The Shift in Synonymous Codon Usage Reveals Similar Genomic Variation during Domestication of Asian and African Rice
Abstract
1. Introduction
2. Results
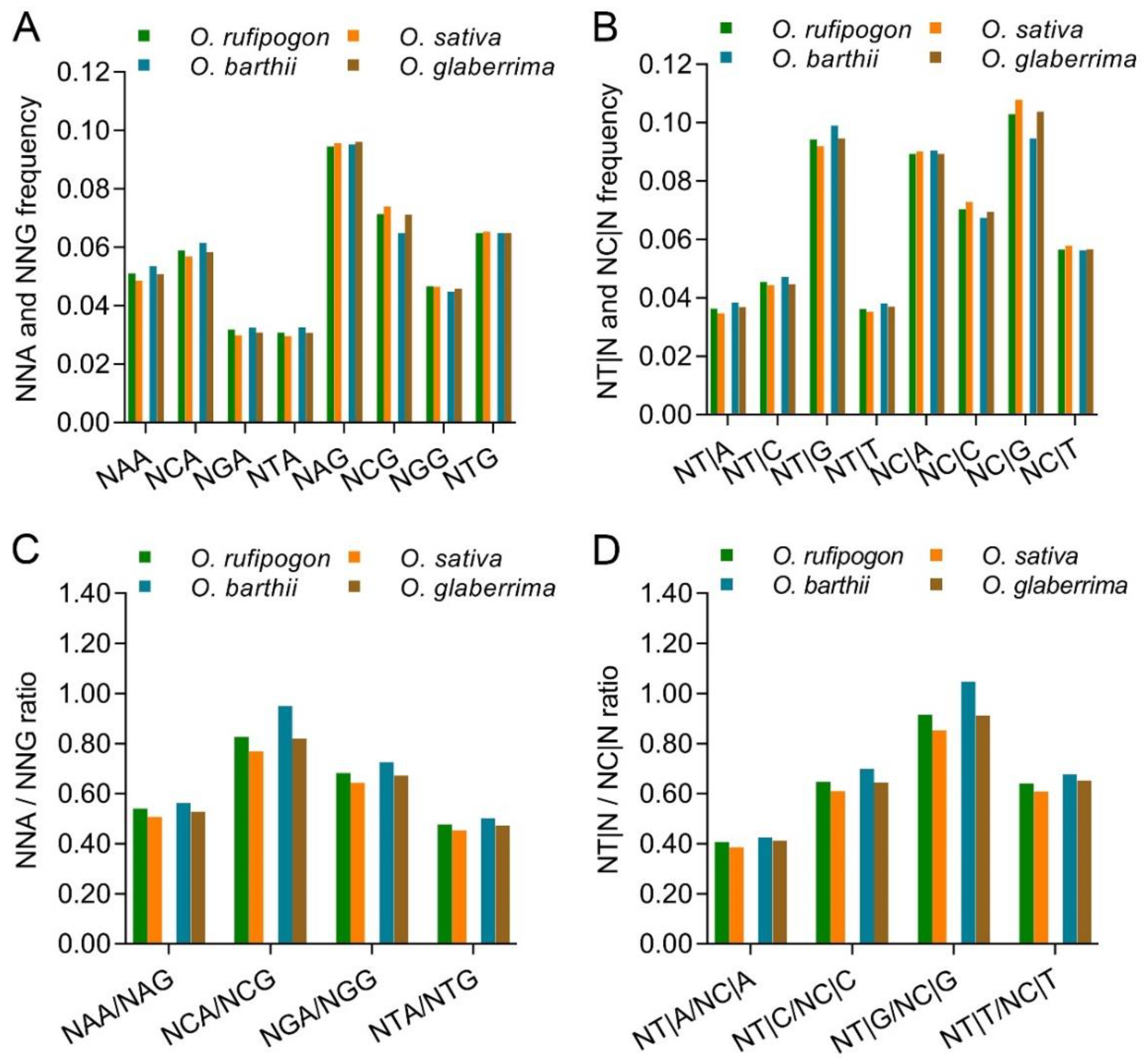
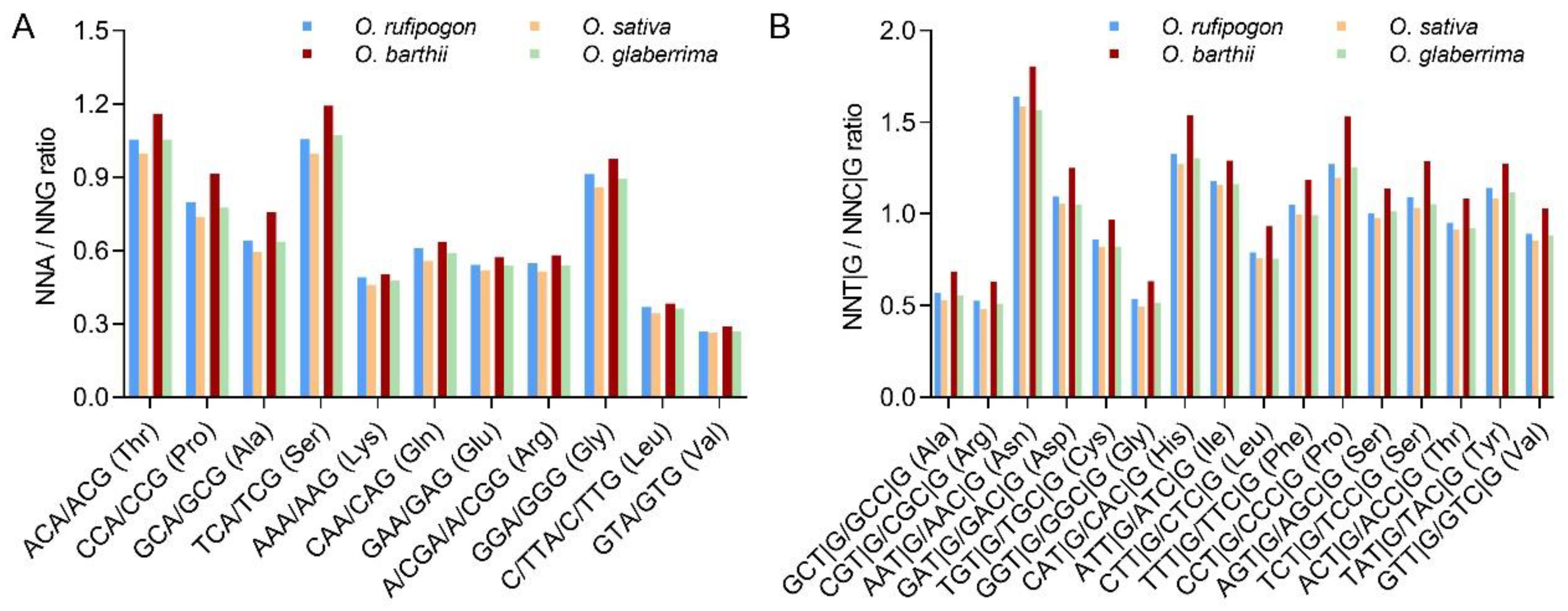
2.1. C/G-Ending Synonymous Codons Are Preferred in Cultivated Rice
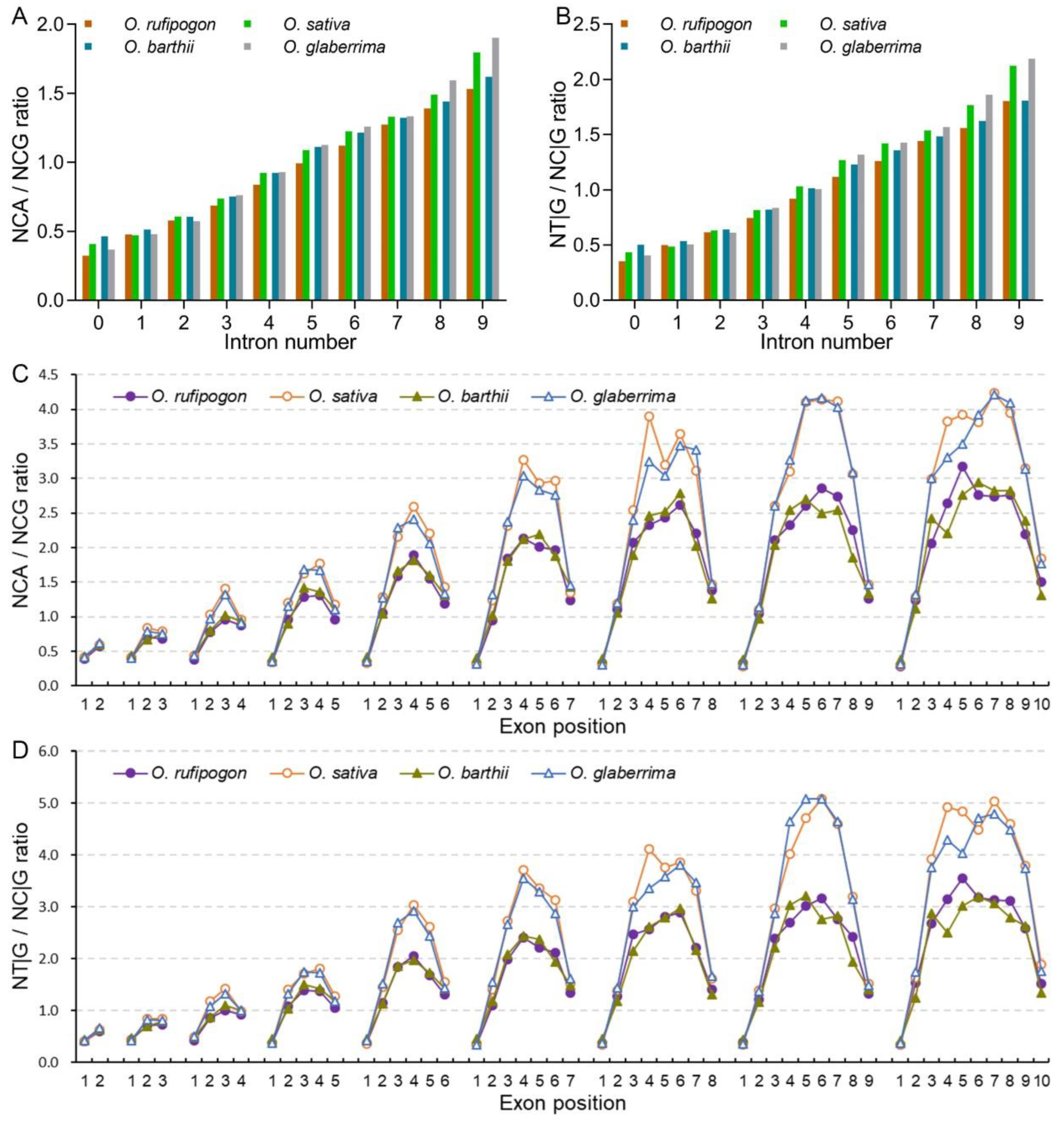
2.2. Cultivated Rice Exhibits Stronger Bias to A/T-Ending Synonymous Codons Following the Rise in Intron Number
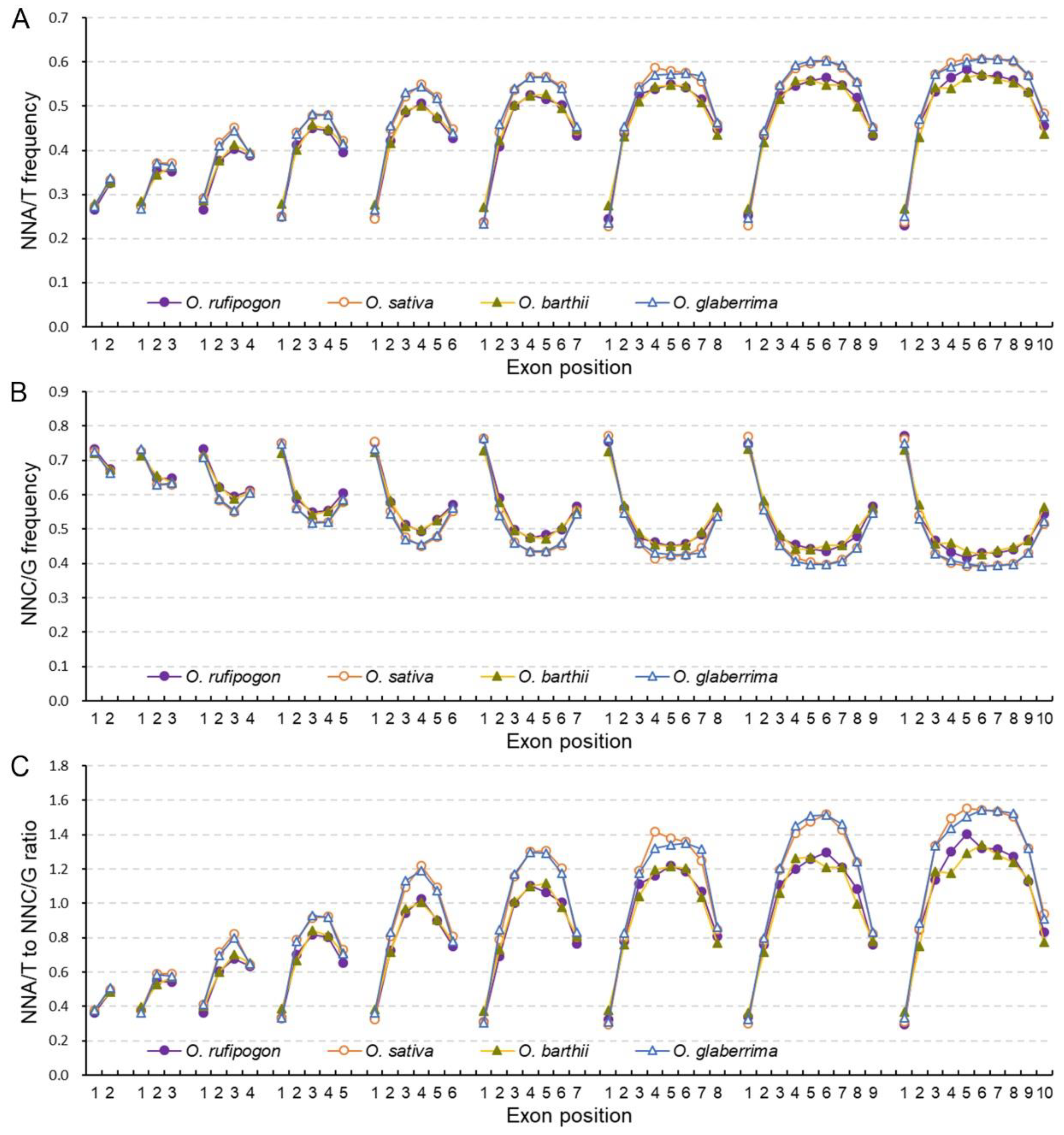
2.3. Cultivated Rice Has Stronger Bias to A/T-Ending Codons in Internal Exons
2.4. SCUB Shift in Cultivated Rice Is Associated with DNA Methylation-Mediated Conversion of Cytosine to Thymine
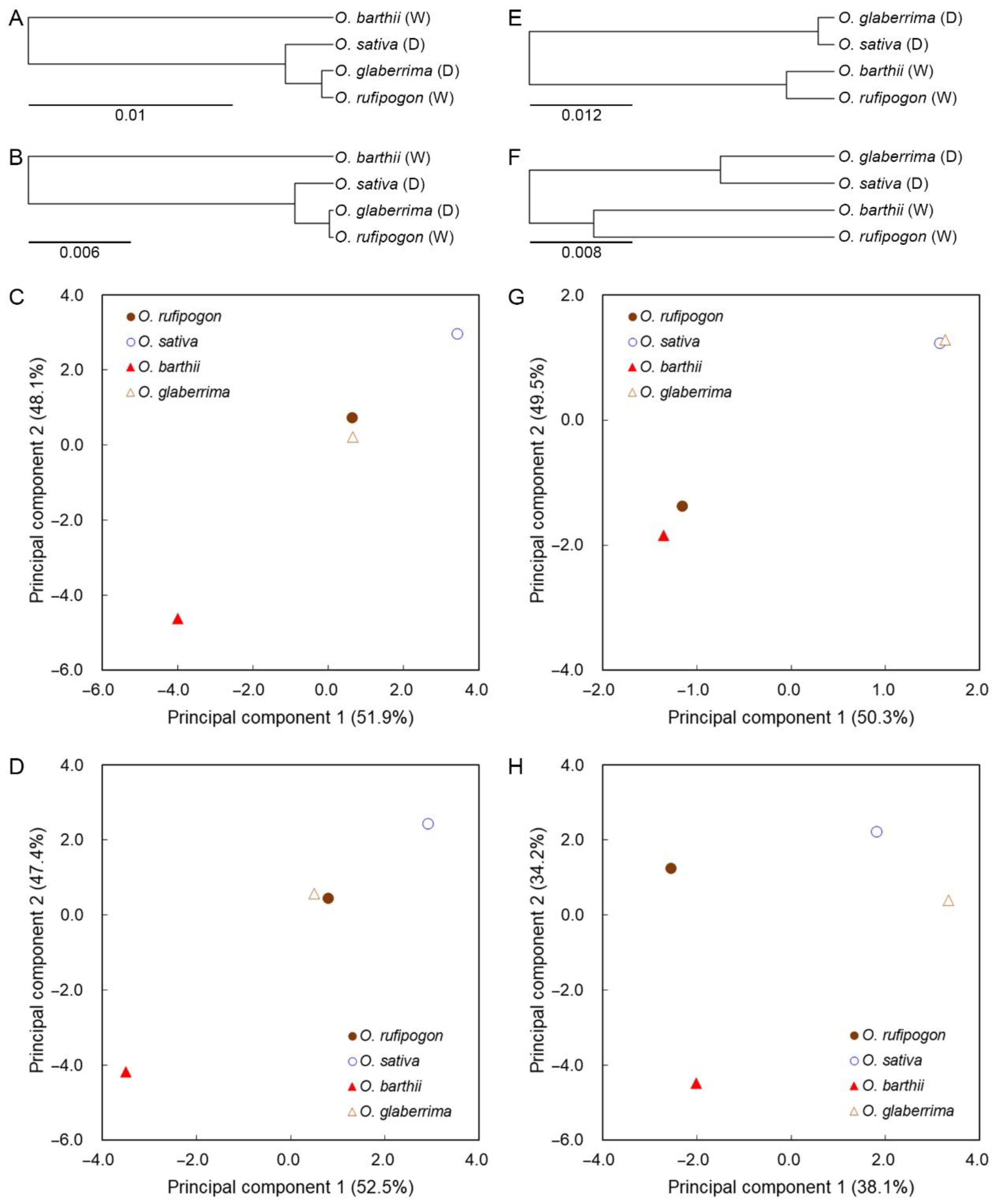
2.5. SCUB Mirrors the Effect of Domestication
3. Discussion
4. Materials and Methods
4.1. Genome Sequences and Codon Count
4.2. Calculation of SCUB Indices
4.3. Calculation of SCUB Frequency
4.4. Cluster Analysis and Principal Component Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuller, D.; Sato, Y.; Castillo, C.; Qin, L.; Weisskopf, A.; Kingwell Banham, E.; Song, J.; Ahn, S.; Van Etten, J. Consilience of genetics and archaeobotany in the entangled history of rice. Archaeol. Anthropol. Sci. 2010, 2, 115–131. [Google Scholar] [CrossRef]
- Wing, R.A.; Purugganan, M.D.; Zhang, Q. The rice genome revolution: From an ancient grain to Green Super Rice. Nat. Rev. Genet. 2018, 19, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kurata, N.; Wei, X.; Wang, Z.-X.; Wang, A.; Zhao, Q.; Zhao, Y.; Liu, K.; Lu, H.; Li, W.; et al. A map of rice genome variation reveals the origin of cultivated rice. Nature 2012, 490, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Feng, Q.; Lu, H.; Li, Y.; Wang, A.; Tian, Q.; Zhan, Q.; Lu, Y.; Zhang, L.; Huang, T.; et al. Pan-genome analysis highlights the extent of genomic variation in cultivated and wild rice. Nat. Genet. 2018, 50, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Liao, Y.; Toivainen, T.; Lv, Y.; Tian, X.; Emerson, J.J.; Gaut, B.S.; Zhou, Y. Evolutionary Genomics of Structural Variation in Asian Rice (Oryza sativa) Domestication. Mol. Biol. Evol. 2020, 37, 3507–3524. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, A.; Sang, T. Rice domestication by reducing shattering. Science 2006, 311, 1936–1939. [Google Scholar] [CrossRef]
- Nei, M.; Gojobori, T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 1986, 3, 418–426. [Google Scholar]
- King, J.; Jukes, T. Non-Darwinian evolution. Science 1969, 165, 788–798. [Google Scholar] [CrossRef]
- Marais, G.; Mouchiroud, D.; Duret, L. Does recombination improve selection on codon usage? Lessons from nematode and fly complete genomes. Proc. Natl. Acad. Sci. USA 2001, 98, 5688–5692. [Google Scholar] [CrossRef]
- Warnecke, T.; Hurst, L. Evidence for a trade-off between translational efficiency and splicing regulation in determining synonymous codon usage in Drosophila melanogaster. Mol. Biol. Evol. 2007, 24, 2755–2762. [Google Scholar] [CrossRef]
- Zhang, G.; Hubalewska, M.; Ignatova, Z. Transient ribosomal attenuation coordinates protein synthesis and co-translational folding. Nat. Struct. Mol. Biol. 2009, 16, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Presnyak, V.; Alhusaini, N.; Chen, Y.; Martin, S.; Morris, N.; Kline, N.; Olson, S.; Weinberg, D.; Baker, K.; Graveley, B.; et al. Codon optimality is a major determinant of mRNA stability. Cell 2015, 160, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Tuller, T.; Carmi, A.; Vestsigian, K.; Navon, S.; Dorfan, Y.; Zaborske, J.; Pan, T.; Dahan, O.; Furman, I.; Pilpel, Y. An evolutionarily conserved mechanism for controlling the efficiency of protein translation. Cell 2010, 141, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Song, S.; Li, C.; Zhang, J. Synonymous mutations in representative yeast genes are mostly strongly non-neutral. Nature 2022, 606, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Akashi, H.; Eyre-Walker, A. Translational selection and molecular evolution. Curr. Opin. Genet. Dev. 1998, 8, 688–893. [Google Scholar] [CrossRef]
- Akashi, H. Gene expression and molecular evolution. Curr. Opin. Genet. Dev. 2001, 11, 660–666. [Google Scholar] [CrossRef]
- Guo, F.B.; Yuan, J.B. Codon usages of genes on chromosome, and surprisingly, genes in plasmid are primarily affected by strand-specific mutational biases in Lawsonia intracellularis. DNA Res. 2009, 16, 91–104. [Google Scholar] [CrossRef]
- Wang, Z.; Lucas, F.; Qiu, P.; Liu, Y. Improving the sensitivity of sample clustering by leveraging gene co-expression networks in variable selection. BMC Bioinform. 2014, 15, 153. [Google Scholar] [CrossRef]
- Knowles, D.G.; McLysaght, A. High Rate of Recent Intron Gain and Loss in Simultaneously Duplicated Arabidopsis Genes. Mol. Biol. Evol. 2006, 23, 1548–1557. [Google Scholar] [CrossRef]
- Sharpton, T.J.; Neafsey, D.E.; Galagan, J.E.; Taylor, J.W. Mechanisms of intron gain and loss in Cryptococcus. Genome Biol. 2008, 9, R24. [Google Scholar] [CrossRef]
- Tarrío, R.; Ayala, F.J.; Rodríguez-Trelles, F. Alternative splicing: A missing piece in the puzzle of intron gain. Proc. Natl. Acad. Sci. USA 2008, 105, 7223–7228. [Google Scholar] [CrossRef] [PubMed]
- Stoltzfus, A. Molecular Evolution: Introns Fall into Place. Curr. Biol. 2004, 14, R351–R352. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Trelles, F.; Tarrío, R.; Ayala, F.J. Origins and Evolution of Spliceosomal Introns. Annu. Rev. Genet. 2006, 40, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Weng, M.-L.; Ruhlman, T.A.; Jansen, R.K. Extensive variation in nucleotide substitution rate and gene/intron loss in mitochondrial genomes of Pelargonium. Mol. Phylogenet. Evol. 2021, 155, 106986. [Google Scholar] [CrossRef]
- Tian, D.; Wang, Q.; Zhang, P.; Araki, H.; Yang, S.; Kreitman, M.; Nagylaki, T.; Hudson, R.; Bergelson, J.; Chen, J.-Q. Single-nucleotide mutation rate increases close to insertions/deletions in eukaryotes. Nature 2008, 455, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Q.; Wu, Y.; Yang, H.; Bergelson, J.; Kreitman, M.; Tian, D. Variation in the Ratio of Nucleotide Substitution and Indel Rates across Genomes in Mammals and Bacteria. Mol. Biol. Evol. 2009, 26, 1523–1531. [Google Scholar] [CrossRef]
- Hershberg, R.; Petrov, D.A. Selection on Codon Bias. Annu. Rev. Genet. 2008, 42, 287–299. [Google Scholar] [CrossRef]
- Coulombe-Huntington, J.; Majewski, J. Characterization of intron loss events in mammals. Genome Res. 2007, 17, 23–32. [Google Scholar] [CrossRef]
- Qin, Z.; Cai, Z.; Xia, G.; Wang, M. Synonymous codon usage bias is correlative to intron number and shows disequilibrium among exons in plants. BMC Genom. 2013, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, Y.; Li, Y.; Liu, C.; Wang, Y.; Xia, G.; Wang, M. Asymmetric Somatic Hybridization Affects Synonymous Codon Usage Bias in Wheat. Front. Genet. 2021, 12, 682324. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Hu, F.; Ge, S.; Ye, M.; Xiang, H.; Zhang, G.; Zheng, X.; Zhang, H.; Zhang, S.; et al. Single-base resolution maps of cultivated and wild rice methylomes and regulatory roles of DNA methylation in plant gene expression. BMC Genom. 2012, 13, 300. [Google Scholar] [CrossRef] [PubMed]
- Ossowski, S.; Schneeberger, K.; Lucas-Lledó, J.I.; Warthmann, N.; Clark, R.M.; Shaw, R.G.; Weigel, D.; Lynch, M. The Rate and Molecular Spectrum of Spontaneous Mutations in Arabidopsis thaliana. Science 2010, 327, 92–94. [Google Scholar] [CrossRef]
- Nabel, C.S.; Manning, S.A.; Kohli, R.M. The Curious Chemical Biology of Cytosine: Deamination, Methylation, and Oxidation as Modulators of Genomic Potential. ACS Chem. Biol. 2012, 7, 20–30. [Google Scholar] [CrossRef]
- Bernardi, G. Codon usage and genome composition. J. Mol. Evol. 1985, 22, 363–365. [Google Scholar] [CrossRef]
- Knight, R.D.; Freeland, S.J.; Landweber, L.F. A simple model based on mutation and selection explains trends in codon and amino-acid usage and GC composition within and across genomes. Genome Biol. 2001, 2, RESEARCH0010. [Google Scholar] [PubMed]
- Zhang, Z.; Yu, J. Modeling compositional dynamics based on GC and purine contents of protein-coding sequences. Biol. Direct. 2010, 5, 63. [Google Scholar] [CrossRef]
- Hanson, G.; Coller, J. Codon optimality, bias and usage in translation and mRNA decay. Nat. Rev. Mol. Cell Biol. 2018, 19, 20–30. [Google Scholar] [CrossRef]
- Galtier, N.; Piganeau, G.; Mouchiroud, D.; Duret, L. GC-content evolution in mammalian genomes: The biased gene conversion hypothesis. Genetics 2001, 159, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, J.B.; Kudla, G. Synonymous but not the same: The causes and consequences of codon bias. Nat. Rev. Genet. 2011, 12, 32–42. [Google Scholar] [CrossRef]
- Fawcett, J.A.; Rouzé, P.; Van de Peer, Y. Higher intron loss rate in Arabidopsis thaliana than A. lyrata is consistent with stronger selection for a smaller genome. Mol. Biol. Evol. 2012, 29, 849–859. [Google Scholar] [CrossRef]
- Singh, N.D.; Arndt, P.F.; Petrov, D.A. Genomic Heterogeneity of Background Substitutional Patterns in Drosophila melanogaster. Genetics 2005, 169, 709–722. [Google Scholar] [CrossRef]
- Xing, Y.; Lee, C. Alternative splicing and RNA selection pressure—Evolutionary consequences for eukaryotic genomes. Nat. Rev. Genet. 2006, 7, 499–509. [Google Scholar] [CrossRef]
- Bernardi, G. Isochores and the evolutionary genomics of vertebrates. Gene 2000, 241, 3–17. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, X.; Yuan, H.; Araki, H.; Wang, J.; Tian, D. The pattern of insertion/deletion polymorphism in Arabidopsis thaliana. Mol. Genet. Genom. 2008, 280, 351–361. [Google Scholar] [CrossRef]
- Laird, P.W. Principles and challenges of genome-wide DNA methylation analysis. Nat. Rev. Genet. 2010, 11, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Bewick, A.J.; Schmitz, R.J. Gene body DNA methylation in plants. Curr. Opin. Plant Biol. 2017, 36, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Mutti, J.S.; Bhullar, R.K.; Gill, K.S. Evolution of Gene Expression Balance Among Homeologs of Natural Polyploids. G3 Genes Genomes Genet. 2017, 7, 1225–1237. [Google Scholar] [CrossRef][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, G.; Zhou, J.; Huo, Z.; Wu, T.; Li, Y.; Li, Y.; Wang, Y.; Wang, M. The Shift in Synonymous Codon Usage Reveals Similar Genomic Variation during Domestication of Asian and African Rice. Int. J. Mol. Sci. 2022, 23, 12860. https://doi.org/10.3390/ijms232112860
Xiao G, Zhou J, Huo Z, Wu T, Li Y, Li Y, Wang Y, Wang M. The Shift in Synonymous Codon Usage Reveals Similar Genomic Variation during Domestication of Asian and African Rice. International Journal of Molecular Sciences. 2022; 23(21):12860. https://doi.org/10.3390/ijms232112860
Chicago/Turabian StyleXiao, Guilian, Junzhi Zhou, Zhiheng Huo, Tong Wu, Yingchun Li, Yajing Li, Yanxia Wang, and Mengcheng Wang. 2022. "The Shift in Synonymous Codon Usage Reveals Similar Genomic Variation during Domestication of Asian and African Rice" International Journal of Molecular Sciences 23, no. 21: 12860. https://doi.org/10.3390/ijms232112860
APA StyleXiao, G., Zhou, J., Huo, Z., Wu, T., Li, Y., Li, Y., Wang, Y., & Wang, M. (2022). The Shift in Synonymous Codon Usage Reveals Similar Genomic Variation during Domestication of Asian and African Rice. International Journal of Molecular Sciences, 23(21), 12860. https://doi.org/10.3390/ijms232112860





