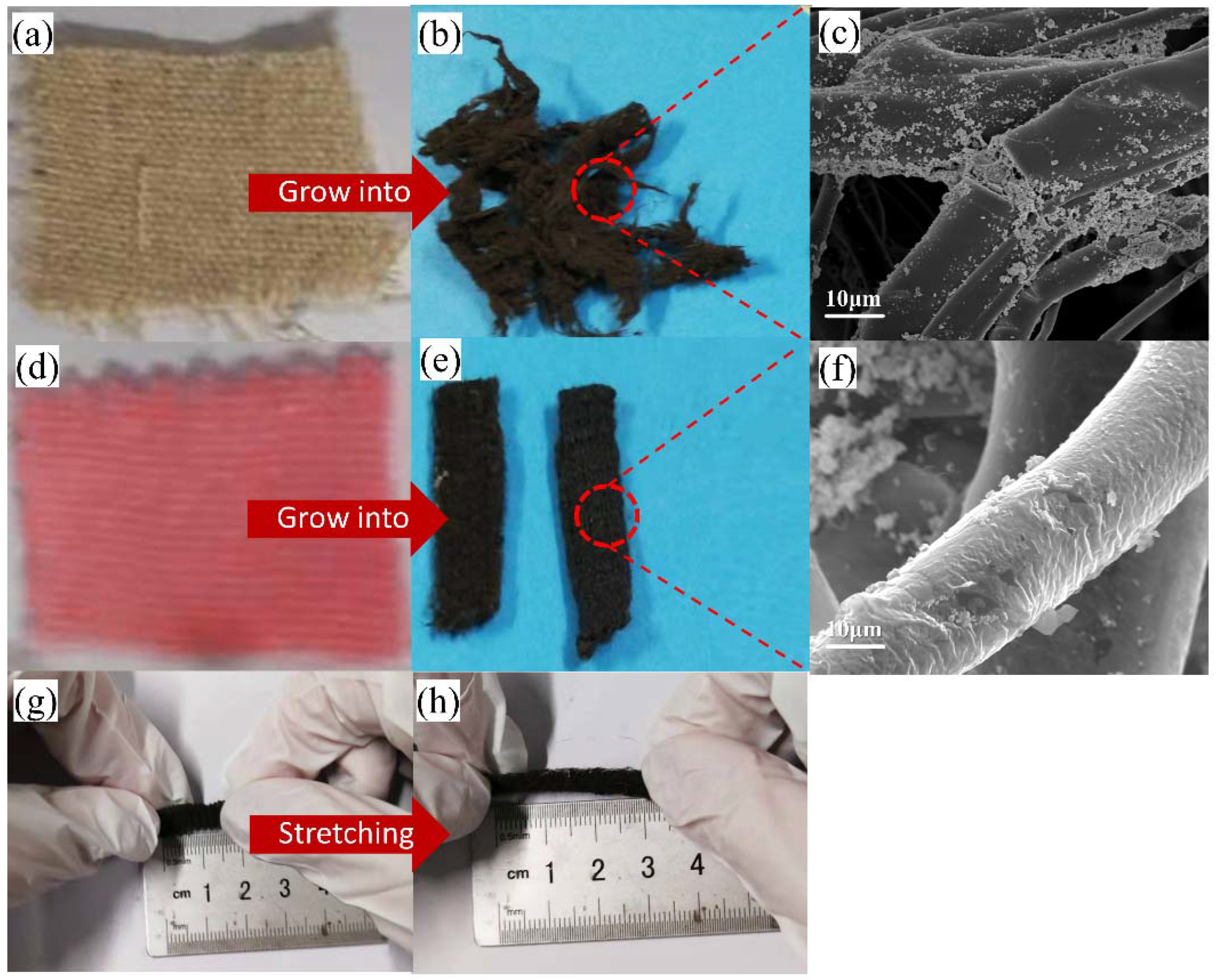
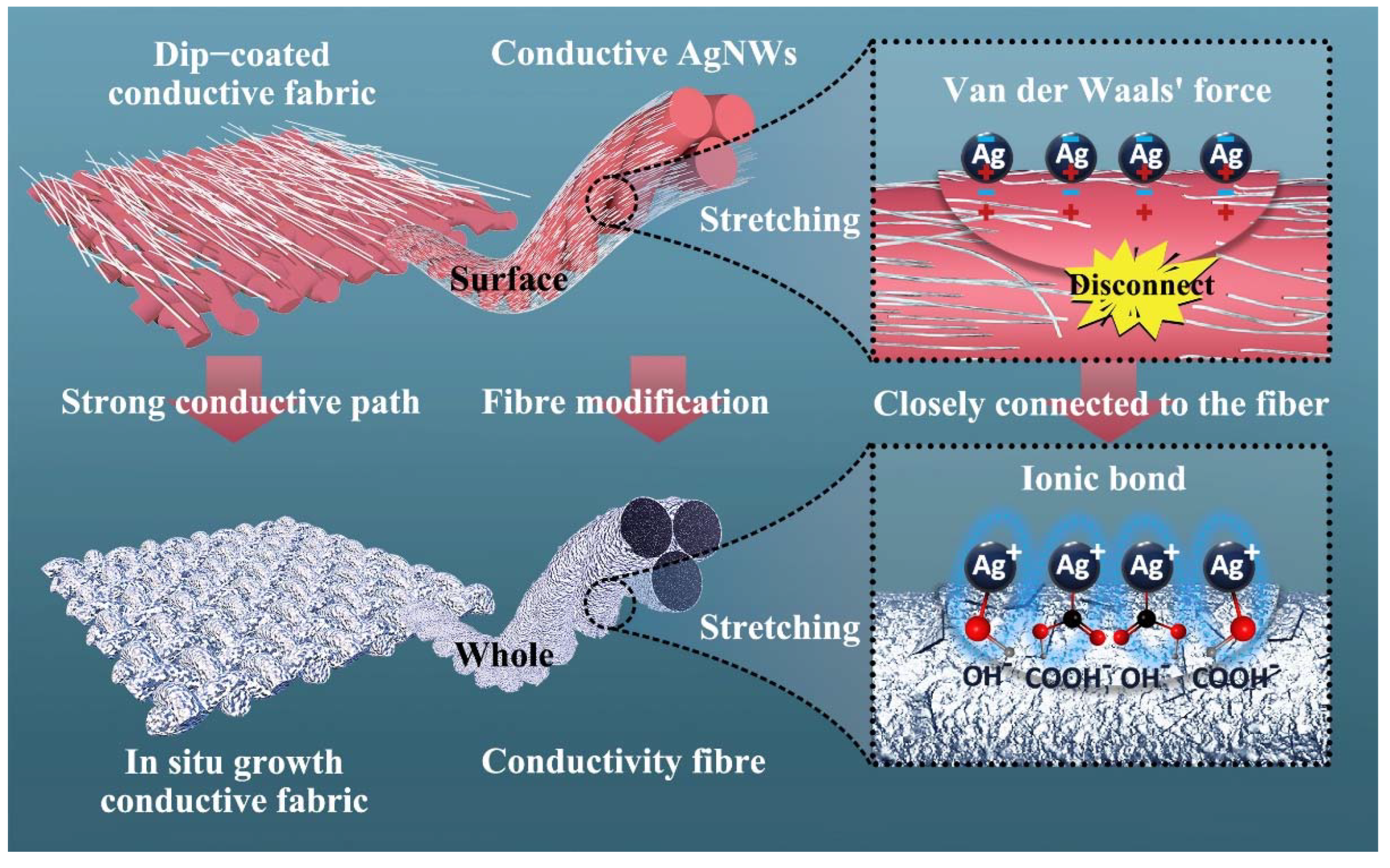
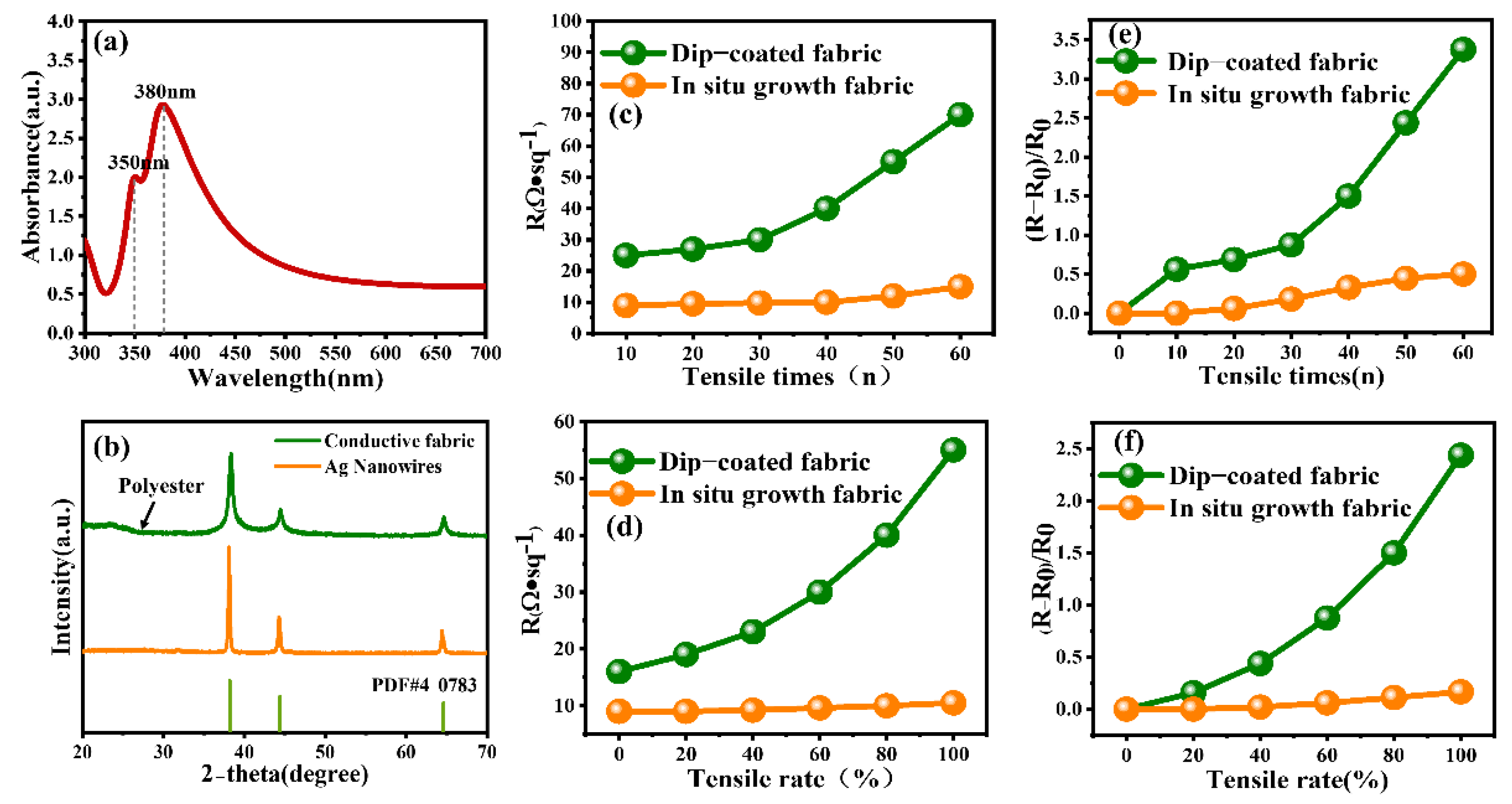
2. Results and Discussions
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, H.; Pang, G.; Xu, K.; Ye, Z.; Yang, H.; Yang, G. A Fully Printed Flexible Sensor Sheet for Simultaneous Proximity–Pressure–Temperature Detection. Adv. Mater. 2021, 6, 2100616. [Google Scholar] [CrossRef]
- Pei, Z.; Yu, Z.; Li, M.; Bai, L.; Wang, W.; Chen, H.; Yang, H.; Wei, D.; Yang, L. Self-healing and toughness cellulose nanocrystals nanocomposite hydrogels for strain-sensitive wearable flexible sensor. Int. J. Biol. Macromol. 2021, 179, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.K.; Jeong, S.Y.; Moon, Y.K.; Jo, Y.M.; Yoon, J.W.; Lee, J.H. Exclusive and ultrasensitive detection of formaldehyde at room temperature using a flexible and monolithic chemiresistive sensor. Nat. Commun. 2021, 12, 4955. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Hou, W.; Liao, K.; Chen, L.; Song, J.; Gao, G.; Qin, L. Solid-phase sintering and vapor-liquid-solid growth of BP@MgO quantum dot crystals with a high piezoelectric response. J. Adv. Ceram. 2022; in press. [Google Scholar]
- Wu, X.; Zhou, Z.; Wang, Y.; Li, J. Syntheses of silver nanowires ink and printable flexible transparent conductive film: A review. Coatings 2020, 10, 865. [Google Scholar] [CrossRef]
- Hao, Y.; Xie, J.; Xu, B.; Hu, B.; Zheng, Y.; Shen, Y. Tunnel elasticity enhancement effect of 3D submicron ceramics (Al2O3, TiO2, ZrO2) fiber on polydimethylsiloxane (PDMS). J. Adv. Ceram. 2021, 10, 502–508. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, H.; Hu, S.; Yue, J. Significant strain-rate dependence of sensing behavior in TiO2@ carbon fibre/PDMS composites for flexible strain sensors. J. Adv. Ceram. 2021, 10, 1350–1359. [Google Scholar] [CrossRef]
- Hou, W.; Liao, Q.; Xie, S.; Song, Y.; Qing, L. Prospects and Challenges of Flexible Stretchable Electrodes for Electronics. Coatings 2022, 12, 558. [Google Scholar] [CrossRef]
- Wang, L.; Fu, X.; He, J.; Shi, X.; Chen, T.; Chen, P.; Wang, B.; Peng, H. Application challenges in fiber and textile electronics. Adv. Mater. 2020, 32, 1901971. [Google Scholar] [CrossRef]
- Guo, L.; Berglin, L.; Mattila, H. Improvement of electro-mechanical properties of strain sensors made of elastic-conductive hybrid yarns. Text. Res. J. 2012, 82, 1937–1947. [Google Scholar] [CrossRef]
- Post, E.R.; Orth, M.; Russo, P.R.; Gershenfeld, N. E-broidery: Design and fabrication of textile-based computing. IBM Syst. J. 2000, 39, 840–860. [Google Scholar] [CrossRef]
- Gimpel, S.; Mohring, U.; Muller, H.; Neudeck, A.; Scheibner, W. Textile-based electronic substrate technology. Journal of industrial textiles. J. Ind. Text 2004, 33, 179–189. [Google Scholar] [CrossRef]
- Dong, C.; Leber, A.; Das Gupta, T.; Chandran, R.; Volpi, M.; Qu, Y.; Nguyen-Dang, T.; Bartolomei, N.; Yan, W.; Sorin, F. High-efficiency super-elastic liquid metal based triboelectric fibers and textiles. Nat. Commun. 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhang, G.; Xu, L.; Sun, W.; Zhong, G.; Lei, J.; Yan, D.; Li, Z. Robustly Superhydrophobic Conductive Textile for Efficient Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2019, 11, 1680–1688. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Wang, K.; Lin, K.; Xie, H.; Zhang, X.; Li, J. Novel Insights into Inkjet Printed Silver Nanowires Flexible Transparent Conductive Films. Int. J. Mol. Sci. 2021, 22, 7719. [Google Scholar] [CrossRef] [PubMed]
- Moazzenchi, B.; Montazer, M. Click electroless plating and sonoplating of polyester with copper nanoparticles producing conductive fabric. Fibers Polym. 2020, 21, 522–531. [Google Scholar] [CrossRef]
- Kang, T.K. Piezoresistive characteristics of nylon thread resistive memories for wearable strain sensors. Coatings 2019, 9, 623. [Google Scholar] [CrossRef]
- Yun, Y.S.; Kim, B.; Park, H.H.; Jin, H.J. Transparent conducting films based on graphene oxide/silver nanowire hybrids with high flexibility. Synth. Met. 2012, 162, 1364–1368. [Google Scholar] [CrossRef]
- Dinu, A.; Apetrei, C. Development of Polypyrrole Modified Screen-Printed Carbon Electrode Based Sensors for Determination of L-Tyrosine in Pharmaceutical Products. Int. J. Mol. Sci. 2021, 22, 7528. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, C.; He, X.; Li, J.; Peng, Q.; Shi, E.; Wang, R.; Du, S.; Cao, A.; Li, Y. Self-stretchable, helical carbon nanotube yarn supercapacitors with stable performance under extreme deformation conditions. Nano Energy 2015, 12, 401–409. [Google Scholar] [CrossRef]
- Zhao, W.; Basnet, B.; Kim, I.J. Carbon nanotube formation using zeolite template and applications. J. Adv. Ceram. 2012, 1, 179–193. [Google Scholar] [CrossRef]
- Liu, L.; Wu, M.; Wu, Q.; Liu, J.; Yang, J.; Zhang, J. Conductive, superhydrophobic, and microwave-absorbing cotton fabric by dip-coating of aqueous silk nanofibers stabilized MWCNTs and octadecanoyl chain bonding. Cellulose 2022, 29, 46874701. [Google Scholar] [CrossRef]
- Görgülüer, H.; Çakıroğlu, B.; Özacar, M. Ag NPs deposited TiO2 coating material for superhydrophobic, antimicrobial and self-cleaning surface fabrication on fabric. J. Coat. Technol. Res. 2021, 18, 569–579. [Google Scholar] [CrossRef]
- Goda, E.S.; Abu Elella, M.H.; Hong, S.E.; Pandit, B.; Yoon, K.R.; Gamal, H. Smart flame retardant coating containing carboxymethyl chitosan nanoparticles decorated graphene for obtaining multifunctional textiles. Cellulose 2021, 28, 5087–5105. [Google Scholar] [CrossRef]
- Si, W.; Liao, Q.; Hou, W.; Chen, L.; Li, X.; Zhang, Z.; Sun, M.; Song, Y.; Qin, L. Low-Frequency Broadband Absorbing Coatings Based on MOFs: Design, Fabrication, Microstructure and Properties. Coatings 2022, 12, 766. [Google Scholar] [CrossRef]
- Koczak, M.J.; Premkumar, M.K. Emerging technologies for the in-situ production of MMCs. Jom 1993, 45, 44–48. [Google Scholar] [CrossRef]
- Lv, J.; Zhou, P.; Zhang, L.; Zhong, Y.; Sui, X.; Wang, B.; Chen, Z.; Xu, H.; Mao, Z. High-performance textile electrodes for wearable electronics obtained by an improved in situ polymerization method. Chem. Eng. J. 2019, 361, 897–907. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Uddin, M.E.; Kim, N.H.; Lau, A.K.; Lee, J.H. In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites. Polymer 2010, 51, 5921–5928. [Google Scholar] [CrossRef]
- Yu, H.; Tong, Z.; Zhang, B.; Chen, Z.; Li, X.; Su, D.; Ji, H. Thermal radiation shielded, high strength, fire resistant fiber/nanorod/aerogel composites fabricated by in-situ growth of TiO2 nanorods for thermal insulation. Chem. Eng. J. 2021, 418, 129342. [Google Scholar] [CrossRef]
- Zhu, Z.; Gao, F.; Zhang, Z.; Zhuang, Q.; Liu, Q.; Yu, H.; Fu, M. In-situ growth of MnCo2O4 hollow spheres on nickel foam as pseudocapacitive electrodes for supercapacitors. J. Colloid Interface Sci. 2021, 587, 56–63. [Google Scholar] [CrossRef]
- Huang, C.; Cai, B.; Zhang, L.; Zhang, C.; Pan, H. Preparation of iron-based metal-organic framework@ cellulose aerogel by in situ growth method and its application to dye adsorption. J. Solid State Chem. 2021, 297, 122030. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Di Girolamo, M.; Sarret, G.; Bureau, S.; Fernandez-Martinez, A.; Lelong, C.; Eymard Vernain, E. In situ formation of silver nanoparticles (Ag-NPs) onto textile fibers. ACS Omega 2021, 6, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Shaqeel, A.; Han, S.; Kang, J.; Yun, J.; Lee, M.; Lee, S.; Kim, J.; Noh, S.; Choi, M. In situ formation of Ag nanoparticles for fiber strain sensors: Toward textile-based wearable applications. ACS Appl. Mater. Interfaces 2021, 13, 39868–39879. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Cao, Y.; Mirkin, C.A.; Kelly, K.L.; Schatz, G.C.; Zheng, J. Photoinduced conversion of silver nanospheres to nanoprisms. Science 2001, 294, 1901–1903. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Y.; Guo, S.; Goebl, J.; Yin, Y. Seeded growth of uniform Ag nanoplates with high aspect ratio and widely tunable surface plasmon bands. Nano Lett. 2010, 10, 5037–5042. [Google Scholar] [CrossRef] [PubMed]
- Kilin, D.S.; Prezhdo, O.V.; Xia, Y. Shape-controlled synthesis of silver nanoparticles: Ab initio study of preferential surface coordination with citric acid. Chem. Phys. Lett. 2008, 458, 113–116. [Google Scholar] [CrossRef]
- Zeng, J.; Xia, X.; Rycenga, M.; Henneghan, P.; Li, Q.; Xia, Y. Successive deposition of silver on silver nanoplates: Lateral versus vertical growth. Angew. Chem. Int. Ed. 2011, 50, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Mayers, B.; Herricks, T.; Xia, Y. Polyol synthesis of uniform silver nanowires: A plausible growth mechanism and the supporting evidence. Nano Lett. 2003, 3, 955960. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Goebl, J.; Lu, Z.; Yin, Y. A systematic study of the synthesis of silver nanoplates: Is citrate a “magic” reagent? J. Am. Chem. Soc. 2011, 133, 18931–18939. [Google Scholar] [CrossRef]
- Washio, I.; Xiong, Y.; Yin, Y.; Xia, Y. Reduction by the end groups of poly (vinyl pyrrolidone): A new and versatile route to the kinetically controlled synthesis of Ag triangular nanoplates. Adv. Mater. 2006, 18, 1745–1749. [Google Scholar] [CrossRef]
- Wang, H.; Wu, H.; Zhong, L.; Zhao, J.; Li, G. Seed-mediated electrochemical growth of silver nanoplates. J. Electrochem. Soc. 2017, 164, D225. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, L.; Yan, A.; Wang, X.; Xie, Y. Controlling synthesis of silver nanowires and dendrites in mixed surfactant solutions. J. Colloid Interface Sci. 2003, 268, 357–361. [Google Scholar] [CrossRef] [PubMed]
- LaMer, V.K.; Dinegar, R.H. Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Montazer, M.; Allahyarzadeh, V. Electroless Plating of Silver Nanoparticles/Nanolayer on Polyester Fabric Using AgNO3/NaOH and Ammonia. Ind. Eng. Chem. Res. 2013, 52, 8436–8444. [Google Scholar] [CrossRef]
- Sungchul, S.; Daniel, F.B. Adhesion mechanisms of nanoparticle silver to substrate materials: Identification. Nanotechnology 2010, 21, 055204. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Q.; Yin, Y.; Zhang, J.; Si, W.; Hou, W.; Qin, L. In Situ Growth of Nanosilver on Fabric for Flexible Stretchable Electrodes. Int. J. Mol. Sci. 2022, 23, 13236. https://doi.org/10.3390/ijms232113236
Liao Q, Yin Y, Zhang J, Si W, Hou W, Qin L. In Situ Growth of Nanosilver on Fabric for Flexible Stretchable Electrodes. International Journal of Molecular Sciences. 2022; 23(21):13236. https://doi.org/10.3390/ijms232113236
Chicago/Turabian StyleLiao, Qingwei, Yuxiang Yin, Jingxin Zhang, Wei Si, Wei Hou, and Lei Qin. 2022. "In Situ Growth of Nanosilver on Fabric for Flexible Stretchable Electrodes" International Journal of Molecular Sciences 23, no. 21: 13236. https://doi.org/10.3390/ijms232113236
APA StyleLiao, Q., Yin, Y., Zhang, J., Si, W., Hou, W., & Qin, L. (2022). In Situ Growth of Nanosilver on Fabric for Flexible Stretchable Electrodes. International Journal of Molecular Sciences, 23(21), 13236. https://doi.org/10.3390/ijms232113236






