Treatments with Liquid Smoke and Certain Chemical Constituents Prevalent in Smoke Reduce Phloem Vascular Sectoriality in the Sunflower with Improvement to Growth
Abstract
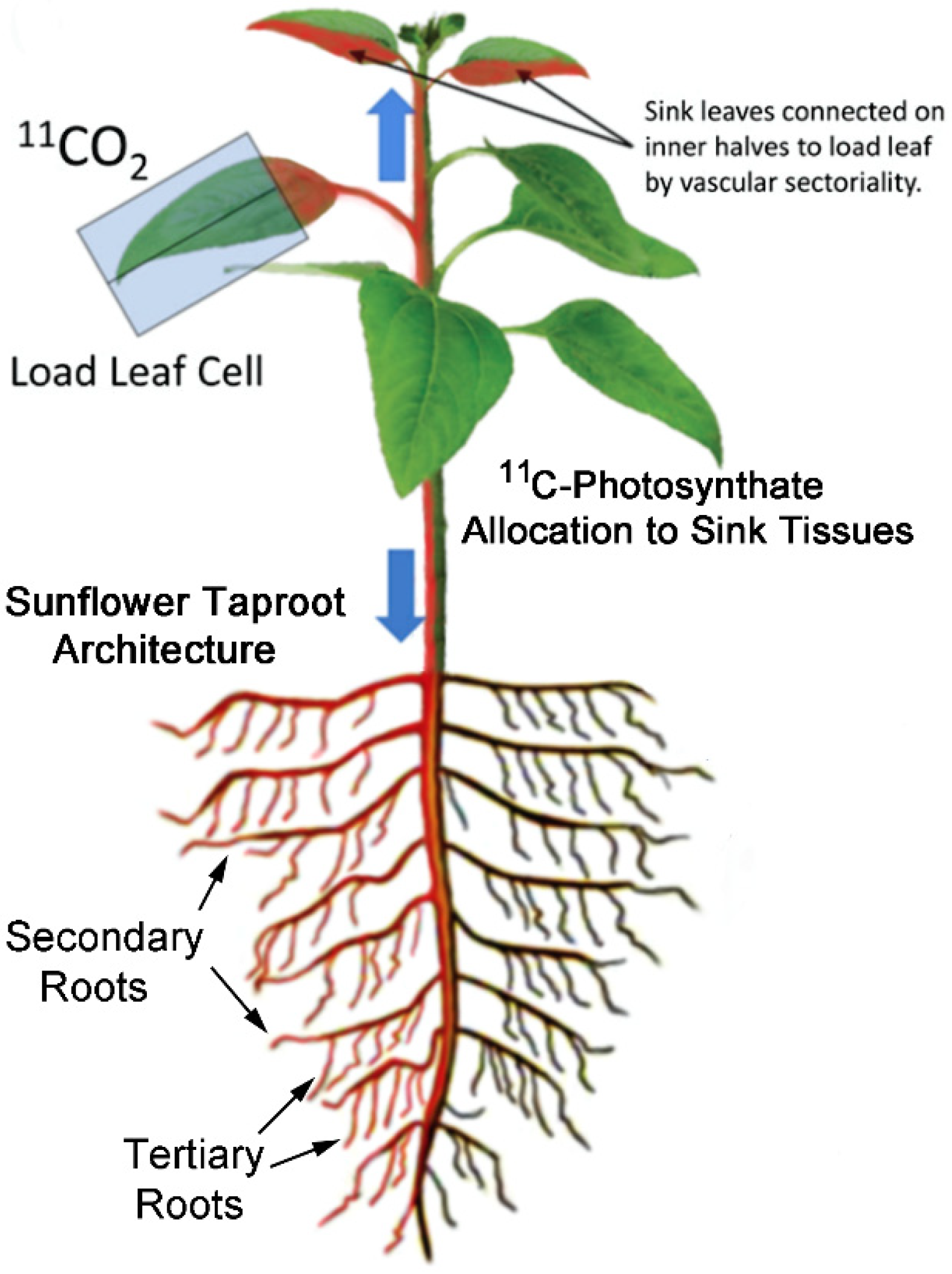
1. Introduction
2. Results
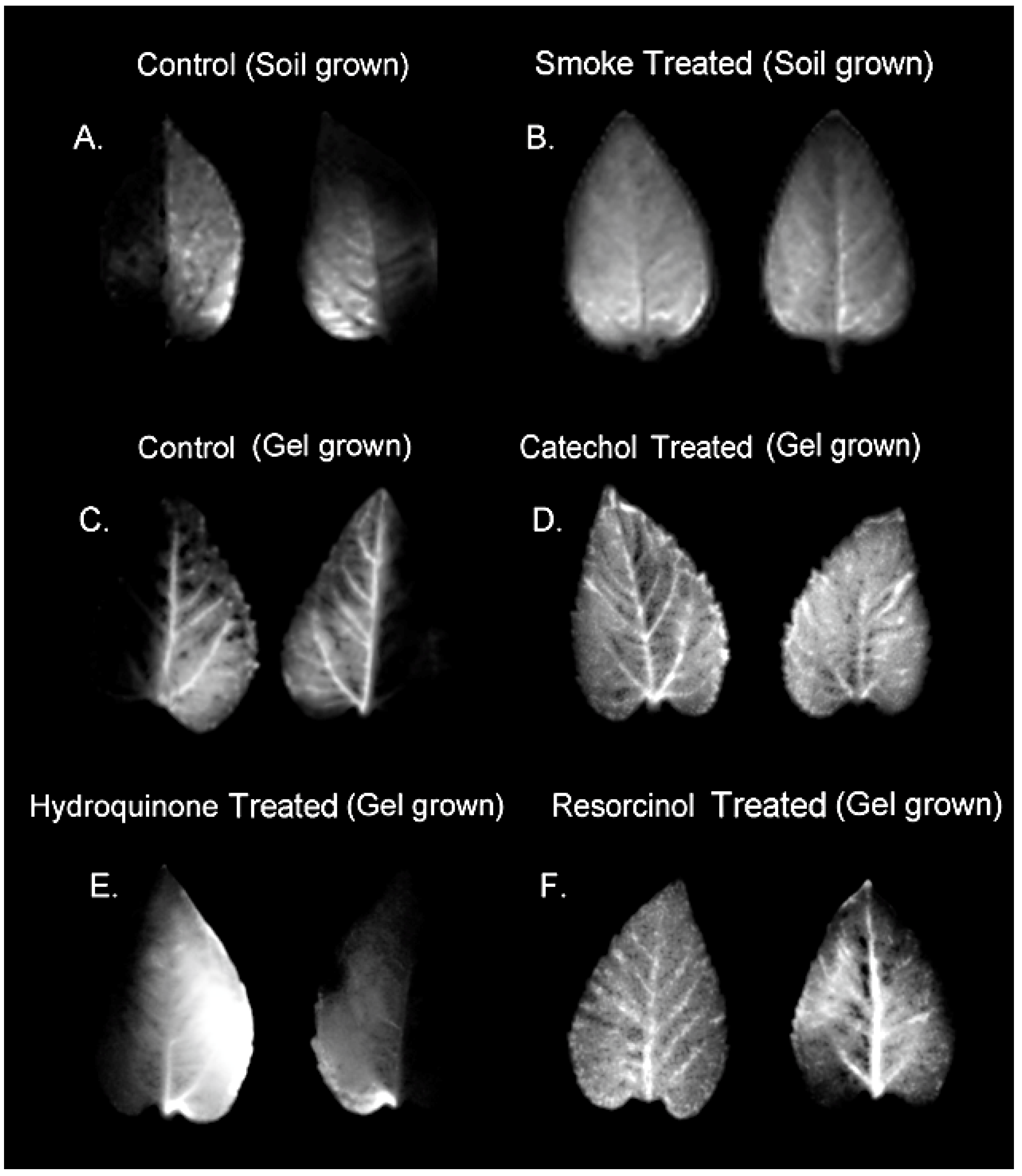
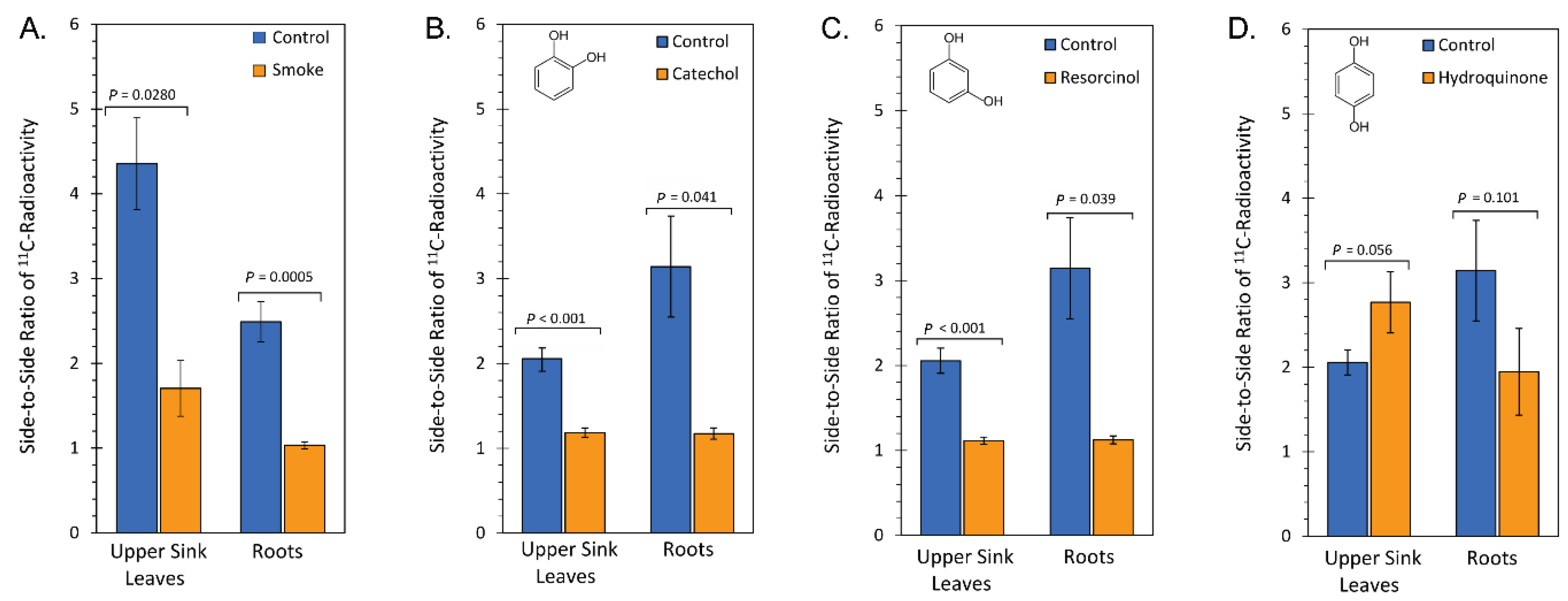
2.1. Treatment with Smoke and Certain Chemical Constituents within Smoke Influence Vascular Sectoriality
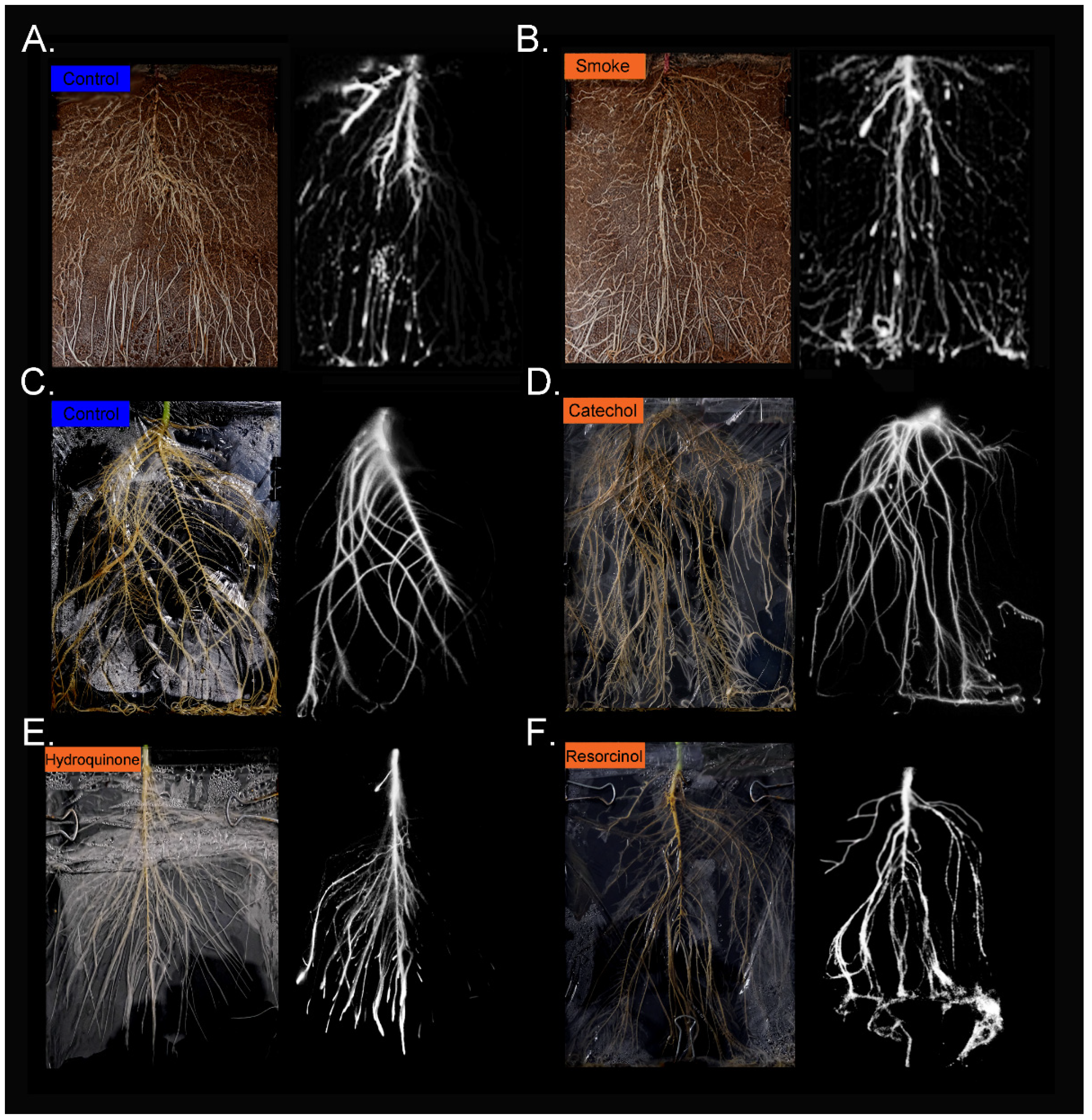
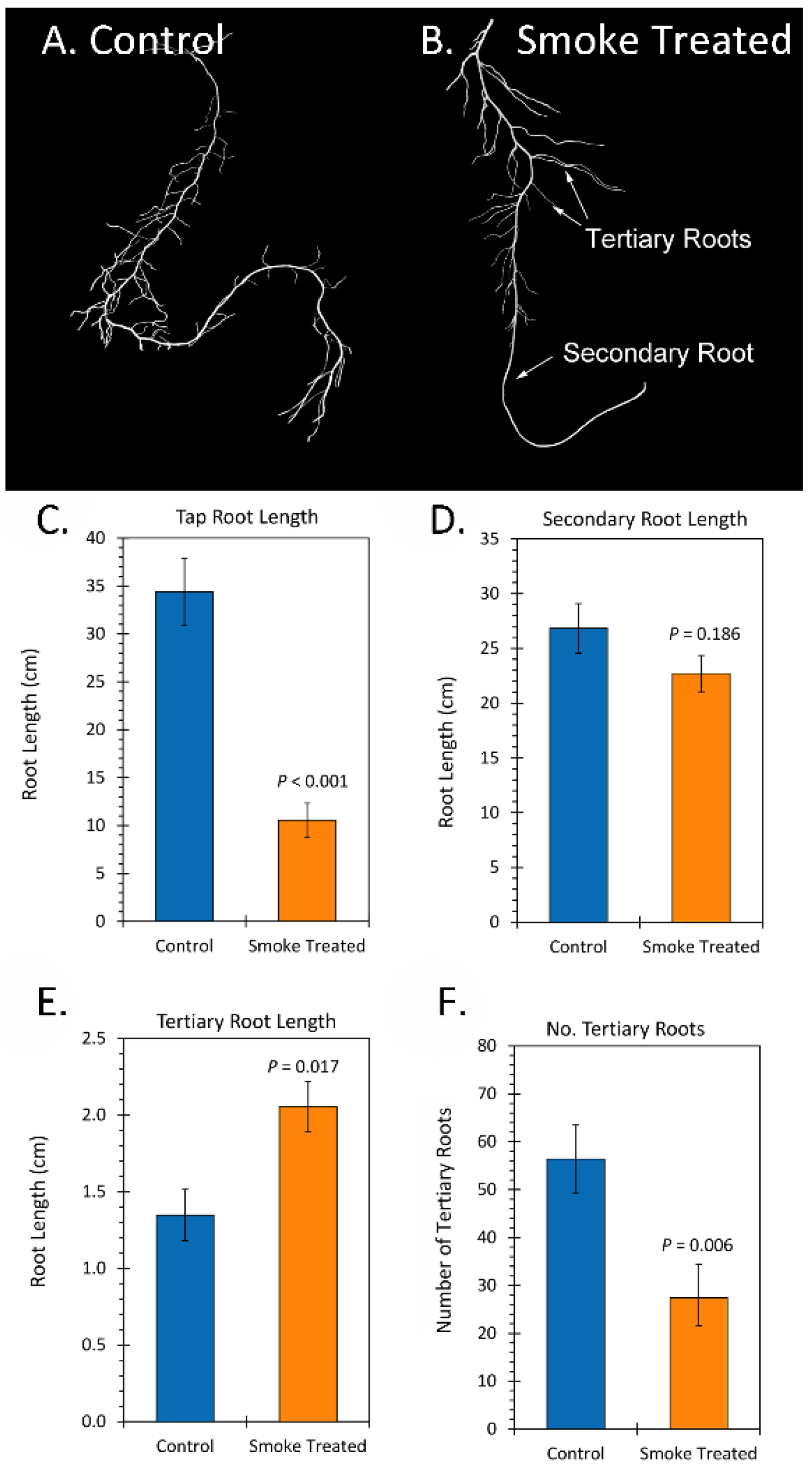
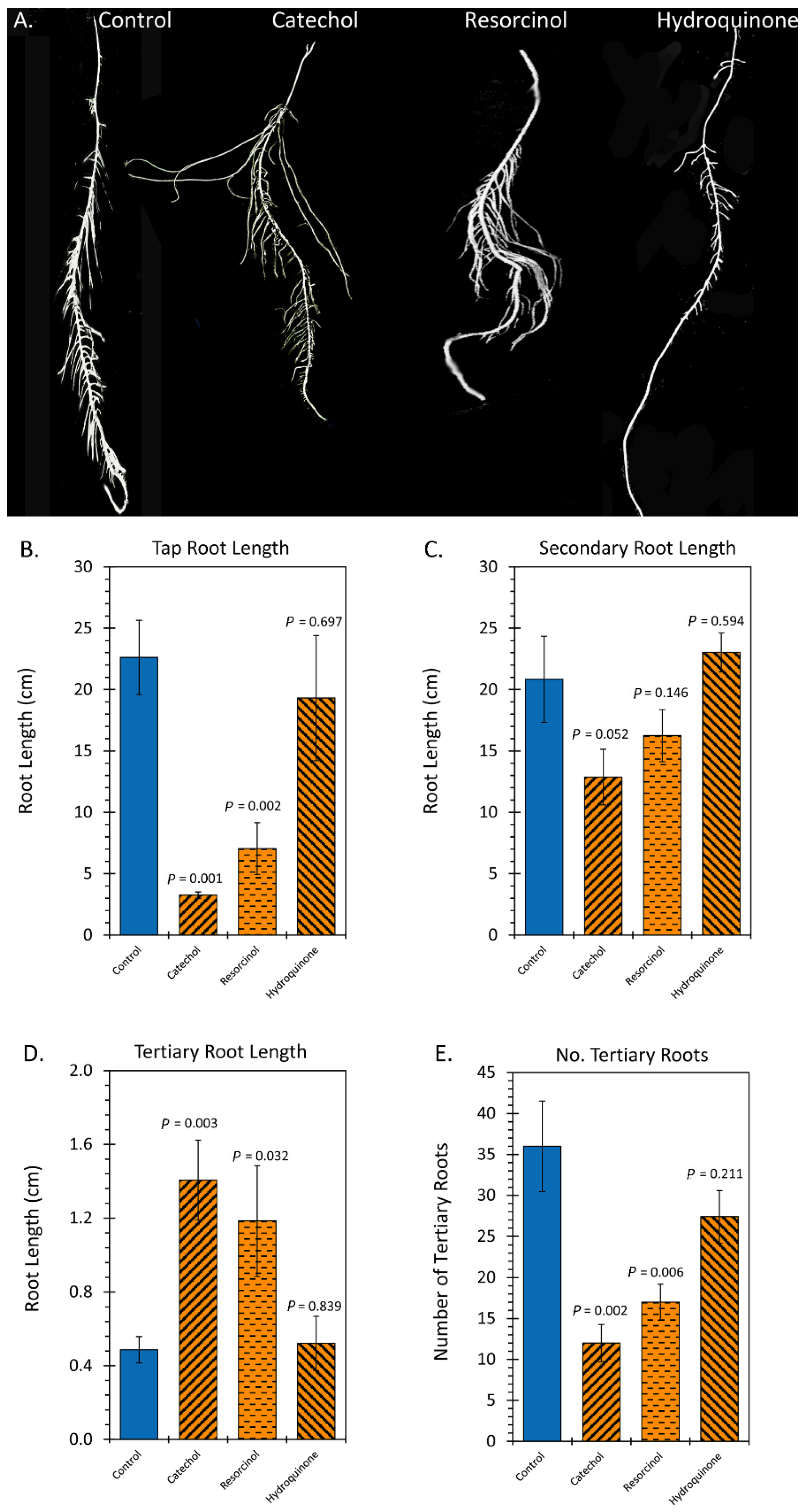
2.2. Treatment with Liquid Smoke and Certain Chemical Constituents within Smoke Influence Root Architecture
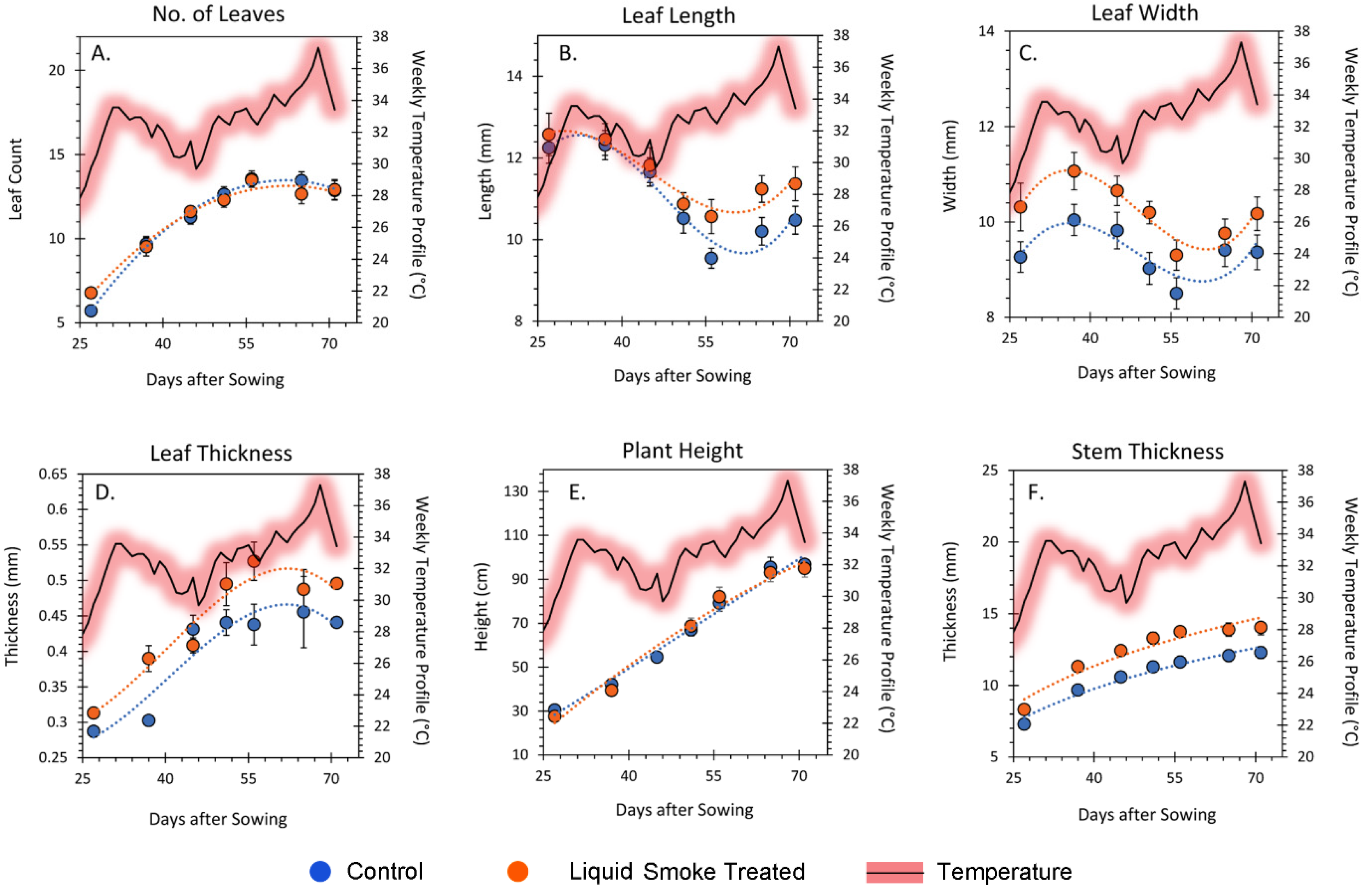
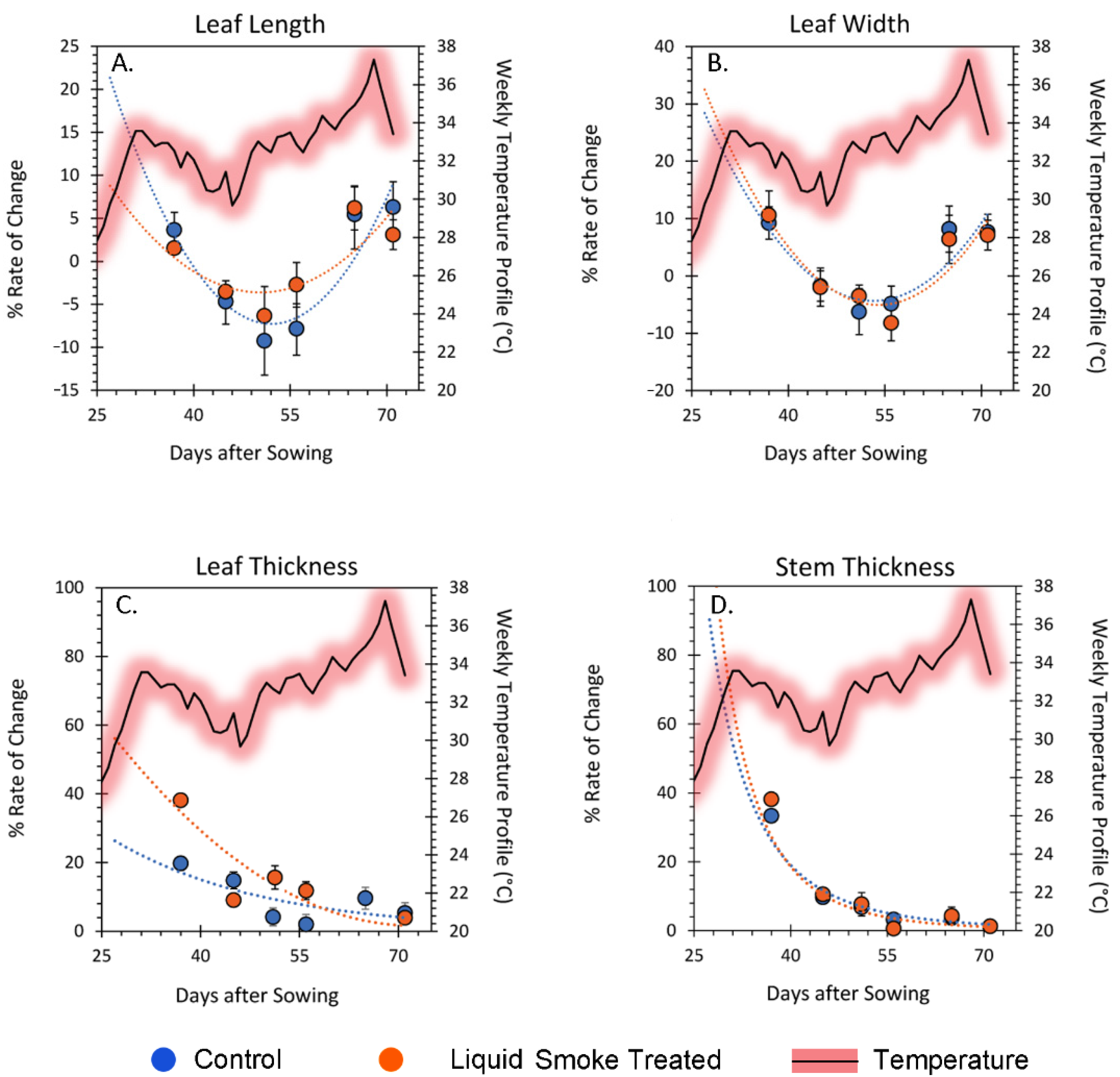
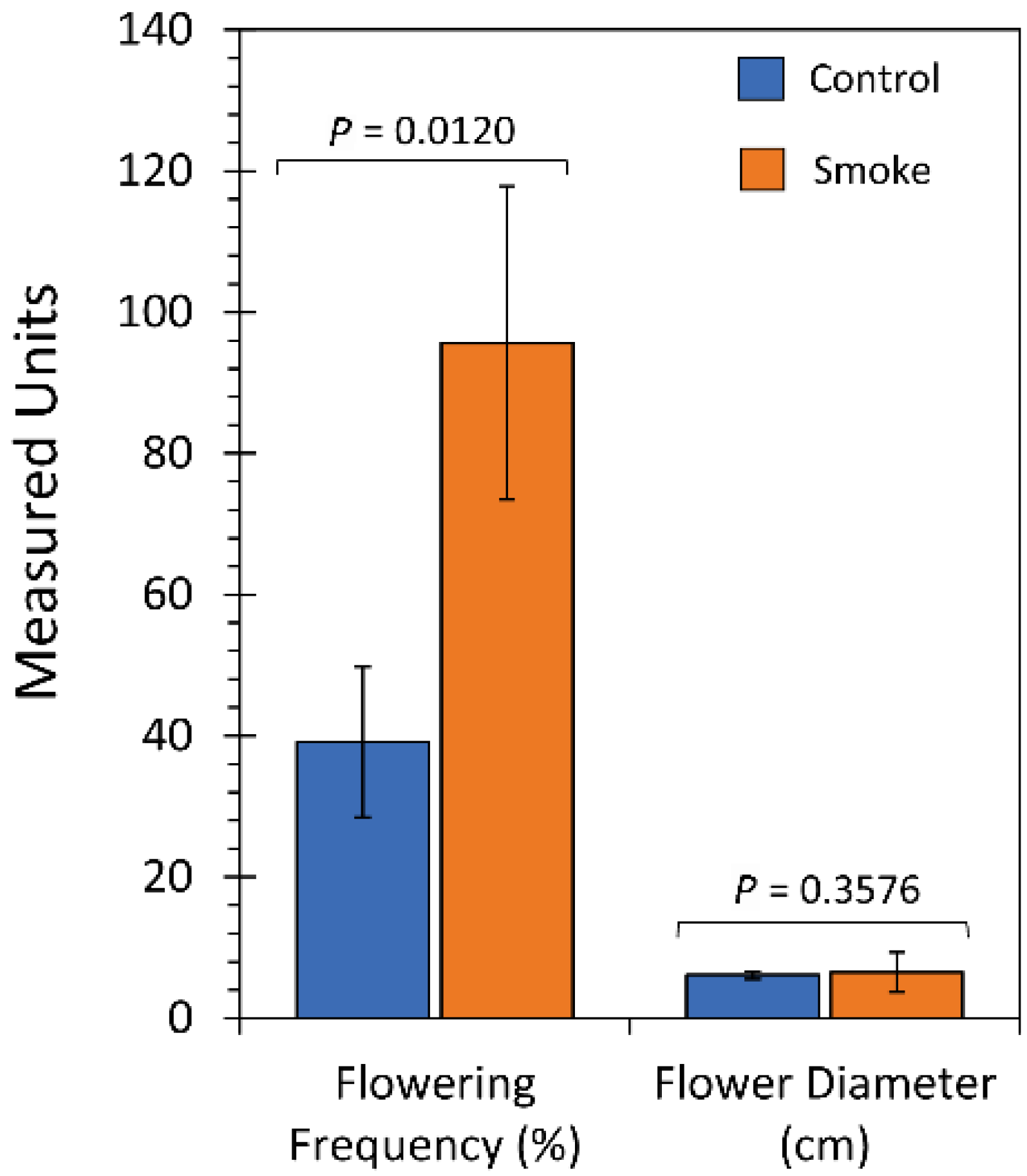
2.3. Chronic Treatment with Liquid Smoke Influences Longer-Term Plant Growth
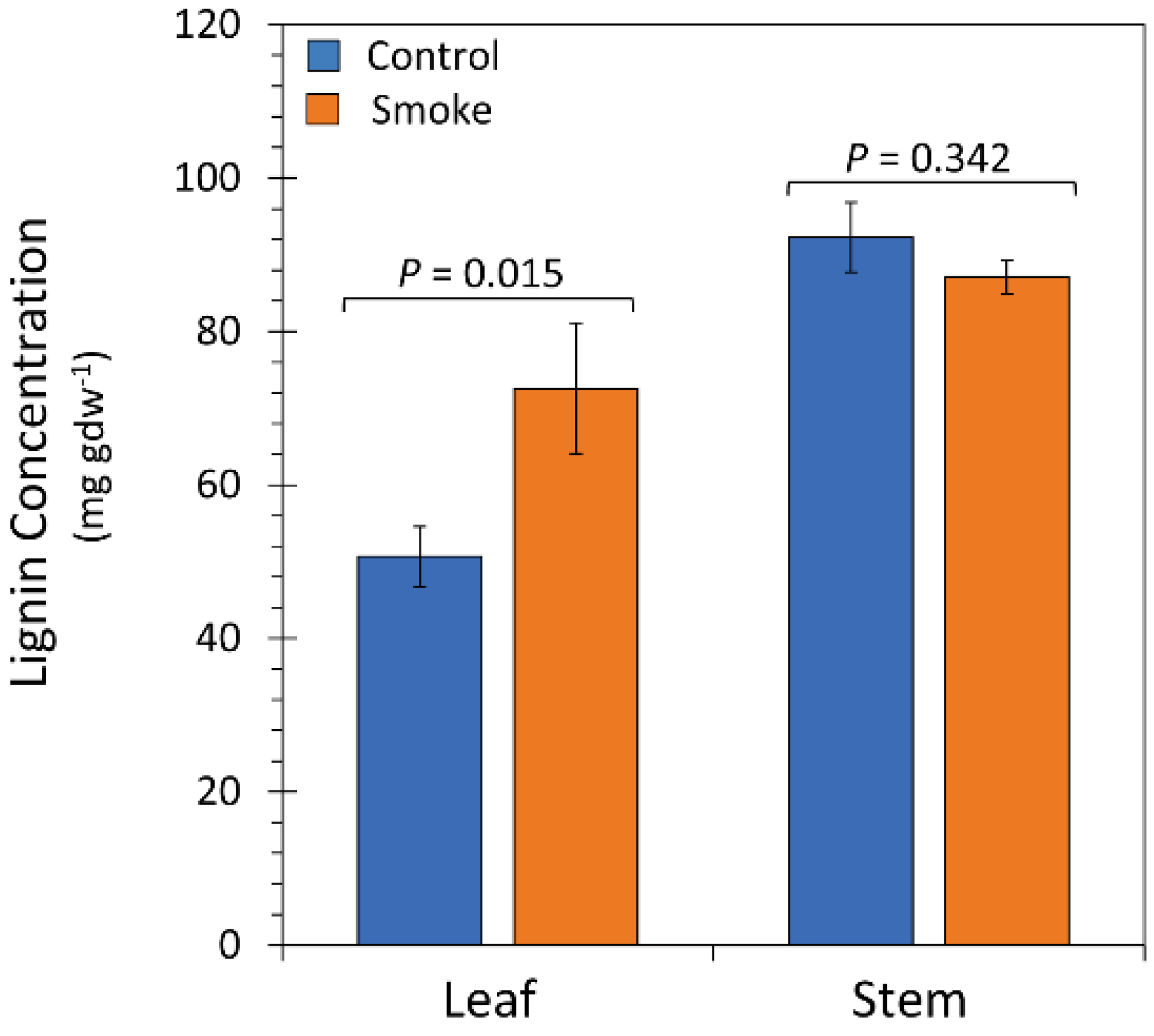
2.4. Treatment with Liquid Smoke Influences Lignin Content in Plant Tissue
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Growth for Laboratory Studies
5.2. Outdoor Plant Growth
5.3. Lignin Analysis
5.4. Measuring Root Growth Trait
5.5. Production and Administration of Radioactive 11CO2
5.6. Autoradiography
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abatzoglou, J.T.; Williams, A.P. Impact of Anthropogenic Climate Change on Wildfire across Western US Forests. Proc. Natl. Acad. Sci. USA 2016, 113, 11770–11775. [Google Scholar] [CrossRef] [PubMed]
- Prescribed Fire: Promoting Better Forest and Wildlife Management. Alabama Cooperative Extension System. Available online: https://www.aces.edu/blog/topics/fire/prescribed-fire-promoting-better-forest-and-wildlife-management/ (accessed on 6 September 2022).
- Rundel, P. Fire as an ecological factor. In Encyclopedia of Pant Physiology; Springer: Berlin, Germany, 1981; Volume 12, pp. 501–538. [Google Scholar]
- Keeley, S.C.; Pizzorno, M. Charred Wood Stimulated Germination of Two Fire-Following Herbs of the California Chaparral and the Role of Hemicellulose. Am. J. Bot. 1986, 73, 1289–1297. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Staszak-Kozinski, L.; Davidson, R. Up in Smoke: I. Smoke-Derived Germination Cues for Postfire Annual, Nicotiana attenuata Torr. Ex. Watson. J. Chem. Ecol. 1994, 20, 2345–2371. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.; Morse, L. Up in Smoke: II. Germination of Nicotiana attenuata in Response to Smoke-Derived Cues and Nutrients in Burned and Unburned Soils. J. Chem. Ecol. 1994, 20, 2373–2391. [Google Scholar] [CrossRef]
- Preston, C.; Baldwin, I. Positive and Negative Signals Regulate Germination in the Post-Fire Annual, Nicotiana attenuata. Ecology 1999, 80, 481–494. [Google Scholar] [CrossRef]
- Van Staden, J.; Brown, N.A.C.; Jäger, A.K.; Johnson, T.A. Smoke as a Germination Cue. Plant Species Biol. 2000, 15, 167–178. [Google Scholar] [CrossRef]
- Nelson, D.C.; Riseborough, J.-A.; Flematti, G.R.; Stevens, J.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Karrikins Discovered in Smoke Trigger Arabidopsis Seed Germination by a Mechanism Requiring Gibberellic Acid Synthesis and Light. Plant Physiol. 2009, 149, 863–873. [Google Scholar] [CrossRef]
- Taylor, J.L.S.; Van Staden, J. Plant-Derived Smoke Solutions Stimulate the Growth of Lycopersicon Esculentum Roots in Vitro. Plant Growth Regul. 1998, 26, 77–83. [Google Scholar] [CrossRef]
- Kulkarni, M.; Sparg, S.; Light, M.; Staden, J. Stimulation of Rice (Oryza sativa L.) Seedling Vigour by Smoke-Water and Butenolide. J. Agron. Crop Sci. 2006, 192, 395–398. [Google Scholar] [CrossRef]
- Soós, V.; Sebestyén, E.; Juhász, A.; Pintér, J.; Light, M.E.; Van Staden, J.; Balázs, E. Stress-Related Genes Define Essential Steps in the Response of Maize Seedlings to Smoke-Water. Funct. Integr. Genom. 2009, 9, 231–242. [Google Scholar] [CrossRef]
- Abdelgadir, H.A.; Kulkarni, M.G.; Arruda, M.P.; Staden, J.V. Enhancing Seedling Growth of Jatropha Curcas—A Potential Oil Seed Crop for Biodiesel. S. Afr. J. Bot. 2011, 78, 88–95. [Google Scholar] [CrossRef]
- Abdollahi, M. Effect of Plant-Derived Smoke on Germination, Seedling Vigour and Growth of Rapeseed (Brassica napus) under Laboratory and Greenhouse Conditions. Seed Sci. Technol. 2012, 40, 437–442. [Google Scholar] [CrossRef]
- Chumpookam, J.; Lin, H.-L.; Shiesh, C.-C. Effect of Smoke-Water on Seed Germination and Seedling Growth of Papaya (Carica papaya Cv. Tainung No. 2). HortScience 2012, 47, 741–744. [Google Scholar] [CrossRef]
- Wang, M.; Schoettner, M.; Xu, S.; Paetz, C.; Wilde, J.; Baldwin, I.T.; Groten, K. Catechol, a Major Component of Smoke, Influences Primary Root Growth and Root Hair Elongation through Reactive Oxygen Species-Mediated Redox Signaling. New Phytol. 2017, 213, 1755–1770. [Google Scholar] [CrossRef]
- Zhong, Z.; Kobayashi, T.; Zhu, W.; Imai, H.; Zhao, R.; Ohno, T.; Rehman, S.U.; Uemura, M.; Tian, J.; Komatsu, S. Plant-Derived Smoke Enhances Plant Growth through Ornithine-Synthesis Pathway and Ubiquitin-Proteasome Pathway in Soybean. J. Proteom. 2020, 221, 103781. [Google Scholar] [CrossRef]
- Giehl, R.; Wirén, N. Root Nutrient Foraging. Plant Physiol. 2014, 166, 509–517. [Google Scholar] [CrossRef]
- Orians, C.M.; Babst, B.; Zanne, A.E. Vascular Constraints and Long-Distance Transport in Dicots. In Vascular Transport in Plants; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 355–371. [Google Scholar]
- Marshall, C. Sectoriality and Physiological Organisation in Herbaceous Plants: An Overview. Vegetatio 1996, 127, 9–16. [Google Scholar] [CrossRef]
- Denno, R.F.; McClure, M.S. Variable Plants and Herbivores in Natural and Managed Systems; Academic Press: New York, NY, USA, 1983. [Google Scholar]
- Baldwin, I.T.; Schmelz, E. Constraints on an Induced Defense: The Role of Leaf Area. Oecologia 1994, 97, 424–430. [Google Scholar] [CrossRef]
- Schittko, U.; Baldwin, I.T. Constraints to Herbivore-Induced Systemic Responses: Bidirectional Signaling Along Orthostichies in Nicotiana attenuata. J. Chem. Ecol. 2003, 29, 763–770. [Google Scholar] [CrossRef]
- Fernández de Bobadilla, M.; Vitiello, A.; Erb, M.; Poelman, E.H. Plant Defense Strategies against Attack by Multiple Herbivores. Trends Plant Sci. 2022, 27, 528–535. [Google Scholar] [CrossRef]
- Orians, C.M.; Pomerleau, J.; Ricco, R. Vascular Architecture Generates Fine Scale Variation in Systemic Induction of Proteinase Inhibitors in Tomato. J. Chem. Ecol. 2000, 26, 471–485. [Google Scholar] [CrossRef]
- Kiefer, I.W.; Slusarenko, A.J. The Pattern of Systemic Acquired Resistance Induction within the Arabidopsis Rosette in Relation to the Pattern of Translocation. Plant Physiol. 2003, 132, 840–847. [Google Scholar] [CrossRef]
- Nooden, L.D.; Rupp, D.C.; Derman, B.D. Separation of Seed Development from Monocarpic Senescence in Soybeans. Nature 1978, 271, 354–357. [Google Scholar] [CrossRef][Green Version]
- Alkio, M.; Diepenbrock, W.; Grimm, E. Evidence for Sectorial Photoassimilate Supply in the Capitulum of Sunflower (Helianthus annuus). New Phytol. 2002, 156, 445–456. [Google Scholar] [CrossRef]
- Kobayashi, M.; Matoh, T.; Azuma, J. Two Chains of Rhamnogalacturonan II Are Cross-Linked by Borate-Diol Ester Bonds in Higher Plant Cell Walls. Plant Physiol. 1996, 110, 1017–1020. [Google Scholar] [CrossRef]
- Loomis, W.D.; Durst, R.W. Chemistry and Biology of Boron. Biofactors 1992, 3, 229–239. [Google Scholar]
- O’Neill, M.A.; Eberhard, S.; Albersheim, P.; Darvill, A.G. Requirement of Borate Cross-Linking of Cell Wall Rhamnogalacturonan II for Arabidopsis Growth. Science 2001, 294, 846–849. [Google Scholar] [CrossRef]
- Housh, A.B.; Matthes, M.S.; Gerheart, A.; Wilder, S.L.; Kil, K.-E.; Schueller, M.; Guthrie, J.M.; McSteen, P.; Ferrieri, R. Assessment of a 18F-Phenylboronic Acid Radiotracer for Imaging Boron in Maize. Int. J. Mol. Sci. 2020, 21, 976. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, A.; O’Neill, M.; Ehwald, R. The Pore Size of Non-Graminaceous Plant Cell Walls Is Rapidly Decreased by Borate Ester Cross-Linking of the Pectic Polysaccharide Rhamnogalacturonan II. Plant Physiol. 1999, 121, 829–838. [Google Scholar] [CrossRef]
- Cakmak, I.; Kurz, H.; Marschner, H. Short-Term Effects of Boron, Germanium and High Light Intensity on Membrane Permeability in Boron Deficient Leaves of Sunflower. Physiol. Plant. 1995, 95, 11–18. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Bretones, G.; Baghour, M.; Ragala, L.; Belakbir, A.; Romero, L. Relationship between Boron and Phenolic Metabolism in Tobacco Leaves. Phytochemistry 1998, 48, 269–272. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; Anzellotti, D.; González-Fontes, A. Changes in Phenolic Metabolism of Tobacco Plants during Short-Term Boron Deficiency. Plant Physiol. Biochem. 2002, 40, 997–1002. [Google Scholar] [CrossRef]
- Watanabe, R.; Chorney, W.; Skok, J.; Wender, S.H. Effect of Boron Deficiency on Polyphenol Production in the Sunflower. Phytochemistry 1963, 3, 391–393. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; Lunar, L.; Lafont, F.; Baumert, A.; González-Fontes, A. Boron Deficiency Causes Accumulation of Chlorogenic Acid and Caffeoyl Polyamine Conjugates in Tobacco Leaves. J. Plant Physiol. 2004, 161, 879–881. [Google Scholar] [CrossRef]
- Karioti, A.; Chatzopoulou, A.; Bilia, A.R.; Liakopoulos, G.; Stavrianakou, S.; Skaltsa, H. Novel Secoiridoid Glucosides in Olea europaea Leaves Suffering from Boron Deficiency. Biosci. Biotechnol. Biochem. 2006, 70, 1898–1903. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; Rexach, J.; González-Fontes, A. Boron in Plants: Deficiency and Toxicity. J. Integr. Plant Biol. 2008, 50, 1247–1255. [Google Scholar] [CrossRef]
- Shelp, B.J.; Marentes, E.; Kitheka, A.M.; Vivekanandan, P. Boron Mobility in Plants. Physiol. Plant. 1995, 94, 356–361. [Google Scholar] [CrossRef]
- Matoh, T.; Ochiai, K. Distribution and Partitioning of Newly Taken-up Boron in Sunflower. Plant Soil 2005, 278, 351–360. [Google Scholar] [CrossRef]
- Brown, P.H.; Shelp, B.J. Boron Mobility in Plants. Plant Soil 1997, 193, 85–101. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global Climatic Drivers of Leaf Size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef]
- Bagniewska-Zadworna, A.; Barakat, A.; Łakomy, P.; Smoliński, D.J.; Zadworny, M. Lignin and Lignans in Plant Defence: Insight from Expression Profiling of Cinnamyl Alcohol Dehydrogenase Genes during Development and Following Fungal Infection in Populus. Plant Sci. 2014, 229, 111–121. [Google Scholar] [CrossRef]
- Gantner, M.; Najda, A.; Piesik, D. Effect of Phenolic Acid Content on Acceptance of Hazel Cultivars by filbert Aphid. Plant Prot. Sci. 2019, 55, 116–122. [Google Scholar] [CrossRef]
- Clarke, S.; Rogiers, S.Y.; Currie, G.M. Sectoriality in Xylem Connections between the Bunch and Leaves of the Grapevine (Vitis vinifera) Shoot. Sci. Hortic. 2014, 168, 229–233. [Google Scholar] [CrossRef]
- Ferrieri, R.A.; Wolf, A.P. The Chemistry of Positron Emitting Nucleogenic (Hot) Atoms with Regard to Preparation of Labelled Compounds of Practical Utility. Radiochim. Acta 1983, 34, 69–83. [Google Scholar] [CrossRef]
- Ferrieri, R.A. Production and application of synthetic precursors labeled with carbon-11 and fluorine-18. In Handbook of Radiopharmaceuticals; John Wiley & Sons, Ltd.: New York, NY, USA, 2003; pp. 229–282. [Google Scholar] [CrossRef]
- Ferrieri, R.A.; Gray, D.W.; Babst, B.A.; Schueller, M.J.; Schlyer, D.J.; Thorpe, M.R.; Orians, C.M.; Lerdau, M. Use of Carbon-11 in Populus Shows That Exogenous Jasmonic Acid Increases Biosynthesis of Isoprene from Recently Fixed Carbon. Plant Cell Environ. 2005, 28, 591–602. [Google Scholar] [CrossRef]
- Powell, A.; Wilder, S.L.; Housh, A.B.; Scott, S.; Benoit, M.; Powell, G.; Waller, S.; Guthrie, J.M.; Schueller, M.J.; Ferrieri, R.A. Examining Effects of Rhizobacteria in Relieving Abiotic Crop Stresses Using Carbon-11 Radiotracing. Physiol. Plant 2022, 174, e13675. [Google Scholar] [CrossRef]
- Wilder, S.L.; Scott, S.; Waller, S.; Powell, A.; Benoit, M.; Guthrie, J.M.; Schueller, M.J.; Awale, P.; McSteen, P.; Matthes, M.S.; et al. Carbon-11 Radiotracing Reveals Physiological and Metabolic Responses of Maize Grown under Different Regimes of Boron Treatment. Plants 2022, 11, 241. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noel, R.; Benoit, M.; Wilder, S.L.; Waller, S.; Schueller, M.; Ferrieri, R.A. Treatments with Liquid Smoke and Certain Chemical Constituents Prevalent in Smoke Reduce Phloem Vascular Sectoriality in the Sunflower with Improvement to Growth. Int. J. Mol. Sci. 2022, 23, 12468. https://doi.org/10.3390/ijms232012468
Noel R, Benoit M, Wilder SL, Waller S, Schueller M, Ferrieri RA. Treatments with Liquid Smoke and Certain Chemical Constituents Prevalent in Smoke Reduce Phloem Vascular Sectoriality in the Sunflower with Improvement to Growth. International Journal of Molecular Sciences. 2022; 23(20):12468. https://doi.org/10.3390/ijms232012468
Chicago/Turabian StyleNoel, Randi, Mary Benoit, Stacy L. Wilder, Spenser Waller, Michael Schueller, and Richard A. Ferrieri. 2022. "Treatments with Liquid Smoke and Certain Chemical Constituents Prevalent in Smoke Reduce Phloem Vascular Sectoriality in the Sunflower with Improvement to Growth" International Journal of Molecular Sciences 23, no. 20: 12468. https://doi.org/10.3390/ijms232012468
APA StyleNoel, R., Benoit, M., Wilder, S. L., Waller, S., Schueller, M., & Ferrieri, R. A. (2022). Treatments with Liquid Smoke and Certain Chemical Constituents Prevalent in Smoke Reduce Phloem Vascular Sectoriality in the Sunflower with Improvement to Growth. International Journal of Molecular Sciences, 23(20), 12468. https://doi.org/10.3390/ijms232012468






