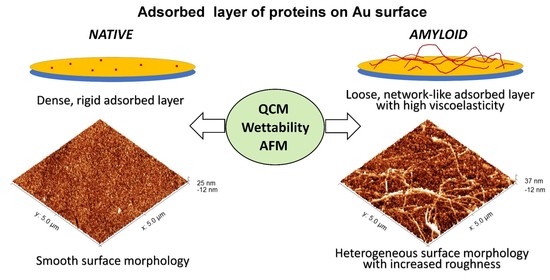
Comparative Study of the Solid-Liquid Interfacial Adsorption of Proteins in Their Native and Amyloid Forms
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Amyloid Formation
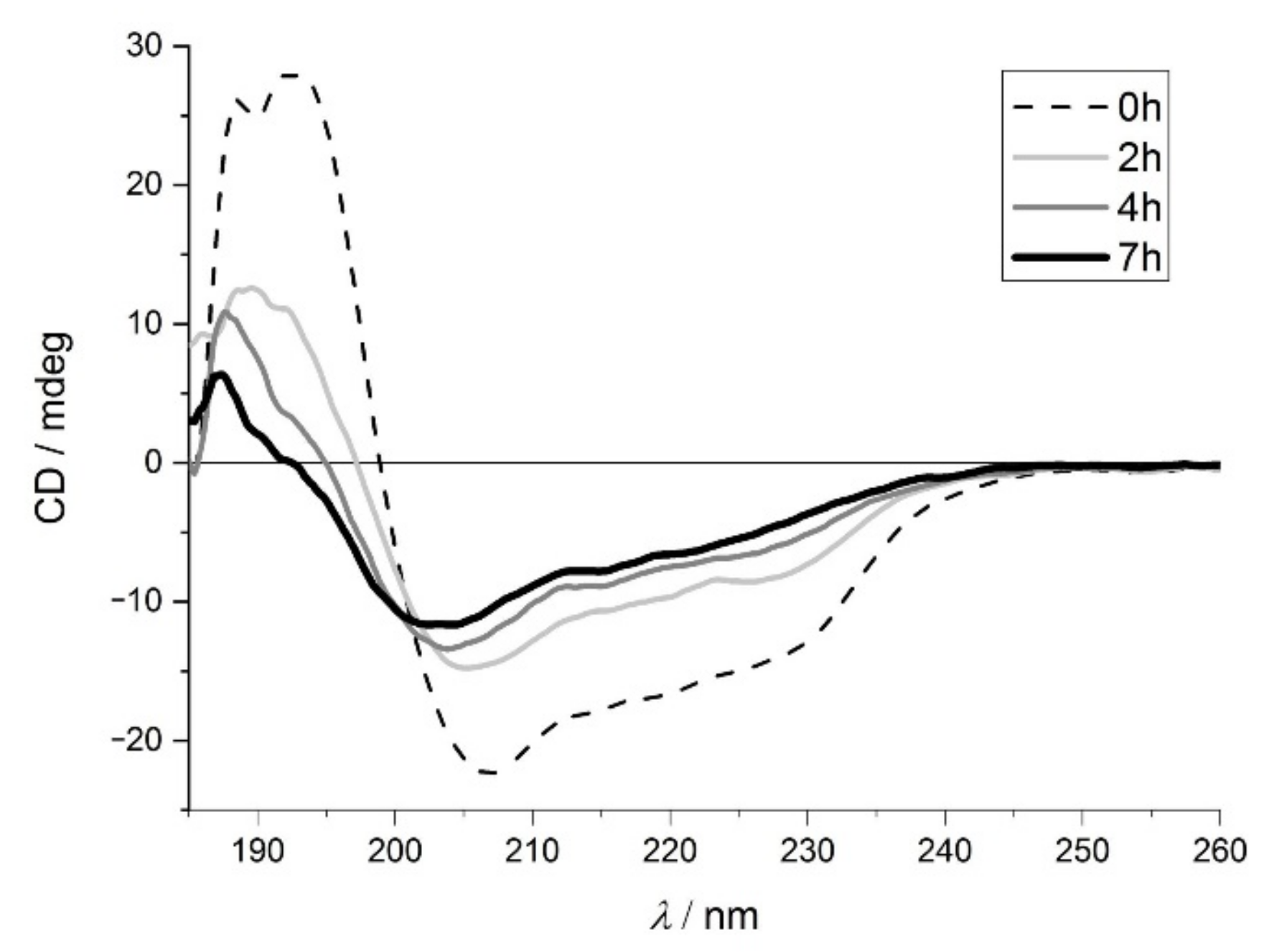
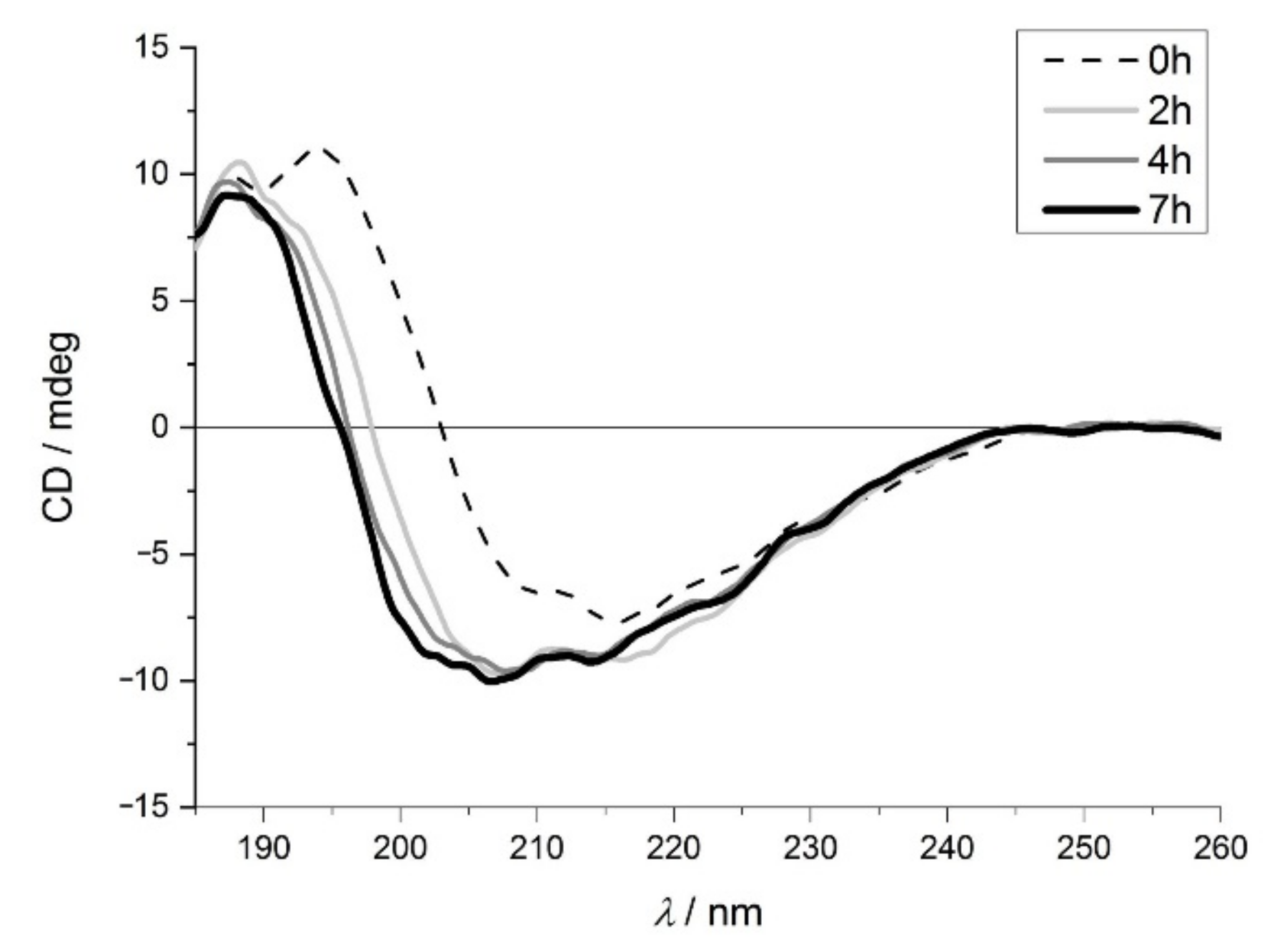
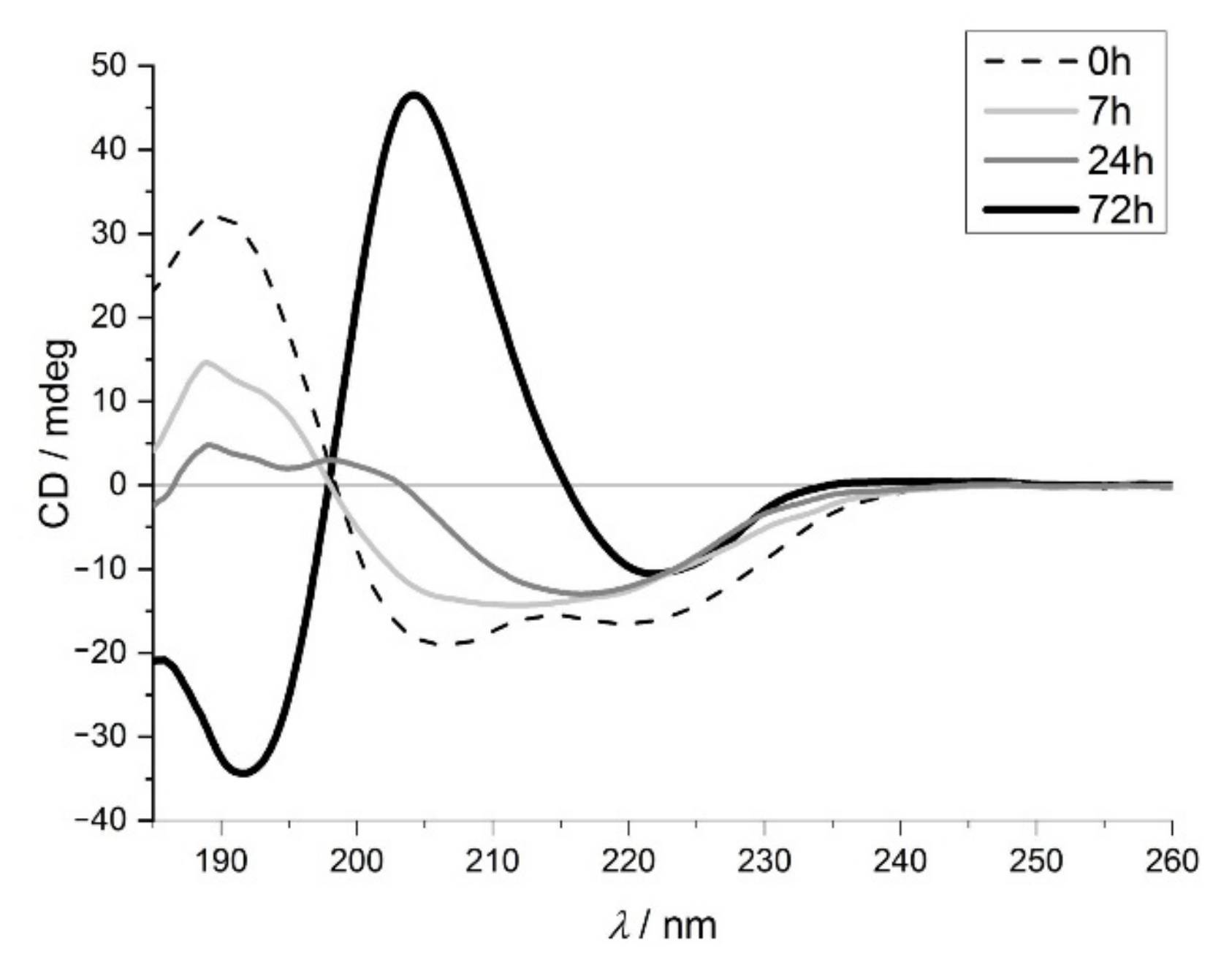
2.1.1. Electronic Circular Dichroism Spectroscopy (ECD) Studies of the Protein Structure
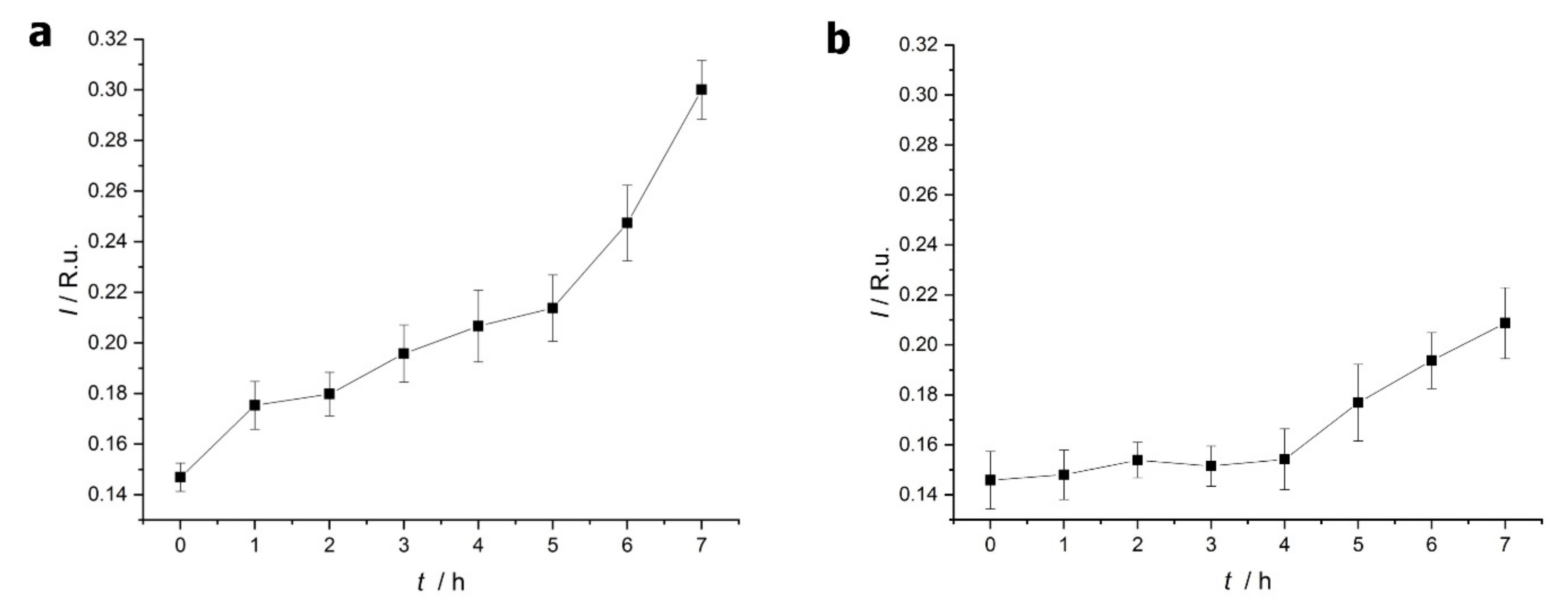
2.1.2. Thioflavin T (ThT) Fluorescence Test
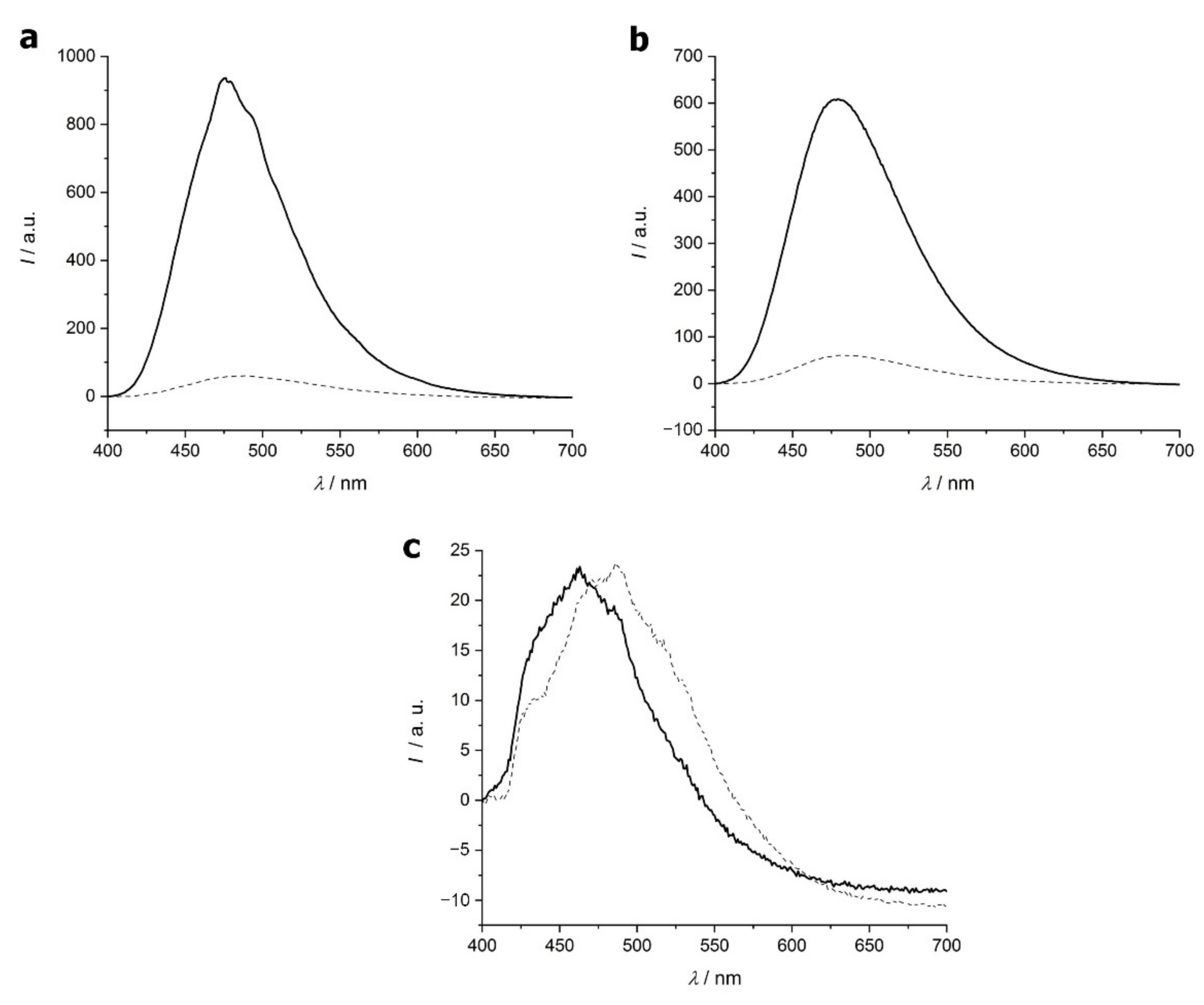
2.1.3. 8-Anilino-1-Naphthalenesulfonic Acid (ANS) Fluorescence Test
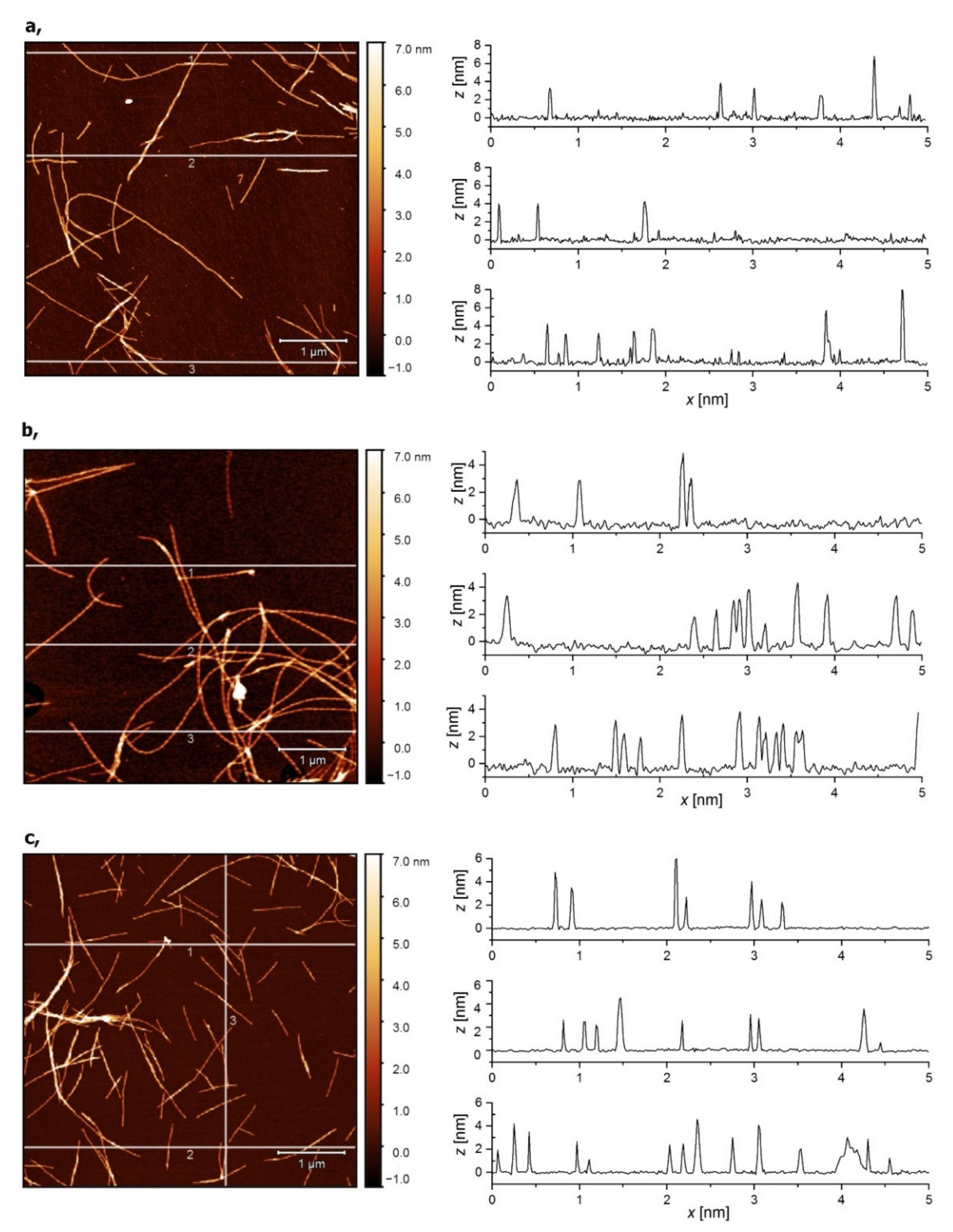
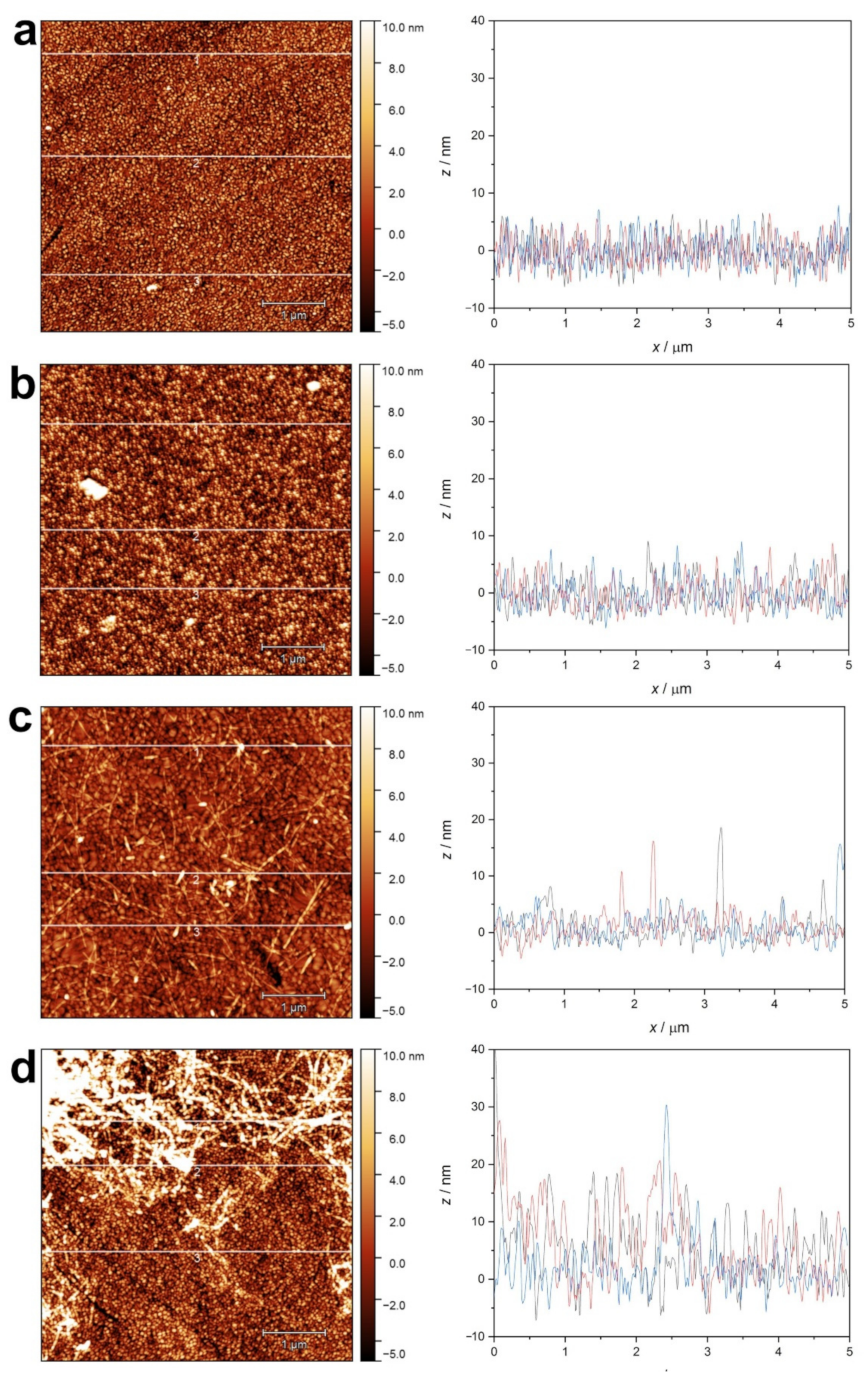
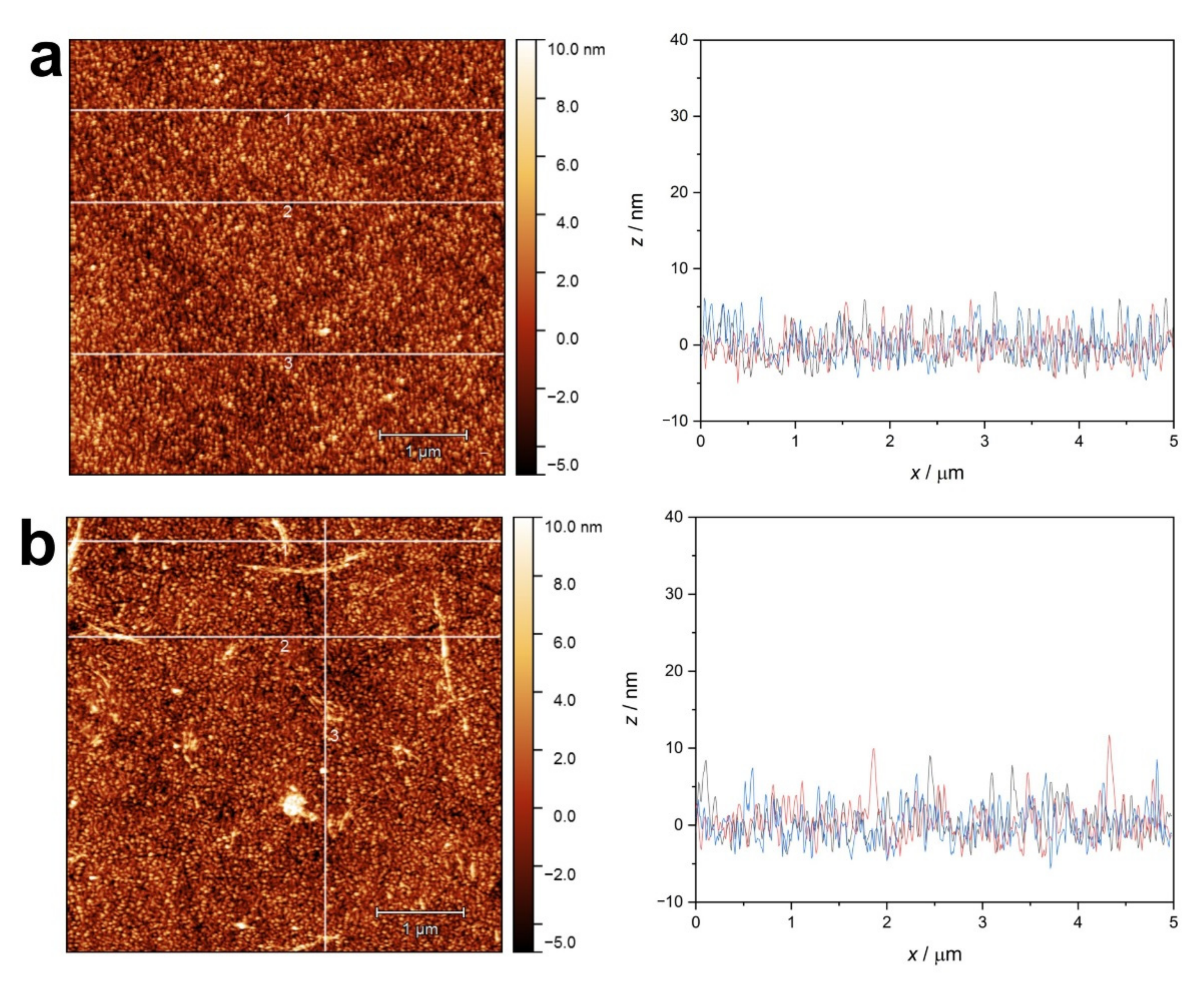
2.1.4. Atomic Force Microscopy (AFM) of Amyloid Fibers
2.1.5. Zeta Potential
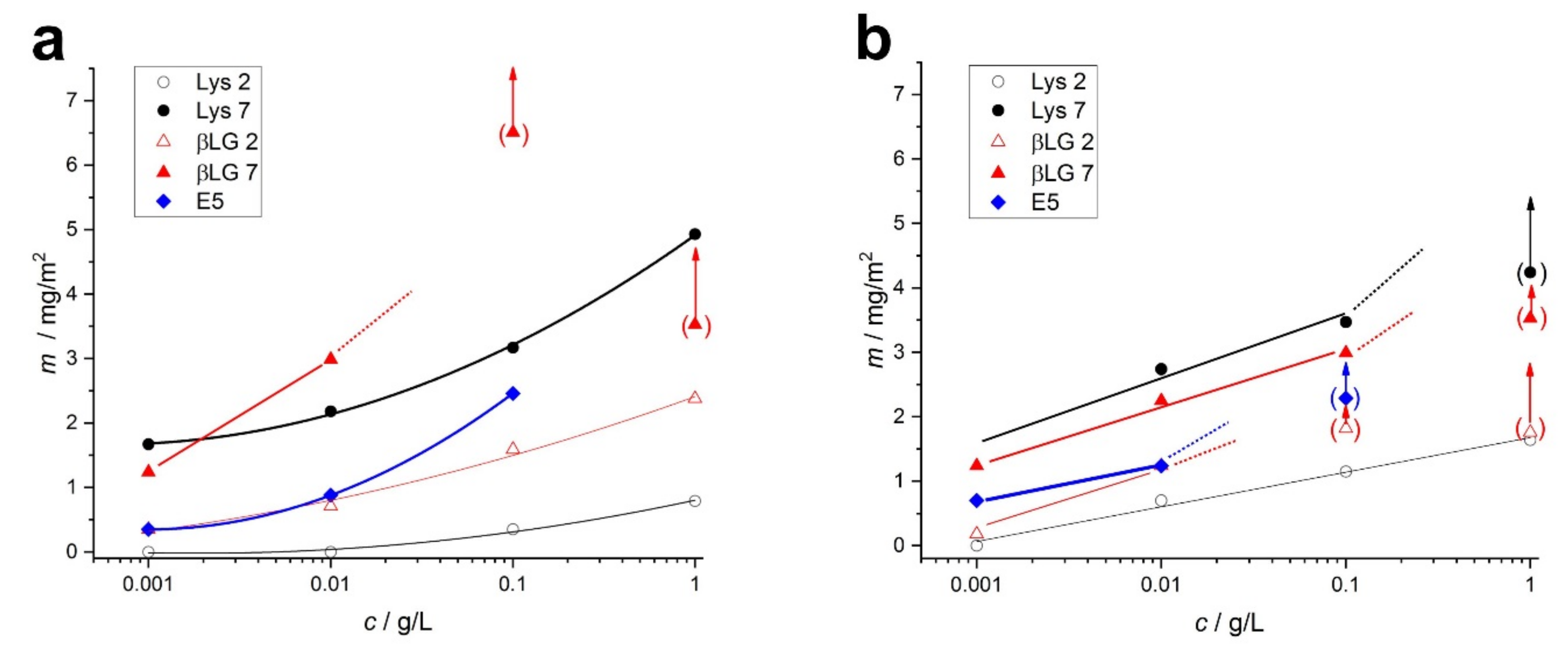
2.2. Protein Adsorption on Gold Surface
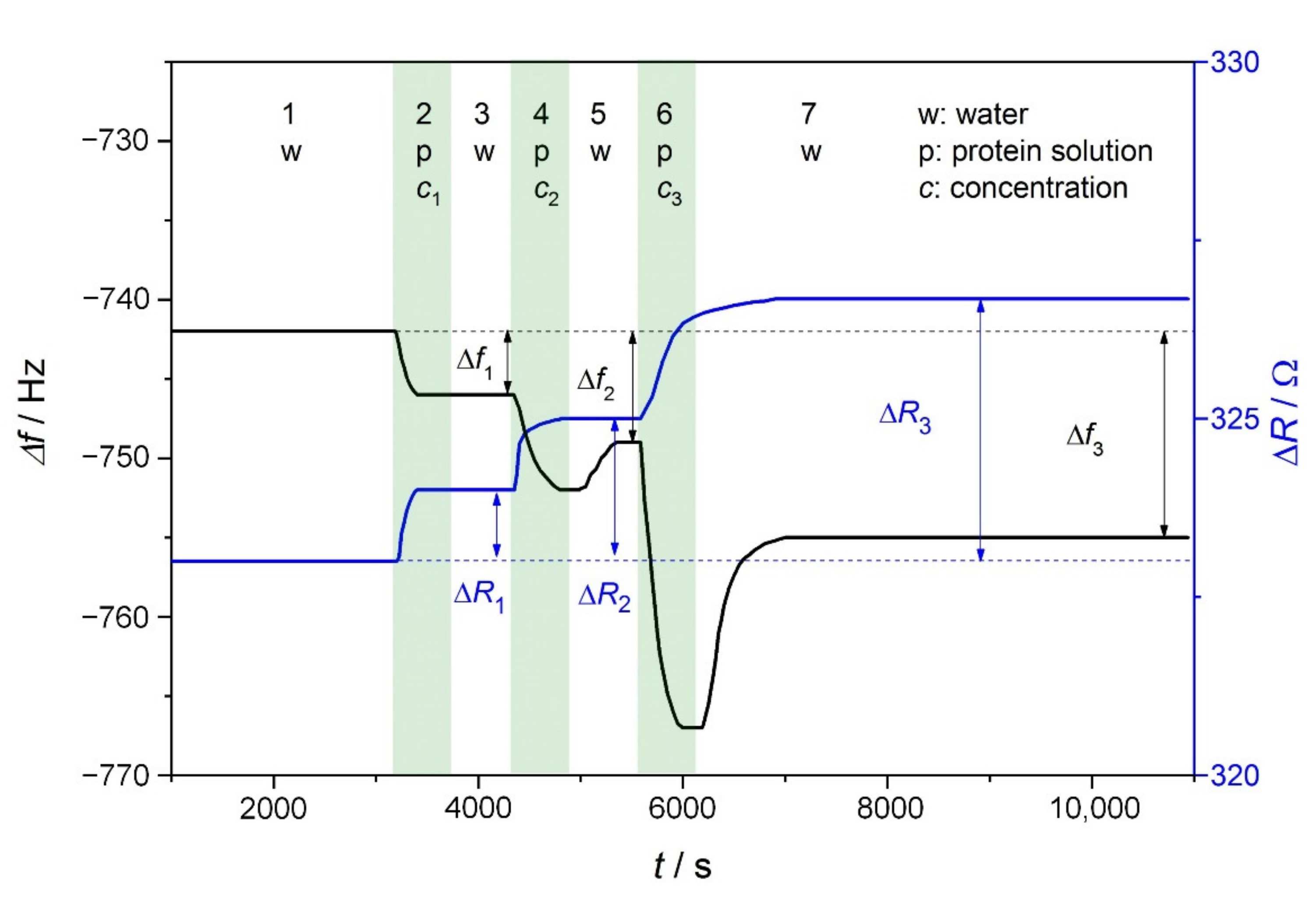
2.2.1. Quartz Crystal Microbalance (QCM) Studies of Adsorption
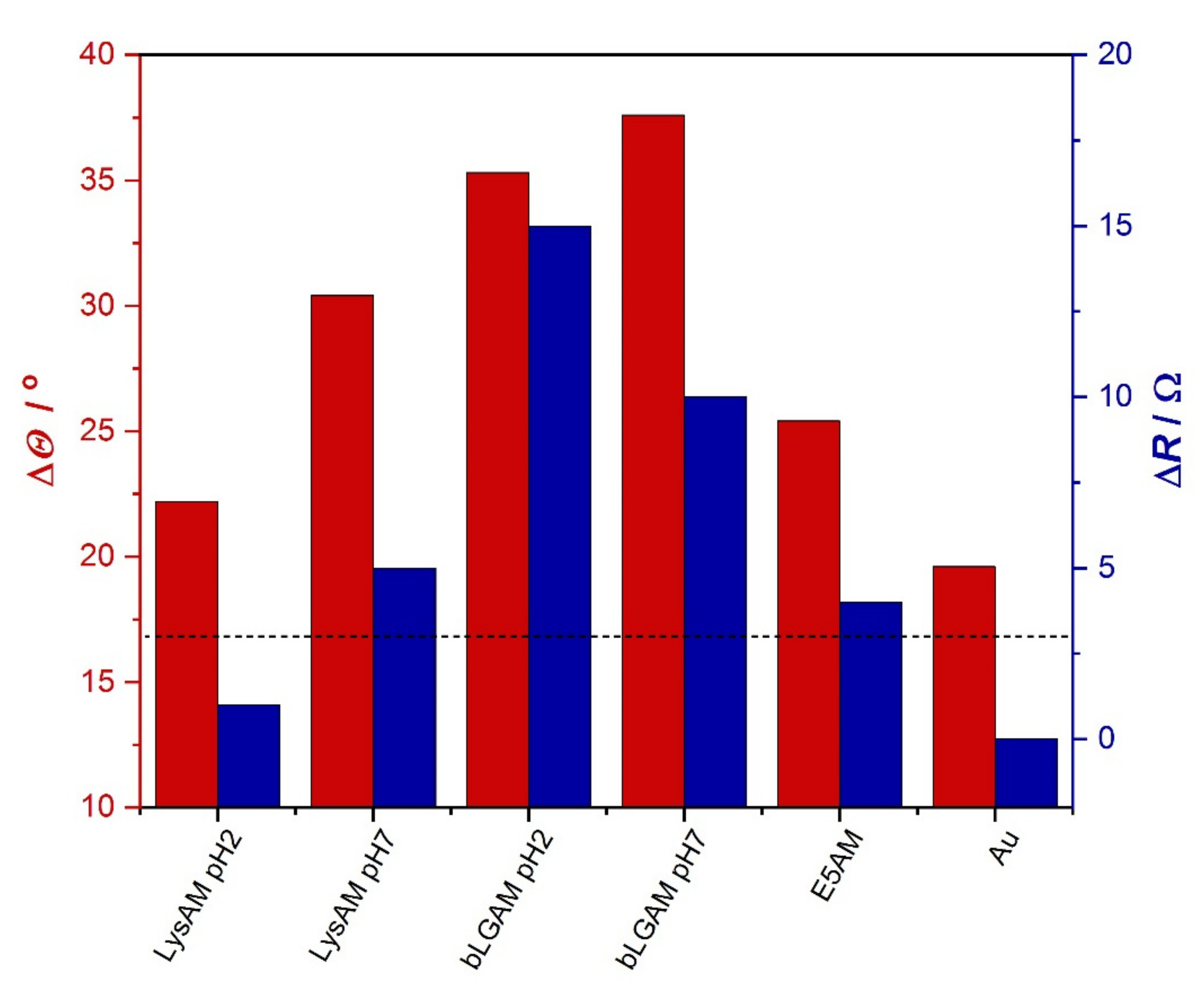
2.2.2. Wettability of the Adsorbed Surfaces
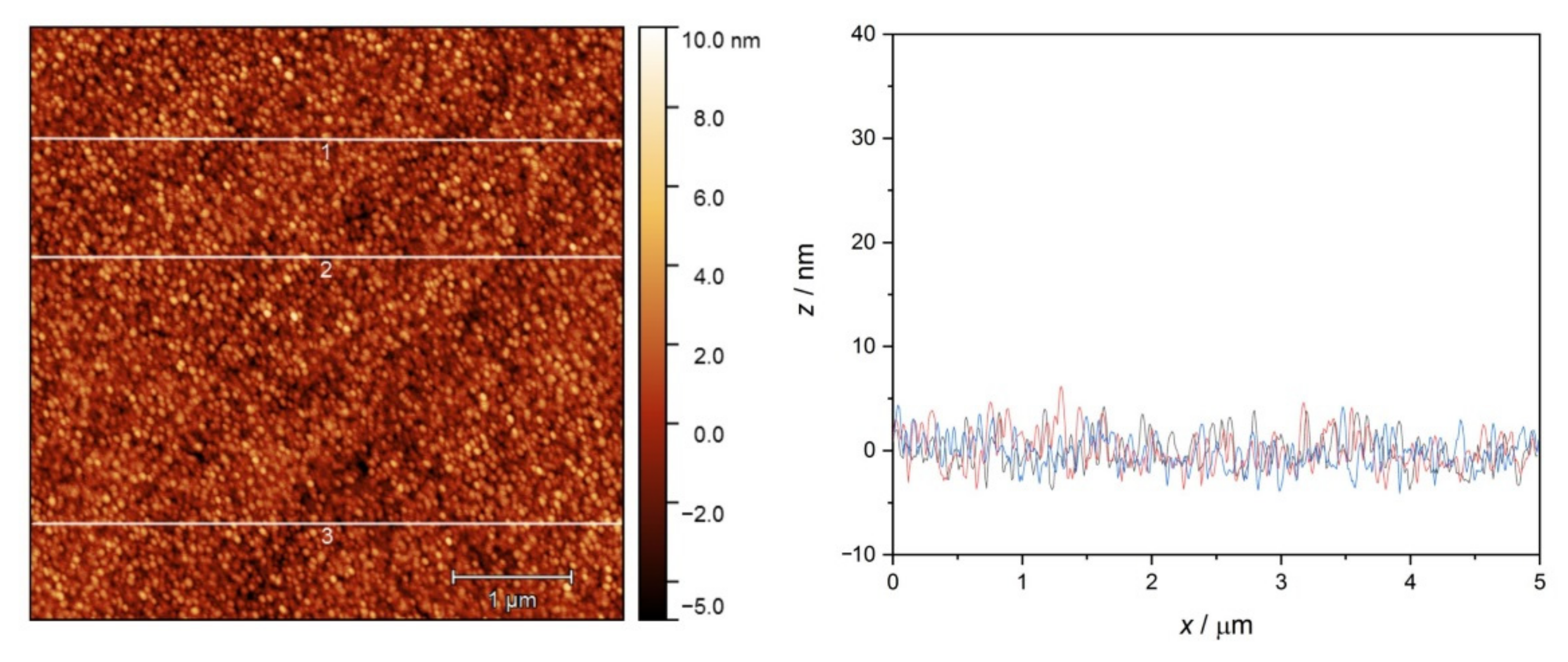
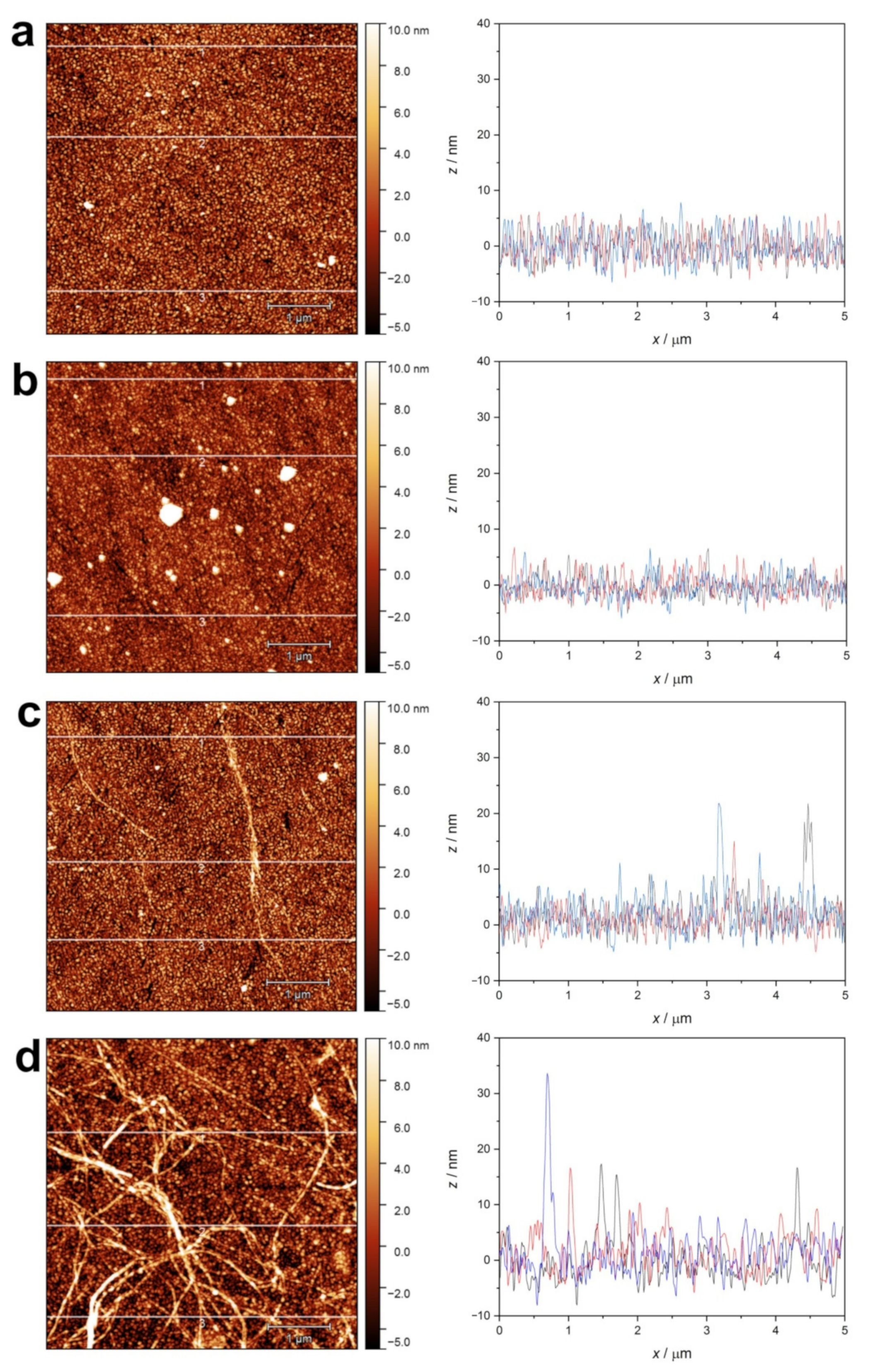
2.2.3. AFM Investigation of the Adsorbed Surfaces
3. Materials and Methods
3.1. Materials
3.2. Preparation of Amyloids
3.3. Quartz Crystal Microbalance (QCM)
3.4. Fluorimetry
3.5. Zeta Potential Measurement
3.6. Electronic Circular Dichroism Spectroscopy (ECD)
3.7. Wettability Measurements
3.8. Atomic Force Microscopy (AFM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nizhnikov, A.A.; Antonets, K.S.; Inge-Vechtomov, S.G. Amyloids: From Pathogenesis to Function. Biochemistry 2015, 80, 1127–1144. [Google Scholar] [CrossRef] [PubMed]
- Otzen, D.; Riek, R. Functional Amyloids. Cold Spring Harb. Perspect. Biol. 2019, 11, a033860. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, N.; Baker, M.O.D.G.; Ball, S.R.; Steain, M.; Pham, C.L.L.; Sunde, M. Microbial Functional Amyloids Serve Diverse Purposes for Structure, Adhesion and Defence. Biophys. Rev. 2019, 11, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, J.; Pan, T.; Wu, R.; Tao, Y.; Lin, H. The Broad-spectrum Antibiofilm Activity of Amyloid-forming Hexapeptides. Microb. Biotechnol. 2021, 14, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Sønderby, T.V.; Najarzadeh, Z.; Otzen, D.E. Functional Bacterial Amyloids: Understanding Fibrillation, Regulating Biofilm Fibril Formation and Organizing Surface Assemblies. Molecules 2022, 27, 4080. [Google Scholar] [CrossRef]
- Maji, S.K.; Perrin, M.H.; Sawaya, M.R.; Jessberger, S.; Vadodaria, K.; Rissman, R.A.; Singru, P.S.; Nilsson, K.P.R.; Simon, R.; Schubert, D.; et al. Functional Amyloids As Natural Storage of Peptide Hormones in Pituitary Secretory Granules. Science 2009, 325, 328–332. [Google Scholar] [CrossRef]
- Taglialegna, A.; Lasa, I.; Valle, J. Amyloid Structures as Biofilm Matrix Scaffolds. J. Bacteriol. 2016, 198, 2579–2588. [Google Scholar] [CrossRef]
- Piscitelli, A.; Cicatiello, P.; Gravagnuolo, A.M.; Sorrentino, I.; Pezzella, C.; Giardina, P. Applications of Functional Amyloids from Fungi: Surface Modification by Class I Hydrophobins. Biomolecules 2017, 7, 45. [Google Scholar] [CrossRef]
- Jackson, M.; Hewitt, E. Why Are Functional Amyloids Non-Toxic in Humans? Biomolecules 2017, 7, 71. [Google Scholar] [CrossRef]
- Nirmalraj, P.N.; List, J.; Battacharya, S.; Howe, G.; Xu, L.; Thompson, D.; Mayer, M. Complete Aggregation Pathway of Amyloid β (1-40) and (1-42) Resolved on an Atomically Clean Interface. Sci. Adv. 2020, 6, eaaz6014. [Google Scholar] [CrossRef]
- Hedayati, M.; Krapf, D.; Kipper, M.J. Dynamics of Long-Term Protein Aggregation on Low-Fouling Surfaces. J. Colloid Interface Sci. 2021, 589, 356–366. [Google Scholar] [CrossRef]
- Rice, L.J.; Ecroyd, H.; van Oijen, A.M. Illuminating Amyloid Fibrils: Fluorescence-Based Single-Molecule Approaches. Comput. Struct. Biotechnol. J. 2021, 19, 4711–4724. [Google Scholar] [CrossRef]
- Gyulai, G.; Pénzes, C.B.; Mohai, M.; Lohner, T.; Petrik, P.; Kurunczi, S.; Kiss, É. Interfacial Properties of Hydrophilized Poly(Lactic-Co-Glycolic Acid) Layers with Various Thicknesses. J. Colloid Interface Sci. 2011, 362, 600–606. [Google Scholar] [CrossRef]
- Horvath, R.; Kobzi, B.; Keul, H.; Moeller, M.; Kiss, É. Molecular Interaction of a New Antibacterial Polymer with a Supported Lipid Bilayer Measured by an in Situ Label-Free Optical Technique. Int. J. Mol. Sci. 2013, 14, 9722–9736. [Google Scholar] [CrossRef]
- Janovák, L.; Dékány, I.; Sebők, D. The Theoretical Concept of Polarization Reflectometric Interference Spectroscopy (PRIFS): An Optical Method to Monitor Molecule Adsorption and Nanoparticle Adhesion on the Surface of Thin Films. Photonics 2019, 6, 76. [Google Scholar] [CrossRef]
- Tarazona, N.A.; Machatschek, R.; Schulz, B.; Prieto, M.A.; Lendlein, A. Molecular Insights into the Physical Adsorption of Amphiphilic Protein PhaF onto Copolyester Surfaces. Biomacromolecules 2019, 20, 3242–3252. [Google Scholar] [CrossRef]
- Keller, A.; Grundmeier, G. Amyloid Aggregation at Solid-Liquid Interfaces: Perspectives of Studies Using Model Surfaces. Appl. Surf. Sci. 2020, 506, 144991. [Google Scholar] [CrossRef]
- Hajiraissi, R.; Hanke, M.; Yang, Y.; Duderija, B.; Gonzalez Orive, A.; Grundmeier, G.; Keller, A. Adsorption and Fibrillization of Islet Amyloid Polypeptide at Self-Assembled Monolayers Studied by QCM-D, AFM, and PM-IRRAS. Langmuir 2018, 34, 3517–3524. [Google Scholar] [CrossRef]
- Migoń, D.; Wasilewski, T.; Suchy, D. Application of QCM in Peptide and Protein-Based Drug Product Development. Molecules 2020, 25, 3950. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tashiro, Y.; Komasa, S.; Miyake, A.; Komasa, Y.; Okazaki, J. Effects of Surface Modification on Adsorption Behavior of Cell and Protein on Titanium Surface by Using Quartz Crystal Microbalance System. Materials 2020, 14, 97. [Google Scholar] [CrossRef]
- Dutta, A.K.; Nayak, A.; Belfort, G. Viscoelastic Properties of Adsorbed and Cross-Linked Polypeptide and Protein Layers at a Solid–Liquid Interface. J. Colloid Interface Sci. 2008, 324, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Hedin, J.; Löfroth, J.-E.; Nydén, M. Adsorption Behavior and Cross-Linking of EHEC and HM-EHEC at Hydrophilic and Hydrophobic Modified Surfaces Monitored by SPR and QCM-D. Langmuir 2007, 23, 6148–6155. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.R.; Ludolph, C.M.; Peppas, N.A. QCM-D Assay for Quantifying the Swelling, Biodegradation, and Protein Adsorption of Intelligent Nanogels. J. Appl. Polym. Sci. 2020, 137, 48655. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, N.; Larson, R.G. Hysteretic Swelling/Deswelling of Polyelectrolyte Brushes and Bilayer Films in Response to Changes in PH and Salt Concentration. Polymers 2021, 13, 812. [Google Scholar] [CrossRef] [PubMed]
- Ábrahám, Á.; Katona, M.; Kasza, G.; Kiss, É. Amphiphilic Polymer Layer—Model Cell Membrane Interaction Studied by QCM and AFM. Eur. Polym. J. 2017, 93, 212–221. [Google Scholar] [CrossRef]
- Willes, K.L.; Genchev, J.R.; Paxton, W.F. Hybrid Lipid-Polymer Bilayers: PH-Mediated Interactions between Hybrid Vesicles and Glass. Polymers 2020, 12, 745. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Buell, A.K.; Wang, X.-Y.; Welland, M.E.; Dobson, C.M.; Knowles, T.P.J.; Perrett, S. Relationship between Prion Propensity and the Rates of Individual Molecular Steps of Fibril Assembly. J. Biol. Chem. 2011, 286, 12101–12107. [Google Scholar] [CrossRef]
- Hovgaard, M.B.; Dong, M.; Otzen, D.E.; Besenbacher, F. Quartz Crystal Microbalance Studies of Multilayer Glucagon Fibrillation at the Solid-Liquid Interface. Biophys. J. 2007, 93, 2162–2169. [Google Scholar] [CrossRef]
- Buell, A.K.; Dobson, C.M.; Knowles, T.P.J.; Welland, M.E. Interactions between Amyloidophilic Dyes and Their Relevance to Studies of Amyloid Inhibitors. Biophys. J. 2010, 99, 3492–3497. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Y.; Tian, X.; Li, C.; Liu, L. Disassembling and Degradation of Amyloid Protein Aggregates Based on Gold Nanoparticle-Modified g-C3N4. Colloids Surf. B Biointerfaces 2020, 192, 111051. [Google Scholar] [CrossRef]
- Hwang, S.S.; Chan, H.; Sorci, M.; van Deventer, J.; Wittrup, D.; Belfort, G.; Walt, D. Detection of Amyloid β Oligomers toward Early Diagnosis of Alzheimer’s Disease. Anal. Biochem. 2019, 566, 40–45. [Google Scholar] [CrossRef]
- Jamerlan, A.; An, S.S.A.; Hulme, J. Advances in Amyloid Beta Oligomer Detection Applications in Alzheimer’s Disease. TrAC Trends Anal. Chem. 2020, 129, 115919. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.-H.; Goto, Y.; Réfrégiers, M.; Kardos, J. Accurate Secondary Structure Prediction and Fold Recognition for Circular Dichroism Spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef]
- Lara, C.; Adamcik, J.; Jordens, S.; Mezzenga, R. General Self-Assembly Mechanism Converting Hydrolyzed Globular Proteins Into Giant Multistranded Amyloid Ribbons. Biomacromolecules 2011, 12, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, C.A.; Melton, L.D.; Gerrard, J.A.; Loveday, S.M. Formation of β-Lactoglobulin Nanofibrils by Microwave Heating Gives a Peptide Composition Different from Conventional Heating. Biomacromolecules 2012, 13, 2868–2880. [Google Scholar] [CrossRef]
- Rovó, P.; Farkas, V.; Stráner, P.; Szabó, M.; Jermendy, Á.; Hegyi, O.; Tóth, G.K.; Perczel, A. Rational Design of α-Helix-Stabilized Exendin-4 Analogues. Biochemistry 2014, 53, 3540–3552. [Google Scholar] [CrossRef][Green Version]
- Taricska, N.; Horváth, D.; Menyhárd, D.K.; Ákontz-Kiss, H.; Noji, M.; So, M.; Goto, Y.; Fujiwara, T.; Perczel, A. The Route from the Folded to the Amyloid State: Exploring the Potential Energy Surface of a Drug-Like Miniprotein. Chem.–A Eur. J. 2020, 26, 1968–1978. [Google Scholar] [CrossRef] [PubMed]
- Biancalana, M.; Koide, S. Molecular Mechanism of Thioflavin-T Binding to Amyloid Fibrils. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2010, 1804, 1405–1412. [Google Scholar] [CrossRef]
- Dzwolak, W.; Pecul, M. Chiral Bias of Amyloid Fibrils Revealed by the Twisted Conformation of Thioflavin T: An Induced Circular Dichroism/DFT Study. FEBS Lett. 2005, 579, 6601–6603. [Google Scholar] [CrossRef]
- Stsiapura, V.I.; Maskevich, A.A.; Kuzmitsky, V.A.; Turoverov, K.K.; Kuznetsova, I.M. Computational Study of Thioflavin T Torsional Relaxation in the Excited State. J. Phys. Chem. A 2007, 111, 4829–4835. [Google Scholar] [CrossRef]
- Voropai, E.S.; Samtsov, M.P.; Kaplevskii, K.N.; Maskevich, A.A.; Stepuro, V.I.; Povarova, O.I.; Kuznetsova, I.M.; Turoverov, K.K.; Fink, A.L.; Uverskii, V.N. Spectral Properties of Thioflavin T and Its Complexes with Amyloid Fibrils. J. Appl. Spectrosc. 2003, 70, 868–874. [Google Scholar] [CrossRef]
- Jin, L.; Gao, W.; Liu, C.; Zhang, N.; Mukherjee, S.; Zhang, R.; Dong, H.; Bhunia, A.; Bednarikova, Z.; Gazova, Z.; et al. Investigating the Inhibitory Effects of Entacapone on Amyloid Fibril Formation of Human Lysozyme. Int. J. Biol. Macromol. 2020, 161, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Younan, N.D.; Viles, J.H. A Comparison of Three Fluorophores for the Detection of Amyloid Fibers and Prefibrillar Oligomeric Assemblies. ThT (Thioflavin T); ANS (1-Anilinonaphthalene-8-Sulfonic Acid); and BisANS (4,4′-Dianilino-1,1′-Binaphthyl-5,5′-Disulfonic Acid). Biochemistry 2015, 54, 4297–4306. [Google Scholar] [CrossRef]
- Haskard, C.A.; Li-Chan, E.C.Y. Hydrophobicity of Bovine Serum Albumin and Ovalbumin Determined Using Uncharged (PRODAN) and Anionic (ANS-) Fluorescent Probes. J. Agric. Food Chem. 1998, 46, 2671–2677. [Google Scholar] [CrossRef]
- Zhao, G.; London, E. An Amino Acid “Transmembrane Tendency” Scale That Approaches the Theoretical Limit to Accuracy for Prediction of Transmembrane Helices: Relationship to Biological Hydrophobicity. Protein Sci. 2006, 15, 1987–2001. [Google Scholar] [CrossRef]
- Protein Data Bank E5. Available online: https://www.rcsb.org/sequence/2MJ9 (accessed on 3 October 2022).
- Komorek, P.; Martin, E.; Jachimska, B. Adsorption and Conformation Behavior of Lysozyme on a Gold Surface Determined by QCM-D, MP-SPR, and FTIR. Int. J. Mol. Sci. 2021, 22, 1322. [Google Scholar] [CrossRef]
- Jachimska, B.; Świątek, S.; Loch, J.I.; Lewiński, K.; Luxbacher, T. Adsorption Effectiveness of β-Lactoglobulin onto Gold Surface Determined by Quartz Crystal Microbalance. Bioelectrochemistry 2018, 121, 95–104. [Google Scholar] [CrossRef]
- Barbalinardo, M.; Antosova, A.; Gambucci, M.; Bednarikova, Z.; Albonetti, C.; Valle, F.; Sassi, P.; Latterini, L.; Gazova, Z.; Bystrenova, E. Effect of Metallic Nanoparticles on Amyloid Fibrils and Their Influence to Neural Cell Toxicity. Nano Res. 2020, 13, 1081–1089. [Google Scholar] [CrossRef]
- Severini, L.; de France, K.J.; Sivaraman, D.; Kummer, N.; Nyström, G. Biohybrid Nanocellulose–Lysozyme Amyloid Aerogels via Electrostatic Complexation. ACS Omega 2022, 7, 578–586. [Google Scholar] [CrossRef]
- Peng, D.; Yang, J.; Li, J.; Tang, C.; Li, B. Foams Stabilized by β-Lactoglobulin Amyloid Fibrils: Effect of PH. J. Agric. Food Chem. 2017, 65, 10658–10665. [Google Scholar] [CrossRef]
- Su, X.-L.; Li, Y. A QCM Immunosensor for Salmonella Detection with Simultaneous Measurements of Resonant Frequency and Motional Resistance. Biosens. Bioelectron. 2005, 21, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Protein Data Bank-Beta-Lactoglobulin. Available online: https://rcsb.org/sequence/2Q2M (accessed on 3 October 2022).
- Johannsmann, D.; Langhoff, A.; Leppin, C. Studying Soft Interfaces with Shear Waves: Principles and Applications of the Quartz Crystal Microbalance (QCM). Sensors 2021, 21, 3490. [Google Scholar] [CrossRef] [PubMed]
- Johannsmann, D. Viscoelastic Analysis of Organic Thin Films on Quartz Resonators. Macromol. Chem. Phys. 1999, 200, 501–516. [Google Scholar] [CrossRef]
- Voinova, M.V.; Jonson, M.; Kasemo, B. ‘Missing Mass’ Effect in Biosensor’s QCM Applications. Biosens. Bioelectron. 2002, 17, 835–841. [Google Scholar] [CrossRef]
- Vida, I.; Fazekas, Z.; Gyulai, G.; Nagy-Fazekas, D.; Pálfy, G.; Stráner, P.; Kiss, É.; Perczel, A. Bacterial Fermentation and Isotope Labelling Optimized for Amyloidogenic Proteins. Microb. Biotechnol. 2021, 14, 1107–1119. [Google Scholar] [CrossRef]
- Norde, W.; Buijs, J.; Lyklema, H. Adsorption Of Globular Proteins. In Fundamentals of Interface and Colloid Science; Academic Press: Cambridge, MA, USA, 2005; p. 3. [Google Scholar]
- Latif, U.; Can, S.; Hayden, O.; Grillberger, P.; Dickert, F.L. Sauerbrey and Anti-Sauerbrey Behavioral Studies in QCM Sensors—Detection of Bioanalytes. Sens. Actuators B Chem. 2013, 176, 825–830. [Google Scholar] [CrossRef]
- Beißner, S.; Thies, J.-W.; Bechthold, C.; Kuhn, P.; Thürmann, B.; Dübel, S.; Dietzel, A. Low-Cost, in-Liquid Measuring System Using a Novel Compact Oscillation Circuit and Quartz-Crystal Microbalances (QCMs) as a Versatile Biosensor Platform. J. Sens. Sens. Syst. 2017, 6, 341–350. [Google Scholar] [CrossRef]
- Martin, S.J.; Granstaff, V. Edwards.; Frye, G.C. Characterization of a Quartz Crystal Microbalance with Simultaneous Mass and Liquid Loading. Anal. Chem. 1991, 63, 2272–2281. [Google Scholar] [CrossRef]
- Ábrahám, Á.; Baranyai, Z.; Gyulai, G.; Pári, E.; Horváti, K.; Bősze, S.; Kiss, É. Comparative Analysis of New Peptide Conjugates of Antitubercular Drug Candidates—Model Membrane and in Vitro Studies. Colloids Surf. B Biointerfaces 2016, 147, 106–115. [Google Scholar] [CrossRef]
- Stanford Research Systems Inc. SRS QCM200 Operation and Service Manual; Stanford Research Systems Inc.: Sunnyvale, CA, USA, 2004. [Google Scholar]
- Nánási, D.E.; Kunfi, A.; Ábrahám, Á.; Mayer, P.J.; Mihály, J.; Samu, G.F.; Kiss, É.; Mohai, M.; London, G. Construction and Properties of Donor–Acceptor Stenhouse Adducts on Gold Surfaces. Langmuir 2021, 37, 3057–3066. [Google Scholar] [CrossRef]

| Protein | ζ/mV | |
|---|---|---|
| pH = 2.0 | pH = 7.0 | |
| Native lysozyme | 29.1 ± 2.2 | 4.1 ± 0.7 |
| Lysozyme amyloid | 48.3 ± 2.2 | 26.4 ± 0.2 |
| Native β-lactoglobulin | 17.5 ± 5.2 | −26.9 ± 1.3 |
| β-lactoglobulin amyloid | 52.5 ± 2.7 | −26.8 ± 0.7 |
| pH | Lysozyme | c/g/L | Δf/Hz | ΔR/Ω |
|---|---|---|---|---|
| 2.0 | NATIVE | 0.001 | 0.00 ± 0.05 | 1.00 ± 0.10 |
| 0.01 | 0.00 ± 0.07 | 1.00 ± 0.15 | ||
| 0.1 | −2.00 ± 0.32 | 1.00 ± 0.12 | ||
| 1 | −4.50 ± 0.50 | 0.50 ± 0.06 | ||
| AMYLOID | 0.001 | 0.00 ± 0.06 | 0.00 ± 0.05 | |
| 0.01 | −4.00 ± 0.42 | 0.50 ± 0.07 | ||
| 0.1 | −6.50 ± 0.71 | 1.00 ± 0.11 | ||
| 1 | −9.30 ± 1.10 | 1.00 ± 0.13 | ||
| 7.0 | NATIVE | 0.001 | −9.50 ± 1.05 | 1.00 ± 0.08 |
| 0.01 | −12.33 ± 1.33 | 1.00 ± 0.12 | ||
| 0.1 | −18.00 ± 1.80 | 1.30 ± 0.15 | ||
| 1 | −28.00 ± 2.10 | 2.30 ± 0.20 | ||
| AMYLOID | 0.001 | −9.00 ± 0.97 | 0.00 ± 0.04 | |
| 0.01 | −15.50 ± 1.25 | 1.00 ± 0.95 | ||
| 0.1 | −19.67 ± 1.74 | 1.67 ± 0.14 | ||
| 1 | −24.00 ± 2.05 | 5.00 ± 0.49 |
| pH | β-Lactoglobulin | c/g/L | Δf/Hz | ΔR/Ω |
|---|---|---|---|---|
| 2.0 | NATIVE | 0.001 | −2.00 ± 0.25 | 1.00 ± 0.07 |
| 0.01 | −4.00 ± 0.53 | 1.00 ± 0.08 | ||
| 0.1 | −9.00 ± 0.95 | 2.00 ± 0.25 | ||
| 1 | −13.50 ± 1.21 | 2.00 ± 0.19 | ||
| AMYLOID | 0.001 | −1.00 ± 0.09 | 0.00 ± 0.04 | |
| 0.01 | −7.00 ± 0.80 | 1.00 ± 0.09 | ||
| 0.1 | −10.33 ± 1.10 | 9.00 ± 0.99 | ||
| 1 | −10.00 ±1.02 | 15.00 ± 1.65 | ||
| 7.0 | NATIVE | 0.001 | −7.00 ± 0.75 | 1.00 ± 0.10 |
| 0.01 | −17.00 ± 1.64 | 0.00 ± 0.07 | ||
| 0.1 | −37.00 ± 4.10 | 11.00 ± 1.52 | ||
| 1 | −20.00 ± 1.83 | 8.00 ± 1.26 | ||
| AMYLOID | 0.001 | −7.00 ± 0.78 | 1.00 ± 0.09 | |
| 0.01 | −12.75 ± 1.30 | 1.00 ± 0.10 | ||
| 0.1 | −17.00 ± 1.60 | 2.30 ± 0.19 | ||
| 1 | −20.00 ± 1.81 | 10.00 ± 0.91 |
| pH | E5 | c/g/L | Δf/Hz | ΔR/Ω |
|---|---|---|---|---|
| 4.1 | NATIVE | 0.001 | −2.00 ± 0.18 | 0.00 ± 0.03 |
| 0.01 | −5.00 ± 0.45 | 1.00 ± 0.11 | ||
| 0.1 | −14.00 ± 1.34 | 2.00 ± 0.18 | ||
| AMYLOID | 0.001 | −4.00 ± 0.42 | 1.00 ± 0.12 | |
| 0.01 | −7.00 ± 0.83 | 2.00 ± 0.21 | ||
| 0.1 | −13.00 ± 1.33 | 4.00 ± 0.47 |
| Protein | Number of AA | Size of Molecule/nm | Number of S-S Bridges | i.e.p./pH | |
|---|---|---|---|---|---|
| Lysozyme * | 14,300 | 129 | 4.5 × 3 × 3 | 4 | 10.7–11.1 |
| β-lactoglobulin * | 18,400 | 162 | 3.6 × 7 | 2 | 5.4 |
| E5 | 2783 | 25 | - | - | 4.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ábrahám, Á.; Massignan, F.; Gyulai, G.; Katona, M.; Taricska, N.; Kiss, É. Comparative Study of the Solid-Liquid Interfacial Adsorption of Proteins in Their Native and Amyloid Forms. Int. J. Mol. Sci. 2022, 23, 13219. https://doi.org/10.3390/ijms232113219
Ábrahám Á, Massignan F, Gyulai G, Katona M, Taricska N, Kiss É. Comparative Study of the Solid-Liquid Interfacial Adsorption of Proteins in Their Native and Amyloid Forms. International Journal of Molecular Sciences. 2022; 23(21):13219. https://doi.org/10.3390/ijms232113219
Chicago/Turabian StyleÁbrahám, Ágnes, Flavio Massignan, Gergő Gyulai, Miklós Katona, Nóra Taricska, and Éva Kiss. 2022. "Comparative Study of the Solid-Liquid Interfacial Adsorption of Proteins in Their Native and Amyloid Forms" International Journal of Molecular Sciences 23, no. 21: 13219. https://doi.org/10.3390/ijms232113219
APA StyleÁbrahám, Á., Massignan, F., Gyulai, G., Katona, M., Taricska, N., & Kiss, É. (2022). Comparative Study of the Solid-Liquid Interfacial Adsorption of Proteins in Their Native and Amyloid Forms. International Journal of Molecular Sciences, 23(21), 13219. https://doi.org/10.3390/ijms232113219