Relationship between Postural Stability, Lead Content, and Selected Parameters of Oxidative Stress
Abstract
1. Introduction
2. Results and Discussion
2.1. Demographic Values and Biochemical Test Results
2.2. Posturography Test
- -
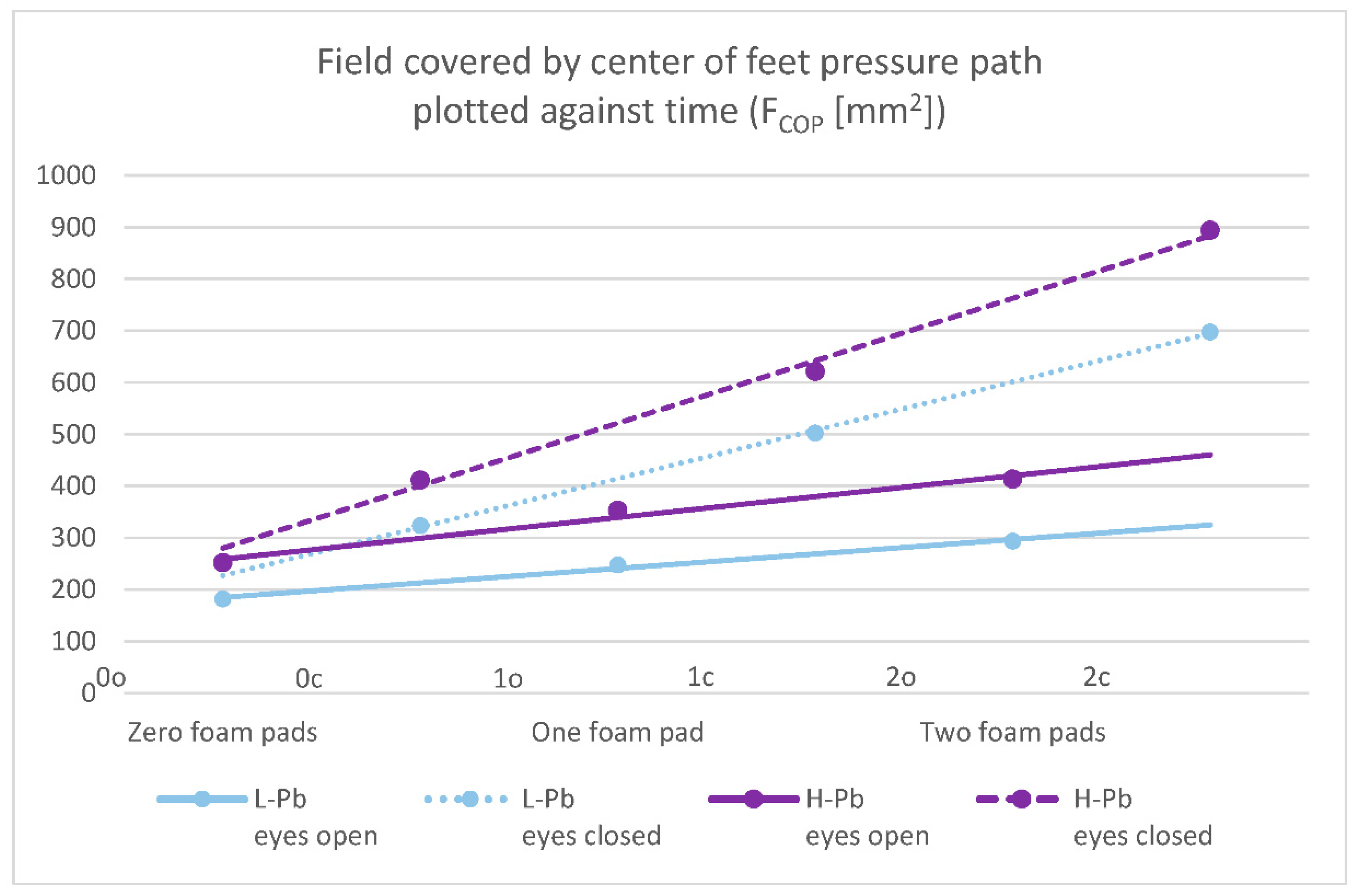
- FCOP—field covered by the center of feet pressure path plotted against time—statistical significance was demonstrated in all test variants (number of foam pads and eye status: 0o, 0c, 1o, 1c, 2o, 2c). The mean results of this parameter show a positive change between the L-Pb and H-Pb subgroups, ranging from 24% to 43% (calculations based on the values in Table 3). The mean values of this parameter show an increasing tendency with increasing difficulty of the test (Figure 1). Moreover, the upward trends (slope of the trend line) show similarity in the L-Pb and H-Pb subgroups in tests with different eye statuses: open/closed.
- -
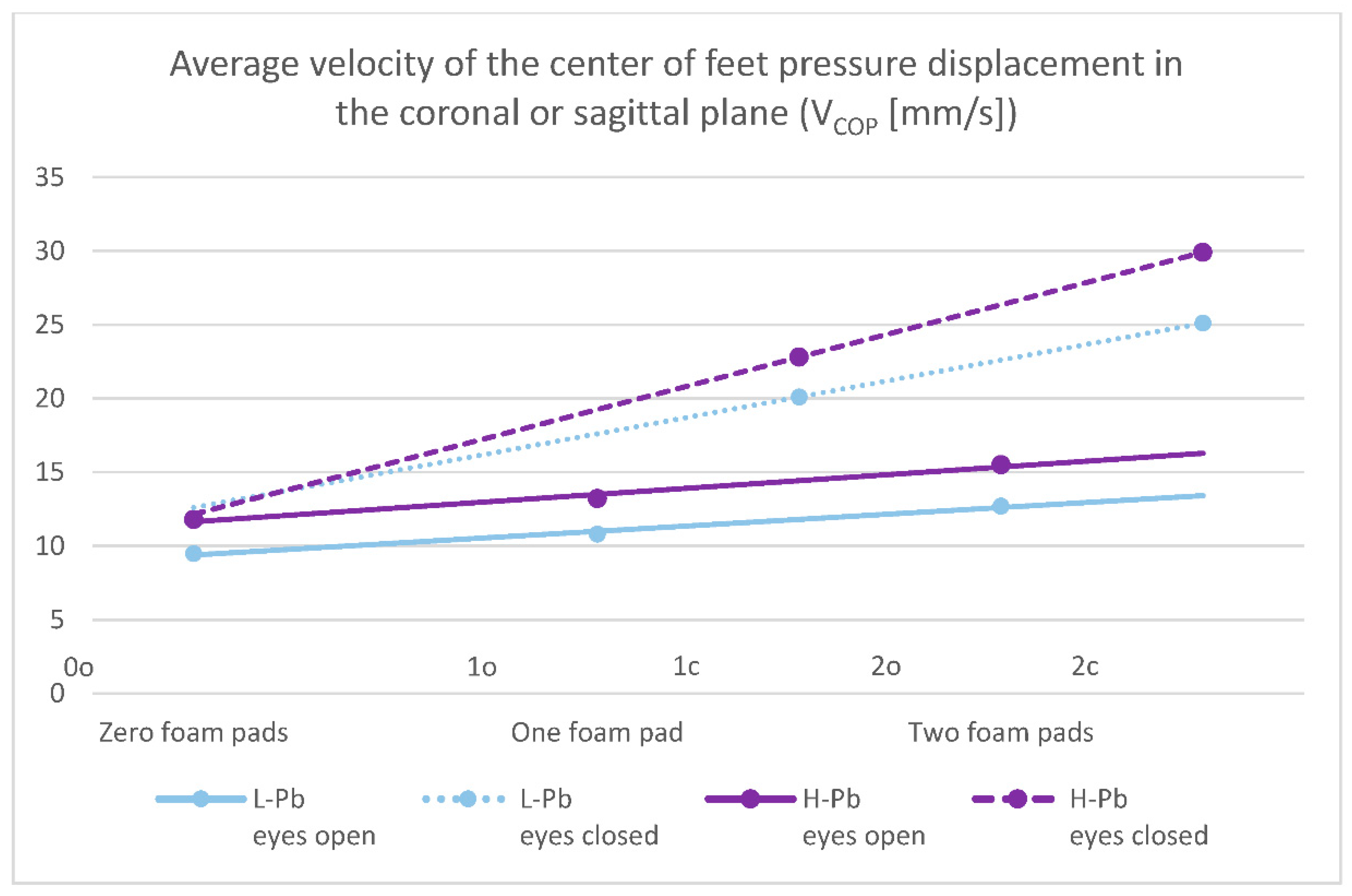
- VCOP—average velocity of the center of feet pressure displacement in the coronal or sagittal plane—the mean values of this parameter show statistical significance in almost all test variants (0o, 1o, 1c, 2o, 2c). The parameter mean values show a positive difference between the subgroups, ranging from 14% to 24% (calculations based on the values in Table 3). The mean values of this parameter show an increasing tendency with increasing difficulty of the test (Figure 2), and there is also an upward trend (slope of the trend line), which shows similarity in subgroups in tests with different eye statuses: open/closed.
- -
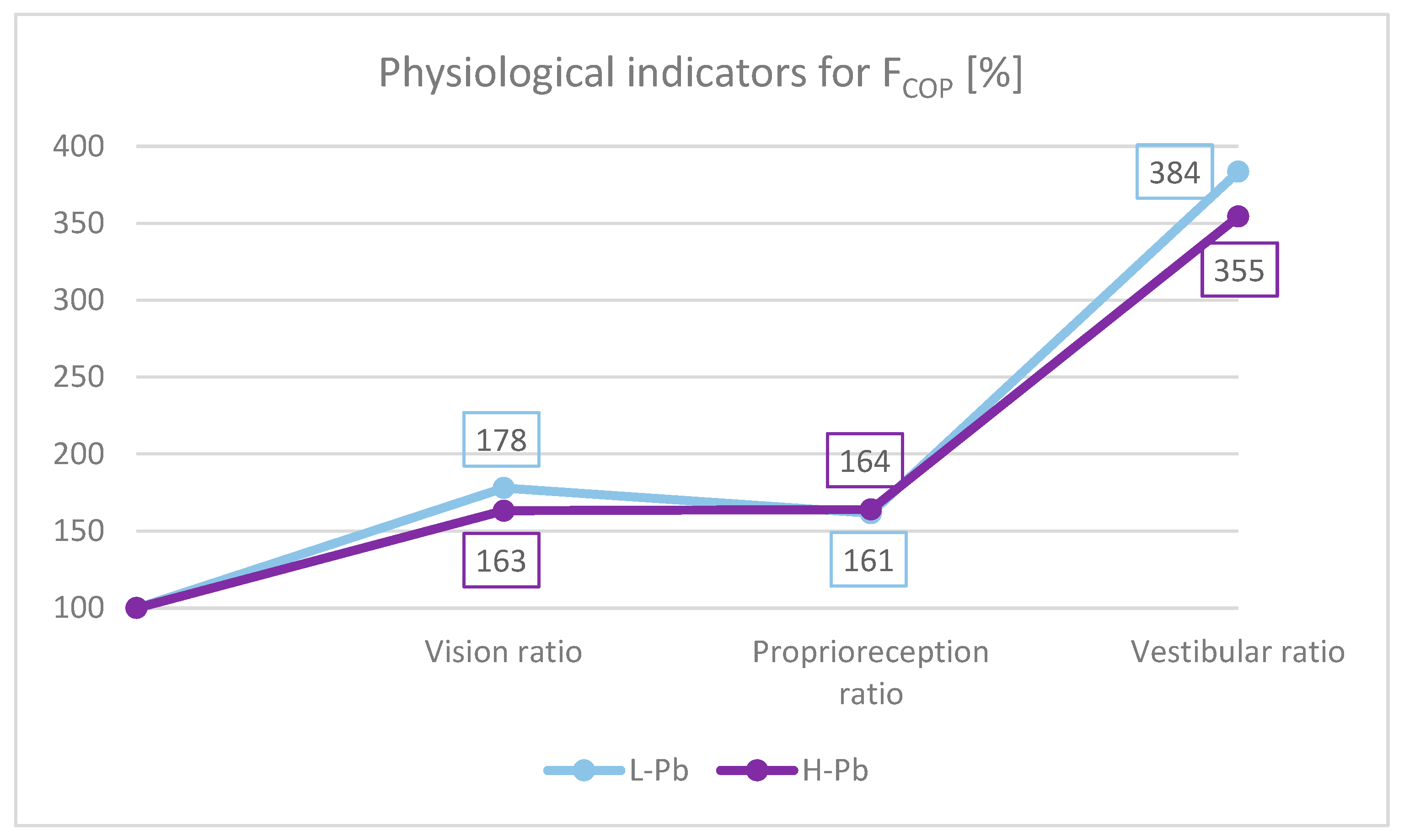
- Effect of the sense of sight on the results: vision ratio = 0c/0o;
- -
- Effect of change in proprioreception of lower extremities: proprioreception ratio = 2o/0o;
- -
- The effect of the greatest influence on the vestibular system (labyrinth in the inner ear and vestibular nuclei in the CNS): vestibular ratio = 2c/0o.
2.3. Oxidative Stress Markers
2.4. Minerals and Metals: Cd, Ca, Fe, Mg, Zn
2.5. Summary, Strengths, and Limitations
- -
- Strong results—lists the results obtained, highlighting the most important of them;
- -
- Results requiring further research—setting out possible directions of projects in the future.
3. Material and Methods
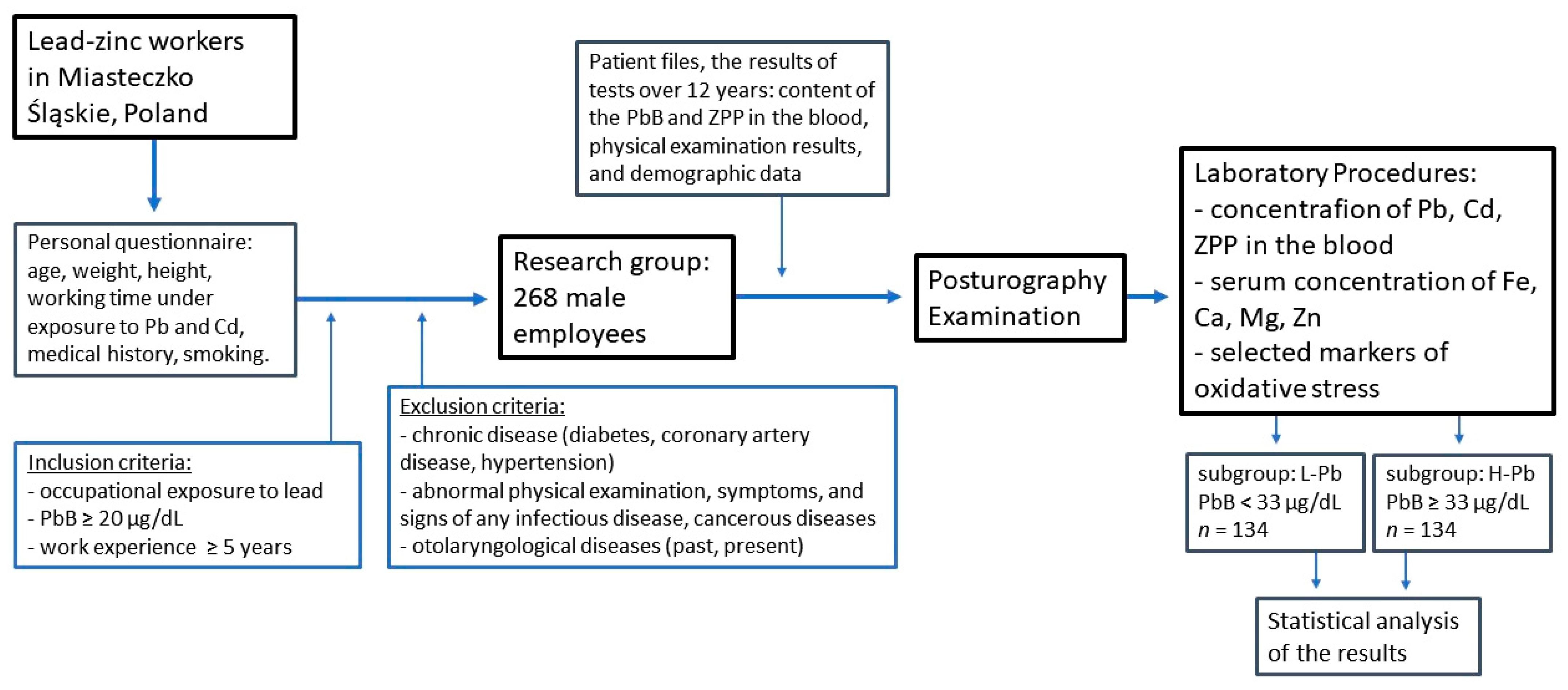
3.1. Study Population
3.2. Posturography Examination
3.3. Laboratory Procedures
3.4. Concentrations of Lead (Pb), Cadmium (Cd), and Zinc Protoporphyrin (ZPP)
3.5. Concentrations of Selected Essential Elements: Iron (Fe), Calcium (Ca), Magnesium (Mg), and Zinc (Zn)
3.6. Biochemical Procedures of Antioxidant Defense and Oxidative Stress Markers
3.7. Time Sequence of the Study
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obeng-Gyasi, E. Sources of lead exposure in various countries. Rev. Environ. Health 2019, 34, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic Effects and Biomarkers of Lead Exposure: A Review. Rev. Environ. Health 2009, 24, 15–45. [Google Scholar] [CrossRef] [PubMed]
- Vorvolakos, T.; Arseniou, S.; Samakouri, M. There is no safe threshold for lead exposure: A literature review. Psychiatriki 2016, 27, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Mandalunis, P.M. A review of metal exposure and its effects on bone health. J. Toxicol. 2018, 11, 4854152. [Google Scholar] [CrossRef]
- Harari, F.; Sallsten, G.; Christensson, A.; Petkovic, M.; Hedblad, B.; Forsgard, N.; Melander, O.; Nilsson, P.M.; Borné, Y.; Engström, G.; et al. Blood Lead Levels and Decreased Kidney Function in a Population-Based Cohort. Am. J. Kidney Dis. 2018, 72, 381–389. [Google Scholar] [CrossRef]
- Pawlas, N.; Broberg, K.; Olewińska, E.; Prokopowicz, A.; Skerfving, S.; Pawlas, K. Modification by the genes ALAD and VDR of lead-induced cognitive effects in children. Neurotoxicology 2012, 33, 37–43. [Google Scholar] [CrossRef]
- Liu, H.-L.; Chuang, H.-Y.; Hsu, C.-N.; Lee, S.-S.; Yang, C.-C.; Liu, K.-T. Effects of Vitamin D Receptor, Metallothionein 1A, and 2A Gene Polymorphisms on Toxicity of the Peripheral Nervous System in Chronically Lead-Exposed Workers. Int. J. Environ. Res. Public Health 2020, 17, 2909. [Google Scholar] [CrossRef]
- Kasperczyk, A.; Machnik, G.; Dobrakowski, M.; Sypniewski, D.; Birkner, E.; Kasperczyk, S. Gene expression and activity of antioxidant enzymes in the blood cells of workers who were occupationally exposed to lead. Toxicology 2012, 301, 79–84. [Google Scholar] [CrossRef]
- Dobrakowski, M.; Boroń, M.; Birkner, E.; Kasperczyk, A.; Chwalińska, E.; Lisowska, G.; Kasperczyk, S. The Effect of a Short-Term Exposure to Lead on the Levels of Essential Metal Ions, Selected Proteins Related to Them, and Oxidative Stress Parameters in Humans. Oxid. Med. Cell. Longev. 2017, 9, 8763793. [Google Scholar] [CrossRef]
- Wyparło-Wszelaki, M.; Machoń-Grecka, A.; Wąsik, M.; Dobrakowski, M. Critical aspects of the physiological interactions between lead and magnesium. J. Biochem. Mol. Toxicol. 2021, 36, e22964. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, M.J.; Fuente, A. The Adverse Effects of Heavy Metals with and without Noise Exposure on the Human Peripheral and Central Auditory System: A Literature Review. Int. J. Environ. Res. Public Health 2016, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Sato, H.; Yokoyama, K.; Murata, K. Subclinical Neurophysiological Effects of Lead: A Review on Peripheral, Central, and Autonomic Nervous System Effects in Lead Workers. Am. J. Ind. Med. 2000, 37, 193–204. [Google Scholar] [CrossRef]
- Min, K.B.; Lee, K.J.; Park, J.B.; Min, J.Y. Lead and Cadmium Levels and Balance and Vestibular Dysfunction among Adult Participants in the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Environ. Health Perspect. 2012, 120, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.S.; Durward, B.R.; Rowe, P.J.; Paul, J.P. What is balance? Clin. Rehabil. 2000, 14, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.D.; Speers, R.A.; Peterka, R.J.; Horak, F.B. Effect of altered sensory conditions on multivariate descriptors of human postural sway. Exp. Brain Res. 1998, 122, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Skalska, A.; Ocetkiewicz, T.; Żak, M.; Grodzicki, T. The influence of age on the parameters of the postural control measured by the computer balance platform. New Med. 2004, 7, 12–19. [Google Scholar]
- Yokoyama, K.; Araki, S.; Nishikitani, M.; Sato, H. Computerized Posturography with Sway Frequency Analysis: Application in Occupational and Environmental Health. Ind. Health 2002, 40, 14–22. [Google Scholar] [CrossRef]
- Park, D.S.; Lee, G.C. Validity and reliability of balance assessment software using the Nintendo Wii balance board: Usability and validation. J. Neuroeng. Rehabil. 2014, 11, 99. [Google Scholar] [CrossRef]
- Hebert, J.R.; Manago, M.M. Reliability and Validity of the Computerized Dynamic Posturography Sensory Organization Test in People with Multiple Sclerosis. Int. J. MS Care 2017, 19, 151–157. [Google Scholar] [CrossRef]
- Morisod, B.; Mermod, M.; Maire, R. Posturographic pattern of patients with chronic subjective dizziness before and after vestibular rehabilitation. J. Vestib. Res. 2018, 27, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Falls, C. Videonystagmography and Posturography. Adv. Otorhinolaryngol. 2019, 82, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Imai, T.; Oya, R.; Okumura, T.; Sato, T.; Osaki, Y.; Ohta, Y.; Inohara, H. Platform posturography of patients with peripheral vestibular dysfunction in the non-acute phase of vertigo. Auris Nasus Larynx 2021, 48, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Patrick, L. Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern. Med. Rev. 2006, 11, 2–22. [Google Scholar]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Chia, S.E.; Chua, L.H.; Ng, T.P.; Foo, S.C.; Jeyaratnam, J. Postural Stability of Workers Exposed to Lead. Occup. Environ. Med. 1994, 51, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Pawlas, N.; Broberg, K.; Skerfving, S.; Pawlas, K. Disturbance of posture in children with very low lead exposure, and modification by VDR FokI genotype. Ann. Agric. Environ. Med. 2014, 21, 739–744. [Google Scholar] [CrossRef]
- Chia, S.E.; Chia, H.P.; Ong, C.N.; Jeyaratnam, J. Cumulative concentrations of blood lead and postural stability. Occup. Environ. Med. 1996, 53, 264–268. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Shukla, R.; Bornschein, R.L.; Dietrich, K.N.; Keitht, R. Lead Effects on Postural Balance of Children. Environ. Health Perspect. 1990, 89, 35–42. [Google Scholar] [CrossRef]
- Nielsen, F.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grandjean, P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [CrossRef]
- Brunk, U.T.; Terman, A. Lipofuscin: Mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med. 2002, 33, 611–619. [Google Scholar] [CrossRef]
- Carocci, A.; Catalano, A.; Lauria, G.; Sinicropi, M.S.; Genchi, G. Lead toxicity, antioxidant defense and environment. In Reviews of Environmental Contamination and Toxicology; Gunther, F.A., de Voogt, P., Eds.; Springer: New York, NY, USA, 2016; pp. 45–67. [Google Scholar] [CrossRef]
- Anetor, J.I.; Ajose, O.A.; Adebiyi, J.A.; Akingbola, T.S.; Iyanda, A.A.; Ebesunu, M.O.; Babalola, O.O.; Aadeniyi, F.A.A. Decreased thiamine and magnesium levels in the potentiation of the neurotoxicity of lead in occupational lead exposure. Biol. Trace Elem. Res. 2007, 116, 43–51. [Google Scholar] [CrossRef]
- Dongre, N.N.; Suryakar, A.N.; Patil, A.J.; Hundekari, I.A.; Devarnavadagi, B.B. Biochemical effects of lead exposure on battery manufacture workers with reference to blood pressure, calcium metabolism and bone mineral density. Indian J. Clin. Biochem. 2013, 28, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, S.S.; Hwangbo, Y.; Ahn, K.D.; Lee, B.K. Cross-sectional study of blood lead effects on iron status in Korean lead workers. Nutrition 2003, 19, 571–576. [Google Scholar] [CrossRef]
- Wright, R.O.; Tsaih, S.W.; Schwartz, J.; Wright, R.J.; Hu, H. Association between iron deficiency and blood lead level in a longitudinal analysis of children followed in an urban primary care clinic. J. Pediatr. 2003, 142, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.I.; Trapani, V.; Simonacci, M.; Ferré, S.; Maier, J.A. Magnesium deficiency and endothelial dysfunction: Is oxidative stress involved? Magnes Res. 2008, 21, 58–64. [Google Scholar]
- Ahamed, M.; Singh, S.; Behari, J.R.; Kumar, A.; Siddiqui, M.K. Interaction of lead with some essential trace metals in the blood of anemic children from Lucknow, India. Clin. Chim. Acta 2007, 377, 92–97. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Jain, S.K. In vivo externalization of phosphatidylserine and phosphatidylethanolamine in the membrane bilayer and hy-percoagulability by the lipid peroxidation of erythrocytes in rats. J. Clin. Investig. 1985, 76, 281–286. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
| L-Pb PbB < 33 (µg/dL) n = 134 | H-Pb PbB ≥ 33 (µg/dL) n = 134 | Variation (%) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD/n | Mean | SD/n | ||||
| Age (years) | 38.91 | 9.83 | 40.57 | 7.53 | 4% | 0.123 | |
| Years of work in exposure to Pb (years) | 10.63 | 9.27 | 13.78 | 7.29 | 30% | 0.002 | |
| Body weight (kg) | 84.62 | 12.63 | 88.11 | 14.74 | 4.1% | 0.083 | |
| Height (cm) | 177.17 | 6.80 | 177.52 | 5.76 | 0.2% | 0.713 | |
| BMI | 26.70 | 4.58 | 27.91 | 4.13 | 4.5% | 0.062 | |
| DM | 1.9% | 5 | 3.5% | 3 | 0.503 | ||
| CAD | 1.9% | 5 | 1.2% | 1 | 0.679 | ||
| HA | 9.7% | 27 | 9.4% | 7 | 0.945 | ||
| Smoking | Yes/No | 22.5% | 63 | 34.2% | 26 | 0.084 | |
| In the past (years) | 13.2 | 7.6 | 13.5 | 7.7 | 2.4% | 0.832 | |
| Currently (number of pieces) | 13.2 | 6.5 | 11.1 | 5.8 | −15.9% | 0.231 | |
| L-Pb PbB < 33 (µg/dL) n = 134 | H-Pb PbB ≥ 33 (µg/dL) n = 134 | Variation (%) | p-Value | |||
|---|---|---|---|---|---|---|
| Mean | SD/n | Mean | SD/n | |||
| PbB (µg/dL) * | 23.0 | 10.3 | 41.5 | 7.6 | 80% | <0.001 |
| PbB (µg/dL) ** | 22.9 | 8.7 | 41.1 | 4.5 | 80% | <0.001 |
| ZPP (µg/g Hb) * | 3.80 | 1.80 | 7.07 | 3.70 | 86% | <0.001 |
| ZPP (µg/g Hb) ** | 3.68 | 1.60 | 6.66 | 2.82 | 81% | <0.001 |
| CdB (µg/L) | 1.99 | 1.70 | 2.51 | 1.95 | 26% | 0.040 |
| Ca (mmol/L) | 2.45 | 0.26 | 2.39 | 0.26 | −3% | 0.011 |
| Fe (µg/dL) | 20.9 | 7.2 | 22.0 | 7.9 | 5% | 0.665 |
| Mg (mmol/L) | 0.82 | 0.15 | 0.81 | 0.11 | −2% | 0.460 |
| Zn (mmol/L) | 14.1 | 4.5 | 13.9 | 4.7 | −1% | 0.862 |
| MDA in serum (µmol/L) | 2.67 | 1.52 | 3.01 | 1.02 | 13% | 0.043 |
| MDA in erythrocytes (nmol/g Hb) | 242.7 | 79.4 | 227.0 | 71.1 | −6% | 0.109 |
| LPS (RF/g Hb) | 665.1 | 340.9 | 683.5 | 293.2 | 3% | 0.657 |
| TOS (µmol/L) | 10.5 | 4.0 | 10.2 | 3.8 | −3% | 0.605 |
| TAC (mmol/L) | 1.12 | 0.13 | 1.14 | 0.11 | 2% | 0.043 |
| OSI (%) | 0.97 | 0.45 | 0.92 | 0.43 | −5% | 0.385 |
| L-Pb PbB < 33 (µg/dL) n = 134 | H-Pb PbB ≥ 33 (µg/dL) n = 134 | Variation (%) | p-Value | |||
|---|---|---|---|---|---|---|
| Mean | SD/n | Mean | SD/n | |||
| Zero foam pads, eyes open (0o): | ||||||
| LCOP (mm) | 4.55 | 1.86 | 5.02 | 1.78 | 10% | 0.041 |
| MAPSCOP (mm) | 2.59 | 1.23 | 2.88 | 1.00 | 11% | 0.040 |
| MLSCOP (mm) | 3.19 | 1.42 | 3.49 | 1.49 | 9% | 0.096 |
| FCOP (mm2) | 181.8 | 171.6 | 252.1 | 209.0 | 39% | 0.003 |
| VCOP (mm/s) | 9.48 | 3.46 | 11.79 | 8.53 | 24% | 0.005 |
| Sinte (mm/s) | 4.44 | 1.75 | 4.81 | 1.77 | 8% | 0.310 |
| Zero foam pads, eyes closed (0c): | ||||||
| LCOP (mm) | 5.68 | 2.21 | 6.12 | 1.93 | 8% | 0.086 |
| MAPSCOP (mm) | 3.38 | 1.52 | 3.67 | 1.32 | 8% | 0.113 |
| MLSCOP (mm) | 4.10 | 2.89 | 4.18 | 1.51 | 2% | 0.795 |
| FCOP (mm2) | 323.5 | 300.4 | 411.3 | 302.1 | 27% | 0.020 |
| VCOP (mm/s) | 17.5 | 24.2 | 18.2 | 8.1 | 4% | 0.758 |
| Sinte (mm/s) | 6.08 | 2.11 | 6.59 | 2.14 | 8% | 0.054 |
| Two foam pads, eyes open (2o): | ||||||
| LCOP (mm) | 5.71 | 2.08 | 6.11 | 2.18 | 7% | 0.136 |
| MAPSCO (mm) | 3.41 | 1.36 | 3.63 | 1.42 | 6% | 0.202 |
| MLSCOP (mm) | 3.82 | 1.82 | 4.21 | 1.65 | 10% | 0.070 |
| FCOP (mm2) | 293.6 | 247.4 | 413.2 | 330.4 | 41% | 0.001 |
| VCOP (mm/s) | 12.7 | 5.1 | 15.5 | 6.6 | 22% | <0.001 |
| Sinte (mm/s) | 5.58 | 1.95 | 6.41 | 2.50 | 15% | 0.003 |
| Two foam pads, eyes closed (2c): | ||||||
| LCOP (mm) | 8.33 | 2.90 | 9.68 | 8.70 | 16% | 0.097 |
| MAPSCOP (mm) | 5.14 | 2.00 | 5.58 | 1.85 | 9% | 0.068 |
| MLSCOP (mm) | 5.44 | 2.05 | 5.80 | 1.86 | 7% | 0.139 |
| FCOP (mm2) | 697.4 | 556.8 | 893.7 | 595.8 | 28% | 0.007 |
| VCOP (mm/s) | 25.1 | 11.5 | 29.9 | 12.3 | 19% | 0.001 |
| Sinte (mm/s) | 8.84 | 3.16 | 10.35 | 9.34 | 17% | 0.029 |
| Age (Years) | Years of Work in Exp. to Pb (Years) | PbB (µg/dL) * | PbB (µg/dL) ** | ZPP (µg/g Hb) * | ZPP (µg/g Hb) ** | MDA in Serum (µmol/L) | MDA in Eryth. (nmol/g Hb) | LPS (RF/g Hb) | TAC (mmol/L) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Zero foam pads, eyes open (0o): | ||||||||||
| LCOP (mm) | 0.20 | 0.16 | 0.19 | 0.17 | 0.08 | 0.05 | 0.04 | 0.06 | 0.16 | −0.06 |
| MAPSCOP (mm) | 0.15 | 0.13 | 0.18 | 0.17 | 0.04 | 0.03 | 0.07 | 0.05 | 0.18 | −0.07 |
| MLSCOP (mm) | 0.19 | 0.15 | 0.17 | 0.15 | 0.09 | 0.06 | 0.04 | 0.06 | 0.12 | −0.05 |
| FCOP (mm2) | 0.17 | 0.16 | 0.23 | 0.22 | 0.14 | 0.13 | 0.06 | 0.02 | 0.15 | −0.05 |
| VCOP (mm/s) | 0.13 | 0.17 | 0.13 | 0.18 | 0.11 | 0.07 | 0.05 | −0.02 | 0.08 | 0.03 |
| Zero foam pads, eyes closed (0c): | ||||||||||
| LCOP (mm) | 0.23 | 0.18 | 0.12 | 0.14 | 0.10 | 0.09 | 0.15 | 0.02 | 0.13 | −0.03 |
| MAPSCOP (mm) | 0.24 | 0.21 | 0.13 | 0.15 | 0.09 | 0.11 | 0.14 | −0.03 | 0.11 | −0.06 |
| MLSCOP (mm) | 0.19 | 0.14 | 0.10 | 0.13 | 0.10 | 0.08 | 0.12 | 0.03 | 0.12 | 0.00 |
| FCOP (mm2) | 0.24 | 0.20 | 0.19 | 0.22 | 0.12 | 0.13 | 0.13 | 0.00 | 0.14 | −0.06 |
| VCOP (mm/s) | 0.21 | 0.21 | 0.12 | 0.16 | 0.11 | 0.10 | 0.00 | −0.01 | 0.05 | −0.02 |
| Two foam pads, eyes open (2o): | ||||||||||
| LCOP (mm) | 0.16 | 0.07 | 0.11 | 0.11 | 0.02 | 0.04 | 0.10 | −0.04 | 0.17 | −0.04 |
| MAPSCOP (mm) | 0.11 | 0.05 | 0.11 | 0.10 | 0.00 | 0.02 | 0.13 | 0.02 | 0.11 | −0.02 |
| MLSCOP (mm) | 0.16 | 0.04 | 0.11 | 0.11 | 0.04 | 0.05 | 0.04 | −0.10 | 0.12 | −0.03 |
| FCOP (mm2) | 0.15 | 0.12 | 0.21 | 0.20 | 0.10 | 0.12 | 0.15 | −0.02 | 0.13 | −0.05 |
| VCOP (mm/s) | 0.17 | 0.15 | 0.17 | 0.21 | 0.10 | 0.11 | 0.14 | −0.01 | 0.12 | 0.04 |
| Two foam pads, eyes closed (2c): | ||||||||||
| LCOP (mm) | 0.16 | 0.09 | 0.14 | 0.18 | 0.04 | 0.05 | 0.02 | 0.07 | 0.08 | −0.06 |
| MAPSCOP (mm) | 0.11 | 0.07 | 0.11 | 0.15 | −0.02 | 0.01 | −0.01 | 0.08 | 0.15 | −0.02 |
| MLSCOP (mm) | 0.19 | 0.11 | 0.15 | 0.18 | 0.11 | 0.09 | 0.03 | 0.04 | 0.01 | −0.06 |
| FCOP (mm2) | 0.17 | 0.15 | 0.18 | 0.22 | 0.05 | 0.10 | 0.09 | 0.11 | 0.20 | −0.06 |
| VCOP (mm/s) | 0.20 | 0.16 | 0.16 | 0.23 | 0.10 | 0.13 | 0.10 | 0.08 | 0.18 | −0.03 |
| Strong Results | Results Requiring Further Research |
|---|---|
|
|
| Strengths | Limitations |
|---|---|
|
|
| Parameter | Description |
|---|---|
| Posturographic Examination | |
| LCOP | Path length—the total distance traveled by the COP in the specified time, described in mm |
| MAPSCOP | Mean sway of the COP from point 0 in the anterior-posterior direction, measured in mm |
| MLSCOP | Mean sway of the COP from point 0 in the lateral direction, in mm |
| FCOP | Field covered by the COP path plotted against time, described in mm2 |
| VCOP | Average velocity of the COP displacement in the coronal or sagittal plane, in mm/s |
| Sinte | Sway intensity—the root mean square of accelerations, recorded in the 0.1 Hz to 10.1 Hz band during the test period, in mm/s |
| SI | Sway index *—factors specified by the manufacturer |
| Test Variants | |
| 0o | Zero foam pads (standing directly on the platform), eyes open |
| 0c | Zero foam pads, eyes closed |
| 1o | One foam pad under the feet, eyes open |
| 1c | One foam pad, eyes closed |
| 2o | Two foam pads, eyes open |
| 2c | Two foam pads, eyes closed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wąsik, M.; Miśkiewicz-Orczyk, K.; Słota, M.; Lisowska, G.; Kasperczyk, A.; Bellanti, F.; Dobrakowski, M.; Błaszczyk, U.; Bułdak, R.J.; Kasperczyk, S. Relationship between Postural Stability, Lead Content, and Selected Parameters of Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 12768. https://doi.org/10.3390/ijms232112768
Wąsik M, Miśkiewicz-Orczyk K, Słota M, Lisowska G, Kasperczyk A, Bellanti F, Dobrakowski M, Błaszczyk U, Bułdak RJ, Kasperczyk S. Relationship between Postural Stability, Lead Content, and Selected Parameters of Oxidative Stress. International Journal of Molecular Sciences. 2022; 23(21):12768. https://doi.org/10.3390/ijms232112768
Chicago/Turabian StyleWąsik, Marta, Katarzyna Miśkiewicz-Orczyk, Michał Słota, Grażyna Lisowska, Aleksandra Kasperczyk, Francesco Bellanti, Michał Dobrakowski, Urszula Błaszczyk, Rafał Jakub Bułdak, and Sławomir Kasperczyk. 2022. "Relationship between Postural Stability, Lead Content, and Selected Parameters of Oxidative Stress" International Journal of Molecular Sciences 23, no. 21: 12768. https://doi.org/10.3390/ijms232112768
APA StyleWąsik, M., Miśkiewicz-Orczyk, K., Słota, M., Lisowska, G., Kasperczyk, A., Bellanti, F., Dobrakowski, M., Błaszczyk, U., Bułdak, R. J., & Kasperczyk, S. (2022). Relationship between Postural Stability, Lead Content, and Selected Parameters of Oxidative Stress. International Journal of Molecular Sciences, 23(21), 12768. https://doi.org/10.3390/ijms232112768









