The Preventive Effect of A Magnetic Nanoparticle-Modified Root Canal Sealer on Persistent Apical Periodontitis
Abstract
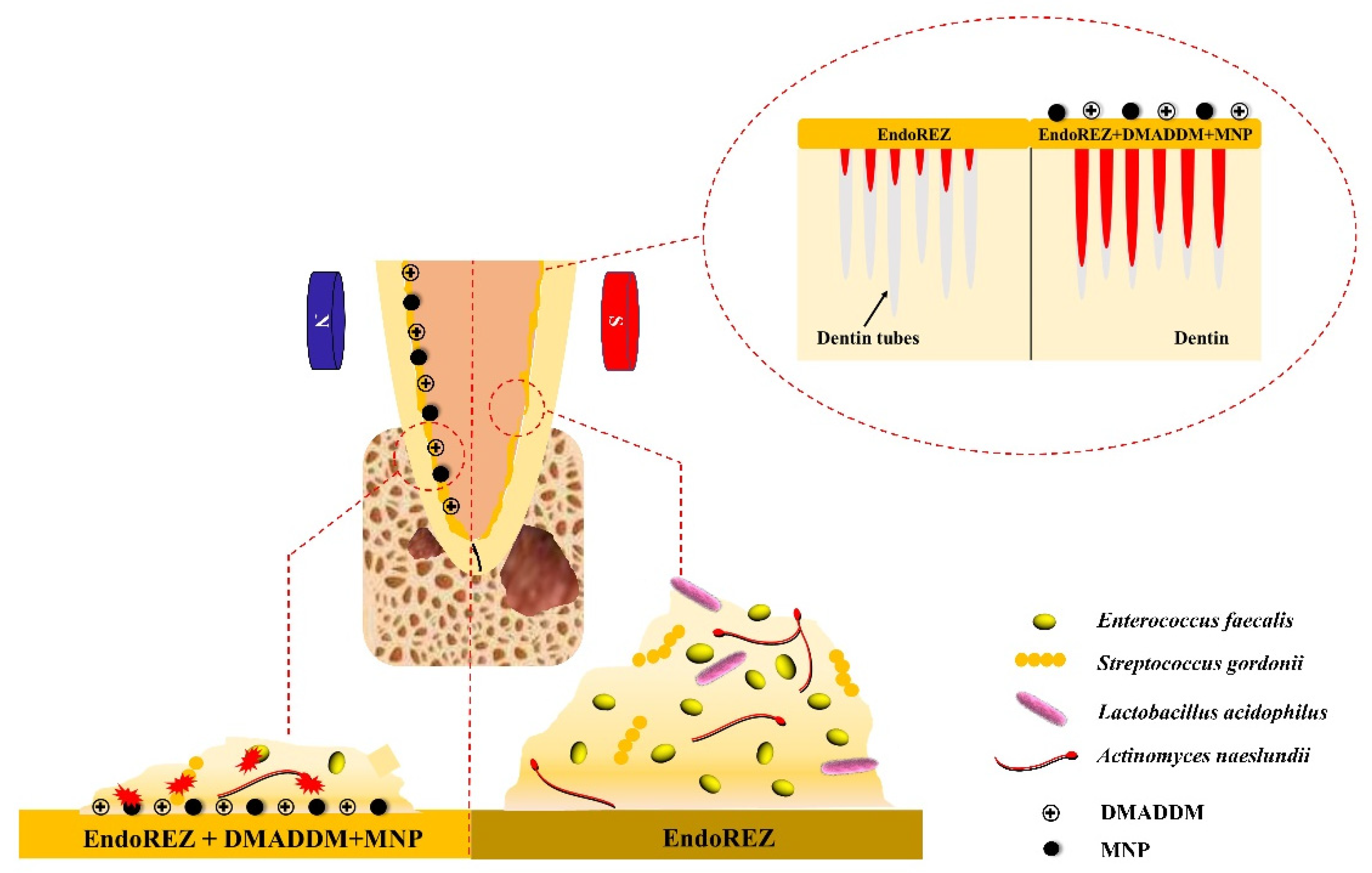
1. Introduction
2. Results
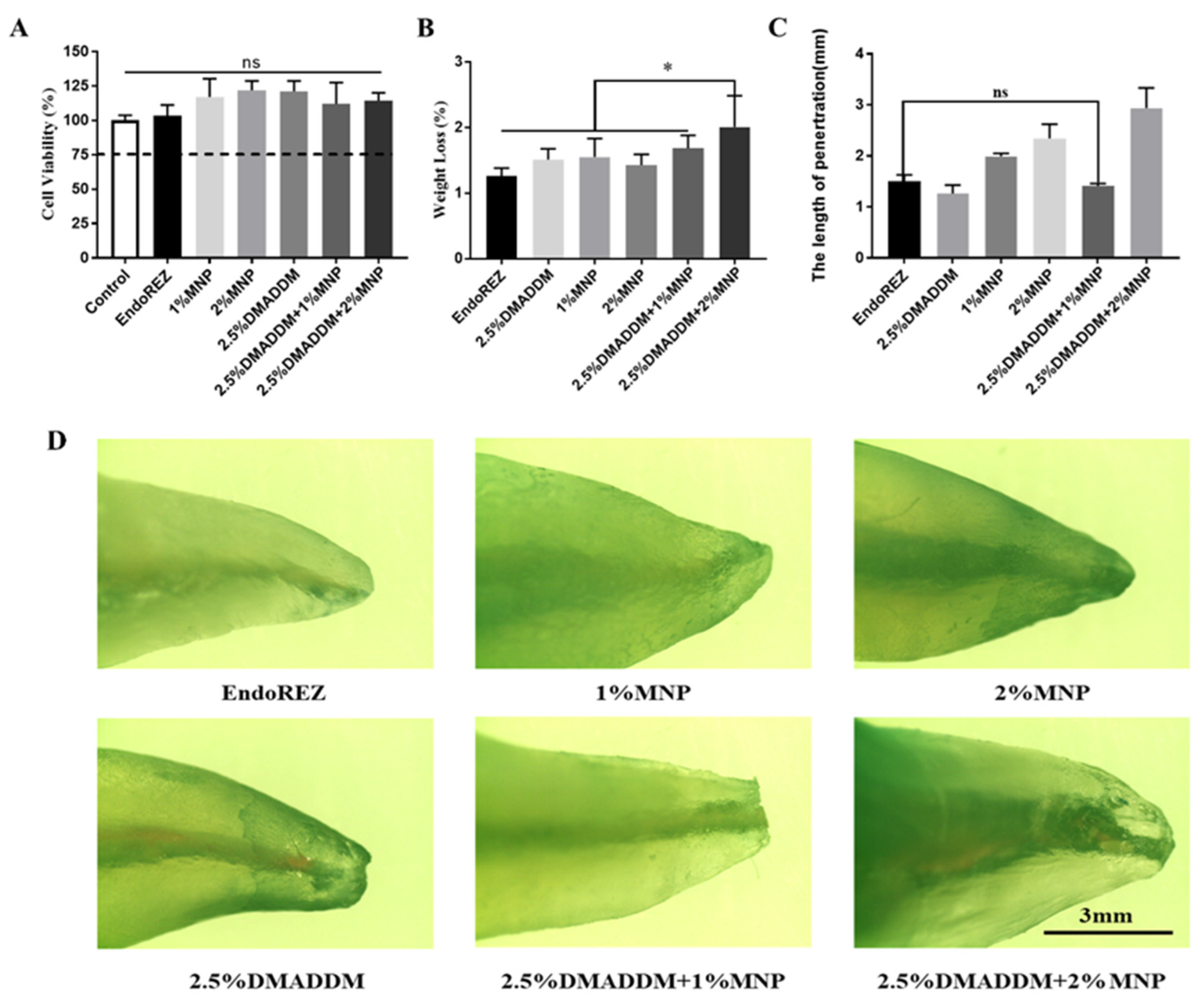
2.1. Cytotoxicity and Material Properties
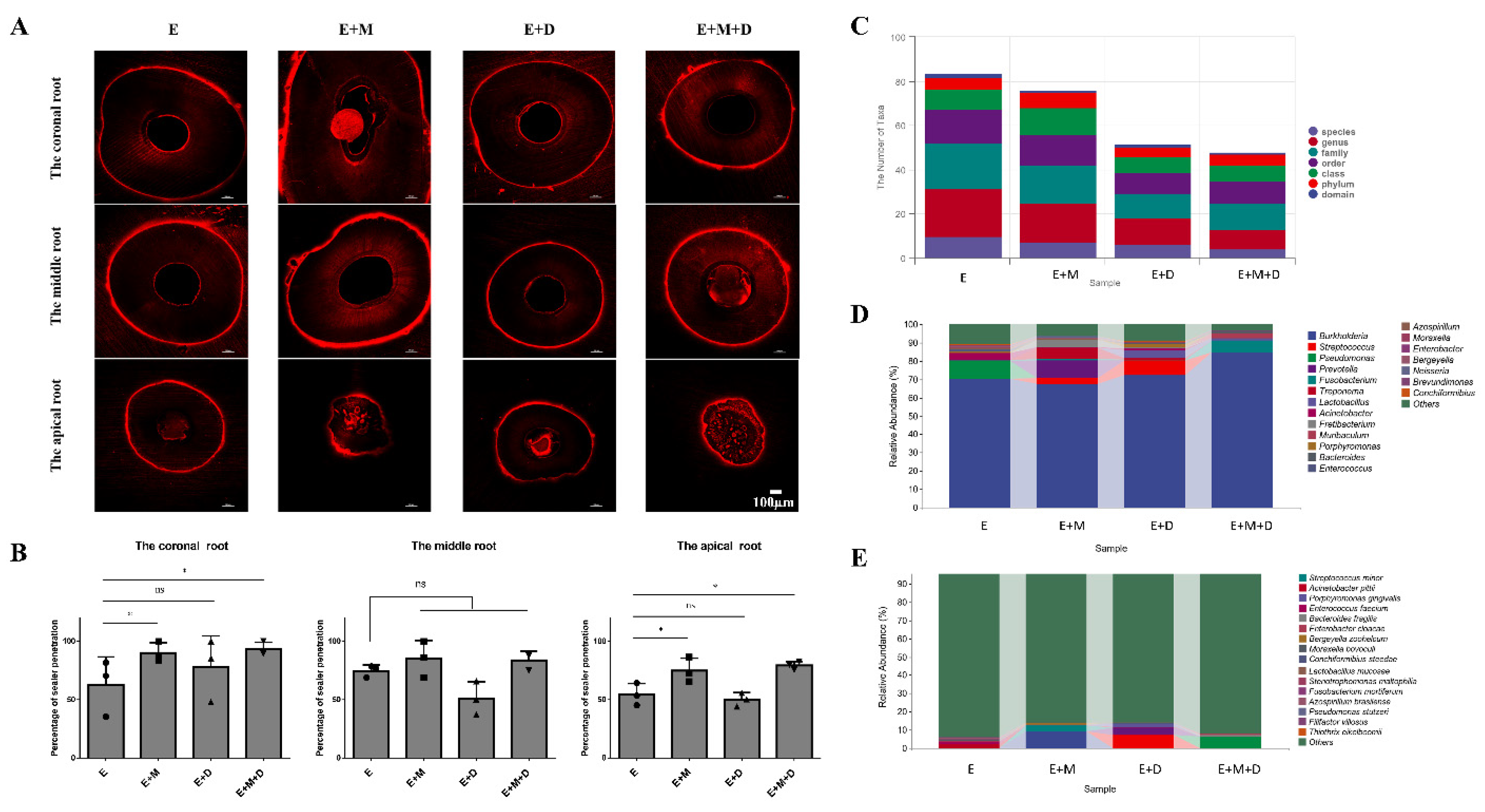
2.2. The Penetration Range of the Sealer
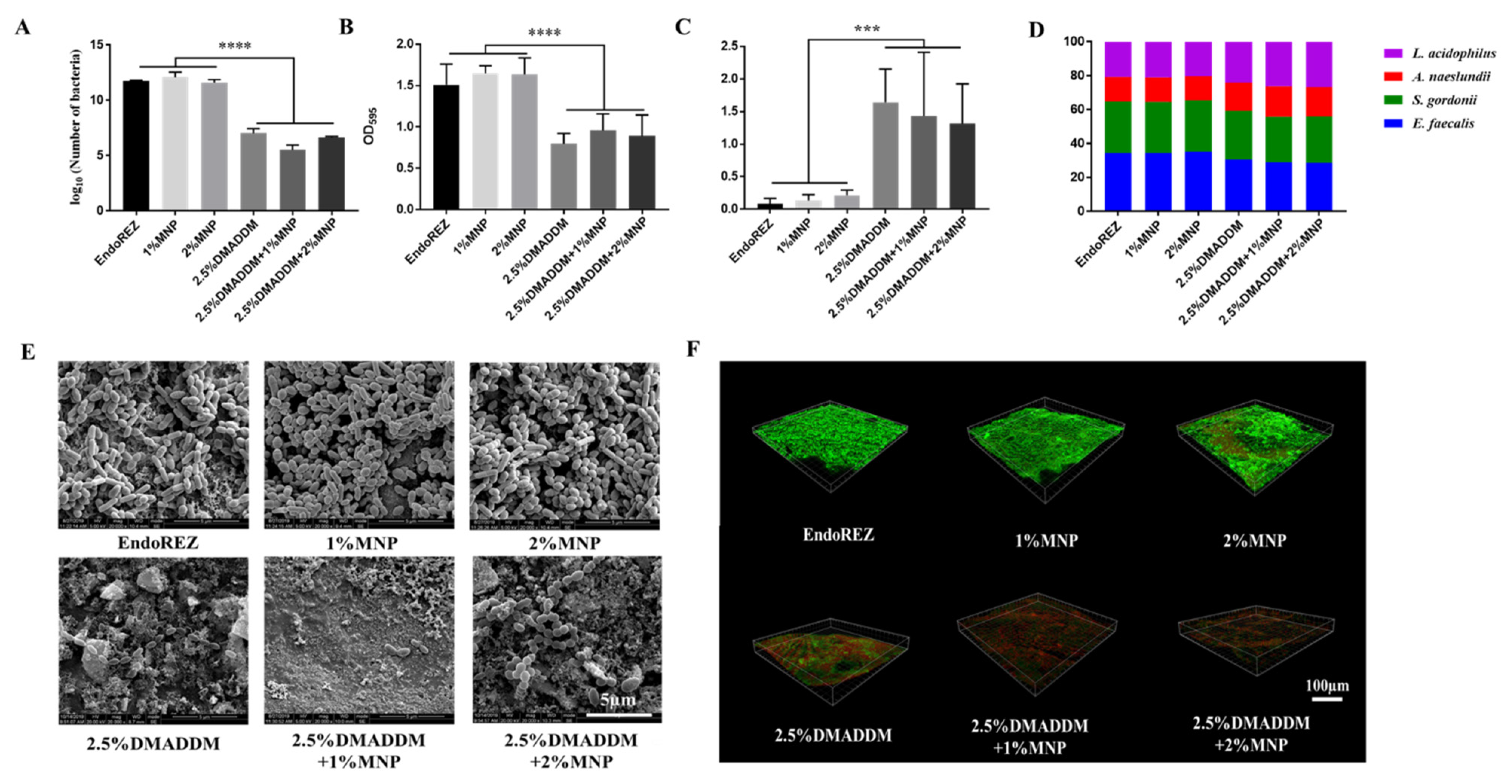
2.3. Antibacterial Effects on Multispecies Biofilms
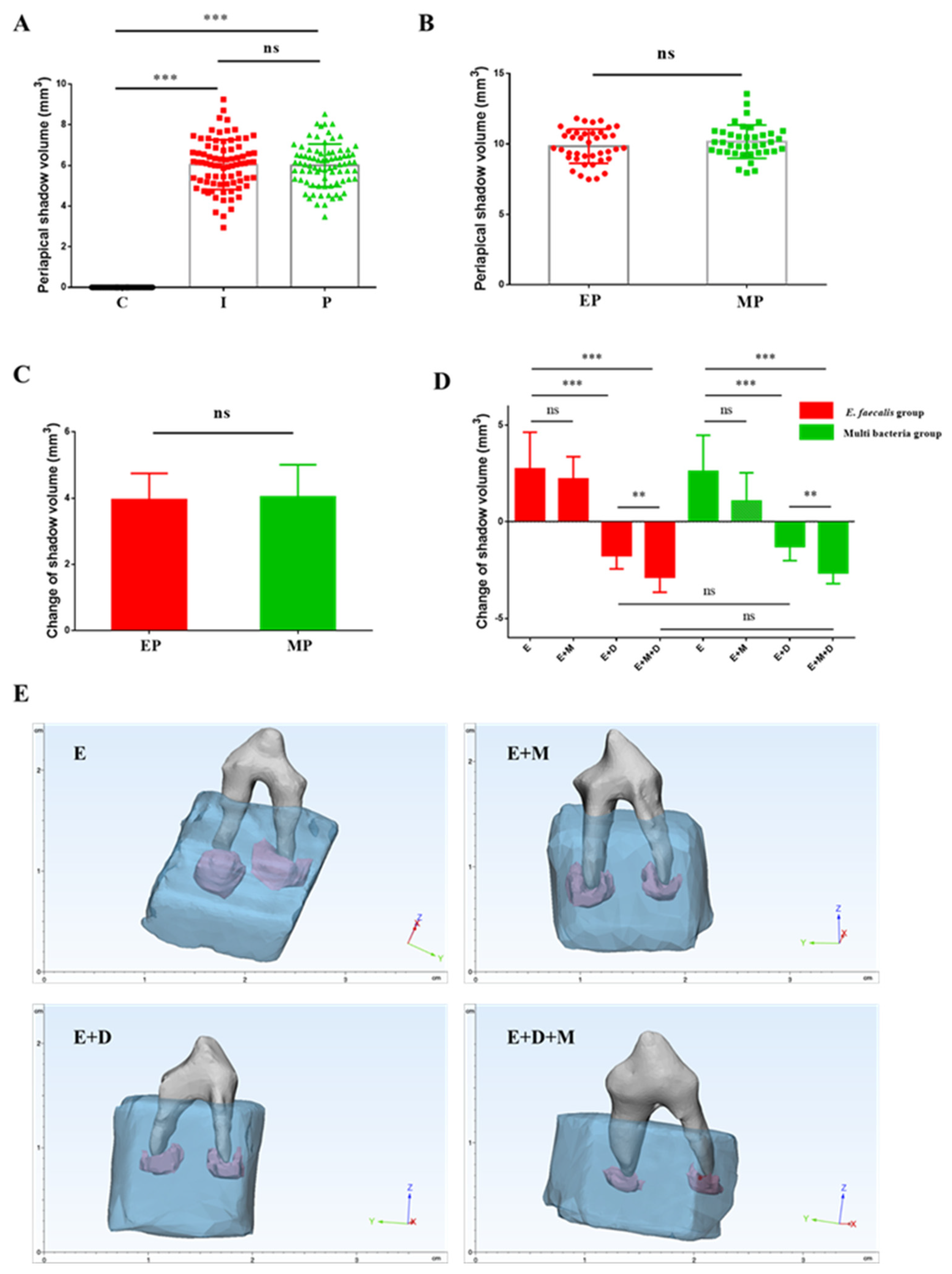
2.4. Radiolucent Zones in the Periapical Region
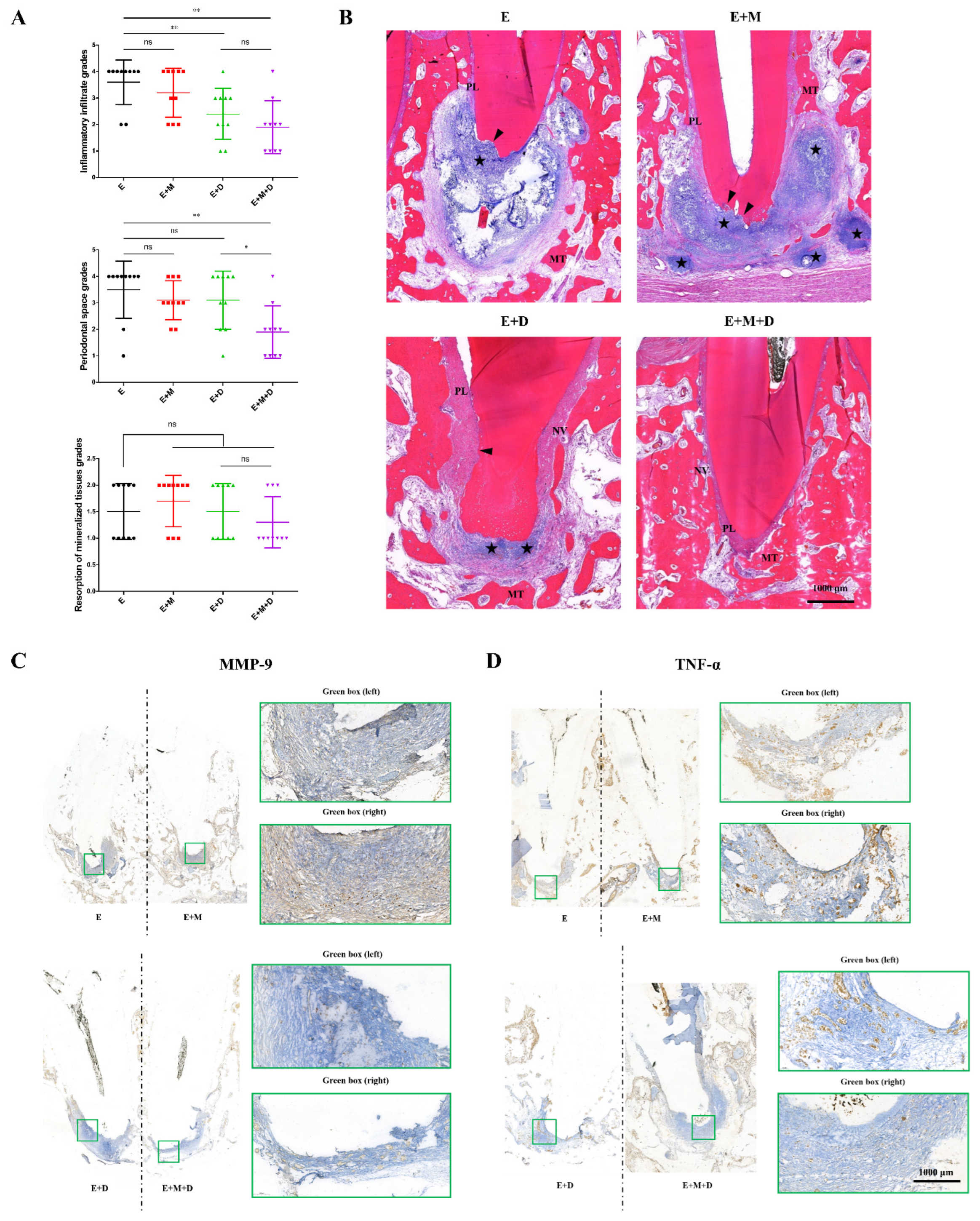
2.5. Histopathological Findings
2.6. Antibacterial Effects and the Penetration Range of the Sealer In Vivo
3. Discussion
4. Materials and Methods
4.1. Synthesis of DMADDM and Specimen Preparation
4.2. Cytotoxicity Test
4.3. Evaluation of Solubility
4.4. Root Canal Preparation and Apical Sealing Ability
4.5. Root Sectioning and Confocal Laser Scanning Microscopy Analysis of the Roots
4.6. Biofilm Formation
4.7. Colony-Forming Units (CFU) and Crystal Violet Assay
4.8. Biofilm Imaging
4.9. Animal Study
4.10. Samples Collection and 16S rRNA Genetic Sequencing
4.11. Hematoxylin-Eosin (HE) Staining
4.12. Immunohistochemical Staining
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laukkanen, E.; Vehkalahti, M.M.; Kotiranta, A.K. Impact of type of tooth on outcome of non-surgical root canal treatment. Clin. Oral Investig. 2019, 23, 4011–4018. [Google Scholar] [CrossRef]
- Nair, P.N.R. On the causes of persistent apical periodontitis: A review. Int. Endod. J. 2006, 39, 249–281. [Google Scholar] [CrossRef] [PubMed]
- Chivatxaranukul, P.; Dashper, S.G.; Messer, H.H. Dentinal tubule invasion and adherence by Enterococcus faecalis. Int. Endod. J. 2008, 41, 873–882. [Google Scholar] [CrossRef]
- Antunes, H.S.; Rôças, I.N.; Alves, F.R.; Siqueira, J.F., Jr. Total and Specific Bacterial Levels in the Apical Root Canal System of Teeth with Post-treatment Apical Periodontitis. J. Endod. 2015, 41, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M.; Dummer, P.M. A new system for classifying tooth, root and canal anomalies. Int. Endod. J. 2018, 51, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Fan, W.; Tay, F.R.; Fan, B. Micro–computed Tomographic Evaluation of the Prevalence, Distribution, and Morphologic Features of Accessory Canals in Chinese Permanent Teeth. J. Endod. 2019, 45, 994–999. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Haapasalo, M. Dentin Extends the Antibacterial Effect of Endodontic Sealers against Enterococcus faecalis Biofilms. J. Endod. 2014, 40, 505–508. [Google Scholar] [CrossRef]
- Ozcan, E.; Eldeniz, A.U.; Ari, H. Bacterial killing by several root filling materials and methods in an ex vivo infected root canal model. Int. Endod. J. 2011, 44, 1102–1109. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Dong, Y. In vitro study of dentinal tubule penetration and filling quality of bioceramic sealer. PLoS ONE 2018, 13, e0192248. [Google Scholar] [CrossRef]
- Mamootil, K.; Messer, H.H. Penetration of dentinal tubules by endodontic sealer cements in extracted teeth and in vivo. Int. Endod. J. 2007, 40, 873–881. [Google Scholar] [CrossRef]
- Sevimay, S.; Kalayci, A. Evaluation of apical sealing ability and adaptation to dentine of two resin-based sealers. J. Oral Rehabil. 2005, 32, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Loushine, R.J.; Monticelli, F.; Weller, R.N.; Breschi, L.; Ferrari, M.; Pashley, D.H. Effectiveness of Resin-Coated Gutta-Percha Cones and a Dual-Cured, Hydrophilic Methacrylate Resin-Based Sealer in Obturating Root Canals. J. Endod. 2005, 31, 659–664. [Google Scholar] [CrossRef]
- Oruçoğlu, H.; Sengun, A.; Yilmaz, N. Apical Leakage of Resin Based Root Canal Sealers with a New Computerized Fluid Filtration Meter. J. Endod. 2005, 31, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, S.; Zhou, X.; Xu, H.; Weir, M.; Ge, Y.; Li, M.; Li, Y.; Xu, X.; Zheng, L.; et al. Effect of Antibacterial Dental Adhesive on Multispecies Biofilms Formation. J. Dent. Res. 2015, 94, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Peng, X.; Ma, Y.; Hu, Y.; Wu, Y.; Lan, F.; Weir, M.D.; Li, M.; Ren, B.; Oates, T.W.; et al. Two-staged time-dependent materials for the prevention of implant-related infections. Acta Biomater. 2020, 101, 128–140. [Google Scholar] [CrossRef]
- Cheng, L.; Weir, M.D.; Zhang, K.; Arola, D.D.; Zhou, X.; Xu, H.H. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 345–355. [Google Scholar] [CrossRef]
- Liu, D.; Peng, X.; Wang, S.; Han, Q.; Li, B.; Zhou, X.; Ren, B.; Xu, H.H.K.; Weir, M.D.; Li, M.; et al. A novel antibacterial resin-based root canal sealer modified by Dimethylaminododecyl Methacrylate. Sci. Rep. 2019, 9, 10632. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, J.; Wang, Z.; Zhou, Y.; Lou, Z.; Chen, B.; Wang, P.; Guo, Z.; Tang, H.; Ma, J.; et al. Magnetic Cell–Scaffold Interface Constructed by Superparamagnetic IONP Enhanced Osteogenesis of Adipose-Derived Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 44279–44289. [Google Scholar] [CrossRef]
- Yun, H.-M.; Ahn, S.-J.; Park, K.-R.; Kim, M.-J.; Kim, J.-J.; Jin, G.-Z.; Kim, H.-W.; Kim, E.-C. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials 2016, 85, 88–98. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opin. Drug Deliv. 2018, 16, 69–78. [Google Scholar] [CrossRef]
- Shamsi, M.; Sedaghatkish, A.; Dejam, M.; Saghafian, M.; Mohammadi, M.; Sanati-Nezhad, A. Magnetically assisted intraperitoneal drug delivery for cancer chemotherapy. Drug Deliv. 2018, 25, 846–861. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, G.; Ren, B.; Gao, Y.; Peng, X.; Li, M.; H.K.Xu, H.; Han, Q.; Li, J.; Zhou, X.; et al. Effect of Antibacterial Root Canal Sealer on Persistent Apical Periodontitis. Antibiotics 2021, 10, 741. [Google Scholar] [CrossRef] [PubMed]
- Flores, D.S.H.; Versiani, M.A.; Guedes, D.F.C.; Pécora, J.D.; Rached-Junior, F.J.A.; Sousa-Neto, M.D. Evaluation of physicochemical properties of four root canal sealers. Int. Endod. J. 2010, 44, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, E.; Bering, N.; Bürklein, S. Selected physicochemical properties of AH Plus, EndoREZ and RealSeal SE root canal sealers. Odontology 2013, 103, 61–65. [Google Scholar] [CrossRef]
- ISO 6876; International Organization for Standardization Dentistry (ISO). Root Canal Sealing Materials. British Standards Institution: London, UK, 2002.
- Zhang, W.; Li, Z.; Peng, B. Assessment of a new root canal sealer’s apical sealing ability. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2009, 107, e79–e82. [Google Scholar] [CrossRef] [PubMed]
- Cotti, E.; Petreucic, V.; Re, D.; Simbula, G. Cytotoxicity Evaluation of a New Resin-based Hybrid Root Canal Sealer: An In Vitro Study. J. Endod. 2014, 40, 124–128. [Google Scholar] [CrossRef]
- Fonseca, D.A.; Paula, A.B.; Marto, C.M.; Coelho, A.; Paulo, S.; Martinho, J.P.; Carrilho, E.; Ferreira, M.M. Biocompatibility of Root Canal Sealers: A Systematic Review of In Vitro and In Vivo Studies. Materials 2019, 12, 4113. [Google Scholar] [CrossRef]
- Jeong, J.W.; DeGraft-Johnson, A.; Dorn, S.O.; Di Fiore, P.M. Dentinal Tubule Penetration of a Calcium Silicate–based Root Canal Sealer with Different Obturation Methods. J. Endod. 2017, 43, 633–637. [Google Scholar] [CrossRef]
- Eymirli, A.; Sungur, D.D.; Uyanik, O.; Purali, N.; Nagas, E.; Cehreli, Z.C. Dentinal Tubule Penetration and Retreatability of a Calcium Silicate–based Sealer Tested in Bulk or with Different Main Core Material. J. Endod. 2019, 45, 1036–1040. [Google Scholar] [CrossRef]
- Patri, G.; Agrawal, P.; Anushree, N.; Arora, S.; Kunjappu, J.J.; Shamsuddin, S.V. A Scanning Electron Microscope Analysis of Sealing Potential and Marginal Adaptation of Different Root Canal Sealers to Dentin: An In Vitro study. J. Contemp. Dent. Pract. 2020, 21, 73–77. [Google Scholar] [CrossRef]
- de Paz, L.E.C. Development of a Multispecies Biofilm Community by Four Root Canal Bacteria. J. Endod. 2012, 38, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rôças, I.N.; Ricucci, D.; Hülsmann, M. Causes and management of post-treatment apical periodontitis. Br. Dent. J. 2014, 216, 305–312. [Google Scholar] [CrossRef]
- Vatkar, N.A.; Hegde, V.; Sathe, S. Vitality of Enterococcus faecalis inside dentinal tubules after five root canal disinfection methods. J. Conserv. Dent. 2016, 19, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Mokashi, P.; Shah, J.; Chandrasekhar, P.; Kulkarniv, G.P.; Podar, R.; Singh, S. Comparison of the penetration depth of five root canal sealers: A confocal laser scanning microscopic study. J. Conserv. Dent. 2021, 24, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.F.; Khan, A.A.; Silva, R.M.; Kang, M.K. Genetic and Epigenetic Characterization of Pulpal and Periapical Inflammation. Front. Physiol. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, Y.; Zhu, X.; Zhang, C.; Li, S.; Jin, L.; Shen, Y.; Haapasalo, M. Role of Polymorphonuclear Neutrophils in the Clearance of Enterococcus faecalis Derived from Saliva and Infected Root Canals. J. Endod. 2011, 37, 346–352. [Google Scholar] [CrossRef]
- Ayre, W.N.; Melling, G.; Cuveillier, C.; Natarajan, M.; Roberts, J.L.; Marsh, L.L.; Lynch, C.D.; Maillard, J.-Y.; Denyer, S.P.; Sloan, A.J. Enterococcus faecalis Demonstrates Pathogenicity through Increased Attachment in an Ex Vivo Polymicrobial Pulpal Infection. Infect. Immun. 2018, 86, e00871-1. [Google Scholar] [CrossRef]
- Sorsa, T.; Tjäderhane, L.; Salo, T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004, 10, 311–318. [Google Scholar] [CrossRef]
- Wan, C.-Y.; Li, L.; Liu, L.-S.; Jiang, C.-M.; Zhang, H.-Z.; Wang, J.-X. Expression of Matrix Metalloproteinases and Tissue Inhibitor of Matrix Metalloproteinases during Apical Periodontitis Development. J. Endod. 2021, 47, 1118–1125. [Google Scholar] [CrossRef]
- Accorsi-Mendonça, T.; Silva, E.J.N.L.; Marcaccini, A.M.; Gerlach, R.F.; Duarte, K.M.R.; Pardo, A.P.S.; Line, S.R.P.; Zaia, A.A. Evaluation of Gelatinases, Tissue Inhibitor of Matrix Metalloproteinase-2, and Myeloperoxidase Protein in Healthy and Inflamed Human Dental Pulp Tissue. J. Endod. 2013, 39, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Belmar, M.J.; Pabst, C.; Martínez, B.; Hernández, M. Gelatinolytic activity in gingival crevicular fluid from teeth with periapical lesions. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontology 2008, 105, 801–806. [Google Scholar] [CrossRef]
- Pereira, M.F.; Pires, F.R.; Armada, L.; Ferreira, D.D.C.; Carrouel, F.; Bourgeois, D.; Gonçalves, L.S. Expression of Inflammatory Markers RANK, MMP-9 and PTHrP in Chronic Apical Periodontitis from People Living with HIV Undergoing Antiretroviral Therapy. J. Clin. Med. 2020, 9, 3611. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rôças, I.N. Clinical Implications and Microbiology of Bacterial Persistence after Treatment Procedures. J. Endod. 2008, 34, 1291–1301.E3. [Google Scholar] [CrossRef]
- Stauffacher, S.; Lussi, A.; Nietzsche, S.; Neuhaus, K.W.; Eick, S. Bacterial invasion into radicular dentine—An in vitro study. Clin. Oral Investig. 2017, 21, 1743–1752. [Google Scholar] [CrossRef]
- Subramanian, K.; Mickel, A.K. Molecular Analysis of Persistent Periradicular Lesions and Root Ends Reveals a Diverse Microbial Profile. J. Endod. 2009, 35, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Du, J.; Peng, Z. Correlation between Enterococcus faecalis and Persistent Intraradicular Infection Compared with Primary Intraradicular Infection: A Systematic Review. J. Endod. 2015, 41, 1207–1213. [Google Scholar] [CrossRef]
- Kakoli, P.; Nandakumar, R.; Romberg, E.; Arola, D.; Fouad, A.F. The Effect of Age on Bacterial Penetration of Radicular Dentin. J. Endod. 2009, 35, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Brosco, V.H.; Bernardineli, N.; Torres, S.A.; Consolaro, A.; Bramante, C.M.; De Moraes, I.G.; Garcia, R.B. Bacterial leakage in root canals obturated by different techniques. Part 1: Microbiologic evaluation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2008, 105, e48–e53. [Google Scholar] [CrossRef]
- Wang, L.; Dong, M.; Zheng, J.; Song, Q.; Yin, W.; Li, J.; Niu, W. Relationship of Biofilm Formation and gelE Gene Expression in Enterococcus faecalis Recovered from Root Canals in Patients Requiring Endodontic Retreatment. J. Endod. 2011, 37, 631–636. [Google Scholar] [CrossRef]
- Taglialegna, A.; Matilla-Cuenca, L.; Dorado-Morales, P.; Navarro, S.; Ventura, S.; Garnett, J.A.; Lasa, I.; Valle, J. The biofilm-associated surface protein Esp of Enterococcus faecalis forms amyloid-like fibers. Npj Biofilms Microbiomes 2020, 6, 15. [Google Scholar] [CrossRef]
- Esteban-Tejeda, L.; Cabal, B.; Torrecillas, R.; Prado, C.; Fernandez-Garcia, E.; López-Piriz, R.; Quintero, F.; Pou, J.; Penide, J.; Moya, J.S. Antimicrobial activity of submicron glass fibres incorporated as a filler to a dental sealer. Biomed. Mater. 2016, 11, 045014. [Google Scholar] [CrossRef]
- Marcuello, C.; Chambel, L.; Rodrigues, M.S.; Ferreira, L.P.; Cruz, M.M. Magnetotactic Bacteria: Magnetism beyond Magnetosomes. IEEE Trans. Nanobiosci. 2018, 17, 555–559. [Google Scholar] [CrossRef]
- Xue, X.; Hanna, K.; Deng, N. Fenton-like oxidation of Rhodamine B in the presence of two types of iron (II, III) oxide. J. Hazard. Mater. 2009, 166, 407–414. [Google Scholar] [CrossRef]
- Tampieri, A.; Landi, E.; Valentini, F.; Sandri, M.; D’Alessandro, T.; Dediu, V.; Marcacci, M. A conceptually new type of bio-hybrid scaffold for bone regeneration. Nanotechnology 2011, 22, 015104. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Sun, Y.; Wang, Z.; Ren, B.; Xu, H.H.K.; Peng, X.; Li, M.; Wang, S.; Wang, H.; Wu, Y.; et al. The Preventive Effect of A Magnetic Nanoparticle-Modified Root Canal Sealer on Persistent Apical Periodontitis. Int. J. Mol. Sci. 2022, 23, 13137. https://doi.org/10.3390/ijms232113137
Guo X, Sun Y, Wang Z, Ren B, Xu HHK, Peng X, Li M, Wang S, Wang H, Wu Y, et al. The Preventive Effect of A Magnetic Nanoparticle-Modified Root Canal Sealer on Persistent Apical Periodontitis. International Journal of Molecular Sciences. 2022; 23(21):13137. https://doi.org/10.3390/ijms232113137
Chicago/Turabian StyleGuo, Xiao, Yan Sun, Zheng Wang, Biao Ren, Hockin H. K. Xu, Xian Peng, Mingyun Li, Suping Wang, Haohao Wang, Yao Wu, and et al. 2022. "The Preventive Effect of A Magnetic Nanoparticle-Modified Root Canal Sealer on Persistent Apical Periodontitis" International Journal of Molecular Sciences 23, no. 21: 13137. https://doi.org/10.3390/ijms232113137
APA StyleGuo, X., Sun, Y., Wang, Z., Ren, B., Xu, H. H. K., Peng, X., Li, M., Wang, S., Wang, H., Wu, Y., Weir, M. D., Zhou, X., Lan, F., & Cheng, L. (2022). The Preventive Effect of A Magnetic Nanoparticle-Modified Root Canal Sealer on Persistent Apical Periodontitis. International Journal of Molecular Sciences, 23(21), 13137. https://doi.org/10.3390/ijms232113137








