Molecular Characterization of Plasma HDL, LDL, and VLDL Lipids Cargos from Atherosclerotic Patients with Advanced Carotid Lesions: A Preliminary Report
Abstract
1. Introduction
2. Results
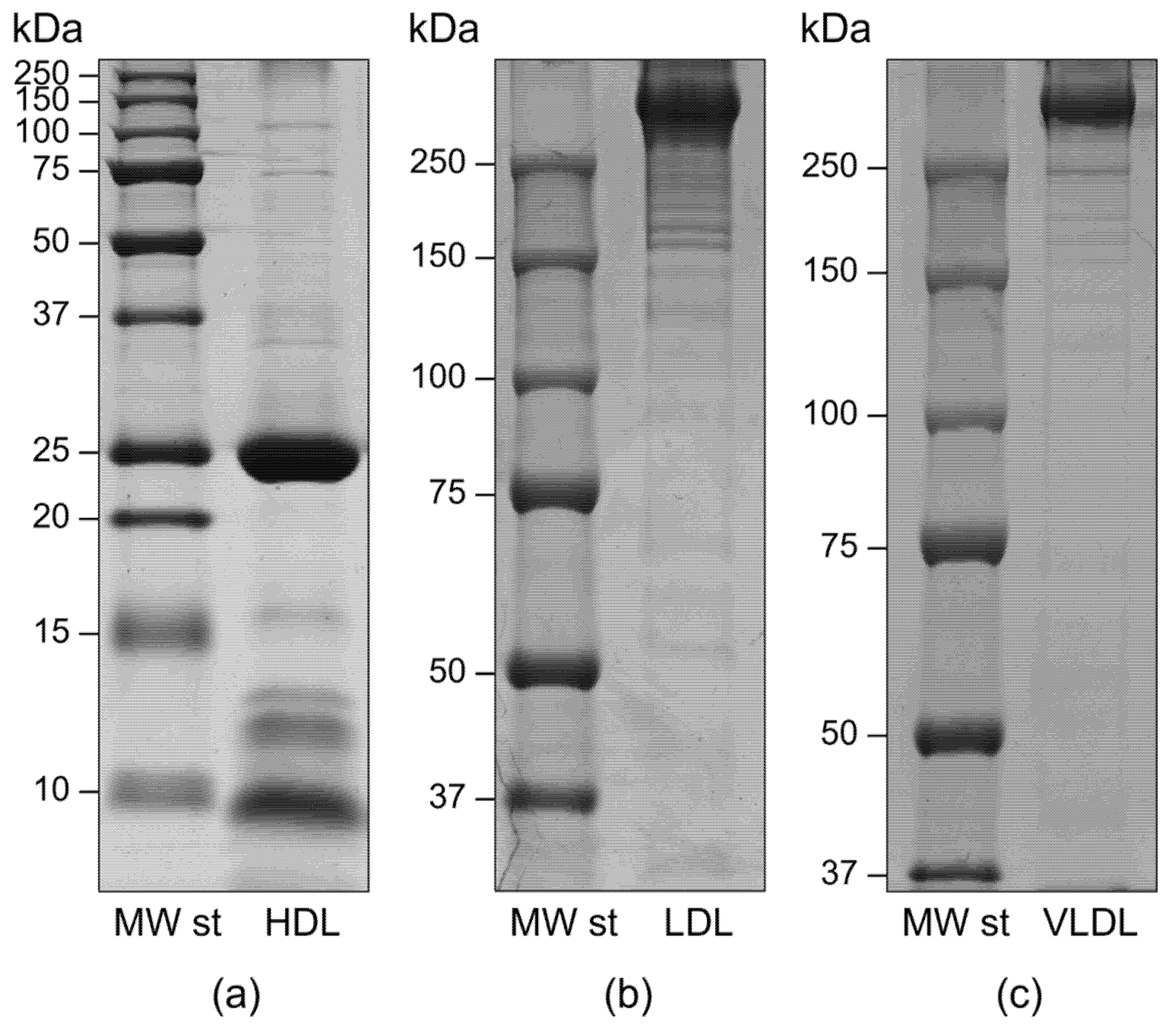
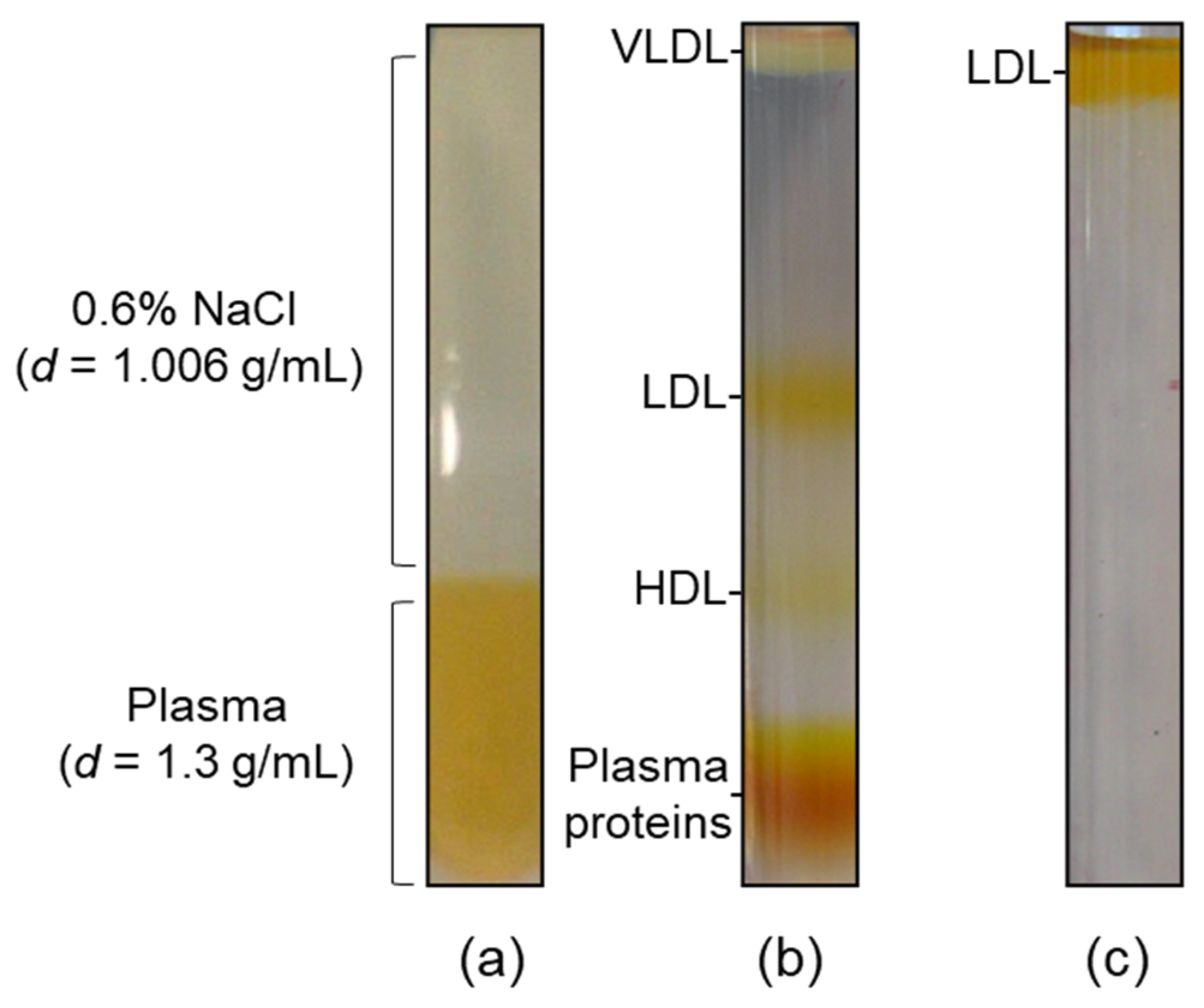
2.1. Lipoproteins Purification
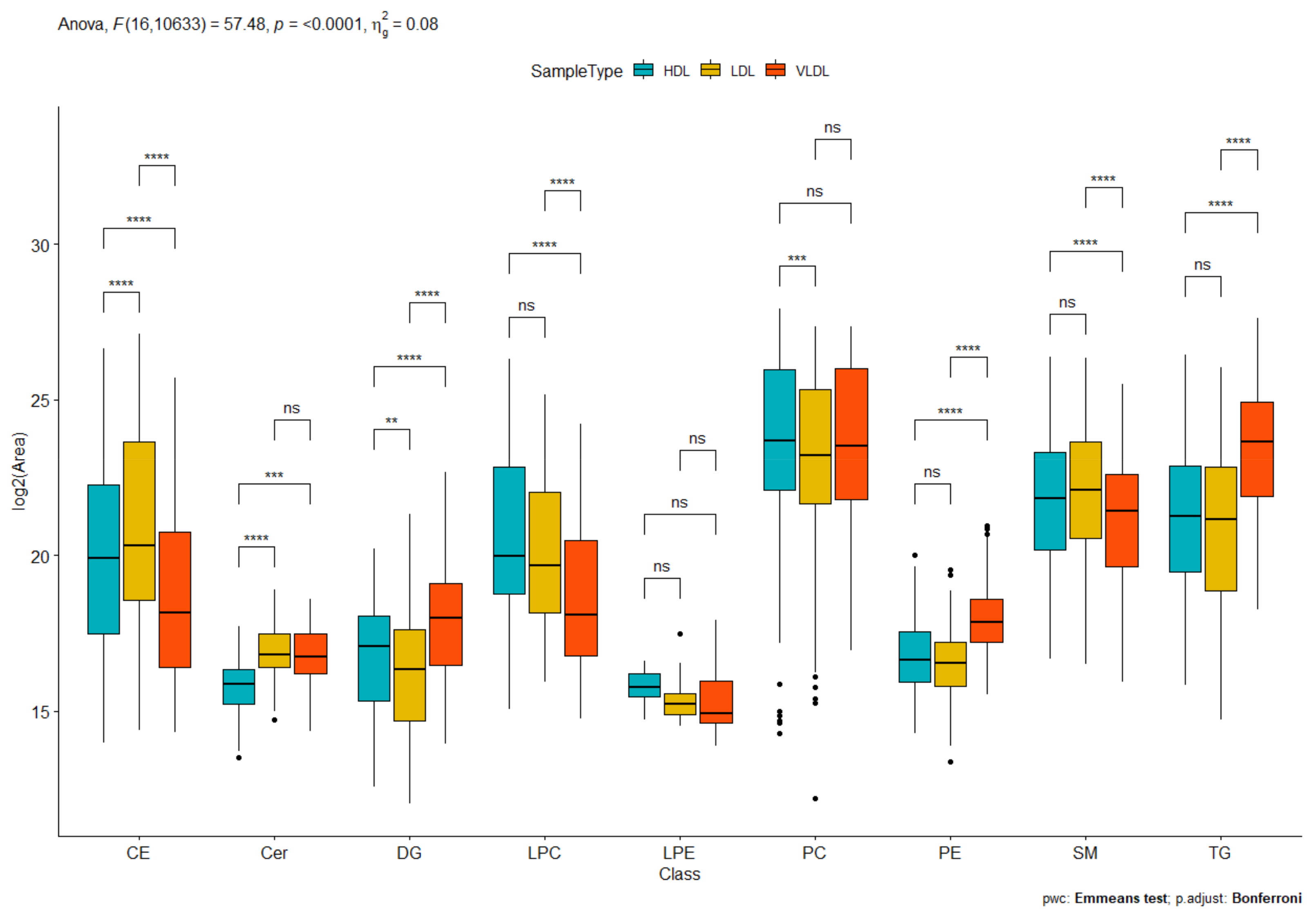
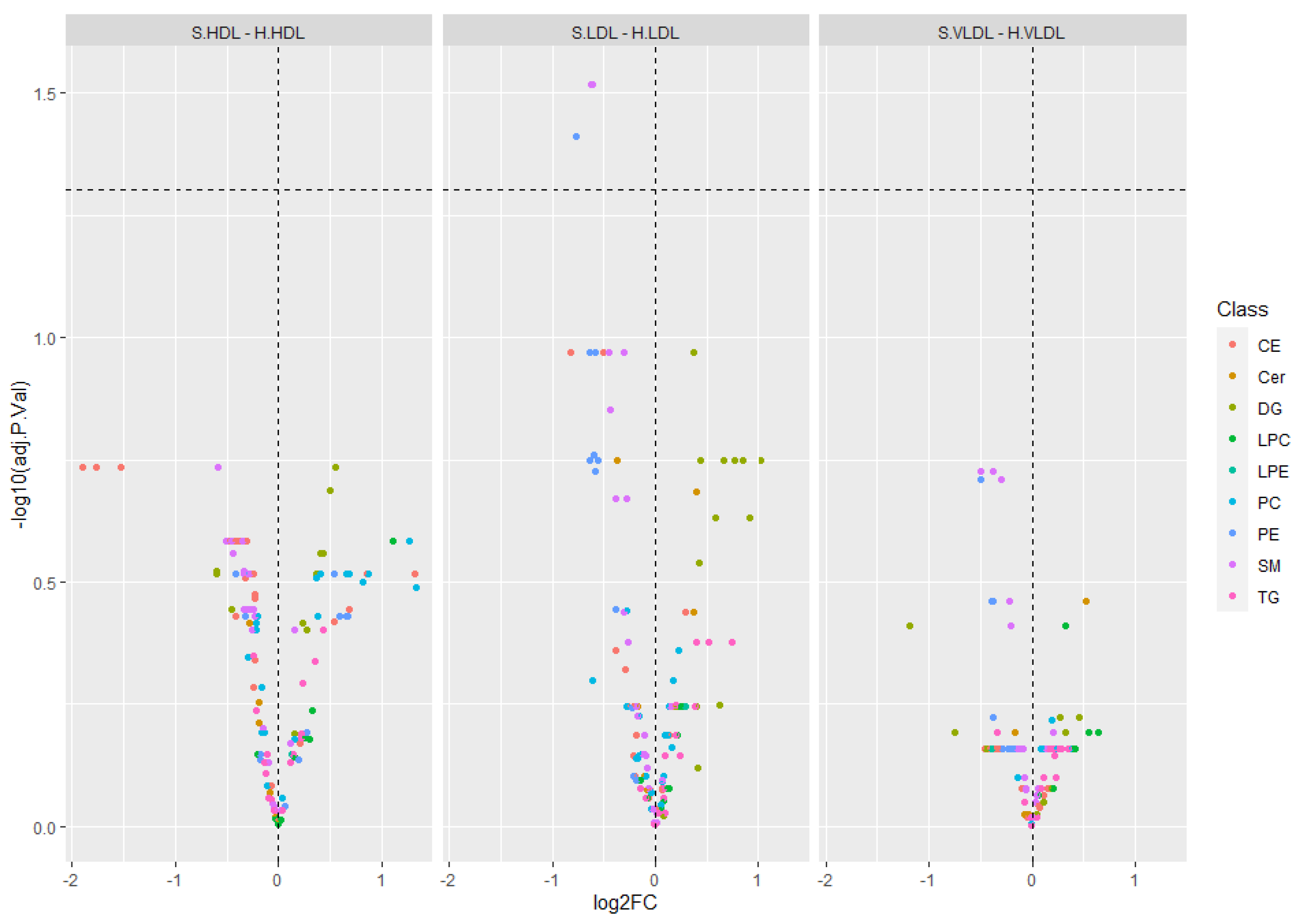
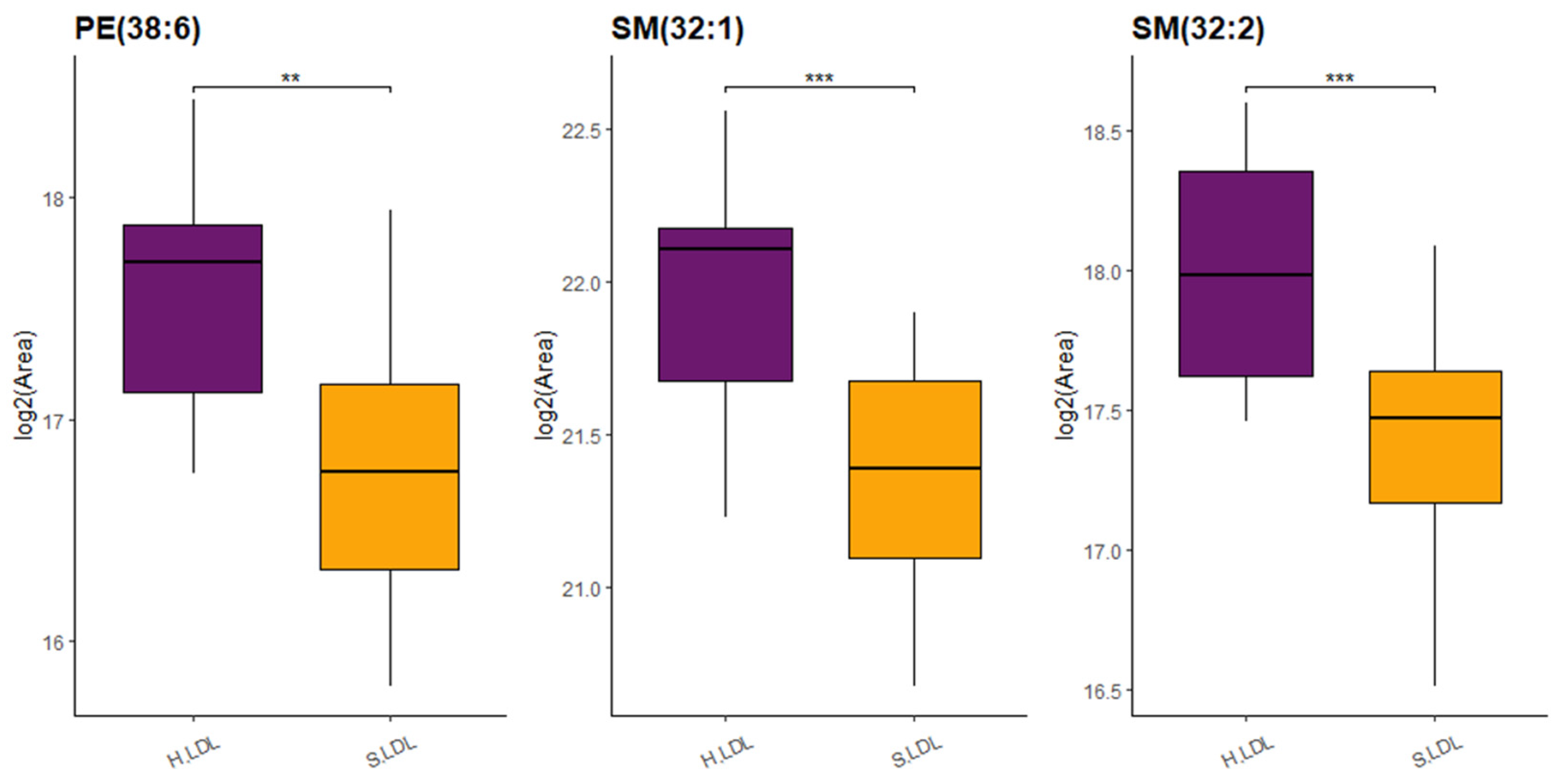
2.2. Targeted Lipidomics
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Lipoprotein Isolation and Purity Assessment
4.3. HDL, LDL, and VLDL Fractions Processing for HPLC-MS/MS Analysis
4.4. HPLC-MS/MS Analysis
4.5. Lipidomics Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hermus, L.; Lefrandt, J.D.; Tio, R.A.; Breek, J.C.; Zeebregts, C.J. Carotid plaque formation and serum biomarkers. Atherosclerosis 2010, 213, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Bonacina, F.; Norata, G.D.; Catapano, A.L. The Interplay of Lipids, Lipoproteins, and Immunity in Atherosclerosis. Curr. Atheroscler. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Finamore, F.; Nieddu, G.; Rocchiccioli, S.; Spirito, R.; Guarino, A.; Formato, M.; Lepedda, A.J. Apolipoprotein Signature of HDL and LDL from Atherosclerotic Patients in Relation with Carotid Plaque Typology: A Preliminary Report. Biomedicines 2021, 9, 1156. [Google Scholar] [CrossRef]
- Lepedda, A.J.; Nieddu, G.; Zinellu, E.; De Muro, P.; Piredda, F.; Guarino, A.; Spirito, R.; Carta, F.; Turrini, F.; Formato, M. Proteomic analysis of plasma-purified VLDL, LDL, and HDL fractions from atherosclerotic patients undergoing carotid endarterectomy: Identification of serum amyloid A as a potential marker. Oxidative Med. Cell Longev. 2013, 2013, 385214. [Google Scholar] [CrossRef]
- Gray-Weale, A.C.; Graham, J.C.; Burnett, J.R.; Byrne, K.; Lusby, R.J. Carotid artery atheroma: Comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J. Cardiovasc. Surg. 1988, 29, 676–681. [Google Scholar]
- Saba, L.; Saam, T.; Jager, H.R.; Yuan, C.; Hatsukami, T.S.; Saloner, D.; Wasserman, B.A.; Bonati, L.H.; Wintermark, M. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019, 18, 559–572. [Google Scholar] [CrossRef]
- Gupta, A.; Kesavabhotla, K.; Baradaran, H.; Kamel, H.; Pandya, A.; Giambrone, A.E.; Wright, D.; Pain, K.J.; Mtui, E.E.; Suri, J.S.; et al. Plaque echolucency and stroke risk in asymptomatic carotid stenosis: A systematic review and meta-analysis. Stroke 2015, 46, 91–97. [Google Scholar] [CrossRef]
- Brinjikji, W.; Rabinstein, A.A.; Lanzino, G.; Murad, M.H.; Williamson, E.E.; DeMarco, J.K.; Huston, J. 3rd. Ultrasound Characteristics of Symptomatic Carotid Plaques: A Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2015, 40, 165–174. [Google Scholar] [CrossRef]
- Jashari, F.; Ibrahimi, P.; Bajraktari, G.; Gronlund, C.; Wester, P.; Henein, M.Y. Carotid plaque echogenicity predicts cerebrovascular symptoms: A systematic review and meta-analysis. Eur. J. Neurol. 2016, 23, 1241–1247. [Google Scholar] [CrossRef]
- Camont, L.; Lhomme, M.; Rached, F.; Le Goff, W.; Negre-Salvayre, A.; Salvayre, R.; Calzada, C.; Lagarde, M.; Chapman, M.J.; Kontush, A. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: Relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2715–2723. [Google Scholar] [CrossRef]
- Ekroos, K.; Janis, M.; Tarasov, K.; Hurme, R.; Laaksonen, R. Lipidomics: A tool for studies of atherosclerosis. Curr. Atheroscler. Rep. 2010, 12, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.T.; Huang, A.; Zhong, L.H.; Shi, Y.; Werstuck, G.H. Comprehensive Plasma Metabolomic Analyses of Atherosclerotic Progression Reveal Alterations in Glycerophospholipid and Sphingolipid Metabolism in Apolipoprotein E-deficient Mice. Sci. Rep. 2016, 6, 35037. [Google Scholar] [CrossRef] [PubMed]
- Borodzicz, S.; Czarzasta, K.; Kuch, M.; Cudnoch-Jedrzejewska, A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis. 2015, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Bonapace, S.; Lunardi, G.; Canali, G.; Dugo, C.; Vinco, G.; Calabria, S.; Barbieri, E.; Laaksonen, R.; Bonnet, F.; et al. Associations between specific plasma ceramides and severity of coronary-artery stenosis assessed by coronary angiography. Diabetes Metab. 2020, 46, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Meikle, P.J.; Pedersen, E.R.; Tell, G.S.; Dhar, I.; Brenner, H.; Schottker, B.; Laaperi, M.; Kauhanen, D.; Koistinen, K.M.; et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J. 2020, 41, 371–380. [Google Scholar] [CrossRef]
- Poss, A.M.; Maschek, J.A.; Cox, J.E.; Hauner, B.J.; Hopkins, P.N.; Hunt, S.C.; Holland, W.L.; Summers, S.A.; Playdon, M.C. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J. Clin. Investig. 2020, 130, 1363–1376. [Google Scholar] [CrossRef]
- Michelucci, E.; Giorgi, N.D.; Finamore, F.; Smit, J.M.; Scholte, A.; Signore, G.; Rocchiccioli, S. Lipid biomarkers in statin users with coronary artery disease annotated by coronary computed tomography angiography. Sci. Rep. 2021, 11, 12899. [Google Scholar] [CrossRef]
- Di Giorgi, N.; Michelucci, E.; Smit, J.M.; Scholte, A.; El Mahdiui, M.; Knuuti, J.; Buechel, R.R.; Teresinska, A.; Pizzi, M.N.; Roque, A.; et al. A specific plasma lipid signature associated with high triglycerides and low HDL cholesterol identifies residual CAD risk in patients with chronic coronary syndrome. Atherosclerosis 2021, 339, 1–11. [Google Scholar] [CrossRef]
- Stegemann, C.; Pechlaner, R.; Willeit, P.; Langley, S.R.; Mangino, M.; Mayr, U.; Menni, C.; Moayyeri, A.; Santer, P.; Rungger, G.; et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 2014, 129, 1821–1831. [Google Scholar] [CrossRef]
- Ellims, A.H.; Wong, G.; Weir, J.M.; Lew, P.; Meikle, P.J.; Taylor, A.J. Plasma lipidomic analysis predicts non-calcified coronary artery plaque in asymptomatic patients at intermediate risk of coronary artery disease. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 908–916. [Google Scholar] [CrossRef]
- Cheng, J.M.; Suoniemi, M.; Kardys, I.; Vihervaara, T.; de Boer, S.P.; Akkerhuis, K.M.; Sysi-Aho, M.; Ekroos, K.; Garcia-Garcia, H.M.; Oemrawsingh, R.M.; et al. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: Results of the ATHEROREMO-IVUS study. Atherosclerosis 2015, 243, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Wong, G.; Tsorotes, D.; Barlow, C.K.; Weir, J.M.; Christopher, M.J.; MacIntosh, G.L.; Goudey, B.; Stern, L.; Kowalczyk, A.; et al. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2723–2732. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, J.P.; Mononen, N.; Hutri-Kahonen, N.; Lehtimaki, M.; Hilvo, M.; Kauhanen, D.; Juonala, M.; Viikari, J.; Kahonen, M.; Raitakari, O.; et al. New evidence from plasma ceramides links apoE polymorphism to greater risk of coronary artery disease in Finnish adults. J. Lipid Res. 2019, 60, 1622–1629. [Google Scholar] [CrossRef]
- Meikle, P.J.; Formosa, M.F.; Mellett, N.A.; Jayawardana, K.S.; Giles, C.; Bertovic, D.A.; Jennings, G.L.; Childs, W.; Reddy, M.; Carey, A.L.; et al. HDL Phospholipids, but Not Cholesterol Distinguish Acute Coronary Syndrome From Stable Coronary Artery Disease. J. Am. Heart Assoc. 2019, 8, e011792. [Google Scholar] [CrossRef]
- Ding, M.; Rexrode, K.M. A Review of Lipidomics of Cardiovascular Disease Highlights the Importance of Isolating Lipoproteins. Metabolites 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Denimal, D.; Pais de Barros, J.P.; Petit, J.M.; Bouillet, B.; Verges, B.; Duvillard, L. Significant abnormalities of the HDL phosphosphingolipidome in type 1 diabetes despite normal HDL cholesterol concentration. Atherosclerosis 2015, 241, 752–760. [Google Scholar] [CrossRef]
- Kostara, C.E.; Ferrannini, E.; Bairaktari, E.T.; Papathanasiou, A.; Elisaf, M.; Tsimihodimos, V. Early Signs of Atherogenic Features in the HDL Lipidomes of Normolipidemic Patients Newly Diagnosed with Type 2 Diabetes. Int. J. Mol. Sci. 2020, 21, 8835. [Google Scholar] [CrossRef]
- Kostara, C.E.; Karakitsou, K.S.; Florentin, M.; Bairaktari, E.T.; Tsimihodimos, V. Progressive, Qualitative, and Quantitative Alterations in HDL Lipidome from Healthy Subjects to Patients with Prediabetes and Type 2 Diabetes. Metabolites 2022, 12, 683. [Google Scholar] [CrossRef]
- Denimal, D.; Nguyen, A.; Pais de Barros, J.P.; Bouillet, B.; Petit, J.M.; Verges, B.; Duvillard, L. Major changes in the sphingophospholipidome of HDL in non-diabetic patients with metabolic syndrome. Atherosclerosis 2016, 246, 106–114. [Google Scholar] [CrossRef]
- Mocciaro, G.; D’Amore, S.; Jenkins, B.; Kay, R.; Murgia, A.; Herrera-Marcos, L.V.; Neun, S.; Sowton, A.P.; Hall, Z.; Palma-Duran, S.A.; et al. Lipidomic Approaches to Study HDL Metabolism in Patients with Central Obesity Diagnosed with Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 6786. [Google Scholar] [CrossRef]
- Orsoni, A.; Therond, P.; Tan, R.; Giral, P.; Robillard, P.; Kontush, A.; Meikle, P.J.; Chapman, M.J. Statin action enriches HDL3 in polyunsaturated phospholipids and plasmalogens and reduces LDL-derived phospholipid hydroperoxides in atherogenic mixed dyslipidemia. J. Lipid Res. 2016, 57, 2073–2087. [Google Scholar] [CrossRef] [PubMed]
- Kostara, C.E.; Tsimihodimos, V.; Elisaf, M.S.; Bairaktari, E.T. NMR-Based Lipid Profiling of High Density Lipoprotein Particles in Healthy Subjects with Low, Normal, and Elevated HDL-Cholesterol. J. Proteome Res. 2017, 16, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, S.; Jiang, M.; Zhu, Y.; Ding, L.; Shi, H.; Dong, P.; Yang, J.; Yang, Y. Atherosclerotic dyslipidemia revealed by plasma lipidomics on ApoE(-/-) mice fed a high-fat diet. Atherosclerosis 2017, 262, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Izumi, Y.; Tamura, S.; Koike, T.; Koike, Y.; Shiomi, M.; Bamba, T. Lipid Profiling of Serum and Lipoprotein Fractions in Response to Pitavastatin Using an Animal Model of Familial Hypercholesterolemia. J. Proteome Res. 2020, 19, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Wong, G.; Tan, R.; Giral, P.; Robillard, P.; Orsoni, A.; Hounslow, N.; Magliano, D.J.; Shaw, J.E.; Curran, J.E.; et al. Statin action favors normalization of the plasma lipidome in the atherogenic mixed dyslipidemia of MetS: Potential relevance to statin-associated dysglycemia. J. Lipid Res. 2015, 56, 2381–2392. [Google Scholar] [CrossRef]
- Khan, A.A.; Mundra, P.A.; Straznicky, N.E.; Nestel, P.J.; Wong, G.; Tan, R.; Huynh, K.; Ng, T.W.; Mellett, N.A.; Weir, J.M.; et al. Weight Loss and Exercise Alter the High-Density Lipoprotein Lipidome and Improve High-Density Lipoprotein Functionality in Metabolic Syndrome. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 438–447. [Google Scholar] [CrossRef]
- Chapman, M.J.; Orsoni, A.; Tan, R.; Mellett, N.A.; Nguyen, A.; Robillard, P.; Giral, P.; Therond, P.; Meikle, P.J. LDL subclass lipidomics in atherogenic dyslipidemia: Effect of statin therapy on bioactive lipids and dense LDL. J. Lipid Res. 2020, 61, 911–932. [Google Scholar] [CrossRef]
- Padro, T.; Vilahur, G.; Sanchez-Hernandez, J.; Hernandez, M.; Antonijoan, R.M.; Perez, A.; Badimon, L. Lipidomic changes of LDL in overweight and moderately hypercholesterolemic subjects taking phytosterol- and omega-3-supplemented milk. J. Lipid Res. 2015, 56, 1043–1056. [Google Scholar] [CrossRef]
- Sutter, I.; Velagapudi, S.; Othman, A.; Riwanto, M.; Manz, J.; Rohrer, L.; Rentsch, K.; Hornemann, T.; Landmesser, U.; von Eckardstein, A. Plasmalogens of high-density lipoproteins (HDL) are associated with coronary artery disease and anti-apoptotic activity of HDL. Atherosclerosis 2015, 241, 539–546. [Google Scholar] [CrossRef]
- Lee, J.H.; Yang, J.S.; Lee, S.H.; Moon, M.H. Analysis of lipoprotein-specific lipids in patients with acute coronary syndrome by asymmetrical flow field-flow fractionation and nanoflow liquid chromatography-tandem mass spectrometry. J. Chromatography. B Anal. Technol. Biomed. Life Sci. 2018, 1099, 56–63. [Google Scholar] [CrossRef]
- Hancock-Cerutti, W.; Lhomme, M.; Dauteuille, C.; Lecocq, S.; Chapman, M.J.; Rader, D.J.; Kontush, A.; Cuchel, M. Paradoxical coronary artery disease in humans with hyperalphalipoproteinemia is associated with distinct differences in the high-density lipoprotein phosphosphingolipidome. J. Clin. Lipidol. 2017, 11, 1192–1200.e1193. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yu, B.; Li, Q.; Guo, Y.; Koike, T.; Koike, Y.; Wu, Q.; Zhang, J.; Mao, L.; Tang, X.; et al. HDL quality features revealed by proteomelipidome connectivity are associated with atherosclerotic disease. J. Mol. Cell Biol. 2022, 14, mjac004. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Kontush, A.; Lhomme, M.; Chapman, M.J. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 2013, 54, 2950–2963. [Google Scholar] [CrossRef]
- Meyer zu Heringdorf, D.; Jakobs, K.H. Lysophospholipid receptors: Signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim. Biophys. Acta 2007, 1768, 923–940. [Google Scholar] [CrossRef]
- Tani, M.; Ito, M.; Igarashi, Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell. signalling 2007, 19, 229–237. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Alewijnse, A.E.; Peters, S.L. Sphingolipid signalling in the cardiovascular system: Good, bad or both? Eur. J. Pharmacol. 2008, 585, 292–302. [Google Scholar] [CrossRef]
- Saito, H.; Arimoto, I.; Tanaka, M.; Sasaki, T.; Tanimoto, T.; Okada, S.; Handa, T. Inhibition of lipoprotein lipase activity by sphingomyelin: Role of membrane surface structure. Biochim. Biophys. Acta 2000, 1486, 312–320. [Google Scholar] [CrossRef]
- Subbaiah, P.V.; Liu, M. Role of sphingomyelin in the regulation of cholesterol esterification in the plasma lipoproteins. Inhibition of lecithin-cholesterol acyltransferase reaction. J. Biol. Chem. 1993, 268, 20156–20163. [Google Scholar] [CrossRef]
- Rye, K.A.; Hime, N.J.; Barter, P.J. The influence of sphingomyelin on the structure and function of reconstituted high density lipoproteins. J. Biol. Chem. 1996, 271, 4243–4250. [Google Scholar] [CrossRef] [PubMed]
- Bolin, D.J.; Jonas, A. Sphingomyelin inhibits the lecithin-cholesterol acyltransferase reaction with reconstituted high density lipoproteins by decreasing enzyme binding. J. Biol. Chem. 1996, 271, 19152–19158. [Google Scholar] [CrossRef] [PubMed]
- Hoofnagle, A.N.; Vaisar, T.; Mitra, P.; Chait, A. HDL lipids and insulin resistance. Curr. Diabetes Rep. 2010, 10, 78–86. [Google Scholar] [CrossRef]
- Schissel, S.L.; Jiang, X.; Tweedie-Hardman, J.; Jeong, T.; Camejo, E.H.; Najib, J.; Rapp, J.H.; Williams, K.J.; Tabas, I. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J. Biol. Chem. 1998, 273, 2738–2746. [Google Scholar] [CrossRef]
- Yancey, P.G.; de la Llera-Moya, M.; Swarnakar, S.; Monzo, P.; Klein, S.M.; Connelly, M.A.; Johnson, W.J.; Williams, D.L.; Rothblat, G.H. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J. Biol. Chem. 2000, 275, 36596–36604. [Google Scholar] [CrossRef] [PubMed]
- Yetukuri, L.; Soderlund, S.; Koivuniemi, A.; Seppanen-Laakso, T.; Niemela, P.S.; Hyvonen, M.; Taskinen, M.R.; Vattulainen, I.; Jauhiainen, M.; Oresic, M. Composition and lipid spatial distribution of HDL particles in subjects with low and high HDL-cholesterol. J. Lipid Res. 2010, 51, 2341–2351. [Google Scholar] [CrossRef]
- Papathanasiou, A.; Kostara, C.; Cung, M.T.; Seferiadis, K.; Elisaf, M.; Bairaktari, E.; Goudevenos, I.A. Analysis of the composition of plasma lipoproteins in patients with extensive coronary heart disease using 1H NMR spectroscopy. Hell. J. Cardiol. HJC Hell. Kardiol. Ep. 2008, 49, 72–78. [Google Scholar]
- Ozerova, I.N.; Perova, N.V.; Shchel’tsyna, N.V.; Mamedov, M.N. Parameters of high-density lipoproteins in patients with arterial hypertension in combination with other components of metabolic syndrome. Bull. Exp. Biol. Med. 2007, 143, 320–322. [Google Scholar] [CrossRef]
- Ruuth, M.; Nguyen, S.D.; Vihervaara, T.; Hilvo, M.; Laajala, T.D.; Kondadi, P.K.; Gistera, A.; Lahteenmaki, H.; Kittila, T.; Huusko, J.; et al. Susceptibility of low-density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. Eur. Heart J. 2018, 39, 2562–2573. [Google Scholar] [CrossRef]
- Wiesner, P.; Leidl, K.; Boettcher, A.; Schmitz, G.; Liebisch, G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 2009, 50, 574–585. [Google Scholar] [CrossRef]
- Sigruener, A.; Kleber, M.E.; Heimerl, S.; Liebisch, G.; Schmitz, G.; Maerz, W. Glycerophospholipid and sphingolipid species and mortality: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. PLoS ONE 2014, 9, e85724. [Google Scholar] [CrossRef] [PubMed]
- Lepedda, A.J.; Lobina, O.; Rocchiccioli, S.; Nieddu, G.; Ucciferri, N.; De Muro, P.; Idini, M.; Nguyen, H.Q.; Guarino, A.; Spirito, R.; et al. Identification of differentially expressed plasma proteins in atherosclerotic patients with type 2 diabetes. J. Diabetes Its Complicat. 2016, 30, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Molendijk, J.; Hill, M.M. lipidr: A Software Tool for Data Mining and Analysis of Lipidomics Datasets. J. Proteome Res. 2020, 19, 2890–2897. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
| “Hard” (n = 12) | “Soft” (n = 16) | |
|---|---|---|
| Age (Years) * | 66 ± 7.5 | 72.2 ± 7.3 |
| Male Gender | 8 (66.7%) | 13 (81.2%) |
| BMI | 26.5 ± 3.1 | 25.6 ± 3.4 |
| Triglycerides (mg/dL) | 102.1 ± 24 | 125.2 ± 43.8 |
| Total Cholesterol (mg/dL) | 190.3 ± 41.9 | 178.6 ± 47.4 |
| HDL Cholesterol (mg/dL) | 44.8 ± 9.3 | 44.1 ± 17.1 |
| nonHDL Cholesterol (mg/dL) | 145.5 ± 40.2 | 134.5 ± 46.1 |
| LDL Cholesterol (mg/dL) | 125.3 ± 37.5 | 106.8 ± 37.1 |
| TG/HDL-C * | 2.3 ± 0.4 | 3.2 ± 1.6 |
| Cholesterol Lowering Therapy | 9 (75%) | 15 (93.8%) |
| Glycemia (mg/dL) | 105.6 ± 14.3 | 115.6 ± 25.1 |
| HbA1C | 5.84 ± 0.472 | 6.482 ± 0.875 |
| Diabetes | 2 (16.7%) | 5 (41.7%) |
| Glucose Lowering Therapy | 1 (8.3%) | 5 (31.2%) |
| Systolic Blood Pressure (mmHg) | 139.1 ± 11.6 | 135.5 ± 19.4 |
| Diastolic Blood Pressure (mmHg) | 76.2 ± 11.2 | 72.2 ± 8.6 |
| Anti-hypertensive Therapy | 9 (75%) | 12 (75%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieddu, G.; Michelucci, E.; Formato, M.; Ciampelli, C.; Obino, G.; Signore, G.; Di Giorgi, N.; Rocchiccioli, S.; Lepedda, A.J. Molecular Characterization of Plasma HDL, LDL, and VLDL Lipids Cargos from Atherosclerotic Patients with Advanced Carotid Lesions: A Preliminary Report. Int. J. Mol. Sci. 2022, 23, 12449. https://doi.org/10.3390/ijms232012449
Nieddu G, Michelucci E, Formato M, Ciampelli C, Obino G, Signore G, Di Giorgi N, Rocchiccioli S, Lepedda AJ. Molecular Characterization of Plasma HDL, LDL, and VLDL Lipids Cargos from Atherosclerotic Patients with Advanced Carotid Lesions: A Preliminary Report. International Journal of Molecular Sciences. 2022; 23(20):12449. https://doi.org/10.3390/ijms232012449
Chicago/Turabian StyleNieddu, Gabriele, Elena Michelucci, Marilena Formato, Cristina Ciampelli, Gabriele Obino, Giovanni Signore, Nicoletta Di Giorgi, Silvia Rocchiccioli, and Antonio Junior Lepedda. 2022. "Molecular Characterization of Plasma HDL, LDL, and VLDL Lipids Cargos from Atherosclerotic Patients with Advanced Carotid Lesions: A Preliminary Report" International Journal of Molecular Sciences 23, no. 20: 12449. https://doi.org/10.3390/ijms232012449
APA StyleNieddu, G., Michelucci, E., Formato, M., Ciampelli, C., Obino, G., Signore, G., Di Giorgi, N., Rocchiccioli, S., & Lepedda, A. J. (2022). Molecular Characterization of Plasma HDL, LDL, and VLDL Lipids Cargos from Atherosclerotic Patients with Advanced Carotid Lesions: A Preliminary Report. International Journal of Molecular Sciences, 23(20), 12449. https://doi.org/10.3390/ijms232012449










