Exploring the Absorption Mechanisms of Imidazolium-Based Ionic Liquids to Epigallocatechin Gallate
Abstract
1. Introduction
2. Results and Discussion
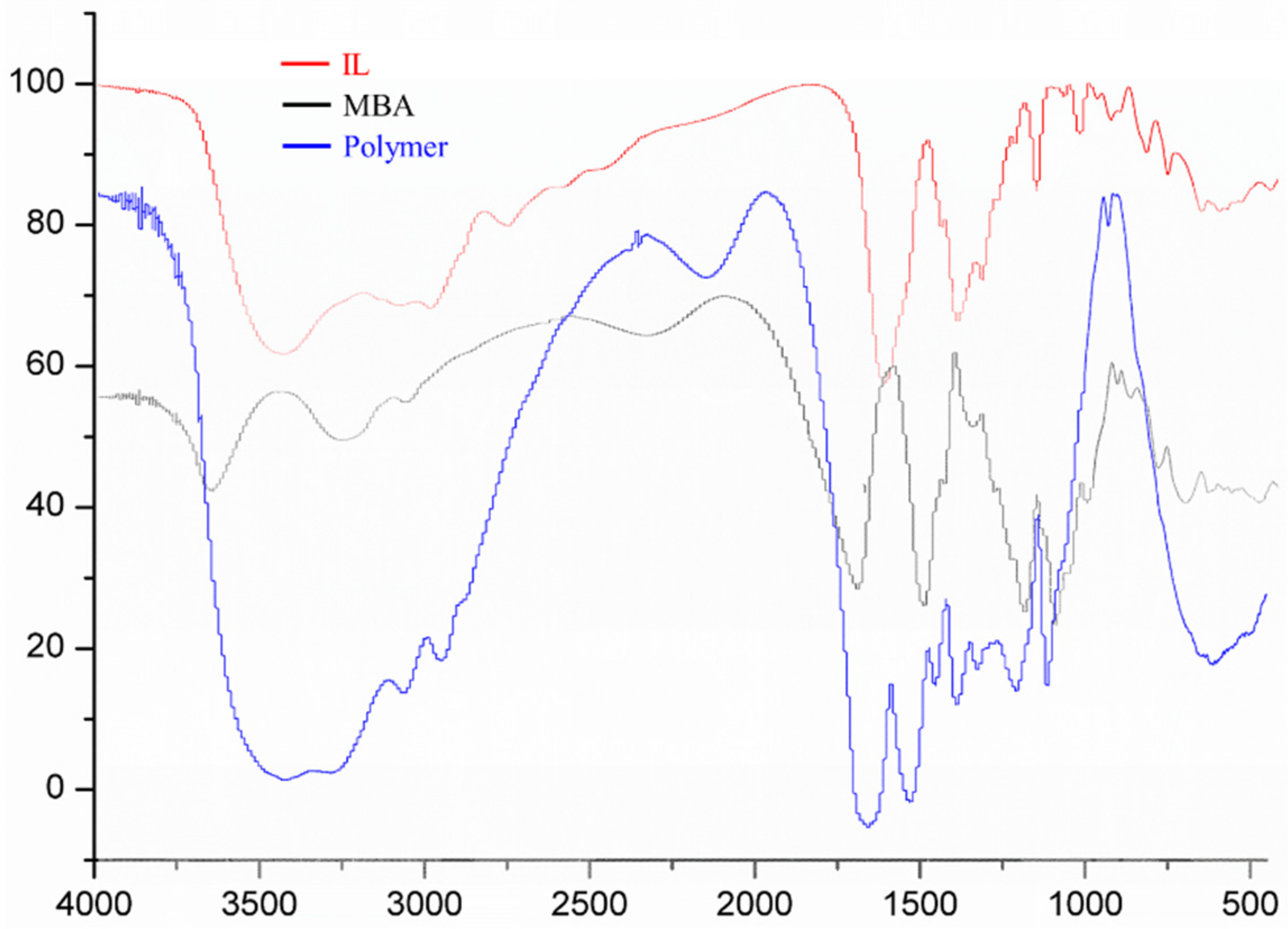
2.1. FT-IR Characterization of (ViIm)2C6(L-Pro)2
2.2. Selective Adsorption of EGCG on ILs
2.3. Optimization of the Adsorption Conditions
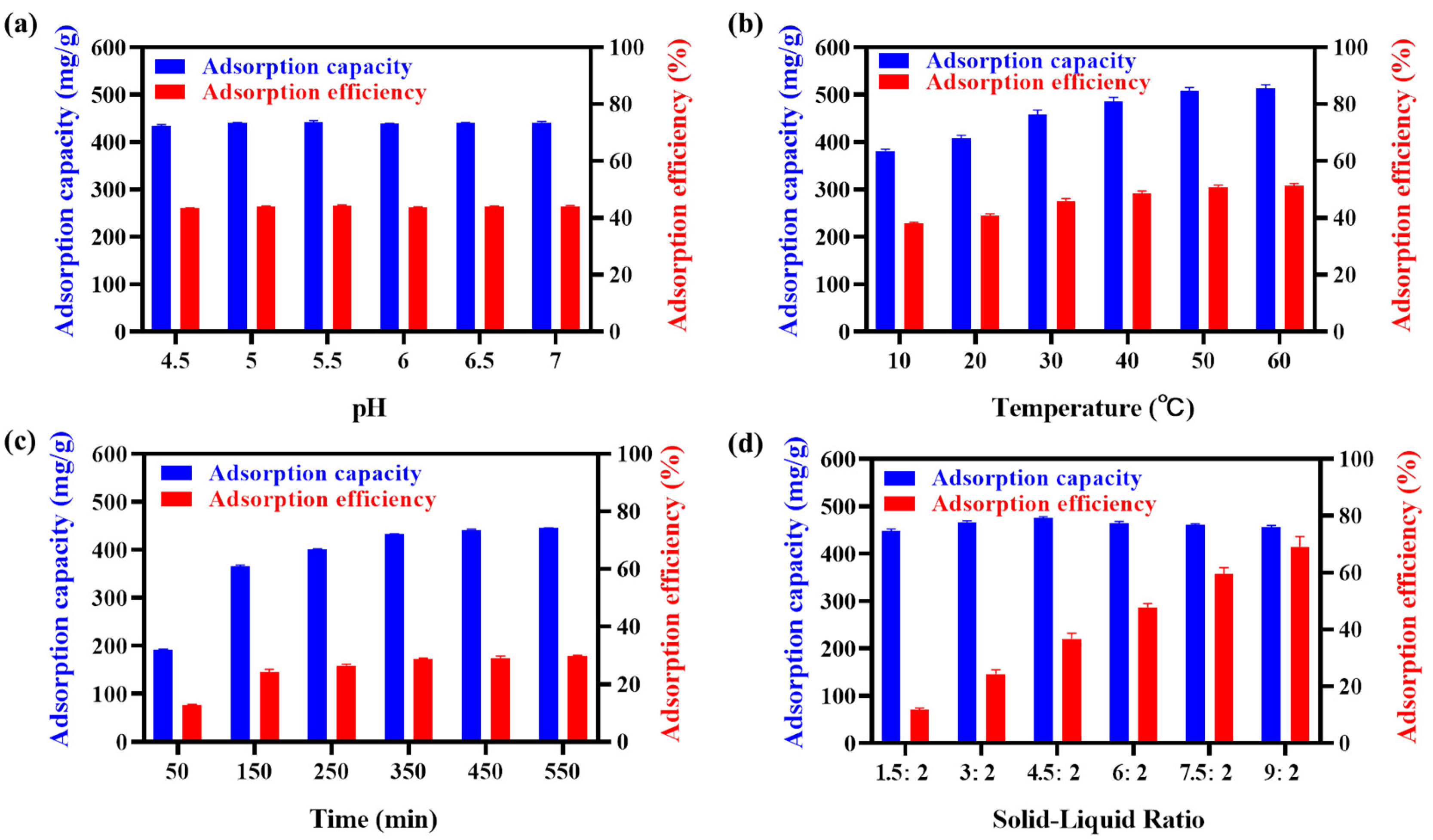
2.3.1. Single-Factor Optimization
2.3.2. RSM Optimization
2.4. Analysis of the Adsorption Process and Mechanism
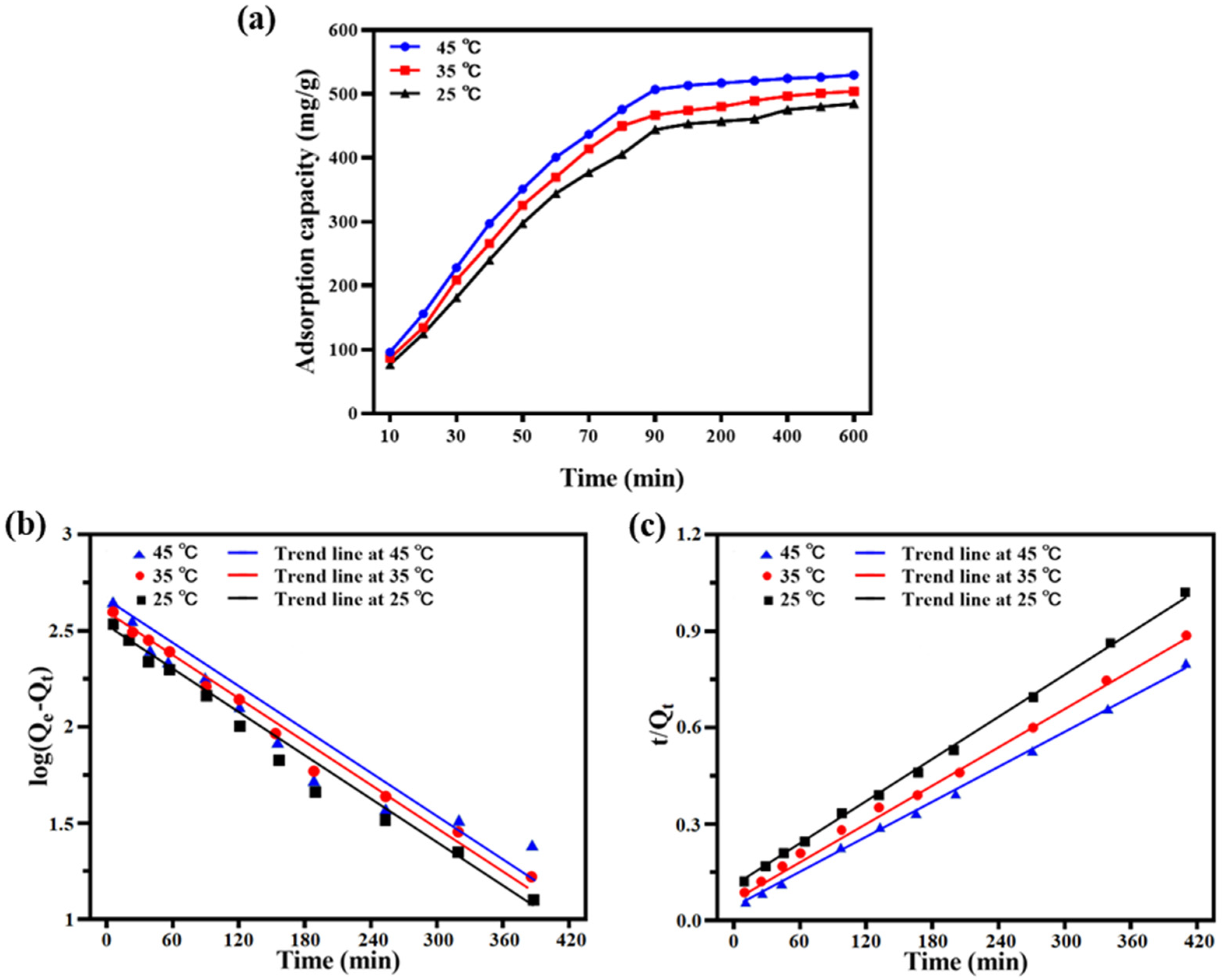
2.4.1. Adsorption Kinetics
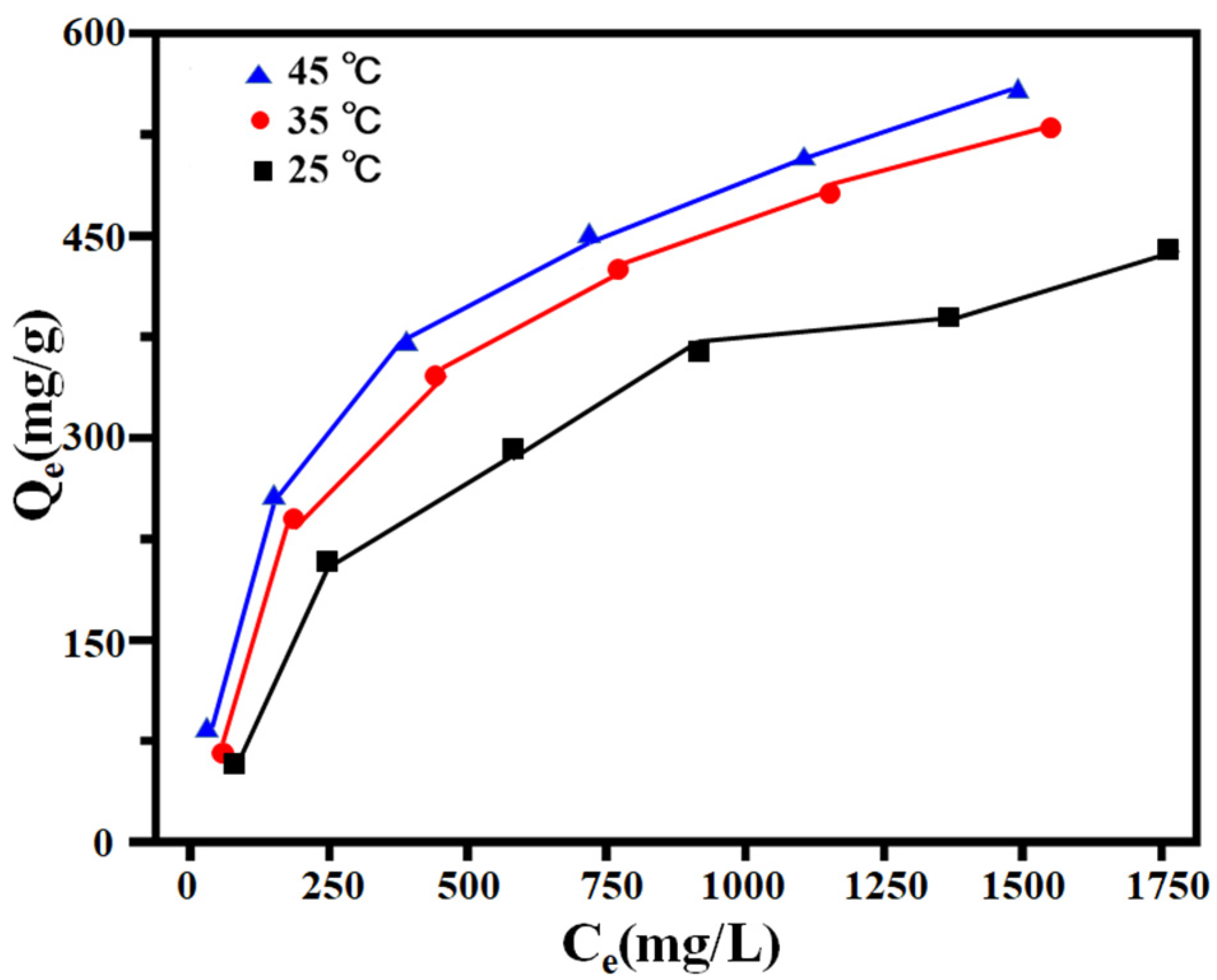
2.4.2. Adsorption Thermodynamics
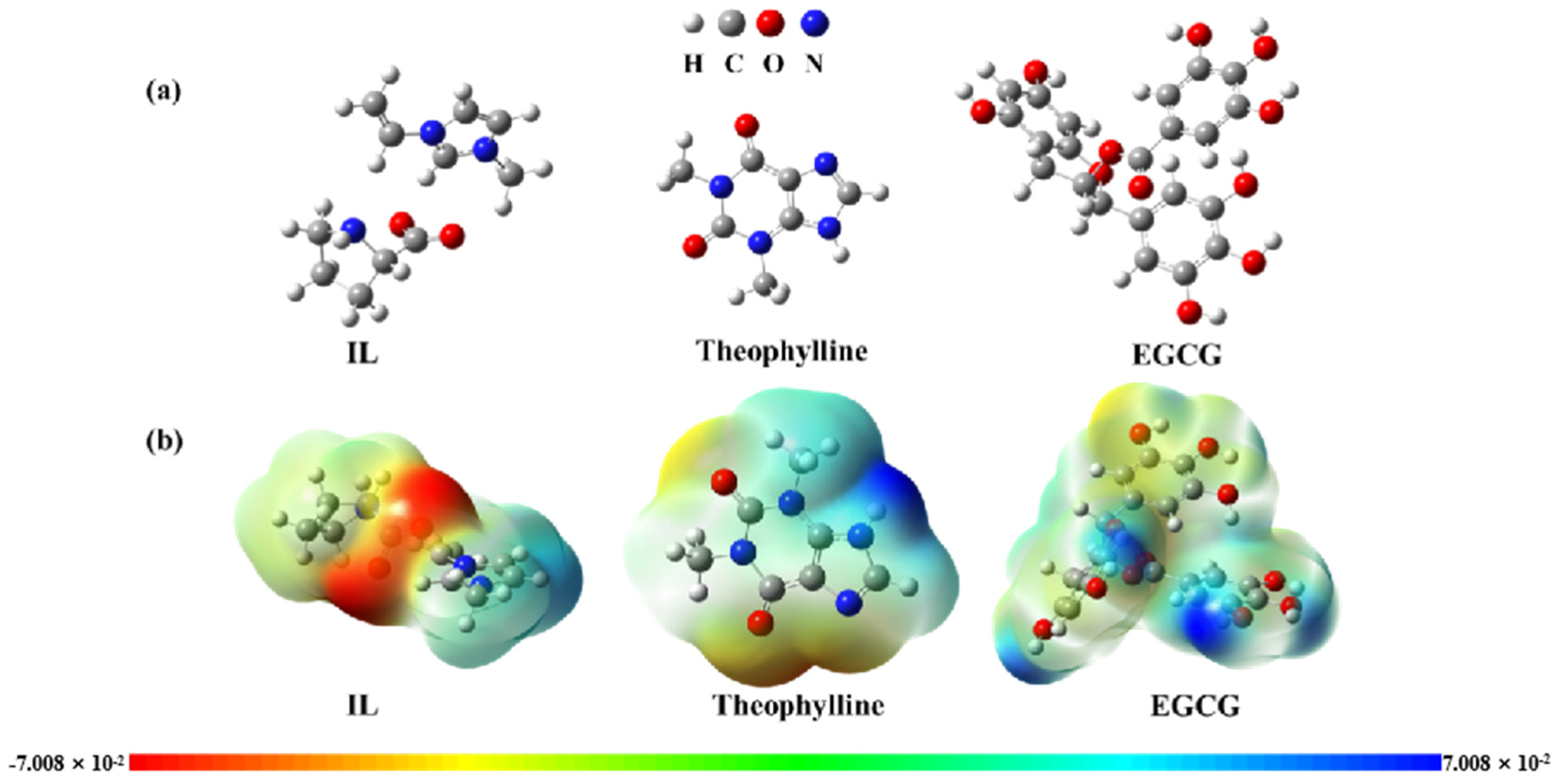
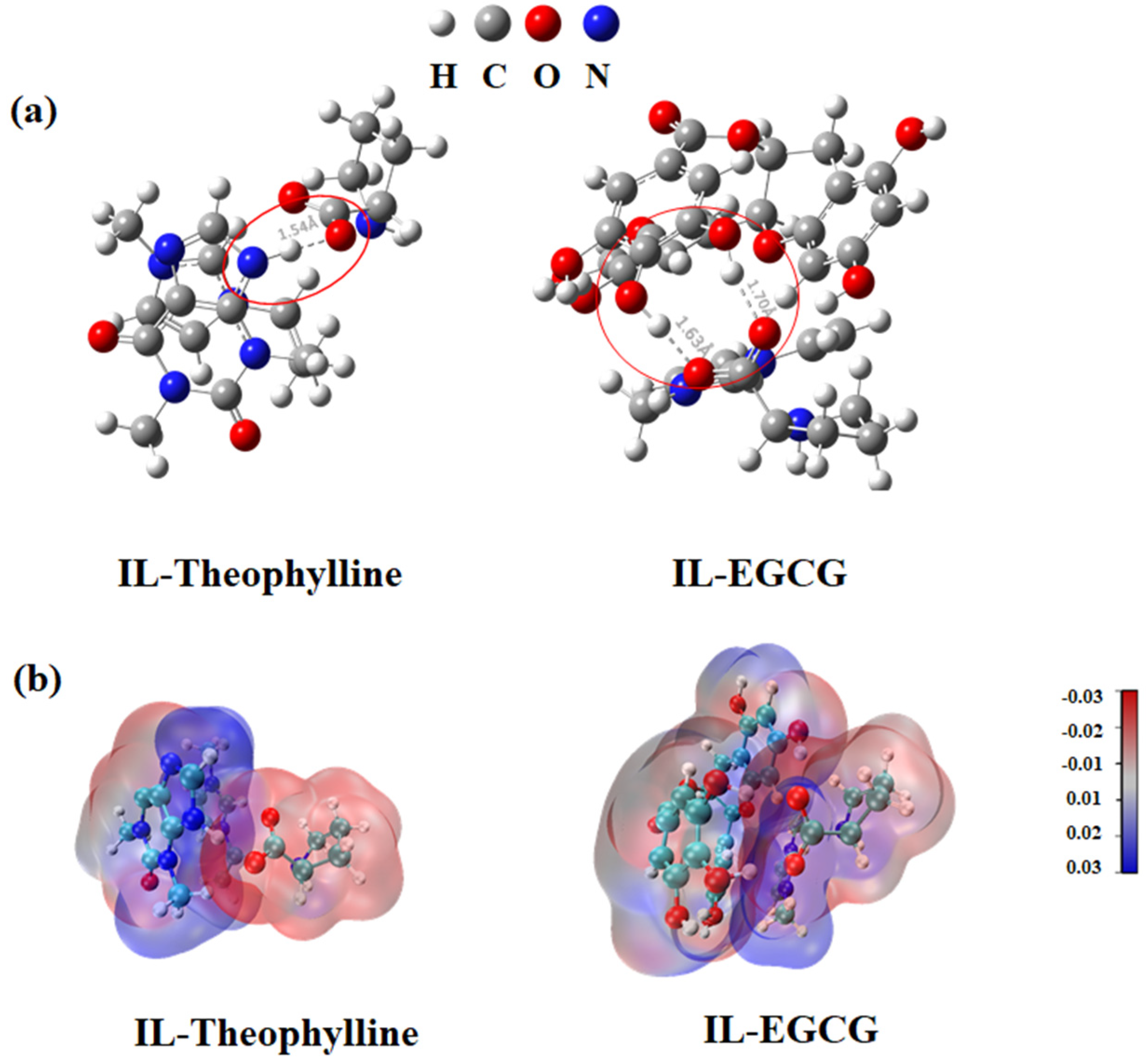
2.4.3. Molecular Docking Study of the Adsorption Mechanism
2.4.4. COSMO-RS-Based EGCG and Theophylline Solubility Calculation
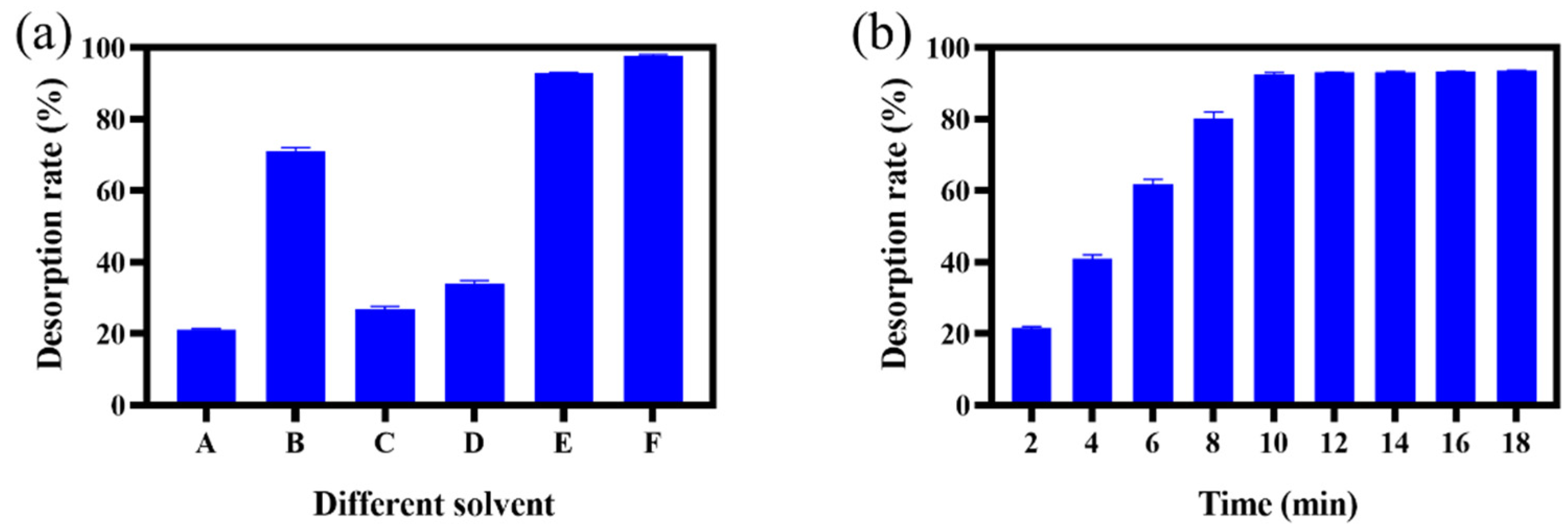
2.5. Desorption Experiment
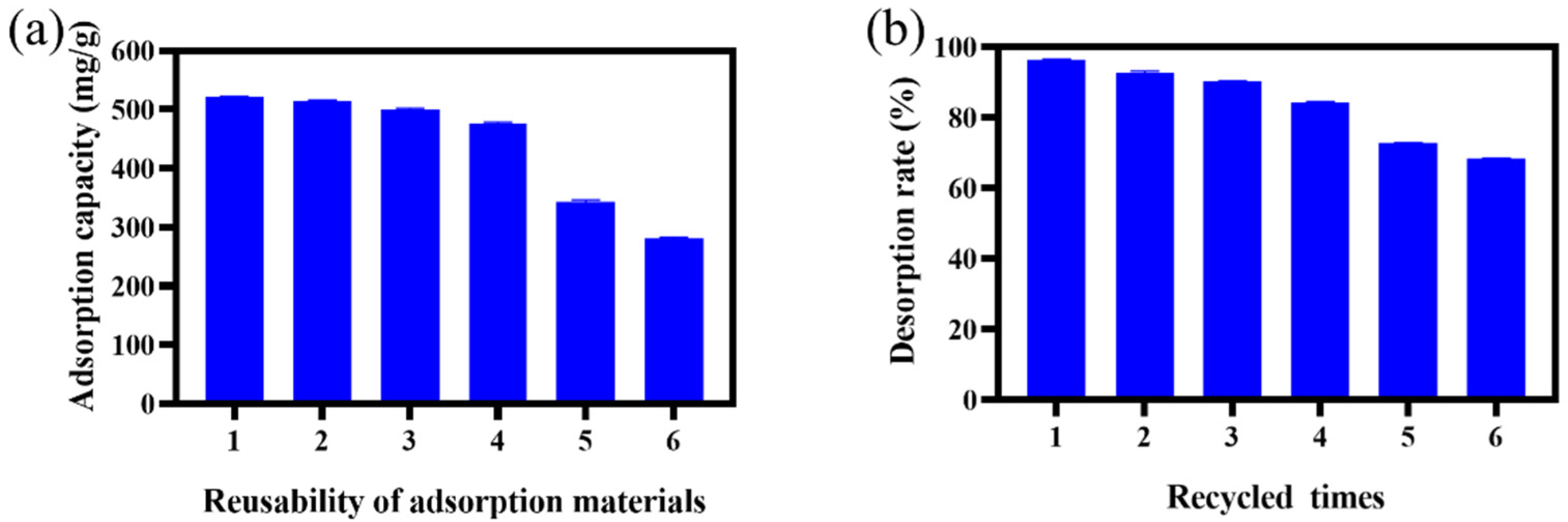
2.6. Reuse Experiment
2.7. SEM Characterization
3. Methods and Materials
3.1. Materials
3.2. Apparatus
3.3. Synthesis of Ionic Liquid Polymers
3.4. Quantification of EGCG
3.5. Adsorption Test of EGCG
3.6. Statistical Analysis
3.7. Kinetics and Thermodynamics Studies
3.7.1. Adsorption Kinetics
3.7.2. Adsorption Thermodynamics
3.7.3. Adsorption Thermodynamic Parameter
3.8. Quantum Chemical Computation
3.9. COSMO-RS Based EGCG and Theophylline Solubility Calculation
3.10. EGCG Desorption Experiment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jin, X.; Liu, M.; Chen, Z.; Mao, R.; Xiao, Q.; Gao, H.; Wei, M. Separation and purification of epigallocatechin-3-gallate (EGCG) from green tea using combined macroporous resin and polyamide column chromatography. J. Chromatogr. B 2015, 1002, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Misra, T.K.; Singh, I.D. Antioxidant activity in the extracts of two edible aroids. Indian J. Pharm. Sci. 2010, 72, 105–108. [Google Scholar] [PubMed]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gong, L.H.; Chen, C.J.; Han, H.B.; Li, H.H. Column-chromatographic extraction and separation of polyphenols, caffeine and theanine from green tea. Food Chem. 2012, 131, 1539–1545. [Google Scholar] [CrossRef]
- Dong, W.B.; Hu, Y.; Zhang, J.H. Study on the Process of Preparation of TPs by Adsorption Resin Method. Food Sci. 2002, 23, 68–73. [Google Scholar]
- Ji, S.; Wang, Y.; Gao, S.; Shao, X.; Cui, W.; Du, Y.; Guo, M.; Tang, D. Highly efficient and selective extraction of minor bioactive natural products using pure ionic liquids: Application to prenylated flavonoids in licorice. J. Ind. Eng. Chem. 2019, 80, 352–360. [Google Scholar] [CrossRef]
- Wang, W.; Li, Q.; Liu, Y.; Chen, B. Ionic liquid-aqueous solution ultrasonic-assisted extraction of three kinds of alkaloids from Phellodendron amurense Rupr and optimize conditions use response surface. Ultrason. Sonochem. 2015, 24, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.Z.; Yu, M.; Ge, J.; Ma, P.X.; Chen, X.F.; Lei, B. Development of a multifunctional platform based on strong, intrinsically photoluminescent and antimicrobial silicapoly(citrates)-based hybrid biodegradable elastomers for bone regeneration. Adv. Funct. Mater. 2015, 25, 5016–5029. [Google Scholar] [CrossRef]
- Liang, J.; Li, J.; Zhou, C.; Jia, W.; Song, H.; Zhang, L.; Zhao, F.; Lee, B.P.; Liu, B. In situ synthesis of biocompatible imidazolium salt hydrogels with antimicrobial activity. Acta Biomater. 2019, 99, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Feng, X.T.; Alula, Y. Bionic multi-tentacled ionic liquid-modified silica gel for adsorption and separation of polyphenols from green tea (Camellia sinensis) leaves. Food Chem. 2017, 230, 637. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Huang, X.; Yao, S.; Peng, L.; Li, F.; Song, H. Synthesis of a New Imidazole Amino Acid Ionic Liquid Polymer and Selective Adsorption Performance for Tea Polyphenols. Polymers 2020, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, Y.; Li, T.; Li, H.; Yang, C.; Sun, D.; Wang, D.; Liu, C.; Che, G. Fabricating magnetic hydrophilic molecularly imprinted resin with enhanced adsorption and recognition performance for targeted detecting chlorophenols in environmental water. Chem. Eng. J. 2021, 420, 129904. [Google Scholar] [CrossRef]
- Francisco, R.; Almeida, C.; Sousa, A.C.A.; Neves, M.C.; Freire, M.G. High Performance of Ionic-Liquid-Based Materials to Remove Insecticides. Int. J. Mol. Sci. 2022, 23, 2989. [Google Scholar] [CrossRef]
- Yuan, K.; Chen, S.; Chen, X.; Yao, S.; Wang, X.; Song, H.; Zhu, M. High effective extraction of selected anthraquinones from Polygonum multiflorum using ionic liquids with ultrasonic assistance. J. Mol. Liq. 2020, 314, 113342. [Google Scholar] [CrossRef]
- Yu, K.; Ding, W.L.; Lu, Y.; Wang, Y.; Liu, Y.; Liu, G.; Huo, F.; He, H. Ionic liquids screening for lignin dissolution: COSMO-RS simulations and experimental characterization. J. Mol. Liq. 2022, 348, 118007. [Google Scholar] [CrossRef]
- Lu, B.; Bo, Y.; Yi, M.; Wang, Z.; Zhang, J.; Zhu, Z.; Zhao, Y.; Zhang, J. Enhancing the Solubility and Transdermal Delivery of Drugs Using Ionic Liquid Microemulsions. Adv. Funct. Mater. 2021, 31, 2102794. [Google Scholar] [CrossRef]
- Qin, C.W.; Gao, H.S.; Xin, X.L.; Yan, L.Y. The dissolution of human hair using ionic liquids through COSMO-RS predication and experimental verification. J. Mol. Liq. 2022, 349, 118094. [Google Scholar] [CrossRef]
- Lippert, J.L.; Robertson, J.A.; Havens, J.R.; Tan, J.S. Structural studies of poly(N-vinylimidazole) complexes by infrared and Raman spectroscopy. Macromolecules 1985, 18, 63–67. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 49–76. [Google Scholar] [CrossRef]
- Huang, X.W. Determination of Epicatechins in compound preparation by UV spectrophotometry. Chin. Trad. Pat. Med. 2002, 180, 197–203. [Google Scholar]
- Chen, P.F.; Song, H.; Zhou, L.; Chen, J.; Liu, J.Y.; Yao, S. Magnetic solid-phase extraction based onferroferric oxide nanoparticles doubly coated withchitosan and b-cyclodextrin in layer-by-layer modefor the separation of ibuprofen. RSC Adv. 2016, 6, 56240–56248. [Google Scholar] [CrossRef]
- Kumar, R. Adsorption of Amaranth Dye onto Alumina Reinforced Polystyrene. Clean Soil Air Water 2011, 39, 74–82. [Google Scholar]
- Ladd, J.; Zhang, Z.; Chen, S.F.; Hower, J.C.; Jiang, S.Y. Zwitterionic polymers exhibiting high resistance to nonspecific protein adsorption from human serum and plasma. Biomacromolecules 2008, 9, 1357–1361. [Google Scholar] [CrossRef]
- Yan, H.; Yang, L.Y.; Yang, Z.; Yang, H.; Li, A.; Cheng, R.S. Preparation of chitosan/poly(acrylic acid) magnetic composite microspheres andapplications in the removal of copper(II) ions from aqueous solutions. J. Hazard. Mater. 2012, 229–230, 371–380. [Google Scholar]
- Ozcan, S.A.; Erdem, B.; Ozcan, A. Adsorption of Acid Blue 193, from aqueous solutions onto BTMA-bentonite. Colloids Surf. A 2005, 266, 73–81. [Google Scholar] [CrossRef]
- Dymińska, L.; Hanuza, J.; Janczak, J.; Ptak, M.; Lisiecki, R. The Structural and Optical Properties of 1,2,4-Triazolo[4,3-a]pyridine-3-amine. Molecules 2022, 27, 721. [Google Scholar] [CrossRef] [PubMed]
- Julie, M.M.; Prabhu, T.; Elamuruguporchelvi, E.; Asif, F.B.; Muthu, S.; Irfan, A. Structural (monomer and dimer), wavefunctional, NCI analysis in aqueous phase, electronic and excited state properties in different solvent atmosphere of 3-{(E)-[(3,4-dichlorophenyl)imino]methyl benzene-1,2-diol. J. Mol. Liq. 2021, 336, 116335. [Google Scholar] [CrossRef]
- Witte, J.; Goldey, M.; Neaton, J.B.; Head-Gordon, M. Beyond Energies: Geometries of Nonbonded Molecular Complexes as Metrics for Assessing Electronic Structure Approaches. J. Chem. Theory Comp. 2015, 11, 1481–1492. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comp. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Pye, C.C.; Ziegler, T.; Lenthe, E.V.; Louwen, J.N. An implementation of the conductor-like screening model of solvation within the Amsterdam density functional package—Part II. COSMO for real solvents 1. Can. J. Chem. 2009, 87, 396–408. [Google Scholar] [CrossRef]
- Xiong, R.; Sandler, S.I.; Burnett, R.I. An Improvement to COSMO-SAC for Predicting Thermodynamic Properties. Ind. Eng. Chem. Res. 2014, 53, 8265–8278. [Google Scholar] [CrossRef]
- Klamt, A.; Jonas, V.; Bürger, T.; Lohrenz, J.C. Refinement and Parametrization of COSMO-RS. J. Phys. Chem. A 1998, 102, 5074–5085. [Google Scholar] [CrossRef]
- Klamt, A. COSMO-RS, from Quantum Chemistry to Fluid Phase Thermodynamics and Drug Design; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Lin, S.T.; Sandler, S.I. A Priori Phase Equilibrium Prediction from a Segment Contribution Solvation Model. Ind. Eng. Chem. Res. 2002, 41, 899–913. [Google Scholar] [CrossRef]





| Source | Sum of Squares | d f | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 99,747.65 | 14 | 7124.83 | 610.51 | <0.0001 | significant |
| X1 | 7500 | 1 | 7500 | 642.66 | <0.0001 | |
| X2 | 23,496.75 | 1 | 23,496.75 | 2013.39 | <0.0001 | |
| X3 | 10.08 | 1 | 10.08 | 0.86 | 0.3684 | |
| X4 | 62,496.33 | 1 | 62,496.33 | 5355.19 | <0.0001 | |
| X1X2 | 1 | 1 | 1 | 0.086 | 0.774 | |
| X1X3 | 1 | 1 | 1 | 0.086 | 0.774 | |
| X1X4 | 25 | 1 | 25 | 2.14 | 0.1654 | |
| X2X3 | 6.25 | 1 | 6.25 | 0.54 | 0.4764 | |
| X2X4 | 1 | 1 | 1 | 0.086 | 0.774 | |
| X3X4 | 4 | 1 | 4 | 0.34 | 0.5676 | |
| X12 | 1771.3 | 1 | 1771.3 | 151.78 | <0.0001 | |
| X22 | 4021.69 | 1 | 4021.69 | 344.61 | <0.0001 | |
| X32 | 72.79 | 1 | 72.79 | 6.24 | 0.0256 | |
| X42 | 90 | 1 | 90 | 7.71 | 0.0148 | |
| Lack of Fit | 88.58 | 10 | 8.85 | 0.47 | 0.8459 | not significant |
| Pure Error | 74.8 | 4 | 18.7 | |||
| Cor Total | 99,911.03 | 28 |
| Project | Data | Project | Data |
|---|---|---|---|
| Standard deviation | 3.42 | R2 | 0.9984 |
| Average | 438.59 | Adjusted R2 | 0.9967 |
| Coefficient of variation | 0.78 | Predicted R2 | 0.9937 |
| T (°C) | Level One | Level Two | |||||
|---|---|---|---|---|---|---|---|
| Qe,exp (mg/g) | Qe,cal (mg/g) | K1 (min−1) | R2 | Qe,cal (mg/g) | K2 g/(mg·min) | R2 | |
| 25 | 428.3 | 330.065 | 0.009903 | 0.9721 | 454.5 | 0.00003872 | 0.9989 |
| 35 | 491.1 | 3332.583 | 0.009673 | 0.9596 | 526.3 | 0.00003785 | 0.9985 |
| 45 | 548.1 | 367.621 | 0.009212 | 0.9224 | 574.7 | 0.00004385 | 0.9988 |
| T (°C) | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|
| n | KF (min−1) | R2 | Qe,cal (mg/g) | KL (min−1) | R2 | |
| 25 | 2.69324 | 34.50485 | 0.9635 | 454 | 0.004794 | 0.9942 |
| 35 | 3.011141 | 36.94019 | 0.9743 | 525 | 0.004737 | 0.9966 |
| 45 | 3.161555 | 53.35139 | 0.9768 | 555 | 0.006868 | 0.9945 |
| C0 (mg/L) | ∆H (kJ·/mol) | ∆S (J/mol·K) | ∆G (kJ/mol) | ||
|---|---|---|---|---|---|
| 293 K | 303 K | 313 K | |||
| 500 | 30.34 | 106.55 | −1.65 | −2.08 | −3.77 |
| 1000 | 24.94 | 83.10 | −0.09 | −0.97 | −1.76 |
| 1500 | 22.13 | 69.51 | 1.49 | 0.55 | 0.11 |
| 2000 | 15.17 | 43.73 | 2.21 | 1.57 | 1.33 |
| 2500 | 16.26 | 44.48 | 3.10 | 2.35 | 2.21 |
| 3000 | 13.83 | 34.93 | −3.51 | 2.90 | 2.80 |
| Molecular | Energy (kcal/mol) |
|---|---|
| (ViIm)2C6(L-Pro)2 & Theophylline | −50.60 |
| (ViIm)2C6(L-Pro)2 & EGCG | −898.89 |
| Factor | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| time (min) | 150 | 350 | 550 |
| solid–liquid ratio (mL/mg) | 3/2 | 6/2 | 9/2 |
| pH | 4 | 5.5 | 7 |
| temperature (°C) | 20 | 40 | 60 |
| Number | Time (min) | Solid–Liquid Ratio (mL/mg) | pH | Temperature (°C) | Adsorption Capacity (mg/g) |
|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 452 |
| 2 | 1 | 0 | 0 | −1 | 388 |
| 3 | 0 | −1 | −1 | 0 | 472 |
| 4 | 0 | 1 | 1 | 0 | 385 |
| 5 | −1 | 0 | −1 | 0 | 413 |
| 6 | 0 | 0 | −1 | 1 | 538 |
| 7 | 0 | −1 | 0 | 1 | 546 |
| 8 | −1 | 0 | 0 | −1 | 346 |
| 9 | 1 | 0 | 0 | 1 | 533 |
| 10 | 0 | 1 | 0 | 1 | 458 |
| 11 | 0 | 0 | 1 | −1 | 389 |
| 12 | 0 | 0 | 0 | 0 | 446 |
| 13 | −1 | 0 | 1 | 0 | 414 |
| 14 | 0 | −1 | 0 | −1 | 406 |
| 15 | 0 | 0 | 0 | 0 | 457 |
| 16 | 0 | −1 | 1 | 0 | 476 |
| 17 | −1 | −1 | 0 | 0 | 432 |
| 18 | 0 | 1 | 0 | −1 | 316 |
| 19 | 1 | 0 | 1 | 0 | 467 |
| 20 | 0 | 0 | −1 | −1 | 385 |
| 21 | −1 | 0 | 0 | 1 | 484 |
| 22 | 0 | 0 | 0 | 0 | 456 |
| 23 | 1 | 0 | −1 | 0 | 464 |
| 24 | 0 | 1 | −1 | 0 | 386 |
| 25 | −1 | 1 | 0 | 0 | 343 |
| 26 | 1 | 1 | 0 | 0 | 395 |
| 27 | 0 | 0 | 0 | 0 | 453 |
| 28 | 1 | −1 | 0 | 0 | 482 |
| 29 | 0 | 0 | 1 | 1 | 536 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Zhang, Y.; Tao, C.; Ni, H.; Liu, X.; Chen, Y.; Wu, Y.; Song, H.; Xu, T. Exploring the Absorption Mechanisms of Imidazolium-Based Ionic Liquids to Epigallocatechin Gallate. Int. J. Mol. Sci. 2022, 23, 12600. https://doi.org/10.3390/ijms232012600
Luo Y, Zhang Y, Tao C, Ni H, Liu X, Chen Y, Wu Y, Song H, Xu T. Exploring the Absorption Mechanisms of Imidazolium-Based Ionic Liquids to Epigallocatechin Gallate. International Journal of Molecular Sciences. 2022; 23(20):12600. https://doi.org/10.3390/ijms232012600
Chicago/Turabian StyleLuo, Yingjie, Yiwei Zhang, Cimin Tao, Hongfei Ni, Xuesong Liu, Yong Chen, Yongjiang Wu, Hang Song, and Tengfei Xu. 2022. "Exploring the Absorption Mechanisms of Imidazolium-Based Ionic Liquids to Epigallocatechin Gallate" International Journal of Molecular Sciences 23, no. 20: 12600. https://doi.org/10.3390/ijms232012600
APA StyleLuo, Y., Zhang, Y., Tao, C., Ni, H., Liu, X., Chen, Y., Wu, Y., Song, H., & Xu, T. (2022). Exploring the Absorption Mechanisms of Imidazolium-Based Ionic Liquids to Epigallocatechin Gallate. International Journal of Molecular Sciences, 23(20), 12600. https://doi.org/10.3390/ijms232012600





