Plastic and Placenta: Identification of Polyethylene Glycol (PEG) Compounds in the Human Placenta by HPLC-MS/MS System
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
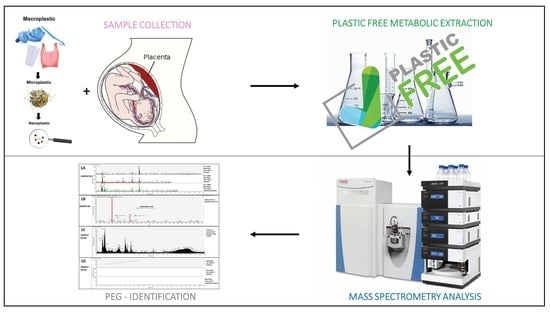
4.1. Enrolment of Patients and Placentas Collection
- -
- Taking special diets prescribed for medical reasons within 4 weeks before childbirth.
- -
- Diarrhea or constipation in the 2 weeks before giving birth.
- -
- Taking antibiotics in the 2 weeks prior to childbirth.
- -
- Taking medications that affect intestinal resorption (e.g., activated charcoal or cholestyramine) in the 2 weeks before childbirth.
- -
- Diagnosis of gastrointestinal disease (e.g., ulcerative colitis or Crohn’s disease, excluding appendectomy), cancer, organ transplant, HIV, or any other serious illness that led to medical treatment.
- -
- Invasive or abrasive dental treatments in the 2 weeks prior to childbirth.
- -
- Current or recent participation (within 4 weeks before delivery) in a clinical trial.
- -
- Alcohol abuse (defined as Alcohol Use Disorder Identification Test Score >10) [31].
4.2. Plastic-Free Extraction Protocol
4.3. UHPLC-MSMS Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Peng, X.; Chen, M.; Chen, S.; Dasgupta, S.; Xu, H.; Ta, K.; Du, M.; Li, J.; Guo, Z.; Bai, S. Microplastics contaminate the deepest part of the world’s ocean. Geochem. Perspect. Lett. 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Napper, I.E.; Davies, B.F.; Clifford, H.; Elvin, S.; Koldewey, H.J.; Mayewski, P.A.; Miner, K.R.; Potocki, M.; Elmore, A.C.; Gajurel, A.P.; et al. Reaching New Heights in Plastic Pollution—Preliminary Findings of Microplastics on Mount Everest. One Earth 2020, 3, 621–630. [Google Scholar] [CrossRef]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the mass of microplastics ingested—A pivotal first step towards human health risk assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef]
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, srep46687. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Fournier, S.B.; D’Errico, J.N.; Adler, D.S.; Kollontzi, S.; Goedken, M.J.; Fabris, L.; Yurkow, E.J.; Stapleton, P.A. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part. Fibre Toxicol. 2020, 17, 55. [Google Scholar] [CrossRef]
- Imik, H.; Gunlu, A. Effects of sodium bicarbonate, polyethylene glycol and methionine added to rations with sorghum (Sorghum vulgare) in fattening ambs on growth performance, wool quality and some blood biochemical markers. Rev. Med. Vet. 2011, 162, 432–439. [Google Scholar]
- Webster, R.; Elliott, V.; Park, B.K.; Walker, D.; Hankin, M.; Taupin, P. PEG and PEG Conjugates Toxicity: Towards an Understanding of the Toxicity of PEG and Its Relevance to PEGylated Biologicals; Veronese, F.M., Ed.; PEGylated Protein Drugs: Basic Science and Clinical Applications; Springer, Birkhäuser: Basel, Switzerland, 2009; pp. 127–146. [Google Scholar]
- Leth, P.M.; Gregersen, M. Ethylene glycol poisoning. Forensic Sci. Int. 2005, 155, 179–184. [Google Scholar] [CrossRef]
- Bhaskar, V.V.; Middha, A.; Tiwari, S.; Shivakumar, S. Liquid chromatography/tandem mass spectrometry method for quantitative estimation of polyethylene glycol 400 and its applications. J. Chromatogr. B 2013, 926, 68–76. [Google Scholar] [CrossRef]
- Thurman, E.M.; Ferrer, I.; Blotevogel, J.; Borch, T. Analysis of Hydraulic Fracturing Flowback and Produced Waters Using Accurate Mass: Identification of Ethoxylated Surfactants. Anal. Chem. 2014, 86, 9653–9661. [Google Scholar] [CrossRef] [PubMed]
- Shimabuku, I.; Chen, D.; Wu, Y.; Miller, E.; Sun, J.; Sutton, R. Occurrence and risk assessment of organophosphate esters and bisphenols in San Francisco Bay, California, USA. Sci. Total Environ. 2021, 813, 152287. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Zhang, Y.; Wang, C.; Wang, X.; Zhou, J.; Shen, M.; Zhao, Y.; Fu, Z.; Jin, Y. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ. Pollut. 2019, 255, 113122. [Google Scholar] [CrossRef]
- Zaheer, J.; Kim, H.; Ko, I.O.; Jo, E.-K.; Choi, E.-J.; Lee, H.-J.; Shim, I.; Woo, H.-J.; Choi, J.; Kim, G.-H.; et al. Pre/post-natal exposure to microplastic as a potential risk factor for autism spectrum disorder. Environ. Int. 2022, 161, 107121. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, P.H.; Krogstad, D.J.; Herwaldt, B.L. Antimalarial agents: Mechanisms of action. Antimicrob. Agents Chemother. 1988, 32, 793–798. [Google Scholar] [CrossRef]
- Arumugasaamy, N.; Navarro, J.; Leach, J.K.; Kim, P.C.W.; Fisher, J.P. In Vitro Models for Studying Transport Across Epithelial Tissue Barriers. Ann. Biomed. Eng. 2019, 47, 1–21. [Google Scholar] [CrossRef]
- Prabhudas, M.; Bonney, E.; Caron, K.; Dey, S.; Erlebacher, A.; Fazleabas, A.; Fisher, S.; Golos, T.; Matzuk, M.; McCune, J.M.; et al. Immune mechanisms at the maternal-fetal interface: Perspectives and challenges. Nat. Immunol. 2015, 16, 328–334. [Google Scholar] [CrossRef]
- Vaughan, O.R.; Fowden, A.L. Placental metabolism: Substrate requirements and the response to stress. Reprod. Domest. Anim. 2016, 51, 25–35. [Google Scholar] [CrossRef]
- Poulsen, M.S.; Rytting, E.; Mose, T.; Knudsen, L.E. Modeling placental transport: Correlation of in vitro BeWo cell permeability and ex vivo human placental perfusion. Toxicol. Vitr. 2009, 23, 1380–1386. [Google Scholar] [CrossRef]
- Verhoef, J.J.F.; Anchordoquy, T.J. Questioning the use of PEGylation for drug delivery. Drug Deliv. Transl. Res. 2013, 3, 499–503. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Walkey, C.; Chan, W.C.W. Polyethylene Glycol Backfilling Mitigates the Negative Impact of the Protein Corona on Nanoparticle Cell Targeting. Angew. Chem. Int. Ed. 2014, 53, 5093–5096. [Google Scholar] [CrossRef] [PubMed]
- Kianpour, E.; Azizian, S. Polyethylene glycol as a green solvent for effective extractive desulfurization of liquid fuel at ambient conditions. Fuel 2014, 137, 36–40. [Google Scholar] [CrossRef]
- Minordi, L.M.; Vecchioli, A.; Mirk, P.; Bonomo, L. CT enterography with polyethylene glycol solution vs CT enteroclysis in small bowel disease. Br. J. Radiol. 2011, 84, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ghirardini, A.V.; Novelli, A.A.; Likar, B.; Pojana, G.; Ghetti, P.F.; Marcomini, A. Sperm cell toxicity test using sea urchin Paracentrotus lividus lamarck (Echinodermata: Echinoidea): Sensitivity and discriminatory ability toward anionic and nonionic surfactants. Environ. Toxicol. Chem. 2001, 20, 644. [Google Scholar] [CrossRef] [PubMed]
- Kassotis, C.D.; Tillitt, D.E.; Davis, J.W.; Hormann, A.M.; Nagel, S.C. Estrogen and Androgen Receptor Activities of Hydraulic Fracturing Chemicals and Surface and Ground Water in a Drilling-Dense Region. Endocrinology 2014, 155, 897–907. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Ilekis, J.V.; Tsilou, E.; Fisher, S.; Abrahams, V.M.; Soares, M.J.; Cross, J.C.; Zamudio, S.; Illsley, N.P.; Myatt, L.; Colvis, C.; et al. Placental origins of adverse pregnancy outcomes: Potential molecular targets: An Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am. J. Obstet. Gynecol. 2016, 215, S1–S46. [Google Scholar] [CrossRef]
- Fujii, H.; Nishimoto, N.; Miyano, M.; Ueda, W.; Oba, H.; Yamaguchi, S.; Aoki, T.; Kurai, O.; Kawada, N.; Okawa, K. The Alcohol Use Disorders. Identification Test (AUDIT) score is useful for predicting alcohol consumption. Nihon Arukoru Yakubutsu Igakkai Zasshi Jpn. J. Alcohol Stud. Drug Depend. 2016, 51, 293–301. [Google Scholar]
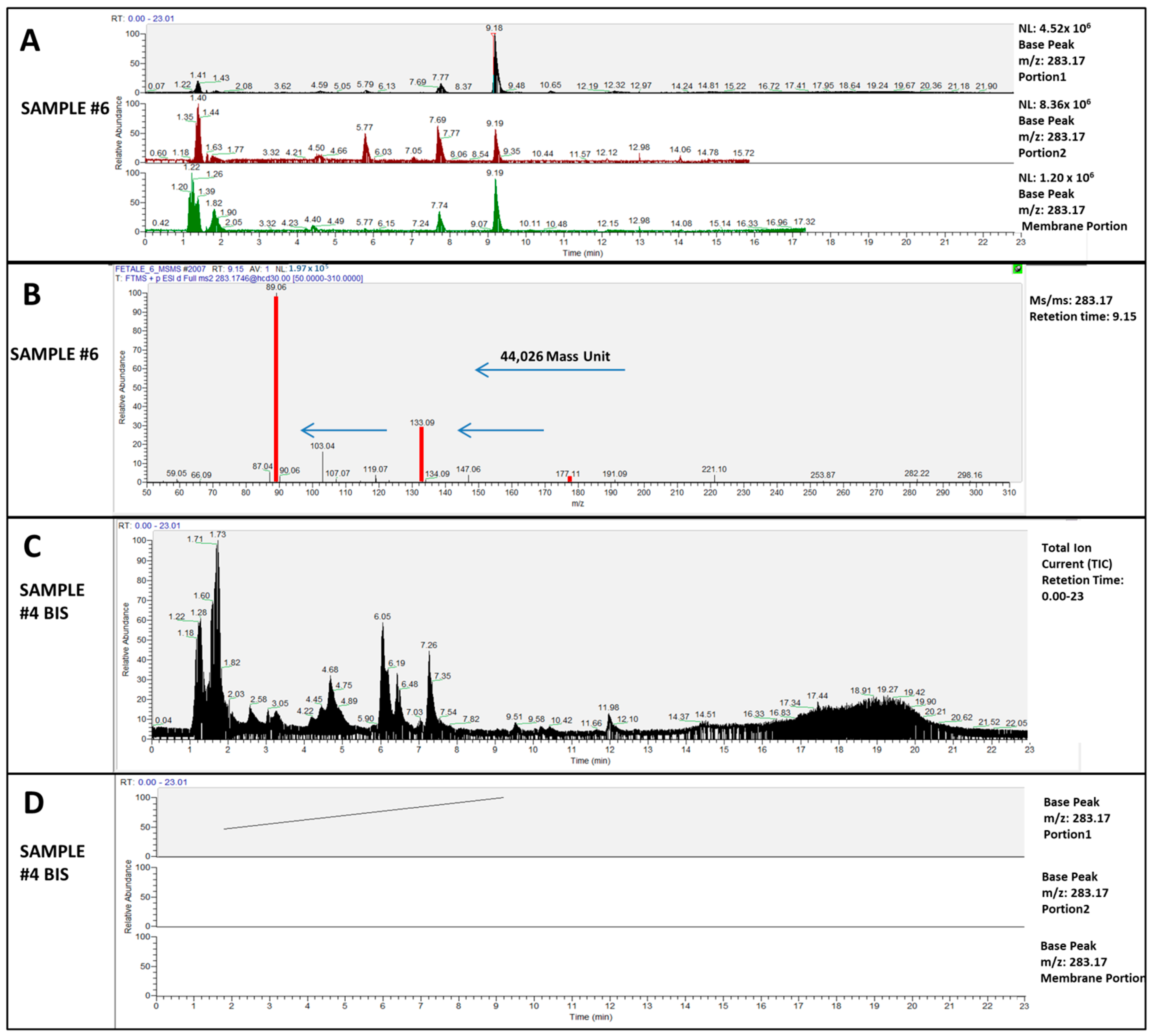
| Putative Formula | Putative Identification | Calculated Exact Mass | Portions | 1bis | 2bis | 3 | 3bis | 4 | 4bis | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C12H26O7 | PEG-EO6 | 283.1752 | Placenta | Portion 1 | * | * | * | * | * | - | * | * | * | * | * | - |
| Portion 2 | * | * | * | * | * | - | - | * | * | * | * | - | ||||
| Chorioamniotic | Membrane Portion | * | * | * | * | * | - | * | * | * | * | - | - | |||
| C14H30O8 | PEG-EO7 | 327.2013 | Placenta | Portion 1 | * | * | - | * | - | - | * | * | * | - | - | - |
| Portion 2 | * | * | * | * | * | - | * | * | * | * | * | - | ||||
| Chorioamniotic | Membrane Portion | - | * | - | * | * | - | * | - | - | - | - | - | |||
| C16H34O9 | PEG-EO8 | 371.2276 | Placenta | Portion 1 | * | * | * | * | - | - | * | * | * | * | - | - |
| Portion 2 | * | * | * | - | * | - | * | * | - | * | * | - | ||||
| Chorioamniotic | Membrane Portion | - | * | - | * | * | - | * | * | * | - | - | - | |||
| C18H38O10 | PEG-EO9 | 415.2538 | Placenta | Portion 1 | - | * | - | - | - | - | * | * | - | * | - | - |
| Portion 2 | - | * | - | * | * | - | * | - | * | * | * | - | ||||
| Chorioamniotic | Membrane Portion | - | - | - | - | * | - | * | - | - | - | * | - | |||
| C20H42O11 | PEG-EO10 | 459.2800 | Placenta | Portion 1 | - | * | - | - | - | - | * | - | * | - | - | - |
| Portion 2 | - | - | - | * | - | - | * | - | * | * | * | - | ||||
| Chorioamniotic | Membrane Portion | - | - | - | - | - | - | * | * | - | - | * | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragusa, A.; Lelli, V.; Fanelli, G.; Svelato, A.; D’Avino, S.; Gevi, F.; Santacroce, C.; Catalano, P.; Rongioletti, M.C.A.; De Luca, C.; et al. Plastic and Placenta: Identification of Polyethylene Glycol (PEG) Compounds in the Human Placenta by HPLC-MS/MS System. Int. J. Mol. Sci. 2022, 23, 12743. https://doi.org/10.3390/ijms232112743
Ragusa A, Lelli V, Fanelli G, Svelato A, D’Avino S, Gevi F, Santacroce C, Catalano P, Rongioletti MCA, De Luca C, et al. Plastic and Placenta: Identification of Polyethylene Glycol (PEG) Compounds in the Human Placenta by HPLC-MS/MS System. International Journal of Molecular Sciences. 2022; 23(21):12743. https://doi.org/10.3390/ijms232112743
Chicago/Turabian StyleRagusa, Antonio, Veronica Lelli, Giuseppina Fanelli, Alessandro Svelato, Sara D’Avino, Federica Gevi, Criselda Santacroce, Piera Catalano, Mauro Ciro Antonio Rongioletti, Caterina De Luca, and et al. 2022. "Plastic and Placenta: Identification of Polyethylene Glycol (PEG) Compounds in the Human Placenta by HPLC-MS/MS System" International Journal of Molecular Sciences 23, no. 21: 12743. https://doi.org/10.3390/ijms232112743
APA StyleRagusa, A., Lelli, V., Fanelli, G., Svelato, A., D’Avino, S., Gevi, F., Santacroce, C., Catalano, P., Rongioletti, M. C. A., De Luca, C., Gulotta, A., Rinalducci, S., & Timperio, A. M. (2022). Plastic and Placenta: Identification of Polyethylene Glycol (PEG) Compounds in the Human Placenta by HPLC-MS/MS System. International Journal of Molecular Sciences, 23(21), 12743. https://doi.org/10.3390/ijms232112743