Nr1d1 Mediated Cell Senescence in Mouse Heart-Derived Sca-1+CD31− Cells
Abstract
1. Introduction
2. Results
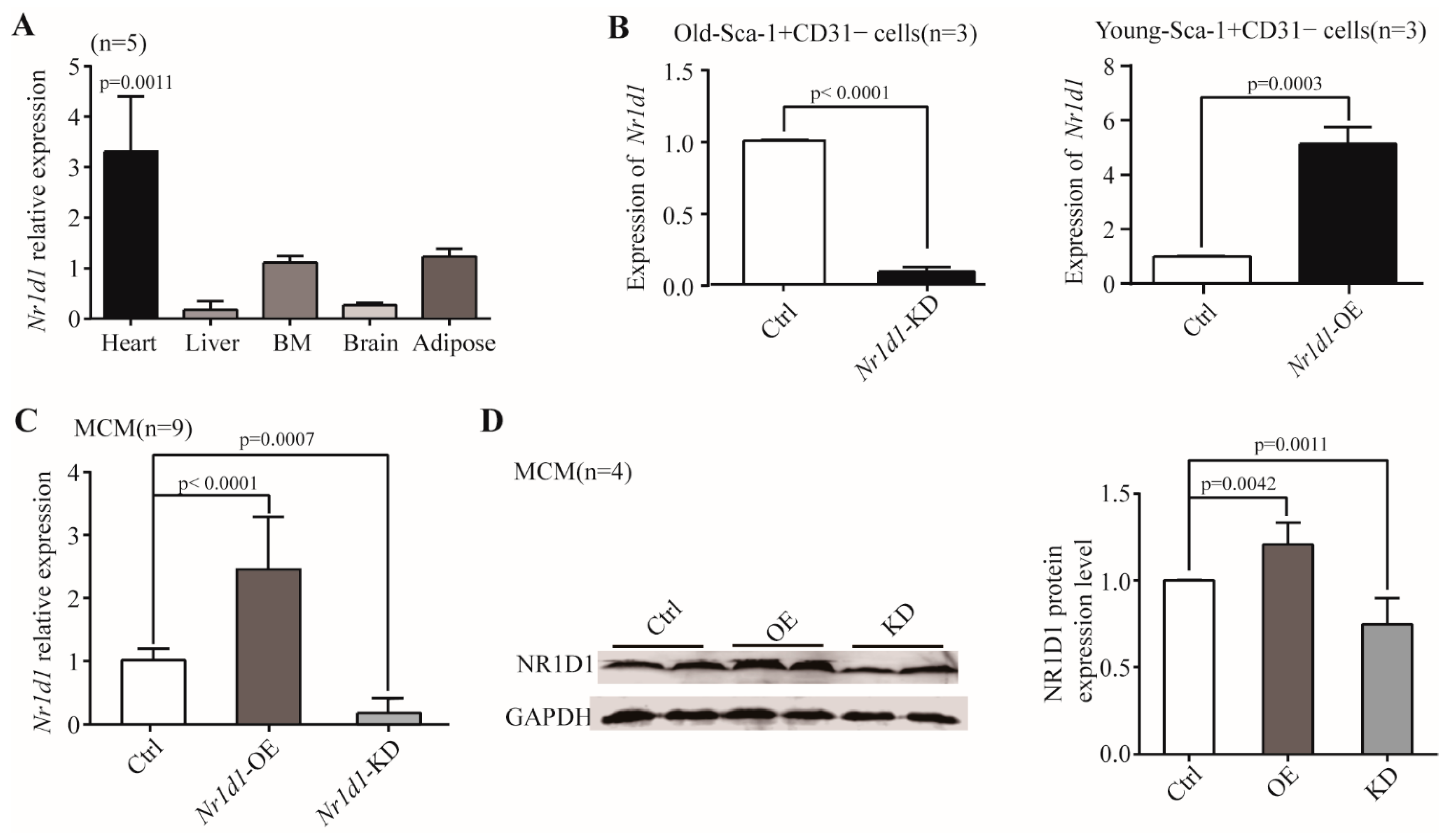
2.1. Nr1d1 mRNA Expression during Aging
2.2. Nr1d1 Was Expressed Predominantly in the Aged Heart-Derived Sca-1+CD31− Cells
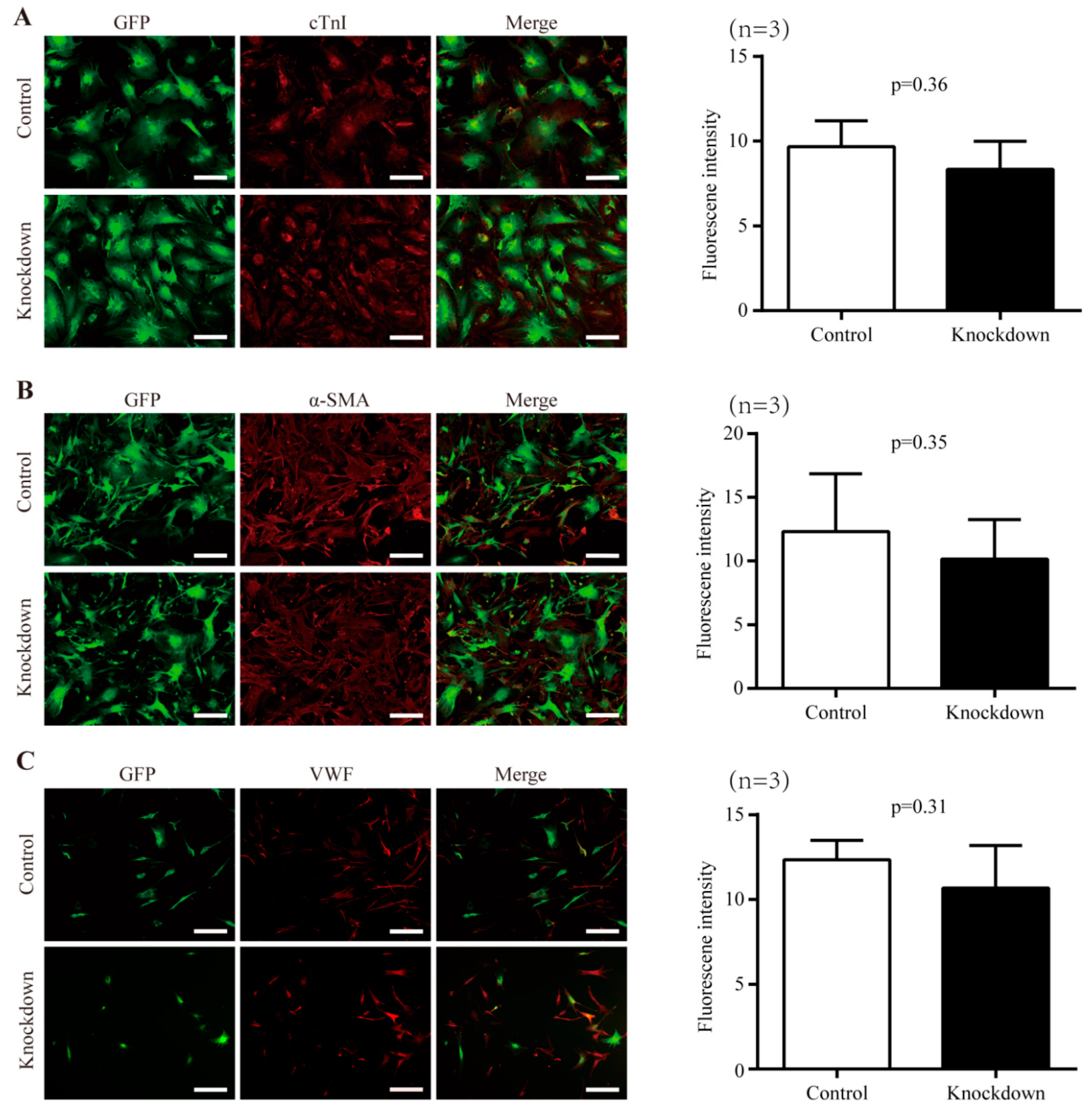
2.3. Impact of Nr1d1 Knockdown on Cell Plasticity
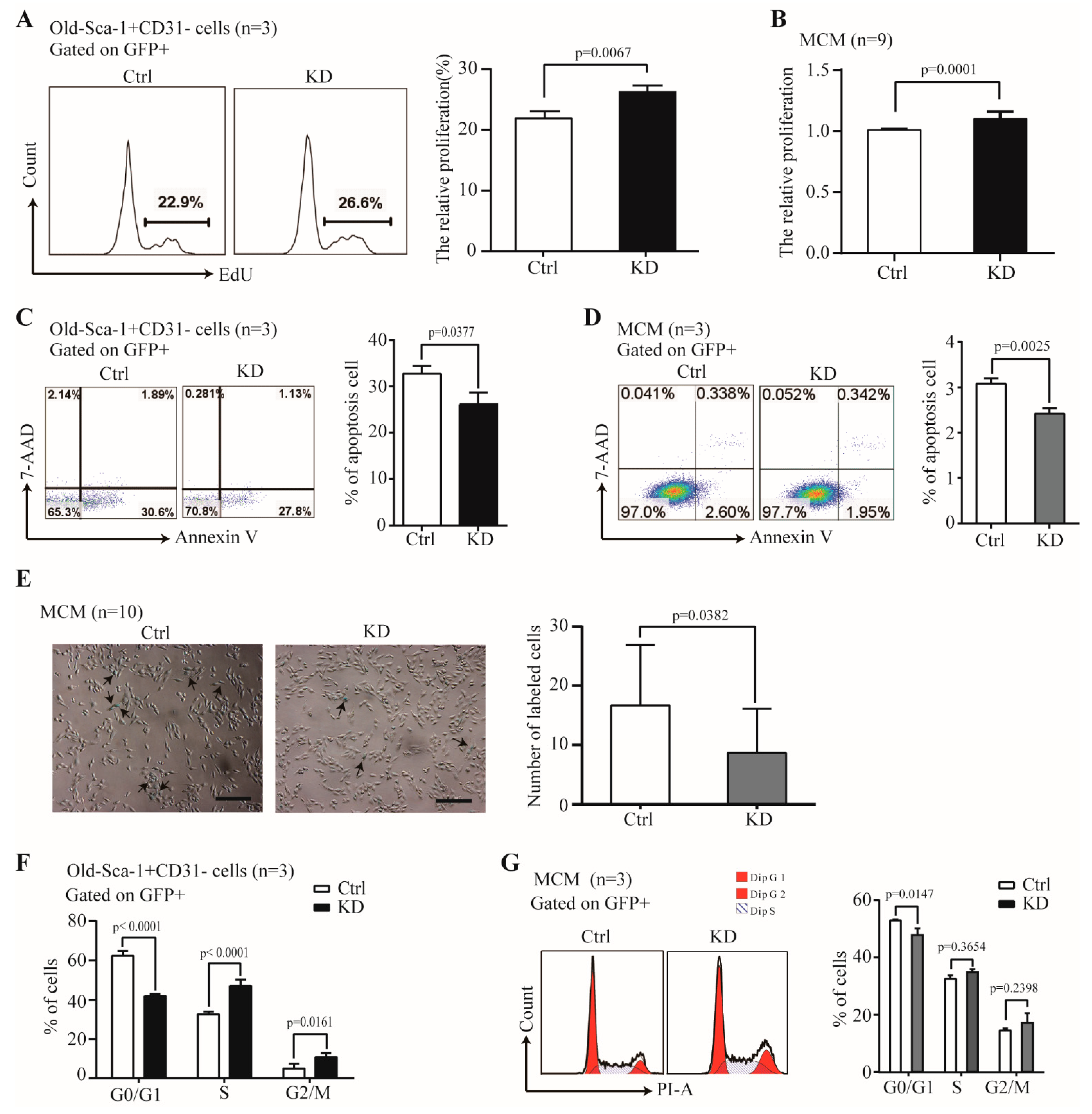
2.4. The Effect of Nr1d1 Knockdown on Cell Senescence
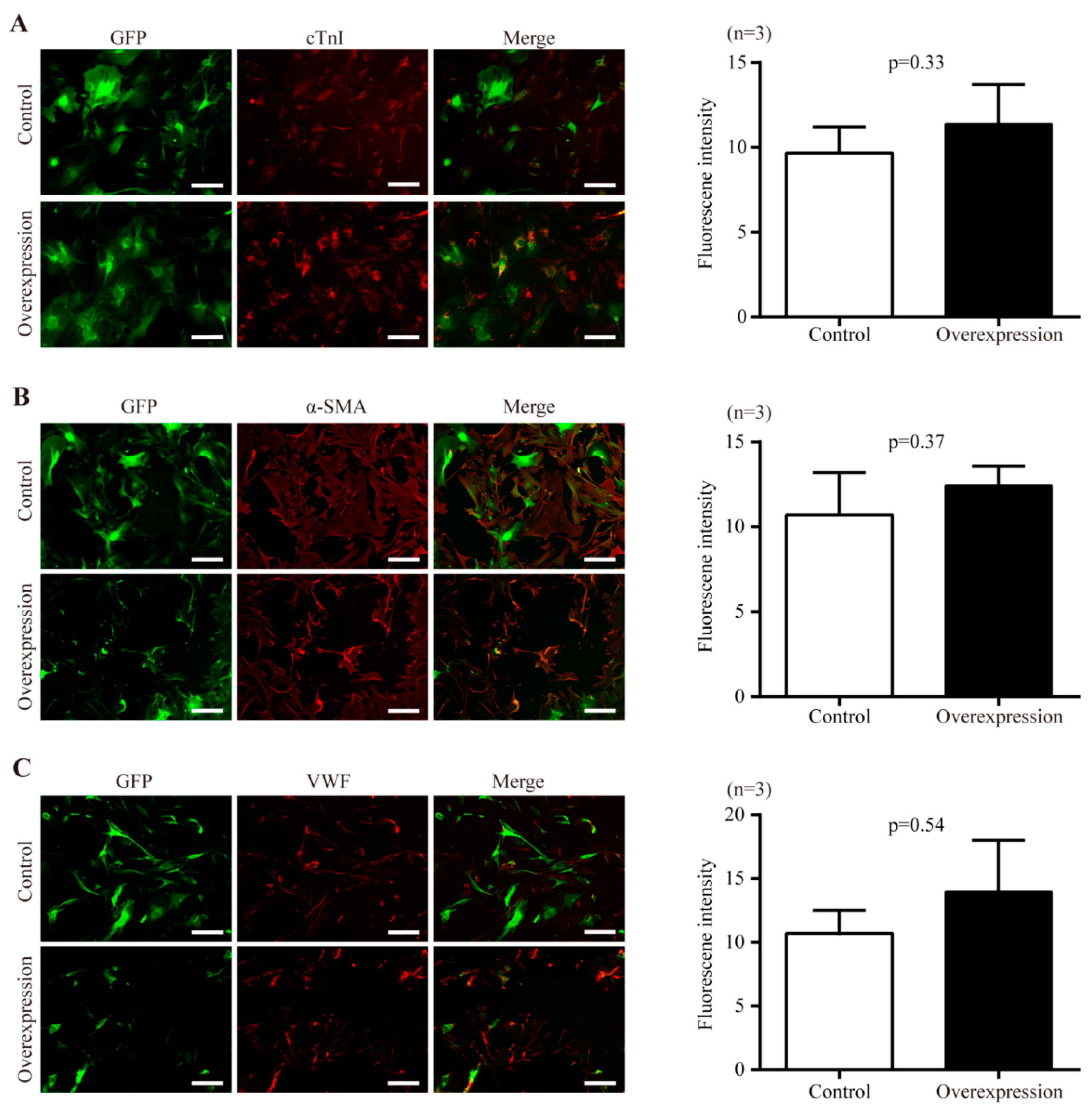
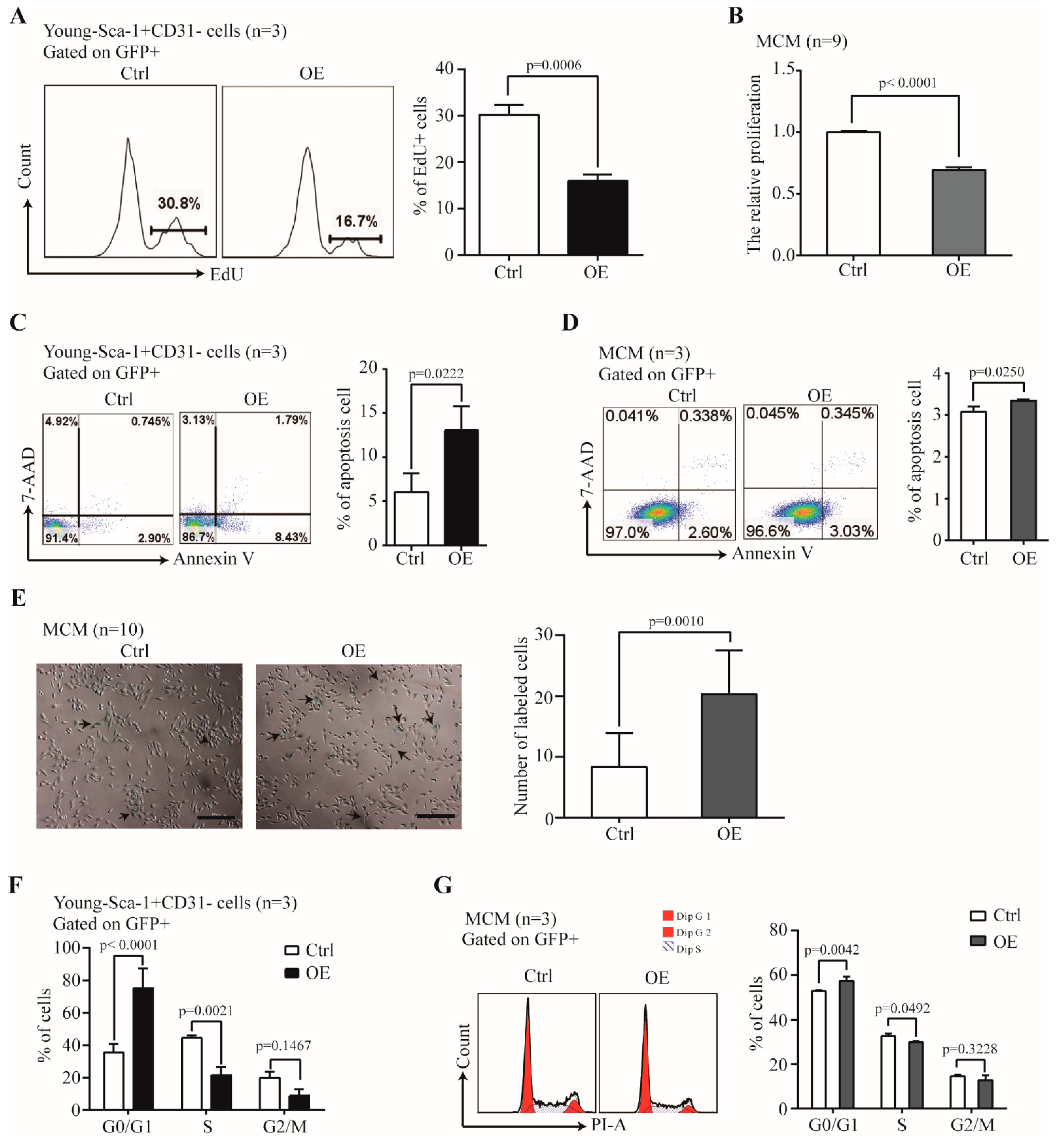
2.5. The Effect of Nr1d1 Overexpression on Cell Plasticity and Senescence
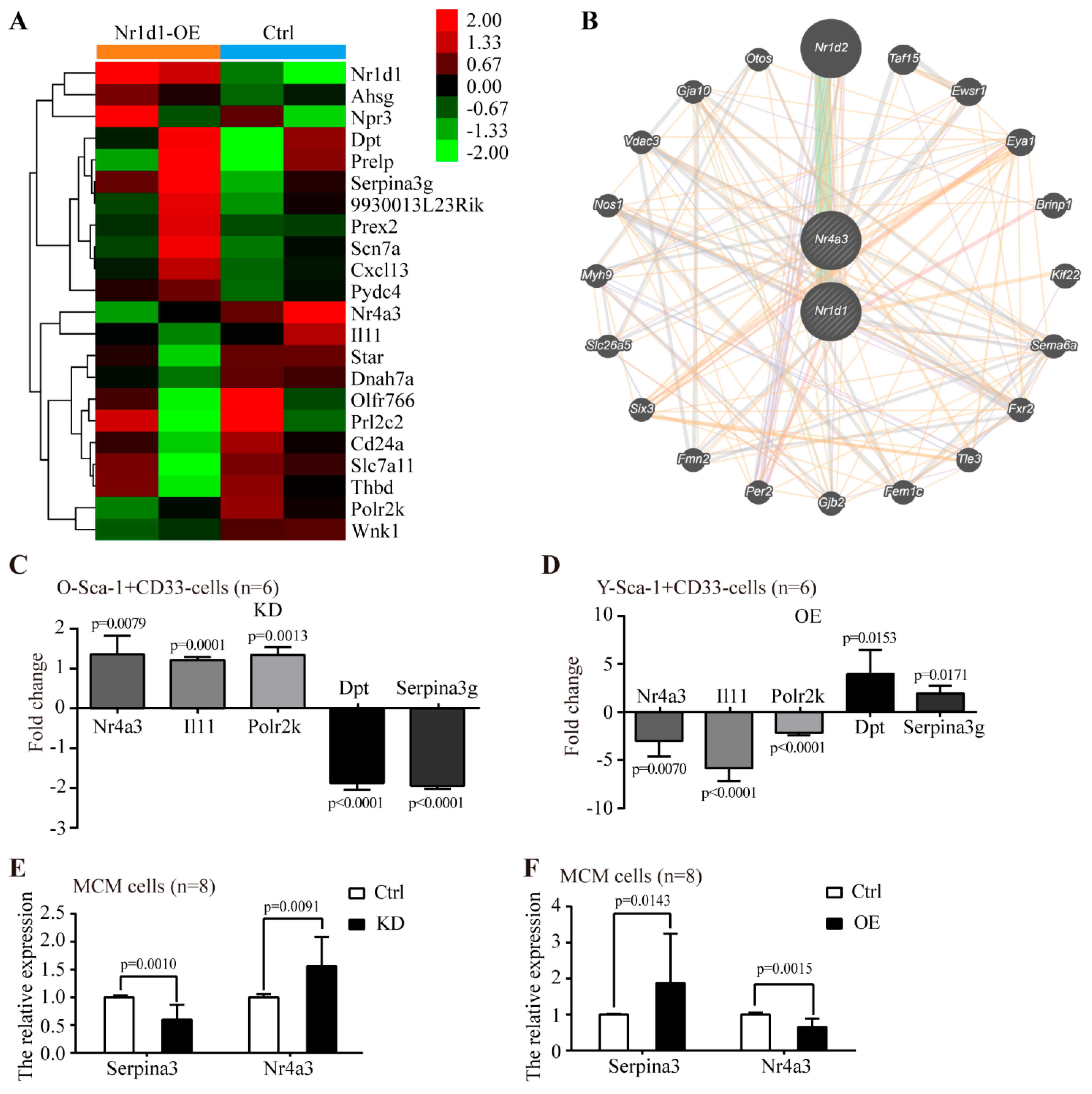
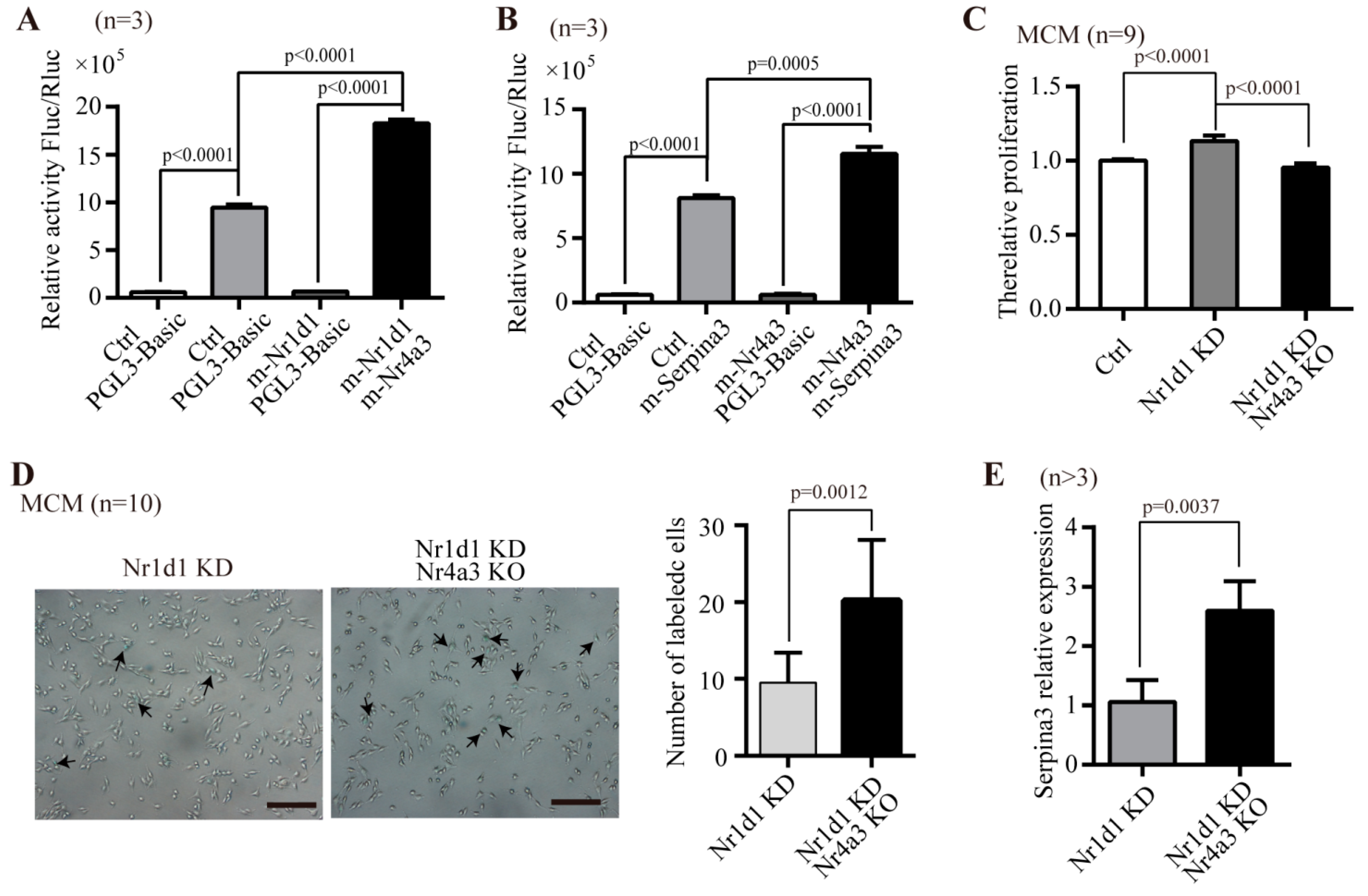
2.6. The Molecular Mechanism Underlying the Regulation of Sca-1+CD31− Cells Aging by Nr1d1
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Cell Culture
4.2. Nr1d1lentivirus Vectors Constructs and Stable Transfection
4.3. Nr4a3 Knockout and Nr1d1 Knockdown
4.4. Cell Differentiation and Immunofluorescence Analysis
4.5. Analysis of Cell Cycle, Proliferation, and Apoptosis
4.6. Microarray Gene Expression Data
4.7. Quantitative Real-Time RT-PCR and Droplet Digital PCR
4.8. Luciferase Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okabe, T.; Chavan, R.; Costa, S.S.F.; Brenna, A.; Ripperger, J.A.; Albrecht, U. REV-ERBalpha influences the stability and nuclear localization of the glucocorticoid receptor. J. Cell Sci. 2016, 129, 4143–4154. [Google Scholar] [PubMed]
- Yin, L.; Wu, N.; Lazar, M.A. Nuclear receptor Rev-erbalpha: A heme receptor that coordinates circadian rhythm and metabolism. Nucl. Recept. Signal 2010, 8, e001. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, Y.; Chu, F.; Ji, S.; Liao, K.; Cui, Z.; Chen, J.; Tang, S. Clock Gene Nr1d1 Alleviates Retinal Inflammation Through Repression of Hmga2 in Microglia. J. Inflamm. Res. 2021, 14, 5901–5918. [Google Scholar] [CrossRef]
- Everett, L.J.; Lazar, M.A. Nuclear receptor Rev-erbalpha: Up, down, and all around. Trends Endocrinol. Metab. 2014, 25, 586–592. [Google Scholar] [CrossRef]
- Yin, L.; Wang, J.; Kleinand, P.S.; Lazar, M.A. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science 2006, 311, 1002–1005. [Google Scholar] [CrossRef]
- Wang, J.; Yin, L.; Lazar, M.A. The orphan nuclear receptor Rev-erb alpha regulates circadian expression of plasminogen activator inhibitor type 1. J. Biol. Chem. 2006, 281, 33842–33848. [Google Scholar] [CrossRef]
- Lee, P.; Bova, R.; Schofield, L.; Bryant, W.; Dieckmann, W.; Slattery, A.; Govendir, M.A.; Emmett, L.; Greenfield, J.R. Brown Adipose Tissue Exhibits a Glucose-Responsive Thermogenic Biorhythm in Humans. Cell Metab. 2016, 23, 602–609. [Google Scholar] [CrossRef]
- Ka, N.-L.; Na, T.-Y.; Na, H.; Lee, M.-H.; Park, H.-S.; Hwang, S.; Kim, I.Y.; Seong, J.K.; Lee, M.-O. NR1D1 Recruitment to Sites of DNA Damage Inhibits Repair and Is Associated with Chemosensitivity of Breast Cancer. Cancer Res. 2017, 77, 2453–2463. [Google Scholar] [CrossRef]
- Woldt, E.; Sebti, Y.; Solt, L.A.; Duhem, C.; Lancel, S.; Eeckhoute, J.; Hesselink, M.K.C.; Paquet, C.; Delhaye, S.; Shin, Y.; et al. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 2013, 19, 1039–1046. [Google Scholar] [CrossRef]
- Pfister, O.; Oikonomopoulos, A.; Sereti, K.-I.; Liao, R. Isolation of Resident Cardiac Progenitor Cells by Hoechst 33342 Staining. Methods Mol. Biol. 2010, 660, 53–63. [Google Scholar]
- Wu, Q.; Zhan, J.; Li, Y.; Wang, X.; Xu, L.; Yu, J.; Pu, S.; Zhou, Z. Differentiation-Associated MicroRNA Alterations in Mouse Heart-Derived Sca-1(+)CD31(−)and Sca-1(+)CD31(+)Cells. Stem. Cells Int. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Noseda, M.; Harada, M.; McSweeney, S.; Leja, T.; Belian, E.; Stuckey, D.J.; Paiva, M.S.A.; Habib, J.; Macaulay, I.; de Smith, A.J.; et al. PDGFRalpha demarcates the cardiogenic clonogenic Sca1+ stem/progenitor cell in adult murine myocardium. Nat. Commun. 2015, 6, 6930. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Huang, X.; He, L.; Zhang, L.; Wang, H.; Yu, W.; Pu, W.; Tian, X.; Nie, Y.; et al. Fate Mapping of Sca1 + Cardiac Progenitor Cells in the Adult Mouse Heart. Circulation 2018, 138, 2967–2969. [Google Scholar] [CrossRef]
- Wu, Q.; Zhan, J.; Pu, S.; Qin, L.; Li, Y.; Zhou, Z. Influence of aging on the activity of mice Sca-1+CD31− cardiac stem cells. Oncotarget 2017, 8, 29–41. [Google Scholar] [CrossRef][Green Version]
- Sulli, G.; Rommel, A.; Wang, X.; Kolar, M.; Puca, F.; Saghatelian, A.; Plikus, M.V.; Verma, I.M.; Panda, S. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 2018, 553, 351–355. [Google Scholar] [CrossRef]
- Song, C.; Tan, P.; Zhang, Z.; Wu, W.; Dong, Y.; Zhao, L.; Liu, H.; Guan, H.; Li, F. REV-ERB agonism suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss partially via FABP4 upregulation. FASEB J. 2018, 32, 3215–3228. [Google Scholar] [CrossRef]
- He, Y.; Lin, F.; Chen, Y.; Tan, Z.; Bai, D.; Zhao, Q. Overexpression of the Circadian Clock Gene Rev-erbalpha Affects Murine Bone Mesenchymal Stem Cell Proliferation and Osteogenesis. Stem. Cells Dev. 2015, 24, 1194–1204. [Google Scholar] [CrossRef]
- Qing, H.; Liu, Y.; Zhao, Y.; Aono, J.; Jones, K.L.; Heywood, E.B.; Howatt, D.; Binkley, C.M.; Daugherty, A.; Liang, Y.; et al. Deficiency of the NR4A Orphan Nuclear Receptor NOR1 in Hematopoietic Stem Cells Accelerates Atherosclerosis. Stem. Cells 2014, 32, 2419–2429. [Google Scholar] [CrossRef]
- Nomiyama, T.; Nakamachi, T.; Gizard, F.; Heywood, E.B.; Jones, K.L.; Ohkura, N.; Kawamori, R.; Conneely, O.M.; Bruemmer, D. The NR4A Orphan Nuclear Receptor NOR1 Is Induced by Platelet-derived Growth Factor and Mediates Vascular Smooth Muscle Cell Proliferation. J. Biol. Chem. 2006, 281, 33467–33476. [Google Scholar] [CrossRef]
- Alonso, J.; Galán, M.; Martí-Pàmies, I.; Romero, J.M.; Camacho, M.; Rodríguez, C.; Martínez-González, J. NOR-1/NR4A3 regulates the cellular inhibitor of apoptosis 2 (cIAP2) in vascular cells: Role in the survival response to hypoxic stress. Sci. Rep. 2016, 6, 34056. [Google Scholar] [CrossRef]
- Wang, C.-G.; Lei, W.; Li, C.; Zeng, D.-X.; Huang, J.-A. Neuron-derived orphan receptor 1 promoted human pulmonary artery smooth muscle cells proliferation. Exp. Lung Res. 2015, 41, 208–215. [Google Scholar] [CrossRef]
- Mizukami, T.; Kuramitsu, M.; Takizawa, K.; Momose, H.; Masumi, A.; Naito, S.; Iwama, A.; Ogawa, T.; Noce, T.; Hamaguchi, I.; et al. Identification of Transcripts Commonly Expressed in Both Hematopoietic and Germ-Line Stem Cells. Stem. Cells Dev. 2008, 17, 67–80. [Google Scholar] [CrossRef]
- Salazar-Olivo, L.A.; Mejia-Elizondo, R.; Alonso-Castro, A.J.; Ponce-Noyola, P.; Maldonado-Lagunas, V.; Melendez-Zajgla, J.; Saavedra-Alanis, V.M. SerpinA3g participates in the antiadipogenesis and insulin-resistance induced by tumor necrosis factor-α in 3T3-F442A cells. Cytokine 2014, 69, 180–188. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; Zhao, J.; Xu, M.; Lou, D.; Tso, P.; Li, Z.; Li, X. Effect of ApoA4 on SERPINA3 mediated by nuclear receptors NR4A1 and NR1D1 in hepatocytes. Biochem. Biophys. Res. Commun. 2017, 487, 327–332. [Google Scholar] [CrossRef]
- Stujanna, E.; Murakoshi, N.; Tajiri, K.; Xu, D.; Kimura, T.; Qin, R.; Feng, D.; Yonebayashi, S.; Ogura, Y.; Yamagami, F.; et al. Rev-erb agonist improves adverse cardiac remodeling and survival in myocardial infarction through an anti-inflammatory mechanism. PLoS ONE 2017, 12, e0189330. [Google Scholar] [CrossRef]
- O’Connell, T.D.; Rodrigo, M.C.; Simpson, P.C. Isolation and Culture of Adult Mouse Cardiac Myocytes. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007, 357, 271–296. [Google Scholar]
- Boon, R.; Iekushi, K.; Lechner, S.; Seeger, T.; Fischer, A.; Heydt, S.; Kaluza, D.; Tréguer, K.; Carmona, G.; Bonauer, A.; et al. MicroRNA-34a regulates cardiac ageing and function. Nature 2013, 495, 107–110. [Google Scholar] [CrossRef]
- Dufour, C.R.; Wilson, B.J.; Huss, J.M.; Kelly, D.P.; Alaynick, W.A.; Downes, M.; Evans, R.M.; Blanchette, M.; Giguère, V. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab. 2007, 5, 345–356. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, Q.; Zhan, J.; Wang, Q.; Zhang, D.; He, S.; Pu, S.; Zhou, Z. Cited2 regulates proliferation and survival in young and old mouse cardiac stem cells. BMC Mol. Cell Biol. 2019, 20, 25. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, S.; Wang, Q.; Liu, Q.; Zhao, H.; Zhou, Z.; Wu, Q. Nr1d1 Mediated Cell Senescence in Mouse Heart-Derived Sca-1+CD31− Cells. Int. J. Mol. Sci. 2022, 23, 12455. https://doi.org/10.3390/ijms232012455
Pu S, Wang Q, Liu Q, Zhao H, Zhou Z, Wu Q. Nr1d1 Mediated Cell Senescence in Mouse Heart-Derived Sca-1+CD31− Cells. International Journal of Molecular Sciences. 2022; 23(20):12455. https://doi.org/10.3390/ijms232012455
Chicago/Turabian StylePu, Shiming, Qian Wang, Qin Liu, Hongxia Zhao, Zuping Zhou, and Qiong Wu. 2022. "Nr1d1 Mediated Cell Senescence in Mouse Heart-Derived Sca-1+CD31− Cells" International Journal of Molecular Sciences 23, no. 20: 12455. https://doi.org/10.3390/ijms232012455
APA StylePu, S., Wang, Q., Liu, Q., Zhao, H., Zhou, Z., & Wu, Q. (2022). Nr1d1 Mediated Cell Senescence in Mouse Heart-Derived Sca-1+CD31− Cells. International Journal of Molecular Sciences, 23(20), 12455. https://doi.org/10.3390/ijms232012455




