Abstract
It is now widely accepted that NK cells can acquire memory, and this makes them more effective to protect against some pathogens. Prior reports indicate memory-like NK cells (mlNKs) in murine model of Mycobacterium tuberculosis (Mtb) as well as in healthy individuals with latent TB infection (LTBI). The increased expression of CD226 was evident in mlNKs from LTBI+ people after stimulation with γ-irradiated Mtb (γ-Mtb). We thus evaluated the contribution of costimulatory CD226 signaling in the functionality of mlNKs in LTBI+ people. We found that blockade of CD226 signaling using the antibody- or CRISPR/Cas9-mediated deletion of the CD226 gene in NK cells diminished the proliferation of mlNKs from LTBI+ people. Blocking CD226 signaling also reduced the phosphorylation of FOXO1 and cMyc expression. Additionally, cMyc inhibition using a chemical inhibitor reduced proliferation by mlNKs from LTBI+ people. Moreover, blocking CD226 signaling reduced glycolysis in NK cells, and the inhibition of glycolysis led to reduced effector function of mlNKs from LTBI+ people. Overall, our results provide a role for CD226 signaling in mlNK responses to Mtb.
1. Introduction
Natural killer (NK) cells contribute to antimicrobial defense and antitumor immunity [1,2,3,4,5]. NK cells perform this function via the secretion of cytokines such as IFN-γ, TNF-α or by the perforin-granzyme-mediated lysis of microbial infected cells and tumor cells. Initially recognized as part of a stereotyped innate immune mechanism, several reports have clearly shown that NK cells can acquire memory characteristics [6,7,8,9]. A role of memory-like NK cells (mlNKs) has been shown for cytomegalovirus [7,10], Zika virus [11,12], simian-human immunodeficiency virus (SHIV) [13], HIV [14], flu virus [15,16] as well as tuberculosis (TB) [17,18]. NK memory has also been reported against haptens [6] as well as a combination of IL12, IL15 and IL18 cytokines [8,9]. NK cell immunotherapy involving mlNKs is currently tested in various human cancers [19,20,21,22,23]. A deeper understanding of molecules and signaling pathways that result in the adoption of the memory program in NK cells could lead to therapeutic maneuvers to optimize the functional response of mlNKs.
NK cell activity is regulated by signals received through activating and inhibitory receptors [24,25]. The signals received through activating receptors such as NKG2D, NKG2C and NKP30 leads to NK cell activation [24,25]. Conversely, signals received through inhibitory receptors such as NKG2A and KLRG1 leads to NK cell inhibition [24,25]. Microbial infected cells may downregulate MHC and upregulate NK cell activating ligands such as MICA, MICB that triggers the NKG2D-mediated activation of NK cells [26]. NK cells become further activated by innate cytokines [21,27] including IL12, IL-15, IL-18, IL21 [17,28,29], type I interferons [30] and costimulatory signaling such as DNAM-1 [31,32,33]. This results in potent anti-pathogen or anti-tumor defense.
CD226 (also known as DNAM-1) signaling contributes to NK cell function including the antitumor activities of NK cells [32,33]. CD226 receptor binding to ligands including moieties from different viruses induces the phosphorylation of Vav1, PLCγ1 and PI3K leading to the activation of ERK and AKT kinases [33]. FOXO1, a negative regulator of NK cell function [34], is directly phosphorylated by AKT and/or SGK1. Recently, it was shown that CD226 signaling leads to the phosphorylation of FOXO1 and the cytoplasmic translocation of phosphorylated FOXO1 and its proteosomal degradation resulting in NK cell activation [33]. An absence of CD226 in Ly49H+ NK cells leads to the suppression of expansion capacity and memory generation after infection with mouse Cytomegalovirus (MCMV) [35]. mlNKs that formed after Zika virus infection displayed higher gene expression of CD226 [11]. Prior reports indicated that CD27+CD56+ NK cells from LTBI+ people proliferated and produced more IFN-γ and this subset constitutes a memory-like NK cell population (mlNKs) [17]. We found the increased expression of CD226 in TB reactive mlNKs. Here, using peripheral blood mononuclear cells (PBMC) from LTBI+ people we investigated the role of costimulatory CD226 signaling in the expansion capacity as well as a function of mlNKs response to Mtb stimulation.
2. Results
2.1. Blockade of CD226 Signaling Reduces Proliferation by mlNKs from LTBI+ People
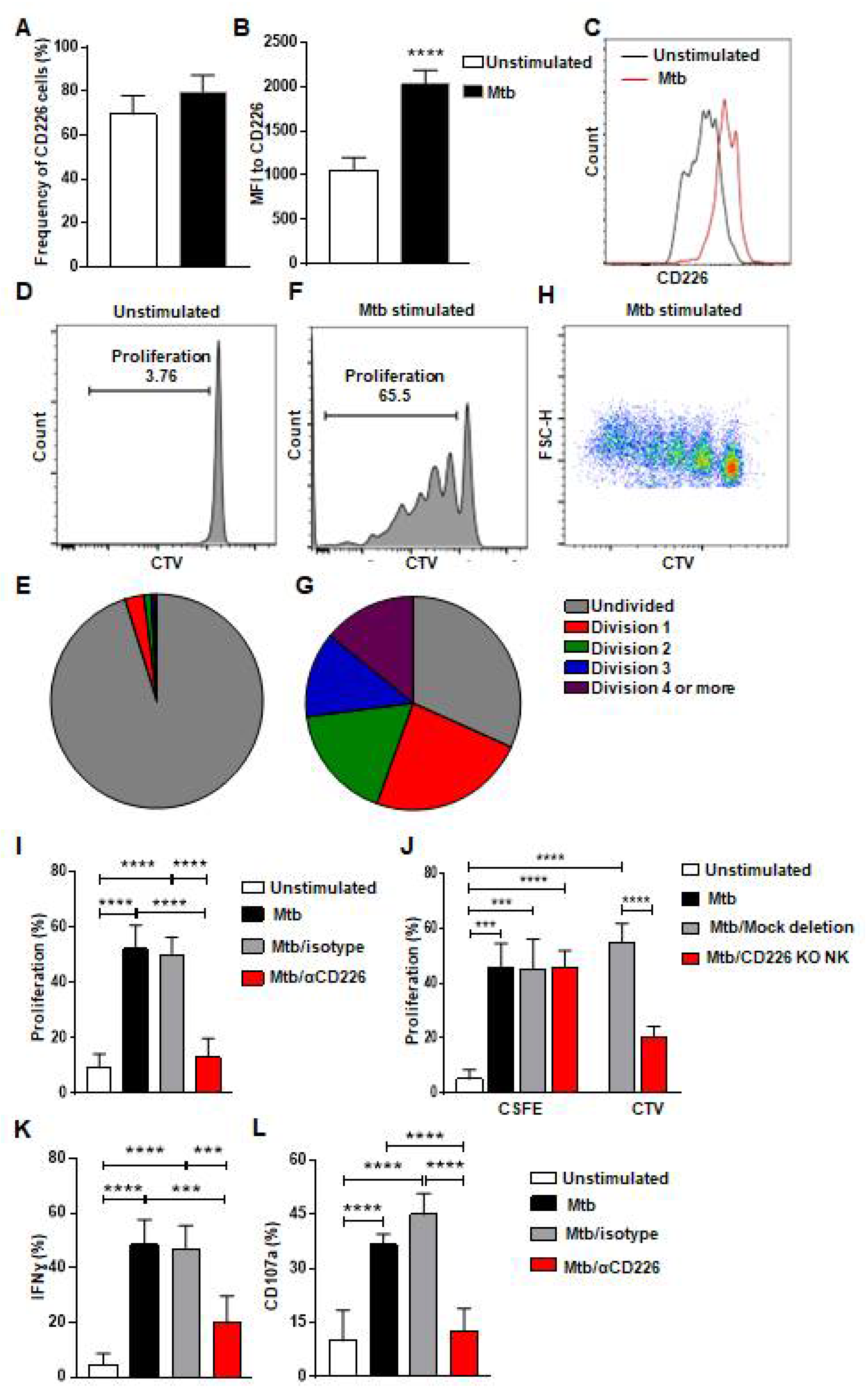
The co-stimulatory CD226 signaling contributes to NK cell function and tumor clearance [32,33]. Previous report indicates that CD226 signaling contributed to the expansion of Ly49H+ NK cells after MCMV infection and to the optimal differentiation of Ly49H+ memory NK cells [35]. It has been previously reported that CD226 expression was more on murine memory-like NK cells in a mouse model of TB [17] and these memory-like NK cells from LTBI+ donors proliferated and showed enhanced degranulation and IFN-γ production [17] (Figure S1A–D). We hypothesized that CD226 signaling contributes to the proliferation of mlNKs from LTBI+ people. To investigate this, we first stimulated peripheral blood mononuclear cells (PBMC) from LTBI+ (n = 5) people with γ-irradiated Mtb (γ-Mtb) for 5 days and assessed CD226 expression on mlNKs (Figure 1A–C). CD56+CD27+ mlNKs from LTBI+ donors (n = 5) proliferated in response to γ-Mtb stimulation (Figure 1D–H) compared to LTBI− (n = 6) people (Figure S1A–H) in accord with published report by others [17]. MFI of CD226 was higher on mlNKs after γ-Mtb stimulation, while frequencies of CD226+ mlNKs were comparable (Figure 1A–C). Next, we inhibited CD226 signaling using CD226 blocking antibody and the NK-cell-specific deletion of CD226 gene using CRISPR/Cas9 (Figure S2A–C). Both approaches led to significantly reduced proliferation of mlNKs from LTBI+ people while isotype antibody had no measurable effect on NK cell proliferation (Figure 1I,J). Blocking CD226 antibody treatment also led to lesser IFN-γ production, degranulation as well as activation profile measured by CD69 and HLA-DR expression by mlNKs from LTBI+ people compared to isotype-antibody-treated counterparts (Figure 1K,L and Figure S1I–K). Blockade of CD226 with antibody also led to reductions in protein levels of IFN-γ, TNF-α, MIP-1β and MCP1 in the culture supernatants on day 5 when measured by ELISA (Figure S3A–E). Together, these results suggest a role of CD226 signaling in the proliferation of mlNKs from LTBI+ people (n = 5).
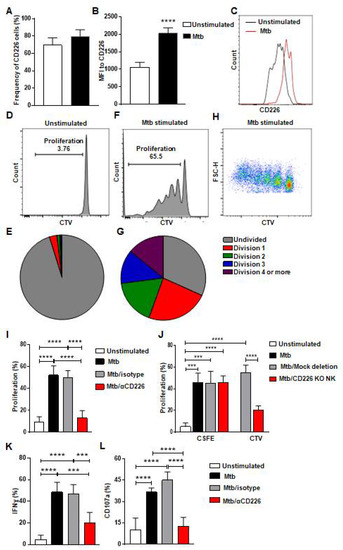
Figure 1.
CD226 blockade reduces proliferation by mlNKs from LTBI+ people after stimulation with Mtb. (A) Frequency of CD226+ cells in CD3−CD45+CD56+CD27+ cells (n = 5). (B) MFI of CD226 in CD3−CD45+CD56+CD27+ cells (n = 5), (C) Comparative histogram. (D–G) Unstimulated CD3−CD45+CD56+CD27+ cells (D,E) CD3−CD45+CD56+CD27+ cells (F–H) were stimulated with γ-Mtb for 5 days. Pie charts show number of divisions in unstimulated (E) and stimulated cells (G). (H) Pseudo color plot showing the number of divisions in CD3−CD45+CD56+CD27+ cells stimulated with γ-irradiated Mtb. (I) Proliferation percentage in CD3−CD45+CD56+CD27+ cells unstimulated or stimulated with γ-Mtb or stimulated with treatments of control isotype or blocking CD226 antibody (n = 7). (J) Proliferation of CD3−CD45+CD56+ cells not deleted or deleted for CD226 gene using CRISPR/Cas9 stained with cell trace violet (CTV) and co-cultured with autologous PBMC stained with CFSE and stimulated with γ-Mtb (n = 6). (K) IFN-γ, (L) CD107a positive cells in CD3−CD45+CD56+CD27+ cells unstimulated or stimulated with γ-Mtb or stimulated and treated with control isotype or blocking CD226 antibody (n = 5). Mean ± s.d. two-sided Student’s t-test or ANOVA. *** p < 0.001, **** p < 0.0001.
2.2. Inhibition of CD226 Signaling Leads to Reduction in FOXO1 Phosphorylation in NK Cells from LTBI+ People
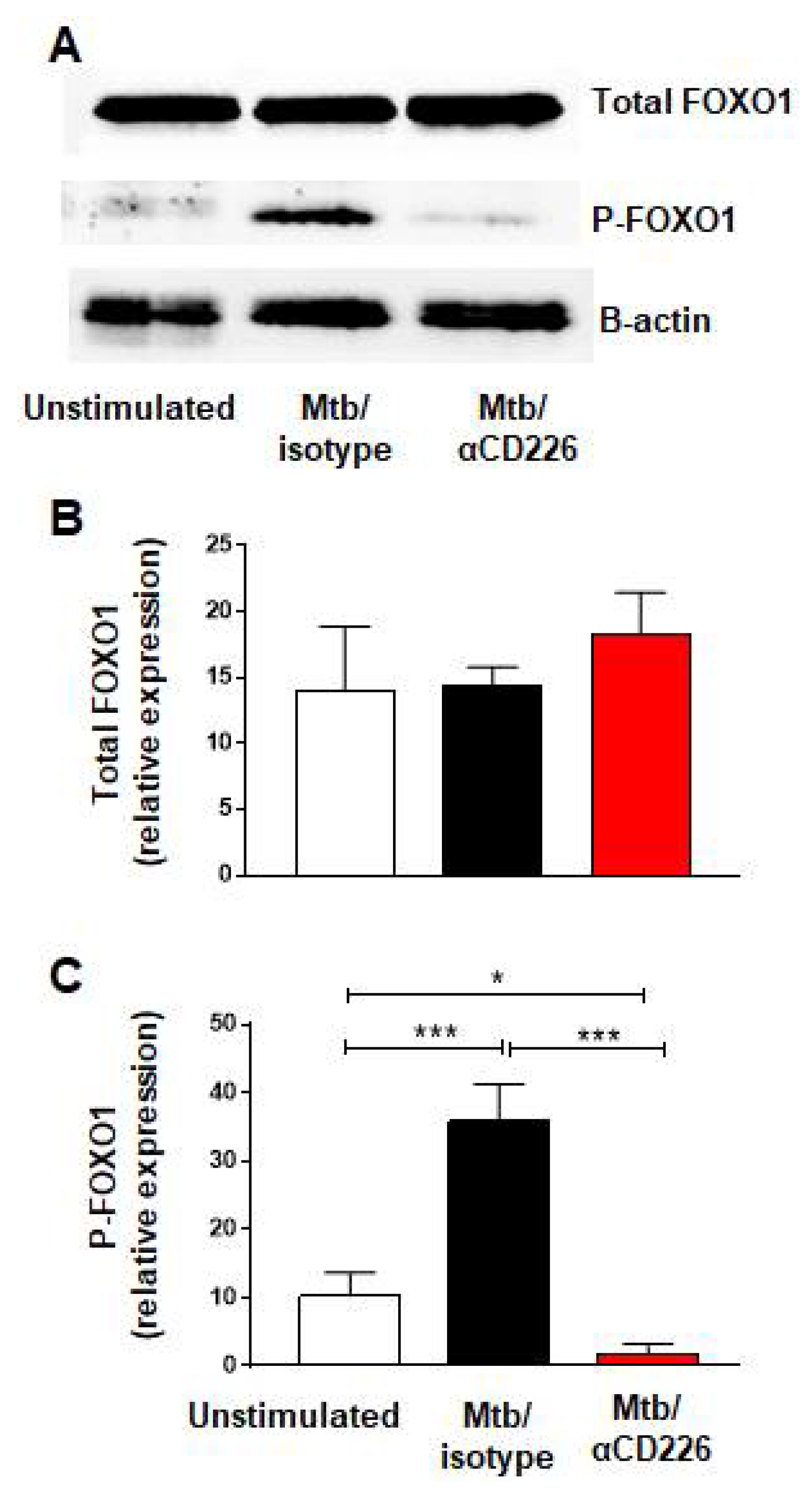
Recent report demonstrated that the activation of CD226 signaling led to the phosphorylation of FOXO1 and its proteasomal degradation [33]. FOXO1 is a negative regulator of NK cell function [34] thus CD226 signaling results in NK cell activation, by removing a negative regulator, FOXO1. We asked whether similar scenario occurs in NK cells from LTBI+ people when CD226 signaling is engaged. PBMC taken from LTBI+ people (n = 5) and stimulated with γ-Mtb were treated with blocking CD226 antibody or isotype matched antibody and NK cells were purified on day 2 to assess the phosphorylation of FOXO1. We noted the increased phosphorylation of FOXO1 in the isotype-antibody-treated group (Figure 2A–C). Conversely, blocking CD226 led to the lower phosphorylation of FOXO1, suggesting the increased presence of negative regulator FOXO1 in this group (Figure 2A–C). Overall, in agreement with a previous report in NK cells [33], these results indicate that blocking CD226 signaling reduced the phosphorylation of FOXO1 in NK cells from LTBI+ people (n = 5).
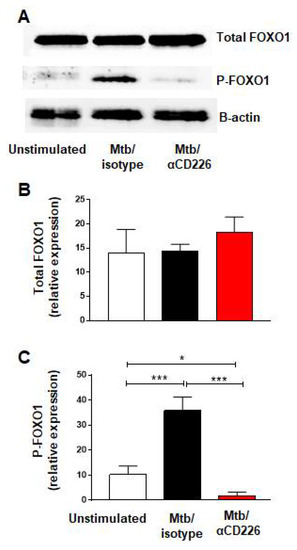
Figure 2.
CD226 blockade reduced FOXO1 phosphorylation in NK cells from LTBI+ people after stimulation with Mtb. (A) Western blot from NK cells unstimulated or stimulated with γ-Mtb for 48 h and treated with isotype or blocking CD226 antibody. (B) Total FOXO1 and (C) phosphorylated FOXO1 (n = 5). Mean ± s.d. ANOVA. * p < 0.05, *** p < 0.001.
2.3. Inhibition of CD226 Signaling Reduces cMyc Levels in NK Cells from LTBI+ People
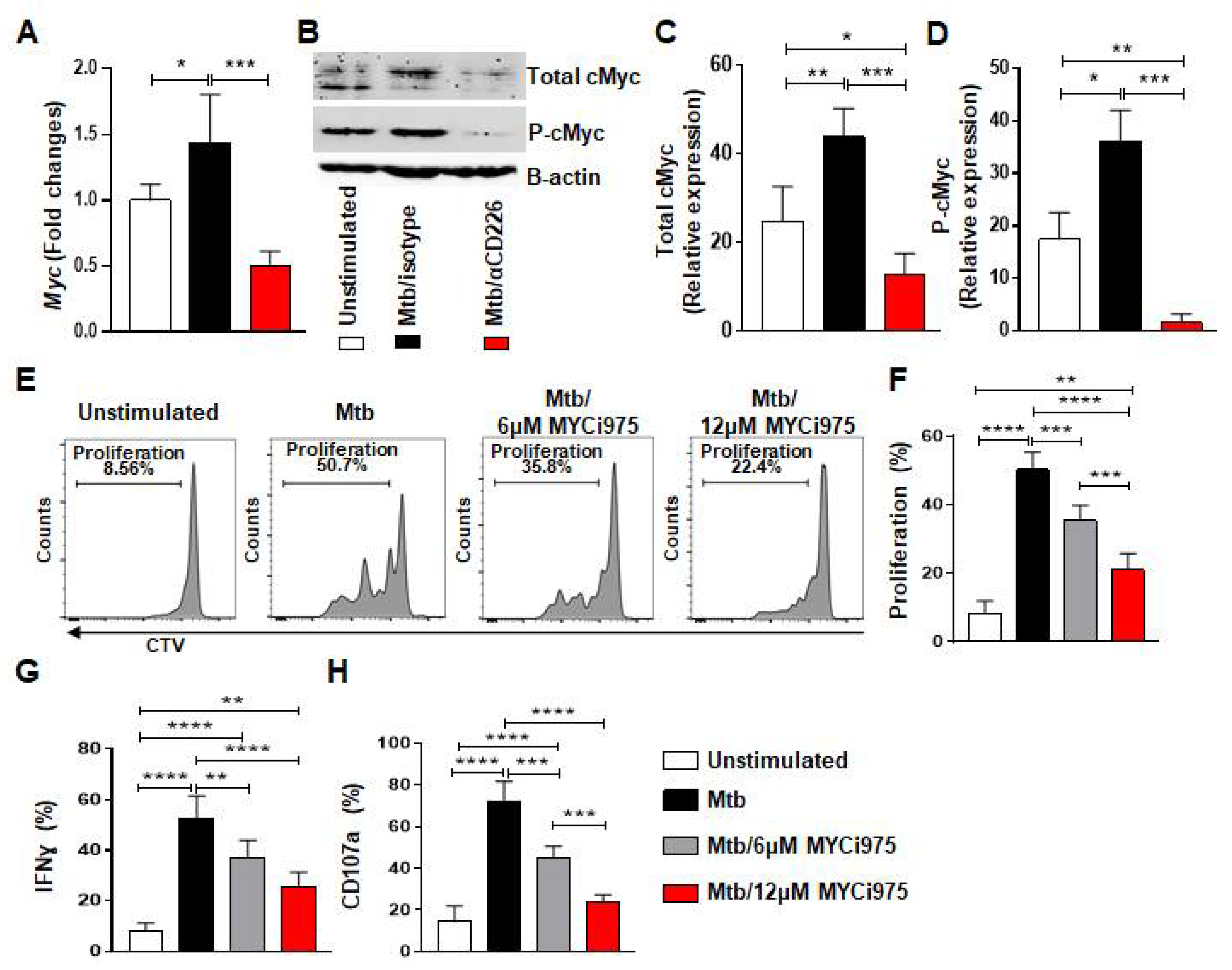
We next explored molecules involved in the proliferation of mlNKs that may be affected by CD226 blockade. cMyc is previously reported to be a target of XBP1s and integrates signals from endoplasmic reticulum (ER) stress to drive NK cell responses after MCMV infection and tumors [36]. PBMC from LTBI+ people (n = 5) were stimulated with γ-Mtb and treated with isotype antibody or CD226 blocking antibody. NK cells were purified at day 1 and day 2 post treatment. When we measured ER stress related genes (day 1 post treatment) such as Ire1α, Xbp1, we did not observe significant differences between isotype antibody and CD226 blocking antibody regimens (Figure S4B). While the expression levels of Chop, Atf4 and Bip were elevated in blocking CD226 antibody groups (Figure S4B). We also measured molecules associated with Wnt signaling [37] and found no differences in Tcf7 gene expression (Figure S4A). Dll1 expression was elevated while Jag1 expression was reduced upon CD226 blocking antibody groups (Figure S4A). Axin 2 expression was reduced in both isotype and CD226 blocking antibody groups (Figure S4A). Notably, Myc gene which is associated with cellular proliferation was higher in isotype antibody group, while Myc expression was reduced when CD226 signaling was blocked (Figure 3A). We also validated these results using Western blotting (Figure 3B–D) performed on day 2 post treatment. Overall, these results indicate lower cMyc levels in NK cells after CD226 blockade.
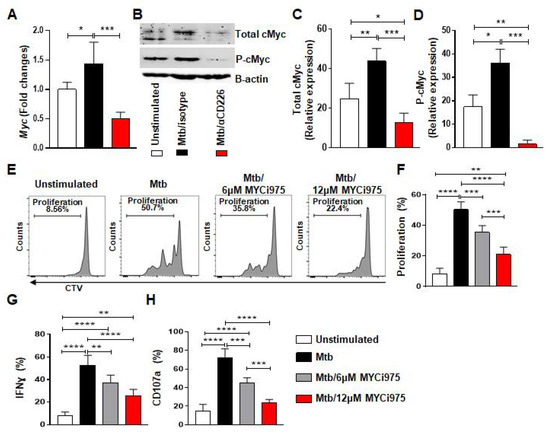
Figure 3.
cMyc inhibition reduces effector function of mlNKs from LTBI+ people after stimulation with Mtb. (A) Myc gene expression by TaqMan PCR in NK cells after 24 h of stimulation with γ-Mtb and treatments with isotype or blocking CD226 antibody (n = 5). (B) Western blot for NK cells unstimulated or stimulated with γ-Mtb for 48 h and treated with isotype or blocking CD226 antibody (n = 5). (C) Total cMyc and (D) phosphorylated cMyc. (E–H) PBMC were stimulated with γ-Mtb or left unstimulated for 5 days. cMyc inhibitor CMYi975 (6 or 12 µM) or DMSO vehicle was added every other day after stimulation with γ-Mtb (E,F) Proliferation of mlNKs from LTBI+ donors (n = 5) stimulated with γ-Mtb and treated with CMYi975 (6 or 12 µM). (G) IFN-γ, (H) CD107a positive cells in CD3−CD45+CD56+CD27+ cells (n = 5) unstimulated or stimulated with γ-Mtb and treated with CMYi975 (6 or 12 µM). Mean ± s.d. ANOVA. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
2.4. Inhibition of cMyc Using a Chemical Inhibitor Reduces Proliferation by mlNKs from LTBI+ People
We investigated whether the inhibition of cMyc using a chemical inhibitor of cMyc (MYCi975) [38] impacts the proliferation of CD56+CD27+ mlNKs from LTBI+ people. To do this, PBMC from LTBI+ people (n = 5) were pretreated with cMyc inhibitor or mock for 30 min and stimulated with γ-irradiated Mtb. The inhibitor and mock were replenished on alternate days and the proliferation of CTV-labeled CD56+CD27+ mlNKs was assessed 5 days later by flow cytometry. The culture supernatants on day 5 were assessed for the quantification of cytokines (Figure S5A–E, n = 5). We observed a significant reduction in the proliferation and effector function of CD56+CD27+ mlNKs from LTBI+ people (n = 5) when cMyc was inhibited compared to mock-treated groups (Figure 3E–H and Figure S6A). cMyc inhibition also led to a reduction in the protein levels of IFN-γ, TNF-α, MIP-1β, MCP1 and IL-10 in the culture supernatants on day 5 when measured by ELISA (Figure S5A–E, n = 5). Overall, these results indicate a role for cMyc in the proliferation of CD56+CD27+ mlNKs from LTBI+ people in response to γ-Mtb stimulation.
2.5. Inhibition of CD226 Signaling Lowered Glycolysis in NK Cells, and Glycolysis Inhibition Reduced Proliferation by mlNKs from LTBI+ People
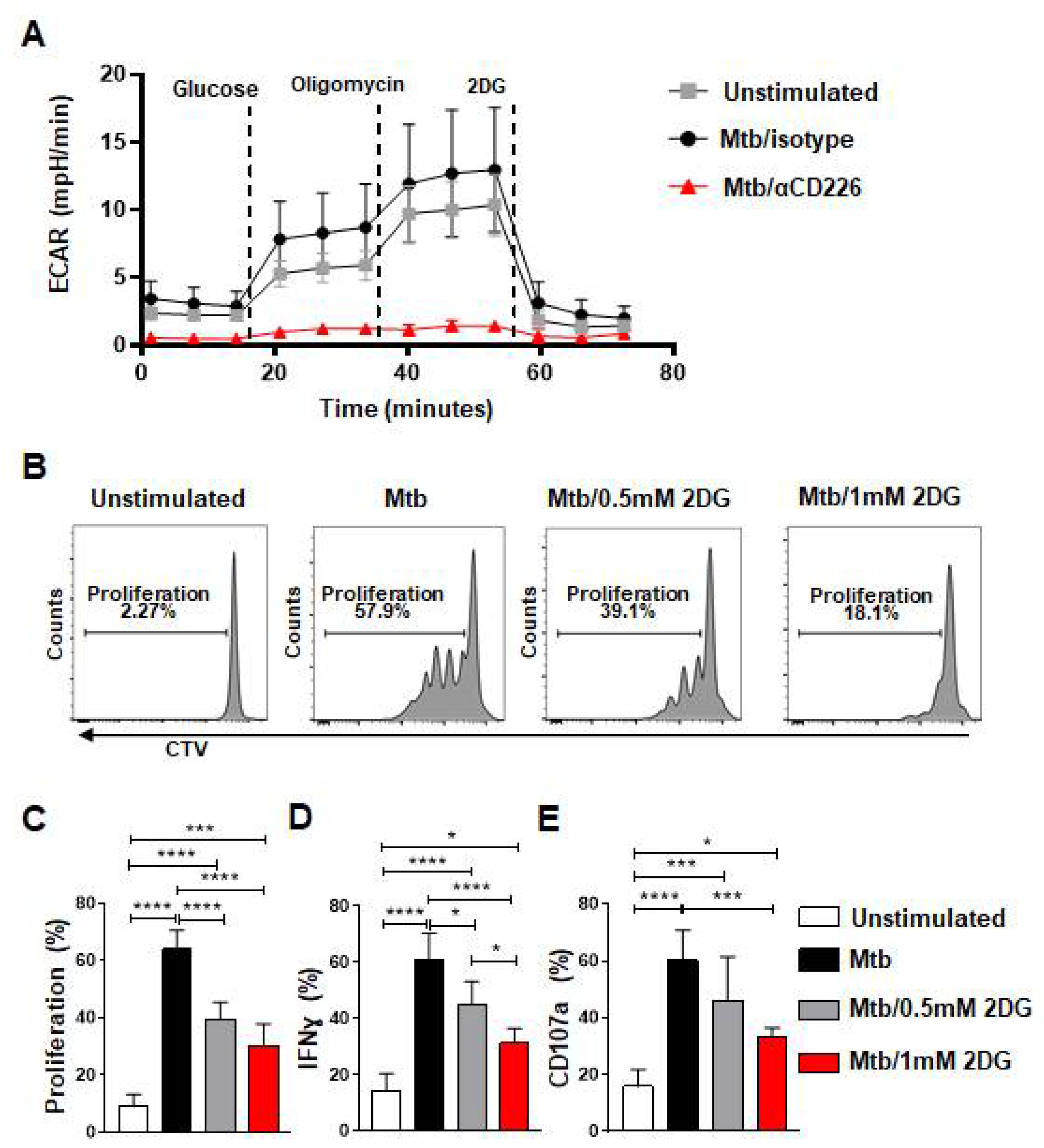
cMyc has been shown to regulate glycolysis in various cells [39,40,41,42]. Since CD226 blockade reduced cMyc levels, we probed whether the inhibition of CD226 signaling modulates the glycolytic metabolism of NK cells from LTBI+ people. PBMC from LTBI+ donors (n = 4) were stimulated with γ-Mtb and treated with isotype antibody or CD226 blocking antibody. NK cells were purified at day 2 post treatment and subjected to measure glycolysis (extracellular acidification rate) by Seahorse analyzer. We found reduced glycolysis in NK cells from LTBI+ people when CD226 signaling was inhibited (Figure 4A and Figure S6C–E). Glycolysis inhibition has been shown previously to impact NK cell proliferation and IFN-γ production [43]. To determine if the inhibition of glycolysis impacts the function of CD56+CD27+ mlNKs from LTBI+ people, PBMC from LTBI+ people (n = 5) were stimulated with γ-Mtb and treated with mock or 2DG (glycolysis inhibitor) [43]. The proliferation and function of CD56+CD27+ mlNKs were assessed 5 days later. We observed a significant reduction in the proliferation of CD56+CD27+ mlNKs from LTBI+ people when glycolysis was inhibited compared to mock-treated groups (Figure 4B,C and Figure S6B). We also observed a reduction in IFN-γ production and degranulation by CD56+CD27+ mlNKs from LTBI+ people from 2DG-treated groups compared to mock treatment (Figure 4D,E). Together, these results indicate a role for glycolysis in the proliferation of CD56+CD27+ mlNKs from LTBI+ people in response to γ-Mtb stimulation.
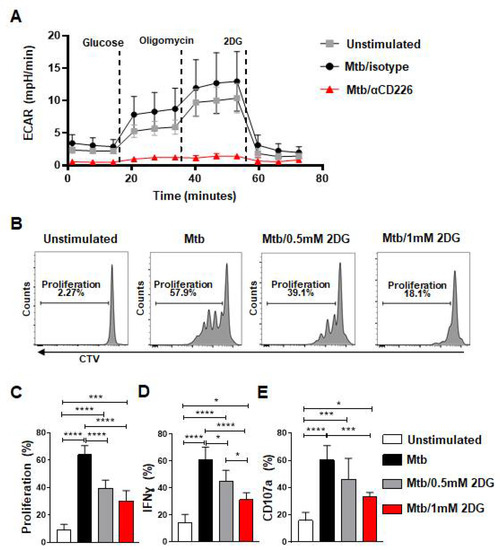
Figure 4.
Blockade of CD226 reduced glycolysis in NK cells and glycolysis inhibition diminished effector function of mlNKs from LTBI+ donors after stimulation with Mtb. (A) PBMC from LTBI+ donors were stimulated with γ-Mtb for 48 h and treated with isotype or blocking CD226 antibody. After this, 5 × 105 purified NK cells (purity > 90%) were plated to perform the glycolysis assay using seahorse analyzer (n = 4). (B) Chemical blockade of glycolysis using 2DG (0.5 or 1 mM every three days) after stimulation with γ-Mtb for 5-day time period was complete. (B–E) Proliferation, frequencies of IFN-γ+ cells and CD107a+ cells among CD3−CD45+CD56+CD27+ cells unstimulated or stimulated with γ-Mtb and treated with 2DG (n = 5). Mean ± s.d. ANOVA. * p < 0.05, *** p < 0.001, **** p < 0.0001.
3. Discussion
In this report, we show that co-stimulatory signaling through CD226 contributes to the function of mlNKs in LTBI+ people. Our results show that the blockade of CD226 signaling using a blocking antibody or CRISPR/Cas9-mediated CD226 deletion reduced proliferation by CD56+CD27+ mlNKs from LTBI+ people in response to γ-Mtb stimulation. Furthermore, our results revealed that blocking CD226 signaling reduced the phosphorylation of FOXO1 and lowered cMyc levels. We also showed that the inhibition of cMyc using a chemical inhibitor reduced proliferation by CD56+CD27+ mlNKs from LTBI+ people in response to γ-Mtb stimulation. Finally, we show that CD226 blockade lowered glycolysis in NK cells and inhibiting glycolysis reduced proliferation by CD56+CD27+ mlNKs from LTBI+ people in response to γ-Mtb stimulation. Overall, our results suggest a role of costimulatory signaling through CD226 in the expansion capacity and function of CD56+CD27+ mlNKs from LTBI+ people and could imply that defects in CD226 signaling may influence mlNKs function in human and possibly Mtb infection outcomes.
Prior report showed a critical role of the ER stress sensor IRE1α and its substrate transcription factor XBP1 in promoting NK cell effector responses to fend off virus infection and to clear tumors [36]. These antiviral and anti-tumor effects were mediated by cMyc, a molecule implicated in cellular proliferation [36]. We noted in LTBI+ people that the expression of Ire1α, Xbp1 did not vary between isotype and CD226 blocking antibody treatment regimens suggesting ER stress may not be involved in the regulation of mlNK responses in LTBI+ persons. Multiple reports have advocated a role for Wnt signaling in proliferation by memory-like NK cells [11,14]. Our results show the equivalent expression of multiple Wnt signaling genes (Axin2, Tcf7) in CD226-antibody-treated versus isotype-treated NK cells from LTBI+ people.
cMyc regulates proliferation in diverse cell types [36,37,44,45,46]. In line with these studies, we report reduced mlNKs proliferation with CD226 blockade due to diminished cMyc levels. Our finding of the inhibition of CD56+CD27+ mlNKs proliferation and effector function from LTBI+ people after treatment with cMyc inhibitor is also consistent with cMyc promoting proliferation and effector function in NK cells as also noted by others [36,47]. However, it is unclear how CD226 signaling promotes cMyc expression in mlNKs cells which we are currently investigating. One likely effect involves effects on aspect of immunometabolism. In fact, some reports do indicate a critical role of cMyc in modulating metabolic process [39,40,41]. Some show that cMyc promotes glycolysis [39,40,41], but others suggest its role in oxidative phosphorylation and mitochondrial metabolism [42]. It was not established if CD226 signaling modulates metabolism in NK cells. Recently, CD226 signaling was reported to promote glycolysis in endothelial cells [48]. Similarly, we noted diminished glycolysis in NK cells from LTBI+ people after CD226 blockade. It is possible that inhibiting CD226 signaling exerts its negative effect on glycolytic process via reduction in cMyc activity. In addition to NK cells, CD226 signaling can also regulate CD8 T cell function [49]. In fact, blocking PD-1 and inhibiting CD226 function compromise tumor clearance by CD8 T cells [50,51,52]. It may be worthwhile to see whether CD226 signaling regulates cMyc expression/glycolytic pathway in CD8 T cells that are actively clearing the tumors. We speculate that the outcome is likely to be positive. Overall, the results from our current NK cell study suggest that CD226 signaling may also play a role in regulating the metabolism of NK or even CD8 T cells in other contexts such as infection or tumor settings.
Limitations
Due to limited numbers of CD56+CD27+mlNKs, our experiments involved probing the protein levels of cMyc, FOXO1 or glycolysis assays in total NK cells after stimulation of the PBMC from LTBI+ people with γ-Mtb and treatments with isotype or blocking CD226 antibodies. We noted reduced CD56+CD27+ mlNKs proliferation from LTBI+ people after stimulation with γ-Mtb and treatments with cMyc inhibitor or glycolysis inhibitor (2DG). Thus, we speculate that CD226 inhibition also reduces FOXO1, cMyc levels or glycolysis in CD56+CD27+ mlNKs from LTBI+ people. Due to human primary cell studies with in vitro nature, the findings from this study require further studies with murine models with CD226 gene deletion where we believe that the absence of the CD226 gene may lead to multiple defects in NK and T cell compartment leading to increased susceptibility to Mtb.
4. Materials and Methods
4.1. Ethics Statement
All human samples studies were approved by the Institutional Review Board of the University of Texas Health Science Center at Tyler with protocol number 2021-033. For this study we collected blood samples from healthy volunteers for the isolation of PBMC.
4.2. Sample Collection
All donors were well-informed about the study before the acquisition of the informed consent. For the study blood from healthy persons positive to QuantiFERON-TB Gold tests and healthy persons negative to QuantiFERON-TB Gold tests were collected. All subjects were between 25 and 55 years old, including both genders.
4.3. Isolation and Culture of PBMC and CD226 Blockade
The blood was mixed with RPMI-1640 (Cytiva, Salt Lake City, UT, USA) complete media with 10% fetal bovine serum (FBS) (HyClone, Salt Lake City, UT, USA) and layered over Ficoll-PaqueTM plus gradient (Cytiva). After 25 min of centrifugation (with minimal acceleration, no brake, 1650 rpm at 25 °C), PBMC were collected and washed 2 times with RPMI-1640 (Cytiva, Salt Lake City, UT, USA) complete media with 10% FBS (HyClone, Salt Lake City, UT, USA). PBMC were cultured at 2.5 × 106 cells/well in 24-well plates with RPMI-1640 (Cytiva, Salt Lake City, UT, USA) with 10% AB heat-inactivated human serum (Sigma-Aldrich, Burlington, MA, USA) at 37 °C and 5% CO2. PBMC were treated with blocking anti-human CD226 mAb (clone 11A8) at 10 µg/mL (BioLegend, San Diego, CA, USA) or isotype control antibody (10 µg/mL, BioLegend) one hour before stimulation with γ-Mtb H37Rv at 10 µg/mL. The control or blocking antibodies were supplemented every other day. After 48 h to 5 days of stimulation, flow cytometry, real time PCR and metabolism assays were performed.
4.4. NK Cell Labelling with Cell Trace Violet/CFSE
PBMC or NK cells were labelled with CellTrace™ Violet (CTV, Invitrogen, Waltham, MA, USA) 1 µM, or CFSE (eBioscience, San Diego, CA, USA) 1 µM for 20 min at 37 °C, followed by multiple washes, and cells were seeded at 2.5 × 106 cells/well.
4.5. c-Myc and Glycolysis Inhibition
PBMC from LTBI+ donors were cultured in 24-well plates (2.5 × 106 cells/well) with RPMI-1640 (Cytiva) complete media with 10% AB heat-inactivated human serum (Sigma-Aldrich, Burlington, MA, USA) at 37 °C and 5% CO2. The cells were treated with MYCi975 (6 µM, 12 µM or 18 µM, MedChemExpress, Monmouth Junction, NJ, USA) or 2-DeoxyGlucose (2DG, 0.5 mM and 1 mM, Sigma-Aldrich) or control DMSO for 30 min. After this time, the cells were stimulated with γ-Mtb (10 µg/mL) for 5 days and analyzed by flow cytometry. cMyc inhibitor was supplemented every other day while 2DG was supplemented every three days.
4.6. Flow Cytometry
PBMC were stained with PerCP anti-human CD45 (clone: HI30, BioLegend), APC-Cy7 anti-human CD3 (clone: HIT3a, BioLegend), BV605 anti-human CD56 (clone: HCD56, BioLegend), PE anti-human/mouse/rat CD27 (clone: LG.3A10, BioLegend), Alexa Fluor 647 anti-human CD226 (DNAM-1) (clone: DX11, BD Biosciences, San Diego, CA, USA) and Zombie Red™ Fixable Viability Kit (live/dead staining) (BioLegend) in 1X PBS at 4 °C for 20–30 min. The cells were washed three times and cell acquisition was performed on LSRFortessa X-20 (BD Biosciences).
For intracellular staining, surface staining was followed by cellular permeabilization with Fixation/Permeabilization solution according to the manufacturer’s protocol (BD biosciences). The cells were washed with Perm/Wash buffer and were stained for FITC anti-human IFN-γ (clone: 45.B3, BioLegend) and APC anti-human CD107a (clone H4A3, BioLegend). In these assays, Brefeldin A (BD Biosciences) was added 4 h prior to surface staining, and CD107a antibody was added 2 h before Brefeldin A. The cellular acquisition was performed on LSRFortessa X-20 and data analysis was conducted using FlowJo v10 software (TreeStar, Ashland, OR, USA).
4.7. Relative Quantification of Genes by Real Time PCR
RNA isolation was performed using RNeasy mini kit (Qiagen, Germantown, MD, USA) according to manufacturer’s protocol and cDNA synthesis was carried out using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA, USA). TaqMan probes (Thermo fisher, Waltham, MA, USA) for endoplasmic reticulum stress genes: Ern1 (Hs00980095_m1), Ddit3 (Hs01090850_m1), Atf4 (Hs00909569_g1), Hspa5 (Hs00607129_gH), Xbp1 (Hs00231936_m1) Xbp1s (Hs03929085_g1), and proliferative genes: Myc (Mm00487804_m1), Axin2 (Mm00443610_m1), Dll1 (Mm01279269_m1), Tcf7 (Mm00493445_m1), and Jag1 (Mm00496902_m1) genes were used. To perform relative quantification, all samples were normalized with Gapdh (Mm99999915_g1) gene. The samples were amplified with TaqMan® gene expression master mix (Applied biosystem) in the QuantStudio 7 flex machine (Applied biosystem).
4.8. Metabolism Assays Using Seahorse Analyzer
Human PBMC from LTBI+ donors were treated with blocking anti-human CD226 clone 11A8 (10 µg/mL, BioLegend) or isotype control antibody (10 µg/mL, BioLegend) for 1 h at 37 °C and 5% CO2. The cells were stimulated with γ-Mtb or left untreated for 48 h at 37 °C and 5% CO2. NK cells were enriched using human NK isolation kit (Miltenyi Biotec, Gaithersburg, MD, USA. purity more than 90%). NK cell glycolytic status or extracellular acidification rate (ECAR) was measured via extracellular flux assay using Seahorse XFe96 Analyzer (Agilent Technologies, Santa Clara, CA, USA). NK cells were washed and resuspended in Agilent XF assay medium supplemented with 1 mM L-Glutamate and then plated for the assay at a density of 5 × 105 NK cells/well in a 96-well Seahorse plate coated with Poly-L-Lysine. During the assay glucose (10 mM), oligomycin (1 µM) and 2DG (50 mM) were injected according to the manufacturer’s protocol. Basal glycolysis, glycolytic capacity and glycolytic reserve were calculated using Seahorse Wave 2.6.3 Desktop Software, Agilent Technologies, USA.
4.9. CRISPR/Cas9-Mediated Deletion of CD226 Gene
NK cells isolated from PBMC were kept with human IL-15 (10 ng/mL) (BioLegend) for 24 h. After this, 2–5 × 106 NK cells were resuspended in 90 microliters NucleofectorTM solution and 10 microliters sgRNA-Cas9 complex was added (0.3 pmol of each sgRNA and 40 pmol spCas9). Four CD226 sgRNAs (Synthego, Redwood City, CA, USA) designed from CD226 human gene (CD226+69947022, CD226+69947028, CD226+69947029, and CD226-69947061) were used. spCas9 protein was also procured from Synthego. NK cells were electroporated using AmaxaTM human NK cell nucleofectorTM kit (Lonza, Bend, OR, USA) according to the manufacturer’s protocol. After electroporation, NK cells were maintained with 10 ng/mL human IL-15 for 5 days, and the deletion of CD226 was verified by Western blotting and flow cytometry. CD226 deleted or control NK cells were stained with CTV and co-cultured with PMBC isolated from the same donor that were stained with CFSE. Proliferation was measured by flow cytometry after the stimulation of co-cultured cells with γ-irradiated Mtb.
4.10. Western Blotting
The protein expression and phosphorylation of c-Myc and FOXO1 were determined by Western blotting using whole cell protein extracts from 106 NK cells lysed with RIPA buffer with protease and phosphatase inhibitors (G-Biosciences, St Louis, MO, USA). The samples were run in 10% SDS-PAGE gel and transferred onto nitrocellulose membranes, which were blocked with 5% skimmed milk in TBST for 1h. After this, the membranes were incubated overnight at 4 °C with primary antibodies for cMyc (Cell signaling, 1:1000 dilution), pcMyc (Cell signaling, 1:1000 dilution), FOXO1 (Cell signaling, 1:1000 dilution), pFOXO1 (Cell signaling, 1:1000 dilution), β-actin (Cat# 4967, Cell signaling, 1:1000 dilution). The membranes were incubated for 1h with secondary antibody conjugated with HRP and proteins bands were visualized by Clarity ECL substrate (Bio-Rad, Hercules, CA, USA) followed by capturing with ChemiDoc™ Imaging System (Bio-Rad).
4.11. Statistical Analysis
GraphPad Prism 7 software was used for experimental analysis. The unpaired or paired t-test or ANOVA was used for determining the statistical significance at * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 and **** p ≤ 0.0001.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232112838/s1.
Author Contributions
O.M. conceptualized the study. O.M., J.D.M., W.K., K.V.-A. and S.S.S. performed experiments. O.M. and J.D.M. did analysis. S.M., N.V.K. and S.S.S. supervised study, wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Department of Pulmonary Immunology, University of Texas Health Science Center at Tyler, Tyler, Texas, USA.
Institutional Review Board Statement
All human samples studies were approved by the Institutional Review Board of the University of Texas Health Science Center at Tyler with protocol number 2021-033.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The manuscript contains all the data including the supplemental figures.
Acknowledgments
We thank Buka Samten for helpful suggestions. We also thank Saptarshi Roy with technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Biron, C.A.; Byron, K.S.; Sullivan, J.L. Severe Herpesvirus Infections in an Adolescent without Natural Killer Cells. N. Engl. J. Med. 1989, 320, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Etzioni, A.; Eidenschenk, C.; Katz, R.; Beck, R.; Casanova, J.L.; Pollack, S. Fatal varicella associated with selective natural killer cell deficiency. J. Pediatr. 2005, 146, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Eidenschenk, C.; Dunne, J.; Jouanguy, E.; Fourlinnie, C.; Gineau, L.; Bacq, D.; McMahon, C.; Smith, O.; Casanova, J.-L.; Abel, L.; et al. A Novel Primary Immunodeficiency with Specific Natural-Killer Cell Deficiency Maps to the Centromeric Region of Chromosome 8. Am. J. Hum. Genet. 2006, 78, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Crespo, Â.C.; Mulik, S.; Dotiwala, F.; Ansara, J.A.; Sen Santara, S.; Ingersoll, K.; Ovies, C.; Junqueira, C.; Tilburgs, T.; Strominger, J.L.; et al. Decidual NK Cells Transfer Granulysin to Selectively Kill Bacteria in Trophoblasts. Cell 2020, 182, 1125–1139.e18. [Google Scholar] [CrossRef] [PubMed]
- Santara, S.S.; Angela, A.C.; Mulik, S.; Ovies, C.; Boulenouar, S.; Strominger, J.L.; Lieberman, J. Decidual NK cells kill Zika virus-infected trophoblasts. Proc. Natl. Acad. Sci. USA 2021, 118, e2115410118. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Goodarzi, M.; Drayton, D.L.; von Andrian, U.H. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 2006, 7, 507–516. [Google Scholar] [CrossRef]
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Adaptive immune features of natural killer cells. Nature 2009, 457, 557–561. [Google Scholar] [CrossRef]
- Cooper, M.A.; Elliott, J.M.; Keyel, P.A.; Yang, L.; Carrero, J.A.; Yokoyama, W.M. Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. USA 2009, 106, 1915–1919. [Google Scholar] [CrossRef]
- Romee, R.; Schneider, S.E.; Leong, J.W.; Chase, J.M.; Keppel, C.R.; Sullivan, R.P.; Cooper, M.A.; Fehniger, T.A. Cytokine activation induces human memory-like NK cells. Blood 2012, 120, 4751–4760. [Google Scholar] [CrossRef]
- Min-Oo, G.; Lanier, L.L. Cytomegalovirus generates long-lived antigen-specific NK cells with diminished bystander activation to heterologous infection. J. Exp. Med. 2014, 211, 2669–2680. [Google Scholar] [CrossRef]
- Kujur, W.; Murillo, O.; Adduri, R.S.R.; Vankayalapati, R.; Konduru, N.V.; Mulik, S. Memory like NK cells display stem cell like properties after zika virus infection. PLoS Pathog. 2020, 16, e1009132. [Google Scholar] [CrossRef] [PubMed]
- Maucourant, C.; Nonato Queiroz, G.A.; Corneau, A.; Leandro Gois, L.; Meghraoui-Kheddar, A.; Tarantino, N.; Bandeira, A.C.; Samri, A.; Blanc, C.; Yssel, H.; et al. NK Cell Responses in Zika Virus Infection Are Biased towards Cytokine-Mediated Effector Functions. J. Immunol. 2021, 207, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.K.; Li, H.; Jost, S.; Blass, E.; Li, H.; Schafer, J.L.; Varner, V.; Manickam, C.; Eslamizar, L.; Altfeld, M.; et al. Antigen-specific NK cell memory in rhesus macaques. Nat. Immunol. 2015, 16, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lifshitz, L.; Gellatly, K.; Vinton, C.L.; Busman-Sahay, K.; McCauley, S.; Vangala, P.; Kim, K.; Derr, A.; Jaiswal, S.; et al. HIV-1-induced cytokines deplete homeostatic innate lymphoid cells and expand TCF7-dependent memory NK cells. Nat. Immunol. 2020, 21, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, J.; Wang, Y.; Chen, Y.; Wei, H.; Sun, R.; Tian, Z. Respiratory Influenza Virus Infection Induces Memory-like Liver NK Cells in Mice. J. Immunol. 2017, 198, 1242–1252. [Google Scholar] [CrossRef]
- Zheng, J.; Wen, L.; Yen, H.; Liu, M.; Liu, Y.; Teng, O.; Wu, W.; Ni, K.; Lam, K.; Huang, C.; et al. Phenotypic and Functional Characteristics of a Novel Influenza Virus Hemagglutinin-Specific Memory NK Cell. J. Virol. 2021, 95, e00165-21. [Google Scholar] [CrossRef]
- Venkatasubramanian, S.; Cheekatla, S.; Paidipally, P.; Tripathi, D.; Welch, E.; Tvinnereim, A.R.; Nurieva, R.; Vankayalapati, R. IL-21-dependent expansion of memory-like NK cells enhances protective immune responses against Mycobacterium tuberculosis. Mucosal Immunol. 2017, 10, 1031–1042. [Google Scholar] [CrossRef]
- Suliman, S.; Geldenhuys, H.; Johnson, J.L.; Hughes, J.E.; Smit, E.; Murphy, M.; Toefy, A.; Lerumo, L.; Hopley, C.; Pienaar, B.; et al. Bacillus Calmette–Guérin (BCG) Revaccination of Adults with Latent Mycobacterium tuberculosis Infection Induces Long-Lived BCG-Reactive NK Cell Responses. J. Immunol. 2016, 197, 1100–1110. [Google Scholar] [CrossRef]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S.; et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef]
- Gang, M.; Wong, P.; Berrien-Elliott, M.M.; Fehniger, T.A. Memory-like natural killer cells for cancer immunotherapy. Semin. Hematol. 2020, 57, 185–193. [Google Scholar] [CrossRef]
- Boieri, M.; Ulvmoen, A.; Sudworth, A.; Lendrem, C.; Collin, M.; Dickinson, A.M.; Kveberg, L.; Inngjerdingen, M. IL-12, IL-15, and IL-18 pre-activated NK cells target resistant T cell acute lymphoblastic leukemia and delay leukemia development in vivo. Oncoimmunology 2017, 6, e1274478. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Fulton, R.J.; Rettman, P.; Sayan, A.E.; Coad, J.; Al-Shamkhani, A.; Khakoo, S.I. Activity of IL-12/15/18 primed natural killer cells against hepatocellular carcinoma. Hepatol. Int. 2019, 13, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Uppendahl, L.D.; Felices, M.; Bendzick, L.; Ryan, C.; Kodal, B.; Hinderlie, P.; Boylan, K.L.M.; Skubitz, A.P.N.; Miller, J.S.; Geller, M.A. Cytokine-induced memory-like natural killer cells have enhanced function, proliferation, and in vivo expansion against ovarian cancer cells. Gynecol. Oncol. 2019, 153, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. Up on the tightrope: Natural killer cell activation and inhibition. Nat. Immunol. 2008, 9, 495–502. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Bauer, S.; Groh, V.; Wu, J.; Steinle, A.; Phillips, J.H.; Lanier, L.L.; Spies, T. Activation of NK cells and T cells by NKG2D, a receptor for stress- inducible MICA. Science 1999, 285, 727–729. [Google Scholar] [CrossRef]
- Freeman, B.E.; Raué, H.-P.; Hill, A.B.; Slifka, M.K. Cytokine-Mediated Activation of NK Cells during Viral Infection. J. Virol. 2015, 89, 7922–7931. [Google Scholar] [CrossRef]
- Skak, K.; Frederiksen, K.S.; Lundsgaard, D. Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology 2008, 123, 575–583. [Google Scholar] [CrossRef]
- Paidipally, P.; Tripathi, D.; Abhinav, V.; Radhakrishnan, R.K.; Dhiman, R.; Venkatasubramanian, S.; Devalraju, K.P.; Tvinnereim, A.R.; Valluri, V.L.; Vankayalapati, R. Interleukin-21 Regulates Natural Killer Cell Responses during Mycobacterium tuberculosis Infection. J. Infect. Dis. 2018, 217, 1323–1333. [Google Scholar] [CrossRef]
- Madera, S.; Rapp, M.; Firth, M.A.; Beilke, J.N.; Lanier, L.L.; Sun, J.C. Type I IFN promotes NK cell expansion during viral infection by protecting NK cells against fratricide. J. Exp. Med. 2016, 213, 225–233. [Google Scholar] [CrossRef]
- Cifaldi, L.; Doria, M.; Cotugno, N.; Zicari, S.; Cancrini, C.; Palma, P.; Rossi, P. DNAM-1 activating receptor and its ligands: How do viruses affect the NK cell-mediated immune surveillance during the various phases of infection? Int. J. Mol. Sci. 2019, 20, 3715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, N.; Lu, Y.; Davidson, D.; Colonna, M.; Veillette, A. DNAM-1 controls NK cell activation via an ITT-like motif. J. Exp. Med. 2015, 212, 2165–2182. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; de Almeida, P.; Manieri, N.; de Almeida Nagata, D.; Wu, T.D.; Harden Bowles, K.; Arumugam, V.; Schartner, J.; Cubas, R.; Mittman, S.; et al. CD226 regulates natural killer cell antitumor responses via phosphorylation-mediated inactivation of transcription factor FOXO1. Proc. Natl. Acad. Sci. USA 2018, 115, E11731–E11740. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Kerdiles, Y.; Chu, J.; Yuan, S.; Wang, Y.; Chen, X.; Mao, H.; Zhang, L.; Zhang, J.; Hughes, T.; et al. Transcription factor foxo1 is a negative regulator of natural killer cell maturation and function. Immunity 2015, 42, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Nabekura, T.; Kanaya, M.; Shibuya, A.; Fu, G.; Gascoigne, N.R.J.; Lanier, L.L. Costimulatory Molecule DNAM-1 Is Essential for Optimal Differentiation of Memory Natural Killer Cells during Mouse Cytomegalovirus Infection. Immunity 2014, 40, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Adams, N.M.; Xu, Y.; Cao, J.; Allan, D.S.J.; Carlyle, J.R.; Chen, X.; Sun, J.C.; Glimcher, L.H. The IRE1 endoplasmic reticulum stress sensor activates natural killer cell immunity in part by regulating c-Myc. Nat. Immunol. 2019, 20, 865–878. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/β-Catenin Signaling in Development and Disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef]
- Han, H.; Jain, A.D.; Truica, M.I.; Izquierdo-Ferrer, J.; Anker, J.F.; Lysy, B.; Sagar, V.; Luan, Y.; Chalmers, Z.R.; Unno, K.; et al. Small-Molecule MYC Inhibitors Suppress Tumor Growth and Enhance Immunotherapy. Cancer Cell 2019, 36, 483–497.e15. [Google Scholar] [CrossRef]
- Bae, S.; Park, P.S.U.; Lee, Y.; Mun, S.H.; Giannopoulou, E.; Fujii, T.; Lee, K.P.; Violante, S.N.; Cross, J.R.; Park-Min, K.H. MYC-mediated early glycolysis negatively regulates proinflammatory responses by controlling IRF4 in inflammatory macrophages. Cell Rep. 2021, 35, 109264. [Google Scholar] [CrossRef]
- Tateishi, K.; Iafrate, A.J.; Ho, Q.; Curry, W.T.; Batchelor, T.T.; Flaherty, K.T.; Onozato, M.L.; Lelic, N.; Sundaram, S.; Cahill, D.P.; et al. Myc-Driven glycolysis is a therapeutic target in glioblastoma. Clin. Cancer Res. 2016, 22, 4452–4465. [Google Scholar] [CrossRef]
- Cargill, K.R.; Stewart, C.A.; Park, E.M.; Ramkumar, K.; Gay, C.M.; Cardnell, R.J.; Wang, Q.; Diao, L.; Shen, L.; Fan, Y.-H.; et al. Targeting MYC-enhanced glycolysis for the treatment of small cell lung cancer. Cancer Metab. 2021, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Goetzman, E.S.; Prochownik, E.V. The role for myc in coordinating glycolysis, oxidative phosphorylation, glutaminolysis, and fatty acid metabolism in normal and neoplastic tissues. Front. Endocrinol. 2018, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Terrén, I.; Orrantia, A.; Mosteiro, A.; Vitallé, J.; Zenarruzabeitia, O.; Borrego, F. Metabolic changes of Interleukin-12/15/18-stimulated human NK cells. Sci. Rep. 2021, 11, 6472. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.V. The role of c-myc in cellular growth control. Oncogene 1999, 18, 2988–2996. [Google Scholar] [CrossRef]
- Dose, M.; Khan, I.; Guo, Z.; Kovalovsky, D.; Krueger, A.; Von Boehmer, H.; Khazaie, K.; Gounari, F. c-Myc mediates pre-TCR-induced proliferation but not developmental progression. Blood 2006, 108, 2669–2677. [Google Scholar] [CrossRef]
- Morrish, F.; Isern, N.; Sadilek, M.; Jeffrey, M.; Hockenbery, D.M. C-Myc activates multiple metabolic networks to generate substrates for cell-cycle entry. Oncogene 2009, 28, 2485–2491. [Google Scholar] [CrossRef]
- Loftus, R.M.; Assmann, N.; Kedia-Mehta, N.; O’Brien, K.L.; Garcia, A.; Gillespie, C.; Hukelmann, J.L.; Oefner, P.J.; Lamond, A.I.; Gardiner, C.M.; et al. Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat. Commun. 2018, 9, 152–160. [Google Scholar] [CrossRef]
- Zhou, S.; Xie, J.; Yu, C.; Feng, Z.; Cheng, K.; Ma, J.; Wang, Y.; Duan, C.; Zhang, Y.; Jin, B.; et al. CD226 deficiency promotes glutaminolysis and alleviates mitochondria damage in vascular endothelial cells under hemorrhagic shock. FASEB J. 2021, 35, e21998. [Google Scholar] [CrossRef]
- Nabekura, T.; Shibuya, K.; Takenaka, E.; Kai, H.; Shibata, K.; Yamashita, Y.; Harada, K.; Tahara-Hanaoka, S.; Honda, S.-I.; Shibuya, A. Critical role of DNAX accessory molecule-1 (DNAM-1) in the development of acute graft-versus-host disease in mice. Proc. Natl. Acad. Sci. USA 2010, 107, 18593–18598. [Google Scholar] [CrossRef]
- Weulersse, M.; Asrir, A.; Pichler, A.C.; Lemaitre, L.; Braun, M.; Carrié, N.; Joubert, M.-V.; Le Moine, M.; Do Souto, L.; Gaud, G.; et al. Eomes-Dependent Loss of the Co-activating Receptor CD226 Restrains CD8+ T Cell Anti-tumor Functions and Limits the Efficacy of Cancer Immunotherapy. Immunity 2020, 53, 824–839.e10. [Google Scholar] [CrossRef]
- Braun, M.; Aguilera, A.R.; Sundarrajan, A.; Corvino, D.; Stannard, K.; Krumeich, S.; Das, I.; Lima, L.G.; Meza Guzman, L.G.; Li, K.; et al. CD155 on Tumor Cells Drives Resistance to Immunotherapy by Inducing the Degradation of the Activating Receptor CD226 in CD8+ T Cells. Immunity 2020, 53, 805–823.e15. [Google Scholar] [CrossRef] [PubMed]
- Banta, K.L.; Xu, X.; Chitre, A.S.; Au-Yeung, A.; Takahashi, C.; O’Gorman, W.E.; Wu, T.D.; Mittman, S.; Cubas, R.; Comps-Agrar, L.; et al. Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8+ T cell responses. Immunity 2022, 55, 512–526.e9. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).