Abstract
Retinoic acid (RA) plays important roles in various biological processes in animals. RA signaling is mediated by two types of nuclear receptors, namely retinoic acid receptor (RAR) and retinoid x receptor (RXR), which regulate gene expression by binding to retinoic acid response elements (RAREs) in the promoters of target genes. Here, we explored the effect of all-trans retinoic acid (ATRA) on the Pacific oyster Crassostera gigas at the transcriptome level. A total of 586 differentially expressed genes (DEGs) were identified in C. gigas upon ATRA treatment, with 309 upregulated and 277 downregulated genes. Bioinformatic analysis revealed that ATRA affects the development, metabolism, reproduction, and immunity of C. gigas. Four tyrosinase genes, including Tyr-6 (LOC105331209), Tyr-9 (LOC105346503), Tyr-20 (LOC105330910), and Tyr-12 (LOC105320007), were upregulated by ATRA according to the transcriptome data and these results were verified by real-time quantitative polymerase chain reaction (RT-qPCR) analysis. In addition, increased expression of Tyr (a melanin-related TYR gene in C. gigas) and Tyr-2 were detected after ATRA treatment. The yeast one-hybrid assay revealed the DNA-binding activity of the RA receptors CgRAR and CgRXR, and the interaction of CgRAR with RARE present in the Tyr-2 promoter. These results provide evidence for the further studies on the role of ATRA and the mechanism of RA receptors in mollusks.
1. Introduction
Retinoic acid (RA), a metabolite of vitamin A, plays several key roles in cell differentiation, embryonic development, growth and reproduction, immune function, and organ regeneration in vertebrates [1,2,3]. There are three main isomeric forms of RA, namely all-trans-RA (ATRA), 13-cis-RA (13cRA), and 9-cis-RA (9cRA). Among these, ATRA is the main biologically active isomer [4]. As a ligand of many nuclear receptors, RA is involved in numerous biological processes through the direct or indirect regulation of hundreds of genes, including those related to transcription factors, enzymes, structural proteins, cell surface receptors, neurotransmitters, neuropeptide hormones, and growth factors [5,6].
Earlier RA signaling was considered unique to vertebrates until the enzymes, nuclear receptors, and binding proteins associated with the RA signaling pathway were discovered in invertebrates [7,8]. The identification of RA signaling components implies that RA in invertebrates may have similar functions to those observed in vertebrates. A growing number of studies have further uncovered the role of RA in mollusks. For example, high concentrations of RA can affect shell structure and have toxic effects on eye formation during developmental stages [9]. In Lymnaea stagnalis, RA can modify electrical synapses of central neurons and have a potential trophic effect during synaptogenesis [10,11]. In addition, RA appears to induce imposex in marine gastropods via an unknown mechanism [12].
Two classes of receptors, retinoic acid receptor (RAR) and retinoid X receptor (RXR), are responsible for the activation of RA signaling. Three RARs (RARα, RARβ, and RARγ) and three RXRs (RXRα, RXRβ, and RXRγ) of different isoforms have been identified in mammals [13]. Both RARs and RXRs belong to the nuclear receptor superfamily and share a highly conserved DNA-binding domain (DBD) and a relatively conserved ligand-binding domain (LBD) [13,14]. RARs form heterodimers with RXRs after RA perception to regulate gene expression by binding to specific DNA sequences in the promoter region of target genes, which are known as retinoic acid response elements (RAREs) [14,15]. The first RARE identified was a direct repeat (DR) of two (A/G)GGTCA half-site core sequences separated by five base pairs, called DR5 [16]. The vast majority of RAREs identified to date consist of two copies of the (A/G)G(G/T)(G/T)(G/C)A core sequence, organized as DRs and separated by a variable number of nucleotides. Of these, RAREs configured as DRs of the (A/G)G(G/T)TCA core sequence are best known [17].
RA receptors have also been identified in several mollusk species. Two RXR isoforms identified in Thais clavigera can form heterodimers with RAR-like proteins and displayed 9c-RA dependent transcriptional activity [18,19]. In Nucella lapillus, the retinoic acid receptor NlRAR combines with the RXR to form a heterodimer that specifically binds to RAREs organized in DRs [20]. In C. gigas, both RXR and RAR have been isolated [21]. Several studies have revealed that CgRXR not only binds to CgRAR [22], but also acts as a co-receptor to form heterodimers with CgTR [23] and CgPPAR2 [24]. Other studies have shown that CgRXR can combine with DRs, with AGGTCA as the core sequence spaced by 0–5 nucleotides in vitro [25].
Tyrosinase, a member of the type-3 copper protein superfamily, is a complex oxidoreductase comprised of multiple subunits [26,27]. Tyrosinase is widely present in plants, microorganisms, and animals and is involved in a variety of biological processes, including oxygen transport, innate immunity, wound healing, and pigmentation [27,28]. Tyrosinase has a well-defined melanogenic enzyme catalytic activity, and is the rate-limiting enzyme that regulates melanin production [29]. In C. gigas, at least 26 genes encode tyrosinase isoforms [30], and these are mainly related to shell formation, prismatic pigmentation, and melanin synthesis [31,32,33].
Our previous study showed that ATRA could activate the expression of the RA receptors CgRAR and CgRXR, and that CgRAR could be found in the nucleus of mammalian cells. Furthermore, CgRAR could combine with CgRXR through the LBD domain. In the current study, transcriptome analysis following ATRA treatment was performed to further investigate the effects of RA in C. gigas. Differentially expressed genes (DEGs) between the ATRA and dimethyl sulfoxide (DMSO) control groups were assayed, and we found that the expression of several TYR genes were significantly upregulated after ATRA treatment. Real-time quantitative polymerase chain reaction (RT-qPCR) analysis revealed that the expression of several TYR genes, including Tyr, Tyr-2, Tyr-6, Tyr-9, Tyr-12, and Tyr-20, were significantly upregulated after ATRA treatment. The yeast one-hybrid assay showed that the RA receptors CgRAR and CgRXR bind to the DR0-DR5 sequence, and that CgRAR can bind to the Tyr-2 promoter.
2. Results
2.1. Transcriptome Analysis upon ATRA Treatment in the Pacific Oyster
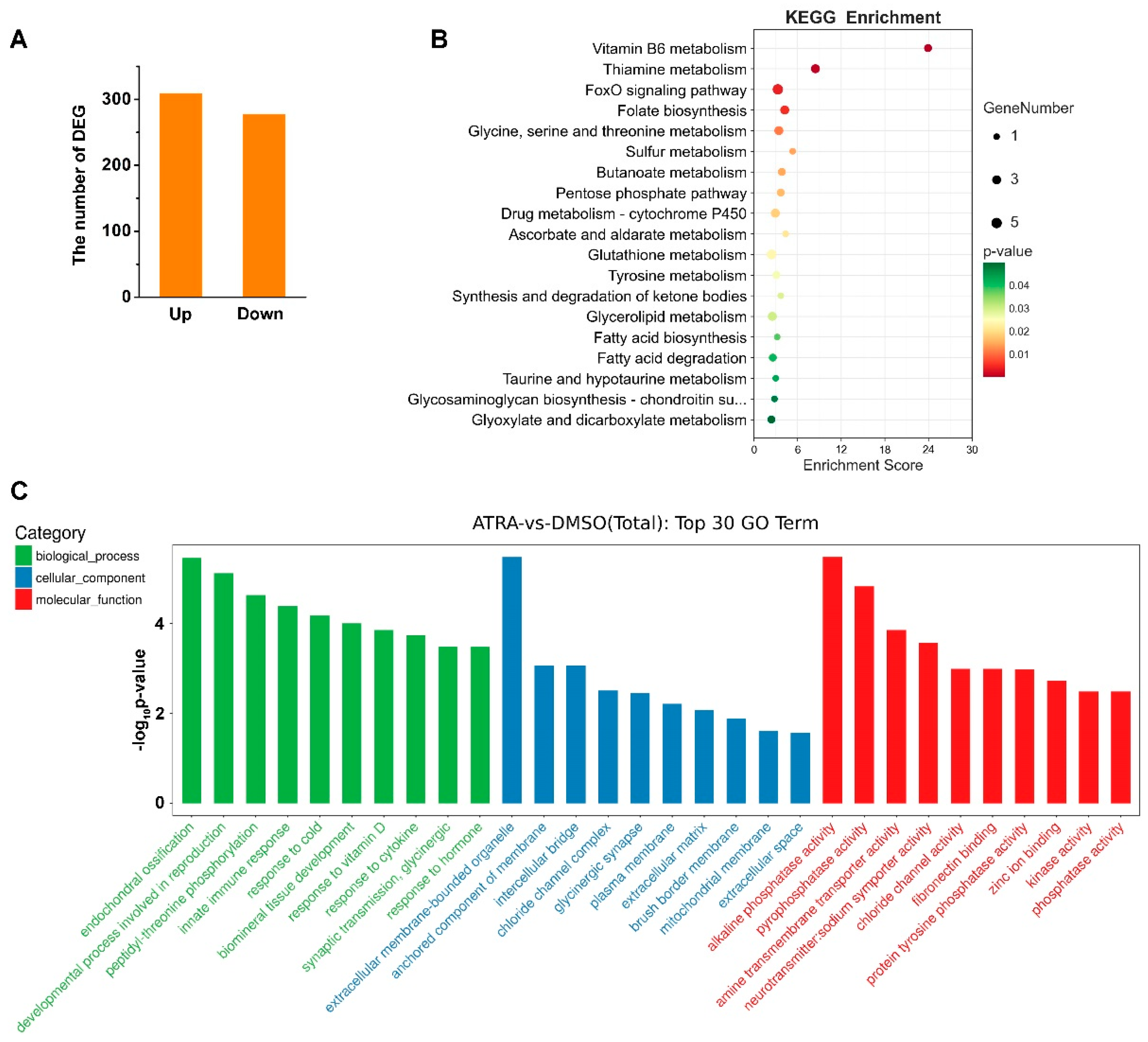
We previously identified the molecular characteristics of two RA receptors, CgRAR and CgRXR, in C. gigas [22]. To further study the genes that might be regulated by RA in C. gigas, transcriptome sequencing was carried out on samples collected from DMSO- and ATRA-treated individuals. A total of 586 DEGs were identified (fold change ≥ 2, p value < 0.05) between ATRA- and DMSO-treated groups (Table S1). Among them, 309 genes were upregulated and 277 were downregulated upon ATRA treatment (Figure 1A). KEGG pathway analysis showed that the 587 DEGs were mainly enriched in several metabolic related pathways, including Vitamin B6 metabolism, thiamine metabolism, glutathione metabolism, and so on (Figure 1B). GO enrichment analysis revealed that the DEGs were significantly enriched in endochondral ossification, the development process involved in reproduction, and innate immune response in the categories of biological process (Figure 1C). Taken together, these results suggested that ATRA is mainly involved in reproductive, developmental, immunity, and metabolic processes in C. gigas.
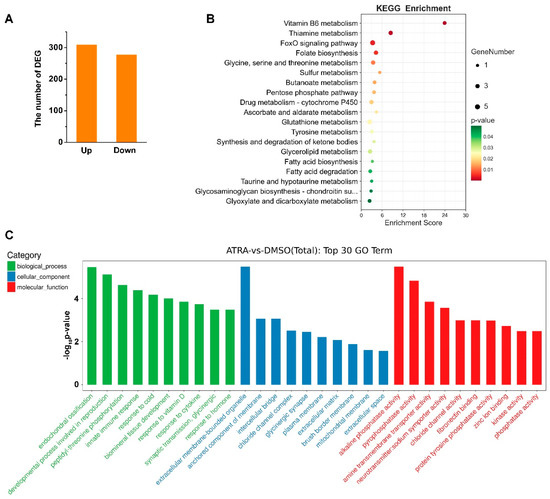
Figure 1.
Transcriptome analysis of C. gigas after ATRA treatment. (A) Numbers of differentially expressed genes (DEGs) in C. gigas after all-trans retinoic acid (ATRA) treatment. Fold change ≥ 2, p values < 0.05. (B) KEGG pathways enriched for the ATRA-regulated genes. p values < 0.05. (C) GO enrichment analysis of DEGs in ATRA-treated oysters. p values < 0.05.
2.2. ATRA Induce Several TYR Genes Expression
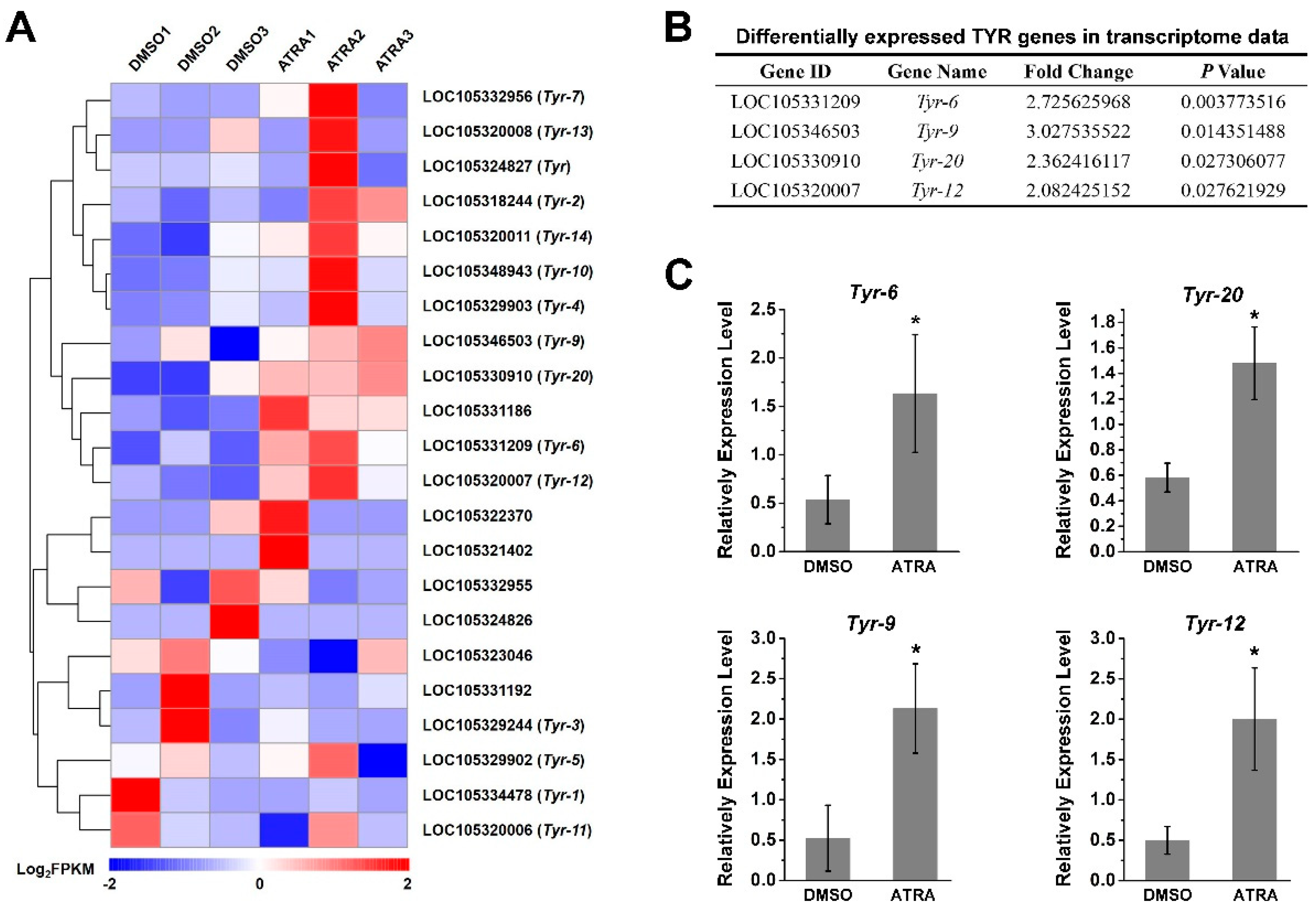
Of interest to us, the tyrosinase metabolic pathways were also enriched according to the result of the KEGG analysis (Figure 1B). It has been reported that RA contributes to melanogenesis and melanin production in vertebrates [34,35]. In order to explore the relationship between RA pathway and melanin production in C. gigas, we screened the expression of tyrosinase (TYR) genes, the key genes for melanin production, from transcriptome data. A total of 22 TYR genes were identified (Figure 2A and Table S2), of which the expression of four TYR genes, including Tyr-6 (LOC105331209), Tyr-9 (LOC105346503), Tyr-20 (LOC105330910), and Tyr-12 (LOC105320007) were differentially expressed (fold change ≥ 2, p value < 0.05) in the ATRA-treated group compared with the DMSO-treated group (Figure 2B). RT-qPCR analysis was performed to verify the RNA sequencing data. The result showed that expression of Tyr-6, Tyr-9, Tyr-20, and Tyr-12 increased significantly after ATRA injection (Figure 2). We hypothesized that RA affects the expression of TYR genes, and therefore affects melanin production in C. gigas.
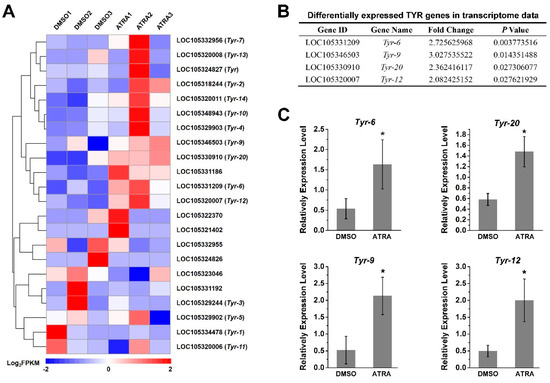
Figure 2.
Expression of TYR gene after ATRA treatment. (A) The expression profiles of 22 TYR genes identified in transcriptome sequencing; (B) differentially expressed TYR genes in transcriptome data (fold change ≥ 2, p values < 0.05); (C) verification of TYR gene expression by RT-qPCR. Relative expression of TYR genes after ATRA treatment. Error bars represent means of three replicates ± SD (standard deviation, n = 6). (* p < 0.05; Student’s t test).
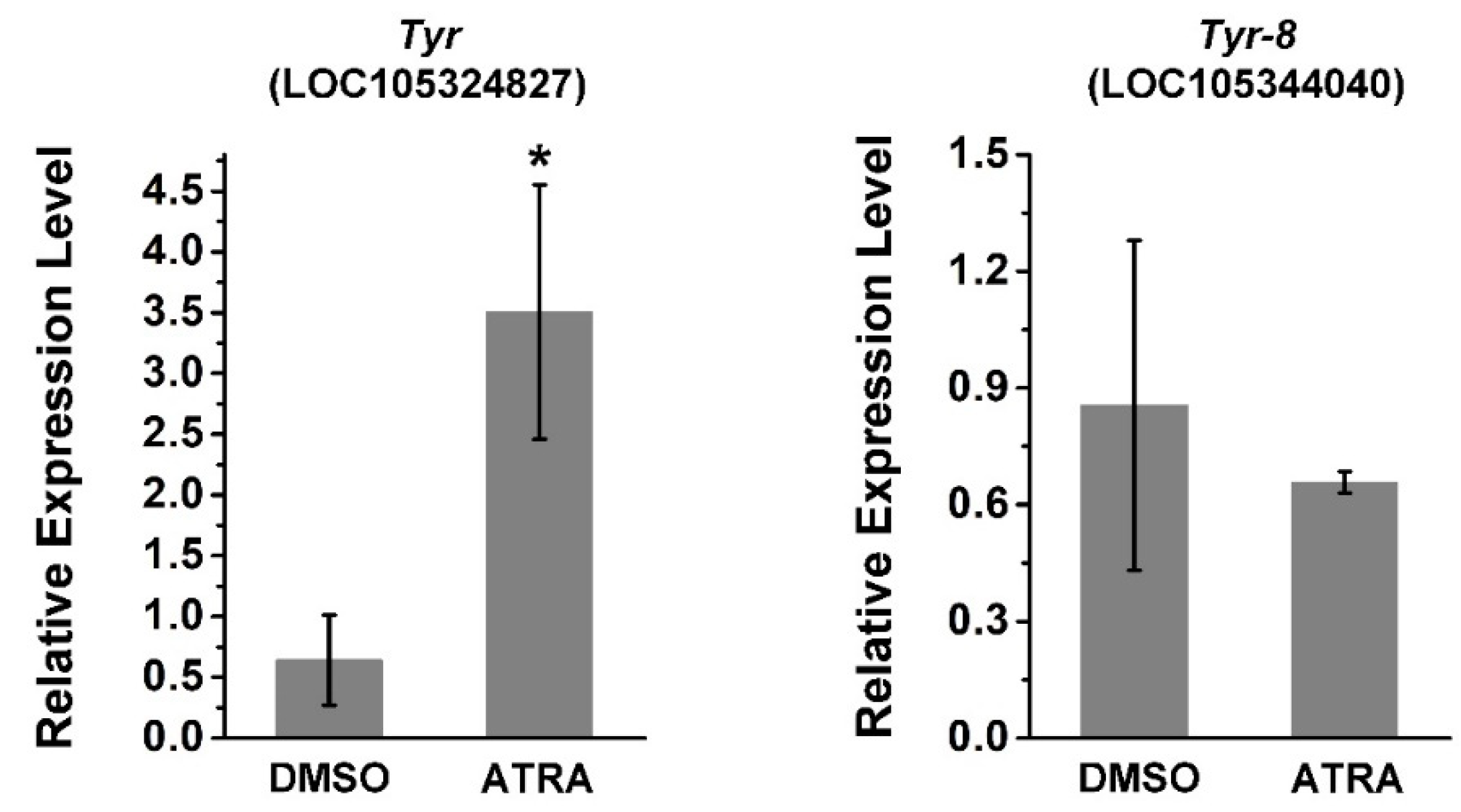
Tyr (LOC105324827) and Tyr-8 (Trp2, LOC105344040) are tyrosinase genes related to melanin production in C. gigas [36,37]. Thus, the relative expression of these two genes was investigated using RT-qPCR after ATRA treatment. As shown in Figure 3, the expression of Tyr was significantly upregulated by ATRA treatment, whereas there was no significant change in the expression of Tyr-8 after ATRA treatment. This suggests that the RA pathway may regulate expression of certain TYR genes in C. gigas.
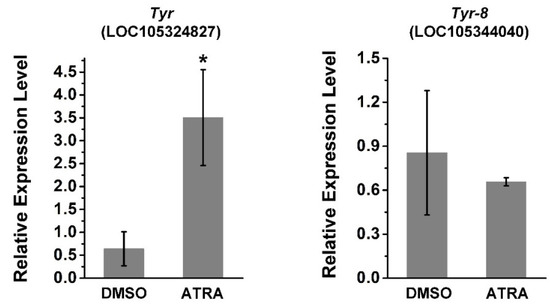
Figure 3.
ATRA upregulates the expression of Tyr and Tyr-8. Error bars represent means of three replicates ± SD (standard deviation, n = 6, * p < 0.05; Student’s t test).
2.3. CgRAR Combines with RARE
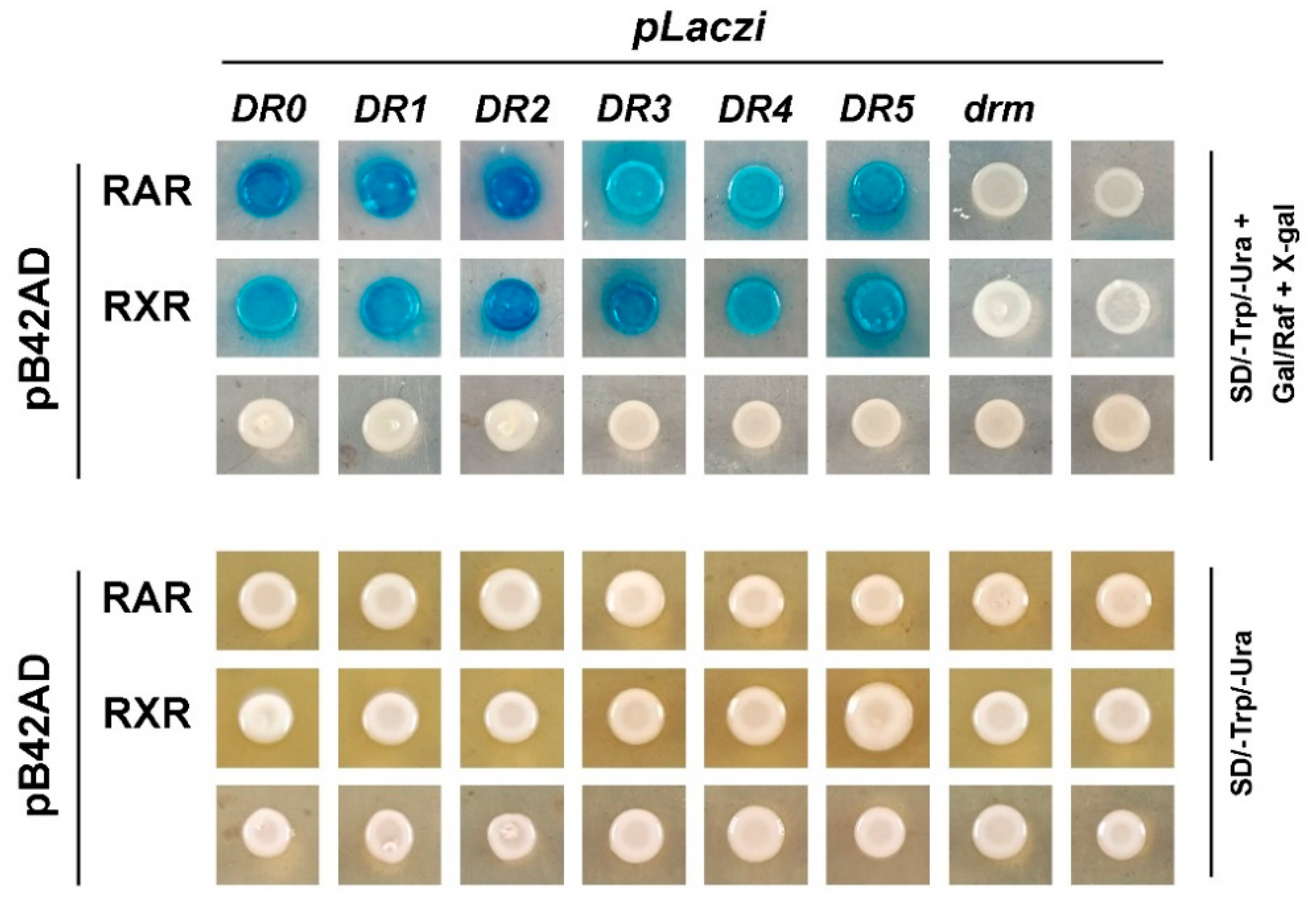
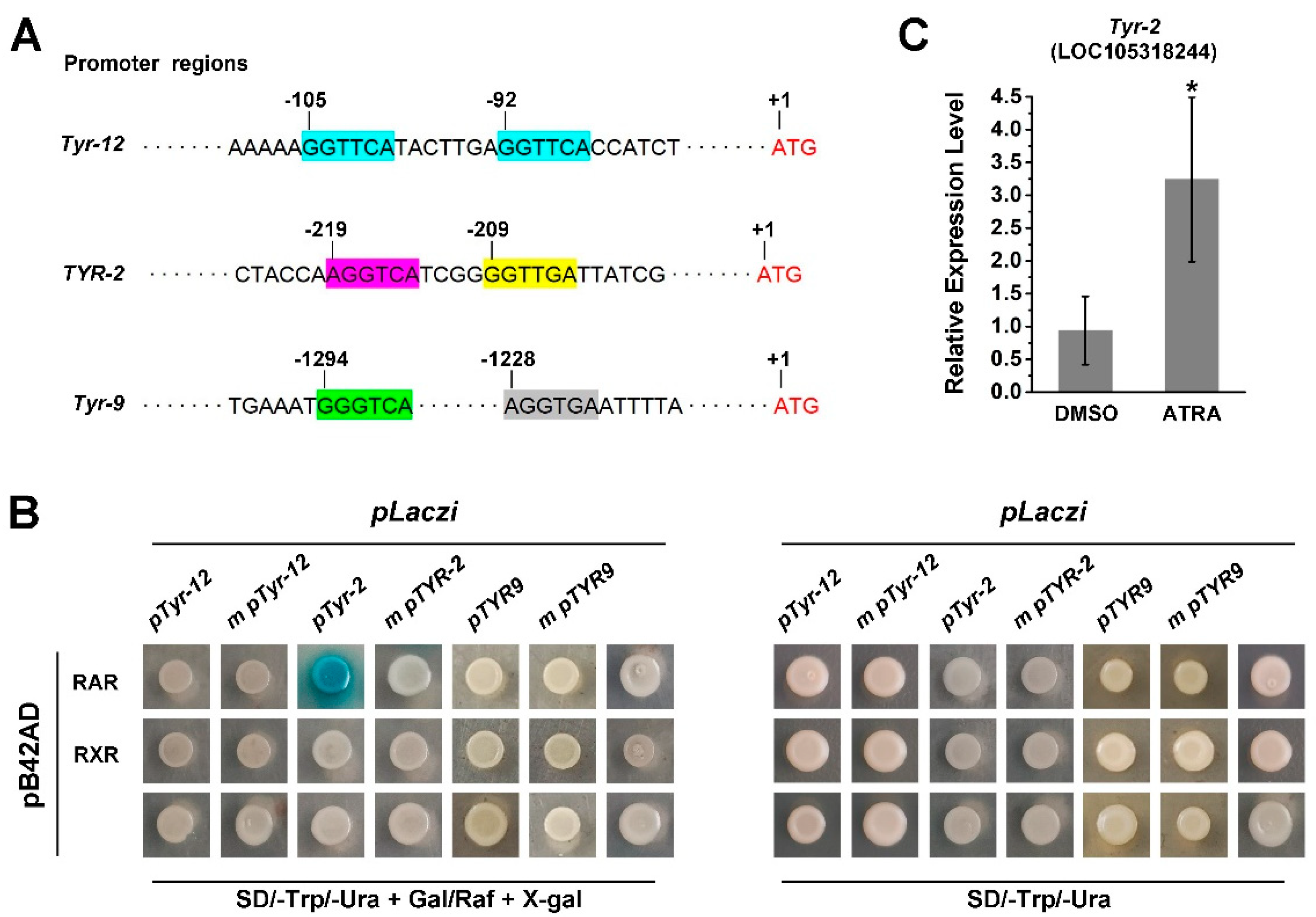
RAR and RXR are members of the nuclear receptor superfamily, which directly bind to the promoter regions of target genes to regulate their expression. Our previous study revealed that both CgRAR-BD and CgRXR-BD fusion vectors exhibited strong self-activation when co-transferred with the pGAD T7 vector in the yeast two-hybrid assay [22]. This suggests that CgRAR and CgRXR may have transcriptional activities. Thus, to detect the binding activity of CgRAR and CgRXR to the DNA sequence, a yeast one-hybrid assay was performed to investigate whether CgRAR or CgRXR interacts with DRs consisting of the (A/G)G(G/T)TCA core sequence. DR0–DR5, containing the RARE core sequence “AGGTCA” separated by 0–5 nucleotides, was produced and inserted into the pLacZi reporter vector to generate a fusion plasmid. CgRAR and CgRXR were then fused to the pB42AD vector. The mutated drm, produced by random mutation of the RARE core sequence “AGTTCA”, was used as a negative control. Yeast stains co-transferred with pB42AD and pLacZi empty vectors were used as blank controls. Yeast stains co-transferred with CgRAR and DR0–DR5 showed obvious positive reactions on a chromogenic medium (Figure 4). CgRXR also showed binding affinity to DR0–DR5, but not to drm (Figure 4). These results indicate that both CgRAR and CgRXR can combine with DR0–DR5 in yeast.
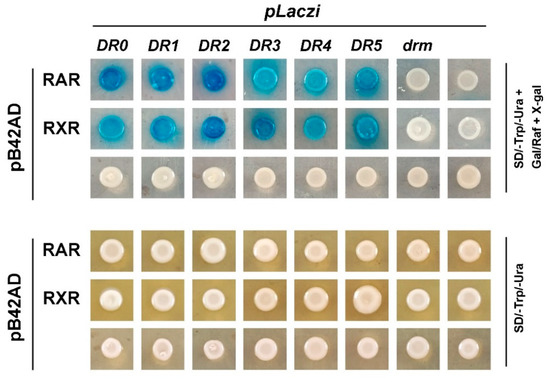
Figure 4.
CgRAR and CgRXR bind DR0–DR5 in yeast. Both CgRAR and CgRXR can successfully bind the artificial triple repeated DR0–DR5 sequences in yeast cells, whereas it did not bind to the drm sequence. Yeast stains co-transferred with fusion plasmids were grown on SD/-Trp/-Ura select medium (lower rows) and were transferred to SD/-Trp/-Ura select medium with Gal/Raf and X-gal (upper rows) to detect the activation of LacZ reporter gene.
2.4. CgRAR and CgRXR Bind the RARE Motif in the Promoter of TYR Genes
Since RA treatment activates the expression of several TYR genes and RA receptors of the Pacific oyster and these have the ability to bind RARE, we further investigated the role of CgRAR and CgRXR in the regulation of TYR gene expression. First, we screened for the RARE motif in the promoter regions of C. gigas TYR genes. A potential RARE, a DR consisting of two “GGTTCA” spaced by seven nucleotides was found in Tyr-12 and a DR consist of (A/G)G(G/T)T(G/C)A core sequence spaced by four nucleotides was found in the promoter region of Tyr-2 (LOC108318244) (Figure 5A). To elucidate whether CgRAR and CgRXR would interact with these potential RAREs in the promoter region of these oyster tyrosinase genes, the 222 bp fragment from the promoter of Tyr-12 (pTyr-12) and 121 bp fragment from the promoter of Tyr-2 (pTyr-2) were amplified. Both pTyr-12 and pTyr-2 contained the potential RARE motifs, and the fragments were fused with the pLacZi vector, respectively, for yeast one-hybrid assays. The same fragments with mutant RARE core sequences (m pTyr-12 and m pTyr-2) were used as negative controls. The Y1H assay revealed that CgRAR interacted with the RARE present in the Tyr-2 promoter, whereas no binding activity of CgRAR to pTyr-12 was detected in yeast (Figure 5B). A mutation of the “AGGTCA” core sequence in the Tyr-2 promoter abolished the LacZ reporter gene activation by CgRAR (Figure 5B). Furthermore, it failed to detect the binding of CgRXR to pTyr-12 or pTyr-2 by Y1H. Taken together, these results illustrate that CgRAR binds the promoter of Tyr-2, and this depends on the DR4 consisting of “AGGTCA” and “CCTTGA” separated by four base pairs. The RT-qPCR was also carried out to detect whether ATRA affects the expression of Tyr-2, and the result reveals an upregulation of Tyr-2 by ATRA treatment (Figure 5C).
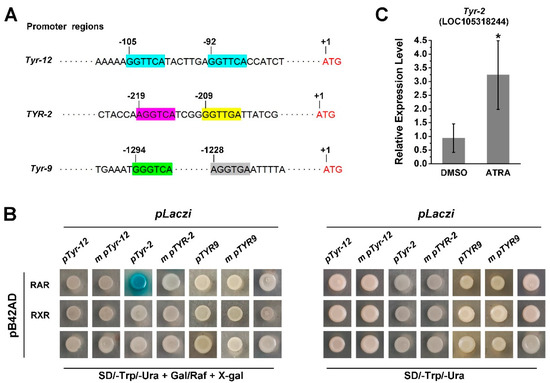
Figure 5.
CgRAR bind the promoter of Tyr-2 in yeast. (A) Schematic structure of RAREs in the TYR promoters. (A/G)G(G/T)(G/T)(G/C)A core sequence are marked in colored frames, and the numbers of scale plates indicate the location of these core sequence upstream from the ATG start codon; (B) CgRAR combine with Tyr-2 promoter; (C) relative expression level of Tyr-2 upon ATRA treatment. Error bars represent ± SD (n = 6, * p < 0.05; Student’s t test).
Two (A/G)G(G/T)T(G/C)A core sequences were found in the promoter of Tyr-9. These were separated by 58 bases and did not form short DR motifs (Figure 5A). RT-qPCR analysis also revealed that Tyr-9 was upregulated by ATRA treatment (Figure 2). Thus, we wonder whether Tyr-9 could be directly regulated by the C. gigas RA receptor. A 173 bp promoter region of Tyr-9 (pTyr-9) was amplified and constructed into the pLacZi vector. Y1H results showed that the LacZ reporter gene could not be activated by CgRAR or CgRXR, suggesting that neither CgRAR nor CgRXR binds to this fragment in the Tyr-9 promoter (Figure 5B).
3. Discussion
3.1. Biological Roles of RA in C. gigas
RA is an important metabolic product derived from vitamin A that functions as a signaling molecule in numerous animals. RA participates in the regulation of many biological processes, including growth and development, reproduction, differentiation, and apoptosis. In invertebrates RA contributes to several processes, including the formation and modulation of central synapses, neuronal differentiation, and shell development [9,10,38]. RA also can affect calcium signaling in the neurons of adult mollusks [39]. Here, we injected ATRA into the adductor muscle of C. gigas to investigate the role of RA in mollusks. Mantle tissue samples were collected for transcriptomic sequencing, and this showed that the expression of 586 genes were affected by ATRA treatment, with 309 upregulated and 277 downregulated genes. GO clustering and KEGG pathway analysis showed that the DEGs were mainly related to development, metabolism, reproduction, and immunity (Figure 1B,C). Other studies in mammals have also shown that RA is associated with mucosal non-specific immunity and can promote antibody production in specific immunity [40,41]. Therefore, RA in C. gigas mainly participate in the biological processes of reproduction, development, and immunity, which is similar to those of mammals.
3.2. The Binding Ability of CgRAR/CgRXR to RAREs and TYR Gene Promoter
As a ligand, RA regulates the expression of different genes through RA receptors. RAR and RXR are two classes of RA receptors belonging to the nuclear receptor superfamily. After the perception of RA, RAR and RXR interact with each other to form heterodimers or interact with themselves to form homodimers [8,14]. RAR and RXR directly bind to specific sequences of target genes and regulate target gene expression following a heterodimer or homodimer formation [5].
Our previous study revealed that the expression of both CgRAR and CgRXR was upregulated upon ATRA treatment [22]. In this study, we found that the expression of four TYR genes were upregulated after ATRA treatment according to the transcriptome data (Figure 2A,B). This result was subsequently conformed by RT-qPCR (Figure 2). The specific binding sites of RAR or RXR in the promoter regions of target genes are mostly RAREs composed of DRs spaced by 0–5 base pairs. Our results also revealed that CgRAR and CgRXR bind DR0–DR5 sequences in yeast (Figure 4). The interaction between CgRXR and DRs has been confirmed using an electrophoresis mobility shift assay (EMSA) [25]. Two possible RAREs were identified in the promoter regions of Tyr-12 and Tyr-2 (Figure 5A). Therefore, we hypothesized that CgRAR and CgRXR can directly bind to the promoters of these TYR genes. The results of the Y1H assay suggest that CgRAR can directly bind to the DR4 consisting of two core sequences, “AGGTCA” and “GGTTGA”, separated by four nucleotides in the Tyr-2 promoter (Figure 5B). Thus, we conclude that CgRAR can directly bind to the promoter region of Tyr-2. ATRA treatment activates the expression of Tyr-2. Further studies are needed to determine whether the binding of CgRAR to the Tyr-2 promoter in vitro directly contributes to the activation of Tyr-2 expression.
Previous studies have reported that RAR may not bind RA directly in some mollusks [20,21]. In Thaisb clavigera, Acanthochitona crinite, Patella vulgate, N. lapillus, and C. gigas, RAR does not activate the transcription of reporter genes in response to stimulation by retinoids [20,42]. In vertebrates, RAR-RXR binds to RAREs in the absence of ligands, whereby unliganded RARs recruit transcriptional co-repressors to negatively regulate the transcription of target genes [43]. One possibility is that in C. gigas, RAR binds to the promoter region to recruit co-repressor factors to inhibit the expression of target genes in the absence of RA. After RA treatment, RAR no longer binds to RARE and dissociates from the promoter region relieving inhibition, and allowing the expression of target genes to reactivate.
Although the expression of Tyr-12 and Tyr-9 was also activated after ATRA treatment (Figure 2), and a DR consisting of two core sequence “GGTTCA” spaced by seven bases was found in the Tyr-12 promoter region, we did not detect the binding activity of CgRAR or CgRXR to the promoter regions of these two genes. Furthermore, the expression of Tyr, Tyr-6, Tyr-9, Tyr-12, and Tyr-20 was upregulated by ATRA treatment; although typical RARE, as defined in vertebrates, was not found in the promoters of these genes. In vertebrates, hundreds of genes have been found to be RA-inducible, but only around 20 have been verified to contain functional RAREs [5]. Except those RA receptors directly binding to target gene promotors to regulate their expression, some target genes can be indirectly expressed by other molecular mechanisms in response to RA [5]. Thus, we suspect that in C. gigas, ATRA activates the expression of these TYR genes in a different manner, other than directly binding to the promoter region of these TYR genes by RA receptor.
3.3. The Relationship between RA Pathway, TYR Genes, and Melanin
We previously compared transcriptomes of black- and white-shelled oyster mantle tissue, and found that expression of the retinaldehyde dehydrogenase gene (which catalyzes the production of RA), as well as the tyrosinase gene of black-shelled oysters was significantly elevated relative to white-shelled oysters [37]. In this study, we found that the expression of Tyr, Tyr-6, Tyr-9, Tyr-12, and Tyr-20 was elevated after ATRA treatment (Figure 2 and Figure 3). Tyrosinase, encoded by the Tyr gene, is a multifunctional copper-containing oxidase that catalyzes the first two steps of melanin production in mammals, and is found in most organisms other than viruses. An expansion of tyrosinase genes has been observed in many bivalves, with more than 26 Tyr genes found in C. gigas [30,44]. In C. gigas, the expression of Tyr-9 and Tyr-12 in black-shelled oyster were significantly higher than that in white-shelled oysters [37]. A recent study revealed that Tyr was strongly expressed in black-shelled oysters, whereas almost no expression was detected in white-shelled oysters [36]. Several studies have linked RA with melanin production in vertebrates. RA influences melanocyte differentiation and proliferation in a dose- and time-dependent manner [45]. In mouse B16F10 melanoma cells, RA increases melanogenesis [34]. In chick RPF cells, RA induces TGF-β, which inhibits RPE cell proliferation and induces melanin synthesis [35]. Therefore, it is possible that the ATRA-induced upregulation of TYR genes contributes to melanin production in C. gigas. In addition, the role that Tyr-6 and Tyr-20 plays in melanin production requires further investigation.
4. Material and Methods
4.1. Experimental Materials
Adult oysters used in this study were collected from a local farm in Yantai, China. Oyster individuals with an average height of 60 mm and average weight of 24.6 g were selected for further studies. In laboratory conditions, all of the selected oysters were acclimated in filtered seawater for 7 days at temperatures of 19–22 °C under aerated conditions before the experiment. During cultivation, the oysters were fed with Isochrysis galbana twice a day and the seawater was changed daily.
4.2. RA Treatment
RA treatment was performed as previously described with a slight modification [22]. Briefly, ATRA (Sigma-aldrich) dissolved in DMSO was injected into the C. gigas individuals via the adductor muscle with a final concentration of 10 μM. Oysters injected with DMSO were used as the control. Injections were carried out every two days for a total of eight days. After treatment, mantle tissues were collected and frozen with liquid nitrogen for transcriptomic sequencing or RT-qPCR analysis.
4.3. Transcriptome Sequencing Analysis and Bioinformatics Analysis
For transcriptome sequencing, 18 individuals were randomly divided into two groups and injected with ATRA and DMSO, respectively. After injection, the mantle tissues of three oyster individuals were weighed in equal quantities and mixed to form one sample, and each group contained three sample replicates. Transcriptome sequencing was performed using the Illumina NovaSeq 6000 PE150 platform at OE Biotech Co., Ltd., (Shanghai, China). More than 46 million clean reads were obtained for each sample and mapped to the reference genome of C. gigas (GCF_902806645.1). The DEGs were identified using the DESeq functions estimateSizeFactors and nbinomTest [46], with a fold change > 2 and p value < 0.05. Gene ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) [47] pathway enrichment analysis of DEGs was performed using R based on hypergeometric distribution.
4.4. RNA Extraction and RT-qPCR
For RT-qPCR analysis, 12 individuals were randomly divided into two groups and, after injection, the mantle tissue RNA was isolated using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s protocol. cDNA was synthesized using the PrimeScript™ RT Master Mix kit (TaKaRa, Japan), and RT-qPCR analysis was performed using SYBR Premix Ex Taq II (TaKaRa, Japan) and a Bio-rad CFX connect PCR instrument. The RS18 gene was used as the reference gene for internal normalization. Relative expression levels of the target genes were calculated with the 2−△△CT method. Primers used for RT-qPCR are listed in Table S3.
4.5. Yeast One-Hybrid (Y1H) Assay
The DNA-binding activity of RA receptors in C. gigas was determined using the typical RARE core sequence as defined in vertebrates. DR0–DR5, formed by triple tandem copies of the RARE core sequence (AGGTCA) separated by 0–5 nucleotides, were produced by primers annealing. The generated DR0–DR5 sequences were then inserted into the pLacZi vector to construct the reporter plasmids. The mutated RARE core sequence was used to form mutated DR (drm) as a negative control. The primers used for the fusion vector construction are listed in Table S4.
To examine the interaction between CgRAR/CgRXR and TYR gene promoters, several 100–250 bp fragments from the promoter regions of the TYR genes containing the RAREs were amplified and inserted into the pLacZi vector. The RARE core sequences were mutated and used as negative controls.
Full-length coding sequences of CgRAR and CgRXR were cloned into the pB42AD vector, respectively. The yeast strain EGY48 was used to assess protein–DNA interactions in this study. Yeast co-transformants were screened on a selective SD/-Trp/-Ura medium, and positive transformants were screened using a SD/-Trp/-Ura chromogenic medium with Gal/Raf and X-gal.
5. Conclusions
Our results show that ATRA may participate in the biological processes of development, metabolism, reproduction, and immunity in oysters, which are similar to those in mammals. Expression of several melanin-related TYR genes were upregulated by ATRA. The RA receptors CgRAR and CgRXR can bind RAREs, and CgRAR interacts with the DR sequence in the promoter of Tyr-2. The expression of six TYR genes (Tyr-6, Tyr-9, Tyr-12, Tyr-20, Tyr, and Tyr-2) were upregulated by ATRA, whereas only the Tyr-2 promoter could be activated by the CgRAR, indicating that ATRA activates the expression of these TYR genes in a different manner. Thus, through the classical RA mechanism, CgRAR directly binds to the promoter region of Tyr-2, and then regulates the transcriptional expression of Tyr-2 in response to ATRA treatment. In addition, ATRA may activate TYR gene expression in other ways without directly binding to the promoters of these target genes by RA receptors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232112840/s1. Table S1. Differently expressed genes identified between ATRA- and DMSO-treated groups. Table S2. The expression profiles of TYR genes in transcriptome data. Table S3. Primers used for RT-qPCR analysis. Table S4. Primers used for fusion vectors construction.
Author Contributions
X.W. and Y.L. designed the experiment. Q.J., K.J., C.H., W.Y., B.H., M.Z., Y.H., X.Z., and Y.L. carried out the experiments; S.C., Y.Z., and L.W. contributed to the oyster collection and oyster tissue collection for transcriptome sequencing; Y.L. and X.W. wrote the manuscript, supervised the study, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. 41876193, 42076088 and 41906088), the Agricultural Variety Improvement Project of Shandong Province (No. 2019LZGC020), the National Key R&D Program of China (No. 2018YFD0901400), the Special Funds for Taishan Scholars Project of Shandong Province, China (No. tsqn201812094), the Shandong Provincial Natural Science Foundation, China (No. ZR2019MC002), the Modern Agricultural Industry Technology System of Shandong Province, China (SDAIT-14-03), and the Plan of Excellent Youth Innovation Team of Colleges and Universities in Shandong Province, China (2019KJF004).
Data Availability Statement
The data of current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| retinoic acid | RA |
| retinoic acid receptor | RAR |
| retinoid x receptor | RXR |
| retinoic acid response element | RARE |
| all-trans retinoic acid | ATRA |
| differentially expressed genes | DEGs |
| yeast one-hybrid | Y1H |
References
- Janesick, A.; Wu, S.C.; Blumberg, B. Retinoic acid signaling and neuronal differentiation. Cell. Mol. Life Sci. CMLS. 2015, 72, 1559–1576. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.J.; Duester, G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell Biol. 2015, 16, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowski, B.; Wragge, J.; Siegenthaler, J.A. Retinoic acid signaling in vascular. Development 2019, 57, e23287. [Google Scholar]
- Ghyselinck, N.B.; Duester, G. Retinoic acid signaling pathways. Development 2019, 146, dev167502. [Google Scholar] [CrossRef]
- Balmer, J.E.; Blomhoff, R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002, 43, 1773–1808. [Google Scholar] [CrossRef]
- Rochette-Egly, C. Retinoic acid-regulated target genes during development: Integrative genomics analysis. Subcell Biochem. 2020, 95, 57–85. [Google Scholar]
- Wang, X.J.; Chen, J.; Lv, Z.B.; Nie, Z.M.; Wang, D.; Shen, H.D.; Wang, X.D.; Wu, X.F.; Zhang, Y.Z. Expression and functional analysis of the cellular retinoic acid binding protein from silkworm pupae (Bombyx mori). J. Cell Biochem. 2007, 102, 970–979. [Google Scholar] [CrossRef]
- Gutierrez-Mazariegos, J.; Schubert, M.; Laudet, V. Evolution of retinoic acid receptors and retinoic acid signaling. Subcell Biochem. 2014, 70, 55–73. [Google Scholar]
- Albalat, R. The retinoic acid machinery in invertebrates: Ancestral elements and vertebrate innovations. Mol. Cell Endocrinol. 2009, 313, 23–35. [Google Scholar] [CrossRef]
- Rothwell, C.M.; De Hoog, E.; Spencer, G.E. The role of retinoic acid in the formation and modulation of invertebrate central synapses. J. Neurophysiol. 2017, 117, 692–704. [Google Scholar] [CrossRef]
- Dmetrichuk, J.M.; Carlone, R.L.; Jones, T.R.; Vesprini, N.D.; Spencer, G.E. Detection of endogenous retinoids in the molluscan CNS and characterization of the trophic and tropic actions of 9-cis retinoic acid on isolated neurons. J. Neurosci. 2008, 28, 13014–13024. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, J.; Mamiya, S.; Kanayama, T.; Nishikawa, T.; Shiraishi, F.; Horiguchi, T. Involvement of the retinoid X receptor in the development of imposex caused by organotins in gastropods. Environ. Sci. Technol. 2004, 38, 6271–6276. [Google Scholar] [CrossRef] [PubMed]
- le Maire, A.; Bourguet, W. Retinoic acid receptors: Structural basis for coregulator interaction and exchange. Subcell Biochem. 2014, 70, 37–54. [Google Scholar] [PubMed]
- le Maire, A.; Teyssier, C.; Balaguer, P.; Bourguet, W.; Germain, P. Regulation of RXR-RAR Heterodimers by RXR- and RAR-Specific Ligands and Their Combinations. Cells 2019, 8, 1392. [Google Scholar] [CrossRef] [PubMed]
- Benbrook, D.M.; Chambon, P.; Rochette-Egly, C.; Asson-Batres, M.A. History of retinoic acid receptors. Subcell Biochem. 2014, 70, 1–20. [Google Scholar] [PubMed]
- Giguère, V. Retinoic acid receptors and cellular retinoid binding proteins: Complex interplay in retinoid signaling. Endocr. Rev. 1994, 15, 61–79. [Google Scholar] [PubMed]
- Balmer, J.E.; Blomhoff, R. A robust characterization of retinoic acid response elements based on a comparison of sites in three species. J. Steroid Biochem. Mol. Biol. 2005, 96, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Urushitani, H.; Katsu, Y.; Ohta, Y.; Shiraishi, H.; Iguchi, T.; Horiguchi, T. Cloning and characterization of the retinoic acid receptor-like protein in the rock shell, Thais clavigera. Aquat. Toxicol. 2013, 142–143, 403–413. [Google Scholar] [CrossRef]
- Urushitani, H.; Katsu, Y.; Ohta, Y.; Shiraishi, H.; Iguchi, T.; Horiguchi, T. Cloning and characterization of retinoid X receptor (RXR) isoforms in the rock shell, Thais clavigera. Aquat. Toxicol. 2011, 103, 101–111. [Google Scholar] [CrossRef]
- Juliana, G.M.; Kumar, N.E.; Daniela, L.; Keely, P.; Jones, J.W.; Maureen, K.; Jun-Ichi, N.; Youhei, H.; Tsuyoshi, N.; Santos, M.M. A mollusk retinoic acid receptor (RAR) ortholog sheds light on the evolution of ligand binding. Endocrinology 2014, 155, 4275–4286. [Google Scholar]
- Vogeler, S.; Galloway, T.S.; Isupov, M.; Bean, T.P. Cloning retinoid and peroxisome proliferator-activated nuclear receptors of the Pacific oyster and in silico binding to environmental chemicals. PLoS ONE 2017, 12, e0176024. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Jin, Q.; Cai, Z.; Huang, B.; Wei, L.; Zhang, M.; Guo, W.; Liu, Y.; Wang, X. Molecular characterization of retinoic acid receptor CgRAR in Pacific oyster (Crassostrea gigas). Front. Physiol. 2021, 12, 666842. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xu, F.; Qu, T.; Zhang, R.; Li, L.; Que, H.; Zhang, G. Identification of thyroid hormones and functional characterization of thyroid hormone receptor in the Pacific oyster Crassostrea gigas provide insight into evolution of the thyroid hormone system. PLoS ONE 2015, 10, e0144991. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Li, Q.; Yu, H.; Liu, S.; Kong, L. A nuclear receptor heterodimer, CgPPAR2-CgRXR, acts as a regulator of carotenoid metabolism in Crassostrea gigas. Gene 2022, 827, 146473. [Google Scholar] [CrossRef]
- Huang, W.; Wu, Q.; Xu, F.; Li, L.; Li, J.; Que, H.; Zhang, G. Functional characterization of retinoid X receptor with an emphasis on the mediation of organotin poisoning in the Pacific oyster (Crassostrea gigas). Gene 2020, 753, 144780. [Google Scholar] [CrossRef]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Invest. Dermatol. 2007, 127, 751–761. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, A.; Rodríguez-López, J.N.; García-Cánovas, F.; García-Carmona, F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Rzepka, Z.; Buszman, E.; Beberok, A.; Wrześniok, D. From tyrosine to melanin: Signaling pathways and factors regulating melanogenesis. Postepy Hig. Med. Dosw. 2016, 70, 695–708. [Google Scholar] [CrossRef]
- Cordero, R.J.B.; Casadevall, A. Melanin. Curr. Biol. 2020, 30, 142–143. [Google Scholar] [CrossRef]
- Zhang, G.; Fang, X.; Guo, X.; Li, L.; Luo, R.; Xu, F.; Yang, P.; Zhang, L.; Wang, X.; Qi, H.; et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef]
- Yu, F.; Qu, B.; Lin, D.; Deng, Y.; Huang, R.; Zhong, Z. Pax3 gene regulated melanin synthesis by tyrosinase pathway in Pteria penguin. Int. J. Mol. Sci. 2018, 19, 3700. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Yano, M.; Morimoto, K.; Miyamoto, H. Tyrosinase localization in mollusc shells. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, X.; Bai, Z.; Zhao, L.; Li, J. HcTyr and HcTyp-1 of Hyriopsis cumingii, novel tyrosinase and tyrosinase-related protein genes involved in nacre color formation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 204, 1–8. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Arcuri, R.; Morgado-Díaz, J.A.; Benchimol, M. Increase of melanogenesis by retinoic acid: An ultrastructural and morphometric study. Tissue Cell 2004, 36, 95–105. [Google Scholar] [CrossRef]
- Kishi, H.; Kuroda, E.; Mishima, H.K.; Yamashita, U. Role of TGF-beta in the retinoic acid-induced inhibition of proliferation and melanin synthesis in chick retinal pigment epithelial cells in vitro. Cell Biol. Int. 2001, 25, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Q.; Yu, H.; Liu, S.; Kong, L. Shell biosynthesis and pigmentation as revealed by the expression of tyrosinase and tyrosinase-like protein genes in Pacific oyster (Crassostrea gigas) with different shell colors. Mar. Biotechnol. 2021, 23, 777–789. [Google Scholar] [CrossRef]
- Wei, L.; Jiang, Q.; Cai, Z.; Yu, W.; He, C.; Guo, W.; Wang, X. Immune-related molecular and physiological differences between black-shelled and white-shelled Pacific oysters Crassostrea gigas. Fish Shellfish. Immunol. 2019, 92, 64–71. [Google Scholar] [CrossRef]
- Carter, C.J.; Rand, C.; Mohammad, I.; Lepp, A.; Vesprini, N.; Wiebe, O.; Carlone, R.; Spencer, G.E. Expression of a retinoic acid receptor (RAR)-like protein in the embryonic and adult nervous system of a protostome species. J. Exp. Zool. Part B Mol. Dev. Evol. 2015, 324, 51–67. [Google Scholar] [CrossRef]
- Vesprini, N.D.; Dawson, T.F.; Yuan, Y.; Bruce, D.; Spencer, G.E. Retinoic acid affects calcium signaling in adult molluscan neurons. J. Neurophysiol. 2015, 113, 172–181. [Google Scholar] [CrossRef]
- Czarnewski, P.; Das, S.; Parigi, S.M.; Villablanca, E.J. Retinoic acid and its role in modulating intestinal innate immunity. Nutrients 2017, 9, 68. [Google Scholar] [CrossRef]
- Kim, C.H. Retinoic acid, immunity, and inflammation. Vitam. Horm. 2011, 86, 83–101. [Google Scholar] [PubMed]
- André, A.; Ruivo, R.; Fonseca, E.; Froufe, E.; Castro, L.F.C.; Santos, M.M. The retinoic acid receptor (RAR) in molluscs: Function, evolution and endocrine disruption insights. Aquat. Toxicol. 2019, 208, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Niederreither, K.; Dolle, P. Retinoic acid in development: Towards an integrated view. Nat. Rev. Genet. 2008, 9, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, F.; McDougall, C.; Degnan, B.M. Evolution of the tyrosinase gene family in bivalve molluscs: Independent expansion of the mantle gene repertoire. Acta Biomater. 2014, 10, 3855–3865. [Google Scholar] [CrossRef]
- VanBuren, C.A.; Everts, H.B. Vitamin A in skin and hair: An update. Nutrients 2022, 14, 2952. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential Expression of RNA-Seq Data at the Gene Level—The DESeq Package; EMBL: Heidelberg, Germany, 2012. [Google Scholar]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic. Acids Res. 2008, 36, 480–484. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).