The Role of Blood-Derived Factors in Protection and Regeneration of Aged Tissues
Abstract
:1. Introduction
2. Parabiosis as Animal Model for Heterochronic Blood Exchange
3. Identification of Blood-Borne Anti-Aging and Pro-Aging Factors
| Factor/Pathway | Effect | References |
|---|---|---|
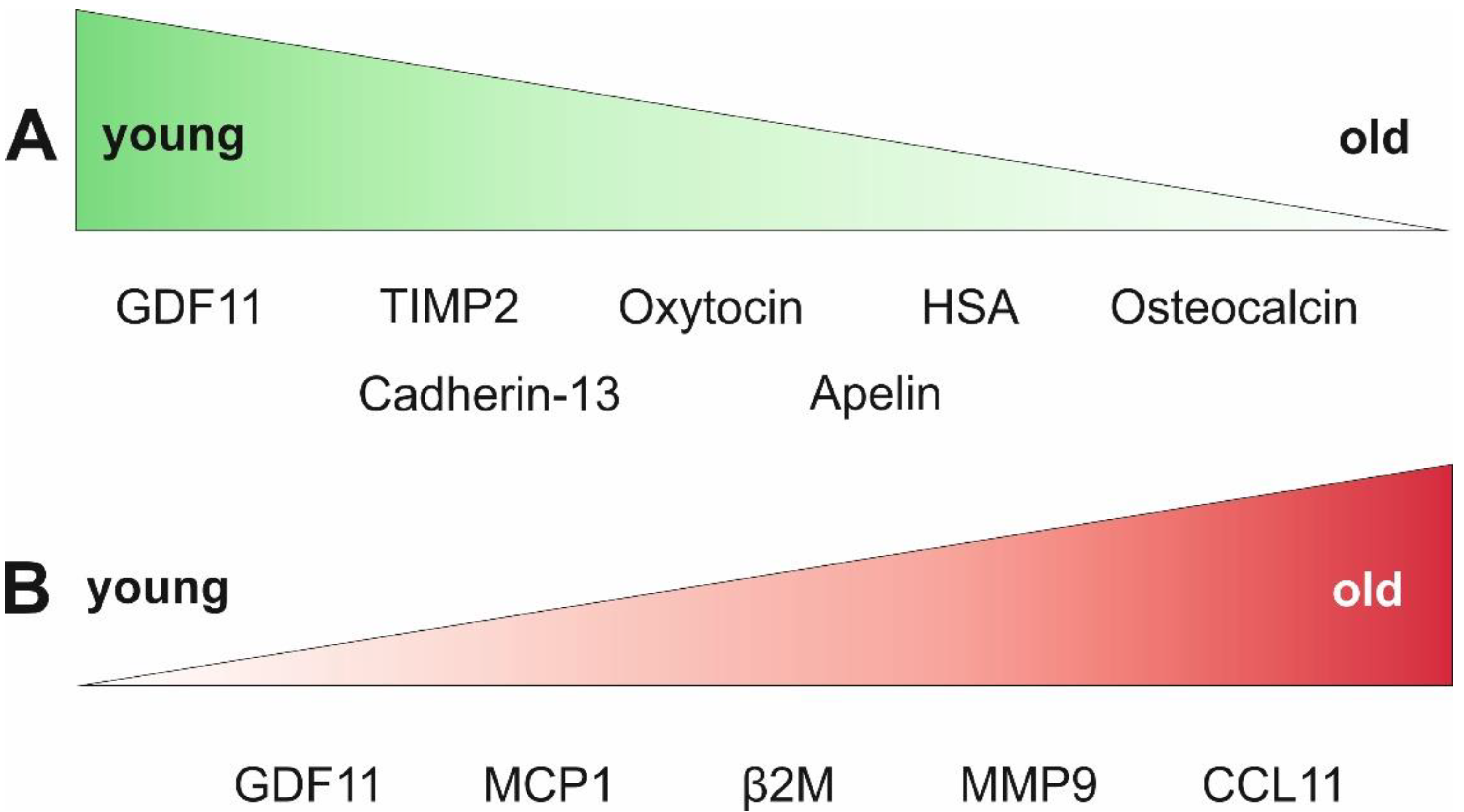
| CCL11 (Eotaxin 1)↑ | Elevated plasma levels are associated with decline in neurogenesis and impaired learning and memory; increases vascular permeability and activates p38-MAPK, Stat3 and NF-kappaB pathways in human coronary artery endothelial cells; no significant differences in Eotaxin 1 plasma levels in healthy elderly and in people with preclinical AD | [63,88,89,90] |
| β2M↑ | Induces impaired neurogenesis and cognitive function in mice; stimulation of cardiac fibroblasts after ischemia-reperfusion injury; elevated plasma levels are associated with frailty | [63,93,94,95,99] |
| GDF11↓↑ | Contradictory results: reduction in the symptoms of age-associated cardiac hypertrophy in old mice, increase in SMAD2- and SMAD3 phosphorylation in cardiomyocytes; reversal of age-related skeletal muscle and stem cell dysfunction; inhibition of skeletal muscle regeneration in rats | [62,100,101,102,104] |
| Oxytocin↓ | Application of oxytocin leads to activated skeletal muscle regeneration, proliferation of mouse satellite cells via MAPK/ERK, antiapoptotic and anti-inflammatory effects on cardiomyocytes; supports myocardial differentiation of adult murine cardiac progenitor cells; high oxytocin serum levels were associated with high bone mineral density in postmenopausal women | [20,105,106,109] |
| Apelin↓ | Decreasing levels in plasma of aging mice and humans accompanied with reduced expression of the apelin receptor in mice; apelin supplementation resulted in restored skeletal muscle function with enhanced biogenesis of mitochondria and reduction of age-associated cardiac hypertrophy in mice; generally lower serum levels of apelin were measured in patients with severe heart failure secondary to MI compared to healthy subjects | [111,112,113] |
| Cadherin-13↓ | Declining plasma levels in aged mice, while intraperitoneal injection of cadherin-13 delays age-associated bone loss; inhibition of the differentiation of bone marrow-derived macrophages to osteoclasts | [122] |
| TIMP2↓ | Application of TIMP2 leads to enhanced synaptic plasticity and improved cognitive function in mice; TIMP2 deficiency inhibits cardiac remodeling processes after myocardial infarction in mice via inhibition of membrane type 1 matrix metalloproteinase; involved in homing mechanisms of human mesenchymal stem cells; elevated plasma levels of TIMP1, TIMP2 and TIMP4 have been associated with higher risk for major adverse cardiac events after acute myocardial injury in humans | [114,115,116,117] |
| Osteocalcin↓ | Decrease in OCN plasma levels is linked to a decrease in cognitive functions; systemic administration of OCN improves the cognitive abilities of old mice; declining OCN serum levels were linked to left ventricular systolic dysfunction in men | [118,119,121] |
| MMP9 ↑ | Biomarker for cardiac aging; knockout leads to enhanced cardiac protection after myocardial infarction in mice; elevated MMP9 concentrations were measured in serum and saliva of patients with cardiovascular disease; increased expression of MMP9 mRNA in the gingiva of old (>60 years) compared to young (17–20 years) patients | [72,73,74,76] |
| MCP1↑ | Biomarker for cardiac aging; proposed to play a major role in the onset of cardiovascular disease; depletion of MCP1 from the blood using the anti-MCP1 antibody Carlumab did not result in a permanent decrease in MCP1 serum levels | [72,75,77,82,83,84] |
| Serum albumin↓ | Low serum albumin levels are associated with decreased antioxidative properties; application of serum albumin leads to protection against oxidative stress in mouse hippocampal slice cultures and in human neurons; low serum albumin levels are associated with increased risk of atrial fibrillation | [136,139,141] |
| Exosomes | Mesenchymal stem cell-derived exosomes reduce apoptosis after hypoxic injury in neonatal rat cardiomyocytes; iPSC-derived exosomes induce proliferation and enhance the cardiac and endothelial differentiation potential of human heart-derived mesenchymal stromal cells; exosomes from serum of young mice significantly improved pathological markers of Huntington’s disease | [131,132,133] |
| P38-MAPK pathway | Increased proliferation and migration of hCSCs | [25,142] |
4. Clinical Trials Assessing the Effects of Young Blood
| Product | Target/Measurements | Outcome | Reference/ ClinicalTrials.gov Identifier |
|---|---|---|---|
| Young plasma (male, 18–30 years) | Safety of intravenously administered young plasma for patients with AD | Safety and feasibility of infusions with young plasma for people with AD have been demonstrated | [145,156] NCT02256306 |
| GRF6019, a plasma-derived product | Safety, tolerability, and feasibility of intravenous infusion in patients with mild to moderate AD | GRF6019 is safe and well tolerated; patients experienced no cognitive decline | [146,157] NCT03520998 |
| Young plasma (male, 18–25 years) | Safety of young plasma for patients with Parkinson’s disease; laboratory makers of PD; progression of cognitive, mood and motor impairments | Primary outcome: safety and feasibility of infusions with young plasma for people with PD. Secondary outcome: slight but significant improvement in phonemic fluency; decreased blood levels of TNFα | [147,158] NCT02968433 |
| Umbilical cord blood plasma | Safety for intravascular administration for patients between 50–80 years; physiological markers of frailty or other age-related biological measures | Recruitment status unknown; No outcome published | [148] NCT03229785 |
| Umbilical cord blood plasma | Umbilical cord blood plasma infusion (50 mL) in elderly adults (65–85 years) with age-related cognitive decline Assessment of: safety, executive function, working memory | Not yet recruiting; No results posted | [150] NCT04566757 |
| Fresh umbilical cord blood, frozen umbilical cord blood, frozen plasma | Fresh cord blood, frozen cord blood and frozen plasma intravenously administered to recipients (>55 years) with a diagnosis of pre-frailty Assessment of: safety, cardiac output, biomarkers for oxidative stress, inflammation and immune response, methylation, mitochondrial DNA copy number, growth factors, antioxidant capacity, hormone status, DNA damage, metabolite | Recruitment status unknown; No results posted | [149] NCT02418013 |
| Plasmapheresis | Safety and feasibility of plasmapheresis/therapeutic plasma exchange with albumin in patients with age-related frailty (50–95 years) | Enrolling by invitation; No results posted (estimated primary completion date: April 2025) | [151] NCT05054894 |
| Young blood plasma (male, 18–30 years) Old blood plasma (male, 40–55 years) | Efficacy and safety of young plasma for patients with acute stroke | Recruitment status unknown; No results posted | [152] NCT02913183 |
| Young blood plasma (16–25 years) | Infusion of young plasma in healthy recipients older than 30 years. Measurement of clinical biomarkers of aging. | No results posted | [153] NCT02803554 |
| Plasma from young donors | Safety and feasibility of plasma infusions in geriatric patients (65–80 years) with heart failure with preserved ejection fraction (HFpEF) | Withdrawn before start | [155] NCT04241159 |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Becker, A.J.; Mc, C.E.; Till, J.E. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 1963, 197, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.B.; Momma, S.; Clarke, D.L.; Risling, M.; Lendahl, U.; Frisén, J. Identification of a Neural Stem Cell in the Adult Mammalian Central Nervous System. Cell 1999, 96, 25–34. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Conboy, I.M.; Conboy, M.J.; Smythe, G.M.; Rando, T.A. Notch-mediated restoration of regenerative potential to aged muscle. Science 2003, 302, 1575–1577. [Google Scholar] [CrossRef]
- de Haan, G.; Lazare, S.S. Aging of hematopoietic stem cells. Blood 2018, 131, 479–487. [Google Scholar] [CrossRef]
- Kuhn, H.G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996, 16, 2027–2033. [Google Scholar] [CrossRef]
- Regulski, M.J. Cellular Senescence: What, Why, and How. Wounds 2017, 29, 168–174. [Google Scholar]
- Anderson, R.; Lagnado, A.; Maggiorani, D.; Walaszczyk, A.; Dookun, E.; Chapman, J.; Birch, J.; Salmonowicz, H.; Ogrodnik, M.; Jurk, D.; et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 2019, 38, e100492. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Fuhrmann-Stroissnigg, H.; Dai, H.M.; Ling, Y.Y.; Stout, M.B.; Pirtskhalava, T.; Giorgadze, N.; Johnson, K.O.; Giles, C.B.; et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 2016, 15, 428–435. [Google Scholar] [CrossRef]
- Marino, F.; Scalise, M.; Salerno, N.; Salerno, L.; Molinaro, C.; Cappetta, D.; Torella, M.; Greco, M.; Foti, D.; Sasso, F.C.; et al. Diabetes-Induced Cellular Senescence and Senescence-Associated Secretory Phenotype Impair Cardiac Regeneration and Function Independently of Age. Diabetes 2022, 71, 1081–1098. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Use of Senolytic and Anti-Fibrotic Agents to Improve the Beneficial Effect of Bone Marrow Stem Cells for Osteoarthritis. Available online: https://clinicaltrials.gov/ct2/show/NCT04815902 (accessed on 15 August 2022).
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K.; et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003, 114, 763–776. [Google Scholar] [CrossRef]
- Bearzi, C.; Rota, M.; Hosoda, T.; Tillmanns, J.; Nascimbene, A.; De Angelis, A.; Yasuzawa-Amano, S.; Trofimova, I.; Siggins, R.W.; Lecapitaine, N.; et al. Human cardiac stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 14068–14073. [Google Scholar] [CrossRef]
- Urbanek, K.; Cesselli, D.; Rota, M.; Nascimbene, A.; De Angelis, A.; Hosoda, T.; Bearzi, C.; Boni, A.; Bolli, R.; Kajstura, J.; et al. Stem cell niches in the adult mouse heart. Proc. Natl. Acad. Sci. USA 2006, 103, 9226–9231. [Google Scholar] [CrossRef]
- Tallini, Y.N.; Greene, K.S.; Craven, M.; Spealman, A.; Breitbach, M.; Smith, J.; Fisher, P.J.; Steffey, M.; Hesse, M.; Doran, R.M.; et al. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc. Natl. Acad. Sci. USA 2009, 106, 1808–1813. [Google Scholar] [CrossRef]
- Zaruba, M.M.; Soonpaa, M.; Reuter, S.; Field, L.J. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation 2010, 121, 1992–2000. [Google Scholar] [CrossRef]
- Cai, C.L.; Liang, X.; Shi, Y.; Chu, P.H.; Pfaff, S.L.; Chen, J.; Evans, S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 2003, 5, 877–889. [Google Scholar] [CrossRef]
- Moretti, A.; Caron, L.; Nakano, A.; Lam, J.T.; Bernshausen, A.; Chen, Y.; Qyang, Y.; Bu, L.; Sasaki, M.; Martin-Puig, S.; et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 2006, 127, 1151–1165. [Google Scholar] [CrossRef]
- Matsuura, K.; Nagai, T.; Nishigaki, N.; Oyama, T.; Nishi, J.; Wada, H.; Sano, M.; Toko, H.; Akazawa, H.; Sato, T.; et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J. Biol. Chem. 2004, 279, 11384–11391. [Google Scholar] [CrossRef]
- Oh, H.; Bradfute, S.B.; Gallardo, T.D.; Nakamura, T.; Gaussin, V.; Mishina, Y.; Pocius, J.; Michael, L.H.; Behringer, R.R.; Garry, D.J.; et al. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. USA 2003, 100, 12313–12318. [Google Scholar] [CrossRef]
- Smits, A.M.; van Vliet, P.; Metz, C.H.; Korfage, T.; Sluijter, J.P.; Doevendans, P.A.; Goumans, M.J. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: An in vitro model for studying human cardiac physiology and pathophysiology. Nat. Protoc. 2009, 4, 232–243. [Google Scholar] [CrossRef]
- Barile, L.; Gherghiceanu, M.; Popescu, L.M.; Moccetti, T.; Vassalli, G. Human cardiospheres as a source of multipotent stem and progenitor cells. Stem Cells Int. 2013, 2013, 916837. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, R.; Daniels, A.; Windmolders, S.; Mees, U.; Macianskiene, R.; Mubagwa, K.; Steels, P.; Jamaer, L.; Dubois, J.; Robic, B.; et al. The cardiac atrial appendage stem cell: A new and promising candidate for myocardial repair. Cardiovasc. Res. 2013, 97, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Höving, A.L.; Schmidt, K.E.; Merten, M.; Hamidi, J.; Rott, A.K.; Faust, I.; Greiner, J.F.W.; Gummert, J.; Kaltschmidt, B.; Kaltschmidt, C.; et al. Blood Serum Stimulates p38-Mediated Proliferation and Changes in Global Gene Expression of Adult Human Cardiac Stem Cells. Cells 2020, 9, 1472. [Google Scholar] [CrossRef]
- Höving, A.L.; Sielemann, K.; Greiner, J.F.W.; Kaltschmidt, B.; Knabbe, C.; Kaltschmidt, C. Transcriptome Analysis Reveals High Similarities between Adult Human Cardiac Stem Cells and Neural Crest-Derived Stem Cells. Biology 2020, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Cianflone, E.; Aquila, I.; Scalise, M.; Marotta, P.; Torella, M.; Nadal-Ginard, B.; Torella, D. Molecular basis of functional myogenic specification of Bona Fide multipotent adult cardiac stem cells. Cell Cycle 2018, 17, 927–946. [Google Scholar] [CrossRef]
- Scalise, M.; Marino, F.; Cianflone, E.; Mancuso, T.; Marotta, P.; Aquila, I.; Torella, M.; Nadal-Ginard, B.; Torella, D. Heterogeneity of Adult Cardiac Stem Cells. Adv. Exp. Med. Biol. 2019, 1169, 141–178. [Google Scholar] [CrossRef]
- van Vliet, P.; Roccio, M.; Smits, A.M.; van Oorschot, A.A.; Metz, C.H.; van Veen, T.A.; Sluijter, J.P.; Doevendans, P.A.; Goumans, M.J. Progenitor cells isolated from the human heart: A potential cell source for regenerative therapy. Neth. Heart J. 2008, 16, 163–169. [Google Scholar] [CrossRef]
- Chimenti, I.; Gaetani, R.; Barile, L.; Forte, E.; Ionta, V.; Angelini, F.; Frati, G.; Messina, E.; Giacomello, A. Isolation and expansion of adult cardiac stem/progenitor cells in the form of cardiospheres from human cardiac biopsies and murine hearts. Methods Mol. Biol 2012, 879, 327–338. [Google Scholar] [CrossRef]
- van Berlo, J.H.; Kanisicak, O.; Maillet, M.; Vagnozzi, R.J.; Karch, J.; Lin, S.C.; Middleton, R.C.; Marban, E.; Molkentin, J.D. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 2014, 509, 337–341. [Google Scholar] [CrossRef]
- Vicinanza, C.; Aquila, I.; Cianflone, E.; Scalise, M.; Marino, F.; Mancuso, T.; Fumagalli, F.; Giovannone, E.D.; Cristiano, F.; Iaccino, E.; et al. Kit(cre) knock-in mice fail to fate-map cardiac stem cells. Nature 2018, 555, E1–E5. [Google Scholar] [CrossRef]
- Aquila, I.; Cianflone, E.; Scalise, M.; Marino, F.; Mancuso, T.; Filardo, A.; Smith, A.J.; Cappetta, D.; De Angelis, A.; Urbanek, K.; et al. c-kit Haploinsufficiency impairs adult cardiac stem cell growth, myogenicity and myocardial regeneration. Cell Death Dis. 2019, 10, 436. [Google Scholar] [CrossRef]
- Vicinanza, C.; Aquila, I.; Scalise, M.; Cristiano, F.; Marino, F.; Cianflone, E.; Mancuso, T.; Marotta, P.; Sacco, W.; Lewis, F.C.; et al. Adult cardiac stem cells are multipotent and robustly myogenic: C-kit expression is necessary but not sufficient for their identification. Cell Death Differ. 2017, 24, 2101–2116. [Google Scholar] [CrossRef]
- Sadek, H.; Olson, E.N. Toward the Goal of Human Heart Regeneration. Cell Stem Cell 2020, 26, 7–16. [Google Scholar] [CrossRef]
- Laflamme, M.A.; Murry, C.E. Heart regeneration. Nature 2011, 473, 326–335. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabe-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; Dos Remedios, C.; et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef]
- Breckwoldt, K.; Weinberger, F.; Eschenhagen, T. Heart regeneration. Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 1749–1759. [Google Scholar] [CrossRef]
- He, L.; Nguyen, N.B.; Ardehali, R.; Zhou, B. Heart Regeneration by Endogenous Stem Cells and Cardiomyocyte Proliferation: Controversy, Fallacy, and Progress. Circulation 2020, 142, 275–291. [Google Scholar] [CrossRef]
- Noiseux, N.; Gnecchi, M.; Lopez-Ilasaca, M.; Zhang, L.; Solomon, S.D.; Deb, A.; Dzau, V.J.; Pratt, R.E. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol. Ther. 2006, 14, 840–850. [Google Scholar] [CrossRef]
- Murry, C.E.; Soonpaa, M.H.; Reinecke, H.; Nakajima, H.; Nakajima, H.O.; Rubart, M.; Pasumarthi, K.B.; Virag, J.I.; Bartelmez, S.H.; Poppa, V.; et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 2004, 428, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.P.; Mielewczik, M.; Zargaran, D.; Cole, G.D. Autologous bone marrow-derived stem cell therapy in heart disease: Discrepancies and contradictions. Int. J. Cardiol. 2013, 168, 3381–3403. [Google Scholar] [CrossRef] [PubMed]
- Strauer, B.E.; Brehm, M.; Schannwell, C.M. The therapeutic potential of stem cells in heart disease. Cell Prolif. 2008, 41 (Suppl. S1), 126–145. [Google Scholar] [CrossRef]
- Khodayari, S.; Khodayari, H.; Amiri, A.Z.; Eslami, M.; Farhud, D.; Hescheler, J.; Nayernia, K. Inflammatory Microenvironment of Acute Myocardial Infarction Prevents Regeneration of Heart with Stem Cells Therapy. Cell. Physiol. Biochem. 2019, 53, 887–909. [Google Scholar] [CrossRef] [PubMed]
- NCT04939077, U.S.National Library of Medicine. Treatment of Heart Failure Using Human Umbilical Cord Mesenchymal Stem Cells (hUC-MSC). Available online: https://clinicaltrials.gov/ct2/show/NCT04939077 (accessed on 17 August 2022).
- NCT04992832, U.S.National Library of Medicine. Multi-intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Heart Failure with Reduced Ejection Fraction (PRIME-HFrEF Study) (PRIME-HFrEF). Available online: https://clinicaltrials.gov/ct2/show/NCT04992832 (accessed on 17 August 2022).
- NCT05043610, U.S.National Library of Medicine. MSCs for Prevention of MI-induced HF (PREVENT-TAHA). Available online: https://clinicaltrials.gov/ct2/show/NCT05043610 (accessed on 17 August 2022).
- NCT05147766, U.S.National Library of Medicine. Safety of Cultured Allogeneic Adult Umbilical Cord Derived Mesenchymal Stem Cells for Heart Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT05147766 (accessed on 17 August 2022).
- Tiburcy, M.; Hudson, J.E.; Balfanz, P.; Schlick, S.; Meyer, T.; Chang Liao, M.L.; Levent, E.; Raad, F.; Zeidler, S.; Wingender, E.; et al. Defined Engineered Human Myocardium with Advanced Maturation for Applications in Heart Failure Modeling and Repair. Circulation 2017, 135, 1832–1847. [Google Scholar] [CrossRef]
- NCT04396899, N.L.o.M.Safety and Efficacy of Induced Pluripotent Stem Cell-derived Engineered Human Myocardium as Biological Ventricular Assist Tissue in Terminal Heart Failure (BioVAT-HF). Available online: https://clinicaltrials.gov/ct2/show/study/NCT04396899 (accessed on 17 August 2022).
- Hofmann, M.; Wollert, K.C.; Meyer, G.P.; Menke, A.; Arseniev, L.; Hertenstein, B.; Ganser, A.; Knapp, W.H.; Drexler, H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation 2005, 111, 2198–2202. [Google Scholar] [CrossRef]
- Hodgkinson, C.P.; Bareja, A.; Gomez, J.A.; Dzau, V.J. Emerging Concepts in Paracrine Mechanisms in Regenerative Cardiovascular Medicine and Biology. Circ. Res. 2016, 118, 95–107. [Google Scholar] [CrossRef]
- Nigro, P.; Bassetti, B.; Cavallotti, L.; Catto, V.; Carbucicchio, C.; Pompilio, G. Cell therapy for heart disease after 15 years: Unmet expectations. Pharmacol. Res. 2018, 127, 77–91. [Google Scholar] [CrossRef]
- Hatzistergos, K.E.; Saur, D.; Seidler, B.; Balkan, W.; Breton, M.; Valasaki, K.; Takeuchi, L.M.; Landin, A.M.; Khan, A.; Hare, J.M. Stimulatory Effects of Mesenchymal Stem Cells on cKit+ Cardiac Stem Cells Are Mediated by SDF1/CXCR4 and SCF/cKit Signaling Pathways. Circ. Res. 2016, 119, 921–930. [Google Scholar] [CrossRef]
- Di Santo, S.; Yang, Z.; Wyler von Ballmoos, M.; Voelzmann, J.; Diehm, N.; Baumgartner, I.; Kalka, C. Novel cell-free strategy for therapeutic angiogenesis: In vitro generated conditioned medium can replace progenitor cell transplantation. PLoS ONE 2009, 4, e5643. [Google Scholar] [CrossRef]
- Wernly, B.; Mirna, M.; Rezar, R.; Prodinger, C.; Jung, C.; Podesser, B.K.; Kiss, A.; Hoppe, U.C.; Lichtenauer, M. Regenerative Cardiovascular Therapies: Stem Cells and Beyond. Int. J. Mol. Sci. 2019, 20, 1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, J.P.; Szalay, K.; Geiger, F.; Kramer, M.; Richter, W.; Kasten, P. Platelet-rich plasma improves expansion of human mesenchymal stem cells and retains differentiation capacity and in vivo bone formation in calcium phosphate ceramics. Platelets 2006, 17, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Walenda, T.; Bokermann, G.; Jost, E.; Galm, O.; Schellenberg, A.; Koch, C.M.; Piroth, D.M.; Drescher, W.; Brummendorf, T.H.; Wagner, W. Serum after autologous transplantation stimulates proliferation and expansion of human hematopoietic progenitor cells. PLoS ONE 2011, 6, e18012. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.F.; Hauser, S.; Widera, D.; Muller, J.; Qunneis, F.; Zander, C.; Martin, I.; Mallah, J.; Schuetzmann, D.; Prante, C.; et al. Efficient animal-serum free 3D cultivation method for adult human neural crest-derived stem cell therapeutics. Eur. Cell Mater. 2011, 22, 403–419. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Hirayama, F.; Wakamoto, S.; Fujihara, M.; Murahashi, H.; Sato, N.; Ikebuchi, K.; Sawada, K.; Koike, T.; Kuwabara, M.; et al. Bone marrow stromal cells prepared using AB serum and bFGF for hematopoietic stem cells expansion. Transfusion 2002, 42, 921–927. [Google Scholar] [CrossRef]
- Loffredo, F.S.; Steinhauser, M.L.; Jay, S.M.; Gannon, J.; Pancoast, J.R.; Yalamanchi, P.; Sinha, M.; Dall’Osso, C.; Khong, D.; Shadrach, J.L.; et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 2013, 153, 828–839. [Google Scholar] [CrossRef]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef]
- Villeda, S.A.; Plambeck, K.E.; Middeldorp, J.; Castellano, J.M.; Mosher, K.I.; Luo, J.; Smith, L.K.; Bieri, G.; Lin, K.; Berdnik, D.; et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014, 20, 659–663. [Google Scholar] [CrossRef]
- Kang, J.S.; Yang, Y.R. Circulating plasma factors involved in rejuvenation. Aging 2020, 12, 23394–23408. [Google Scholar] [CrossRef]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764. [Google Scholar] [CrossRef]
- Salpeter, S.J.; Khalaileh, A.; Weinberg-Corem, N.; Ziv, O.; Glaser, B.; Dor, Y. Systemic regulation of the age-related decline of pancreatic beta-cell replication. Diabetes 2013, 62, 2843–2848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousefzadeh, M.J.; Wilkinson, J.E.; Hughes, B.; Gadela, N.; Ladiges, W.C.; Vo, N.; Niedernhofer, L.J.; Huffman, D.M.; Robbins, P.D. Heterochronic parabiosis regulates the extent of cellular senescence in multiple tissues. Geroscience 2020, 42, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.W.; Zhao, P.; Yung, W.H.; Sheng, Y.; Ke, Y.; Qian, Z.M. Tissue iron is negatively correlated with TERC or TERT mRNA expression: A heterochronic parabiosis study in mice. Aging 2018, 10, 3834–3850. [Google Scholar] [CrossRef] [PubMed]
- Conboy, I.M.; Conboy, M.J.; Rebo, J. Systemic Problems: A perspective on stem cell aging and rejuvenation. Aging 2015, 7, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, S.; Qu, J.; Belmonte, J.C.I.; Liu, G.H. Rejuvenation of Tissue Stem Cells by Intrinsic and Extrinsic Factors. Stem Cells Transl. Med. 2022, 11, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Chiao, Y.A.; Dai, Q.; Zhang, J.; Lin, J.; Lopez, E.F.; Ahuja, S.S.; Chou, Y.M.; Lindsey, M.L.; Jin, Y.F. Multi-analyte profiling reveals matrix metalloproteinase-9 and monocyte chemotactic protein-1 as plasma biomarkers of cardiac aging. Circ. Cardiovasc. Genet. 2011, 4, 455–462. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Ronsivalle, V.; Alibrandi, A.; Palazzo, G.; Lo Giudice, A. Impact of Matrix Metalloproteinase-9 during Periodontitis and Cardiovascular Diseases. Molecules 2021, 26, 1777. [Google Scholar] [CrossRef]
- Romanic, A.M.; Harrison, S.M.; Bao, W.; Burns-Kurtis, C.L.; Pickering, S.; Gu, J.; Grau, E.; Mao, J.; Sathe, G.M.; Ohlstein, E.H.; et al. Myocardial protection from ischemia/reperfusion injury by targeted deletion of matrix metalloproteinase-9. Cardiovasc. Res. 2002, 54, 549–558. [Google Scholar] [CrossRef]
- Niu, J.; Kolattukudy, P.E. Role of MCP-1 in cardiovascular disease: Molecular mechanisms and clinical implications. Clin. Sci 2009, 117, 95–109. [Google Scholar] [CrossRef]
- Kim, S.; Ahn, S.H.; Lee, J.S.; Song, J.E.; Cho, S.H.; Jung, S.; Kim, S.K.; Kim, S.H.; Lee, K.P.; Kwon, K.S.; et al. Differential Matrix Metalloprotease (MMP) Expression Profiles Found in Aged Gingiva. PLoS ONE 2016, 11, e0158777. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Schafer, M.J.; Noren Hooten, N.; Atkinson, E.J.; Evans, M.K.; Baker, D.J.; Quarles, E.K.; Robbins, P.D.; Ladiges, W.C.; LeBrasseur, N.K.; et al. Circulating levels of monocyte chemoattractant protein-1 as a potential measure of biological age in mice and frailty in humans. Aging Cell 2018, 17, e12706. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, B.; Dalmasso, B.; Hatse, S.; Laenen, A.; Kenis, C.; Swerts, E.; Neven, P.; Smeets, A.; Schoffski, P.; Wildiers, H. Biological ageing and frailty markers in breast cancer patients. Aging 2015, 7, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Schwamborn, J.; Lindecke, A.; Elvers, M.; Horejschi, V.; Kerick, M.; Rafigh, M.; Pfeiffer, J.; Prullage, M.; Kaltschmidt, B.; Kaltschmidt, C. Microarray analysis of tumor necrosis factor alpha induced gene expression in U373 human glioblastoma cells. BMC Genom. 2003, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Widera, D.; Holtkamp, W.; Entschladen, F.; Niggemann, B.; Zanker, K.; Kaltschmidt, B.; Kaltschmidt, C. MCP-1 induces migration of adult neural stem cells. Eur. J. Cell Biol. 2004, 83, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Pinke, K.H.; Calzavara, B.; Faria, P.F.; do Nascimento, M.P.; Venturini, J.; Lara, V.S. Proinflammatory profile of in vitro monocytes in the ageing is affected by lymphocytes presence. Immun. Ageing 2013, 10, 22. [Google Scholar] [CrossRef]
- NCT00992186, U.S.National Library of Medicine. A Study of the Safety and Efficacy of Single-agent Carlumab (an Anti-Chemokine Ligand 2 [CCL2]) in Participants with Metastatic Castrate-Resistant Prostate Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00992186 (accessed on 12 August 2022).
- Pienta, K.J.; Machiels, J.P.; Schrijvers, D.; Alekseev, B.; Shkolnik, M.; Crabb, S.J.; Li, S.; Seetharam, S.; Puchalski, T.A.; Takimoto, C.; et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Investig. New Drugs 2013, 31, 760–768. [Google Scholar] [CrossRef]
- Brana, I.; Calles, A.; LoRusso, P.M.; Yee, L.K.; Puchalski, T.A.; Seetharam, S.; Zhong, B.; de Boer, C.J.; Tabernero, J.; Calvo, E. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: An open-label, multicenter phase 1b study. Target. Oncol. 2015, 10, 111–123. [Google Scholar] [CrossRef]
- NCT02732938, U.S.National Library of Medicine. Ph1b/2 Study of PF-04136309 in Combination with Gem/Nab-P in First-line Metastatic Pancreatic Patients (CCR2i). Available online: https://clinicaltrials.gov/ct2/show/NCT02732938 (accessed on 15 August 2022).
- Noel, M.; O’Reilly, E.M.; Wolpin, B.M.; Ryan, D.P.; Bullock, A.J.; Britten, C.D.; Linehan, D.C.; Belt, B.A.; Gamelin, E.C.; Ganguly, B.; et al. Phase 1b study of a small molecule antagonist of human chemokine (C-C motif) receptor 2 (PF-04136309) in combination with nab-paclitaxel/gemcitabine in first-line treatment of metastatic pancreatic ductal adenocarcinoma. Investig. New Drugs 2020, 38, 800–811. [Google Scholar] [CrossRef]
- Hoefer, J.; Luger, M.; Dal-Pont, C.; Culig, Z.; Schennach, H.; Jochberger, S. The “Aging Factor” Eotaxin-1 (CCL11) Is Detectable in Transfusion Blood Products and Increases with the Donor’s Age. Front. Aging Neurosci. 2017, 9, 402. [Google Scholar] [CrossRef]
- Prins, S.; de Kam, M.L.; Teunissen, C.E.; Groeneveld, G.J. Inflammatory plasma biomarkers in subjects with preclinical Alzheimer’s disease. Alzheimer Res. Ther. 2022, 14, 106. [Google Scholar] [CrossRef]
- Wu, J.M.; Hsieh, T.C.; Yang, C.J.; Olson, S.C. Resveratrol and its metabolites modulate cytokine-mediated induction of eotaxin-1 in human pulmonary artery endothelial cells. Ann. N. Y. Acad. Sci. 2013, 1290, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Jamaluddin, M.S.; Wang, X.; Wang, H.; Rafael, C.; Yao, Q.; Chen, C. Eotaxin increases monolayer permeability of human coronary artery endothelial cells. Arter. Thromb. Vasc. Biol. 2009, 29, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.W.; Su, Y.H.; Lin, Y.K.; Wu, Y.C.; Huang, Y.H.; Yang, F.H.; Chiang, H.J.; Yen, Y.; Wang, P.D. Small blood stem cells for enhancing early osseointegration formation on dental implants: A human phase I safety study. Stem Cell Res. Ther. 2021, 12, 380. [Google Scholar] [CrossRef] [PubMed]
- NCT04451486, U.S.National Library of Medicine. A Phase I Study of SB Cell for the Enhanced Osseointegration of Guided Bone Regeneration in Implant Surgery. Available online: https://clinicaltrials.gov/ct2/show/NCT04451486 (accessed on 12 August 2022).
- Smith, L.K.; He, Y.; Park, J.S.; Bieri, G.; Snethlage, C.E.; Lin, K.; Gontier, G.; Wabl, R.; Plambeck, K.E.; Udeochu, J.; et al. beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat. Med. 2015, 21, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Shen, Y.Y.; Ji, L.J.; Jiang, X.Y.; Wang, X.F.; Shi, Y. Association between serum beta2-microglobulin levels and frailty in an elderly Chinese population: Results from RuLAS. Clin. Interv. Aging 2017, 12, 1725–1729. [Google Scholar] [CrossRef]
- Kim, M.; Suzuki, T.; Kojima, N.; Yoshida, H.; Yoshida, Y.; Hirano, H.; Won, C.W.; Kim, H. Association Between Serum beta2 -Microglobulin Levels and Prevalent and Incident Physical Frailty in Community-Dwelling Older Women. J. Am. Geriatr. Soc. 2017, 65, e83–e88. [Google Scholar] [CrossRef]
- You, L.; Xie, R.; Hu, H.; Gu, G.; Zheng, H.; Zhang, J.; Yang, X.; He, X.; Cui, W. High levels of serum beta2-microglobulin predict severity of coronary artery disease. BMC Cardiovasc. Disord. 2017, 17, 71. [Google Scholar] [CrossRef]
- Quesada, J.M.; Alonso, J.; Gonzalez, J.; Munoz, R.; Jans, I.; Martiu, A.; Bouillon, R. Serum beta-2 microglobulin is a marker of high bone remodelling in elderly women. Mech. Ageing Dev. 1998, 102, 293–298. [Google Scholar] [CrossRef]
- Althubiti, M.; Elzubier, M.; Alotaibi, G.S.; Althubaiti, M.A.; Alsadi, H.H.; Alhazmi, Z.A.; Alghamdi, F.; El-Readi, M.Z.; Almaimani, R.; Babakr, A. Beta 2 microglobulin correlates with oxidative stress in elderly. Exp. Gerontol. 2021, 150, 111359. [Google Scholar] [CrossRef]
- Molenaar, B.; Timmer, L.T.; Droog, M.; Perini, I.; Versteeg, D.; Kooijman, L.; Monshouwer-Kloots, J.; de Ruiter, H.; Gladka, M.M.; van Rooij, E. Single-cell transcriptomics following ischemic injury identifies a role for B2M in cardiac repair. Commun. Biol. 2021, 4, 146. [Google Scholar] [CrossRef]
- Sinha, M.; Jang, Y.C.; Oh, J.; Khong, D.; Wu, E.Y.; Manohar, R.; Miller, C.; Regalado, S.G.; Loffredo, F.S.; Pancoast, J.R.; et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 2014, 344, 649–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egerman, M.A.; Cadena, S.M.; Gilbert, J.A.; Meyer, A.; Nelson, H.N.; Swalley, S.E.; Mallozzi, C.; Jacobi, C.; Jennings, L.L.; Clay, I.; et al. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metab. 2015, 22, 164–174. [Google Scholar] [CrossRef]
- Poggioli, T.; Vujic, A.; Yang, P.; Macias-Trevino, C.; Uygur, A.; Loffredo, F.S.; Pancoast, J.R.; Cho, M.; Goldstein, J.; Tandias, R.M.; et al. Circulating Growth Differentiation Factor 11/8 Levels Decline with Age. Circ. Res. 2016, 118, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Gagliano-Juca, T.; Pencina, K.M.; Krishnan, S.; Li, Z.; Tracy, R.P.; Jasuja, R.; Bhasin, S. Age Trends in Growth and Differentiation Factor-11 and Myostatin Levels in Healthy Men, and Differential Response to Testosterone, Measured Using Liquid Chromatography-Tandem Mass Spectrometry. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2022, 77, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.A.; Beatty, A.L.; Heidecker, B.; Regan, M.C.; Brody, E.N.; Foreman, T.; Kato, S.; Mehler, R.E.; Singer, B.S.; Hveem, K.; et al. Association of growth differentiation factor 11/8, putative anti-ageing factor, with cardiovascular outcomes and overall mortality in humans: Analysis of the Heart and Soul and HUNT3 cohorts. Eur. Heart J. 2015, 36, 3426–3434. [Google Scholar] [CrossRef]
- Elabd, C.; Cousin, W.; Upadhyayula, P.; Chen, R.Y.; Chooljian, M.S.; Li, J.; Kung, S.; Jiang, K.P.; Conboy, I.M. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat. Commun. 2014, 5, 4082. [Google Scholar] [CrossRef]
- Jankowski, M.; Broderick, T.L.; Gutkowska, J. The Role of Oxytocin in Cardiovascular Protection. Front. Psychol. 2020, 11, 2139. [Google Scholar] [CrossRef]
- Jankowski, M.; Danalache, B.; Wang, D.; Bhat, P.; Hajjar, F.; Marcinkiewicz, M.; Paquin, J.; McCann, S.M.; Gutkowska, J. Oxytocin in cardiac ontogeny. Proc. Natl. Acad. Sci. USA 2004, 101, 13074–13079. [Google Scholar] [CrossRef]
- Elabd, C.; Basillais, A.; Beaupied, H.; Breuil, V.; Wagner, N.; Scheideler, M.; Zaragosi, L.E.; Massiera, F.; Lemichez, E.; Trajanoski, Z.; et al. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells 2008, 26, 2399–2407. [Google Scholar] [CrossRef]
- Breuil, V.; Panaia-Ferrari, P.; Fontas, E.; Roux, C.; Kolta, S.; Eastell, R.; Ben Yahia, H.; Faure, S.; Gossiel, F.; Benhamou, C.L.; et al. Oxytocin, a new determinant of bone mineral density in post-menopausal women: Analysis of the OPUS cohort. J. Clin. Endocrinol. Metab. 2014, 99, E634–E641. [Google Scholar] [CrossRef]
- Barraza, J.A.; Grewal, N.S.; Ropacki, S.; Perez, P.; Gonzalez, A.; Zak, P.J. Effects of a 10-day oxytocin trial in older adults on health and well-being. Exp. Clin. Psychopharmacol. 2013, 21, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinel, C.; Lukjanenko, L.; Batut, A.; Deleruyelle, S.; Pradere, J.P.; Le Gonidec, S.; Dortignac, A.; Geoffre, N.; Pereira, O.; Karaz, S.; et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 2018, 24, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Ghosh, A.K.; Eren, M.; Mackie, A.R.; Levine, D.C.; Kim, S.Y.; Cedernaes, J.; Ramirez, V.; Procissi, D.; Smith, L.H.; et al. Downregulation of the Apelinergic Axis Accelerates Aging, whereas Its Systemic Restoration Improves the Mammalian Healthspan. Cell Rep. 2017, 21, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.R.; Xu, R.Y.; Zhang, N.K.; Chen, Y.; Wang, Z.G.; Zhu, Z.M.; Fei, Y.X.; Cao, Y.; Xu, H.T.; Yang, Y. Increased apelin following bone marrow mononuclear cell transplantation contributes to the improvement of cardiac function in patients with severe heart failure. Cell Transplant. 2009, 18, 1311–1318. [Google Scholar] [CrossRef]
- Castellano, J.M.; Mosher, K.I.; Abbey, R.J.; McBride, A.A.; James, M.L.; Berdnik, D.; Shen, J.C.; Zou, B.; Xie, X.S.; Tingle, M.; et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature 2017, 544, 488–492. [Google Scholar] [CrossRef]
- Kandalam, V.; Basu, R.; Abraham, T.; Wang, X.; Soloway, P.D.; Jaworski, D.M.; Oudit, G.Y.; Kassiri, Z. TIMP2 deficiency accelerates adverse post-myocardial infarction remodeling because of enhanced MT1-MMP activity despite lack of MMP2 activation. Circ. Res. 2010, 106, 796–808. [Google Scholar] [CrossRef]
- Ries, C.; Egea, V.; Karow, M.; Kolb, H.; Jochum, M.; Neth, P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: Differential regulation by inflammatory cytokines. Blood 2007, 109, 4055–4063. [Google Scholar] [CrossRef]
- Kelly, D.; Squire, I.B.; Khan, S.Q.; Dhillon, O.; Narayan, H.; Ng, K.H.; Quinn, P.; Davies, J.E.; Ng, L.L. Usefulness of plasma tissue inhibitors of metalloproteinases as markers of prognosis after acute myocardial infarction. Am. J. Cardiol. 2010, 106, 477–482. [Google Scholar] [CrossRef]
- Khrimian, L.; Obri, A.; Ramos-Brossier, M.; Rousseaud, A.; Moriceau, S.; Nicot, A.S.; Mera, P.; Kosmidis, S.; Karnavas, T.; Saudou, F.; et al. Gpr158 mediates osteocalcin’s regulation of cognition. J. Exp. Med. 2017, 214, 2859–2873. [Google Scholar] [CrossRef]
- Mera, P.; Laue, K.; Ferron, M.; Confavreux, C.; Wei, J.; Galan-Diez, M.; Lacampagne, A.; Mitchell, S.J.; Mattison, J.A.; Chen, Y.; et al. Osteocalcin Signaling in Myofibers Is Necessary and Sufficient for Optimum Adaptation to Exercise. Cell Metab. 2016, 23, 1078–1092. [Google Scholar] [CrossRef]
- Smith, C.; Voisin, S.; Al Saedi, A.; Phu, S.; Brennan-Speranza, T.; Parker, L.; Eynon, N.; Hiam, D.; Yan, X.; Scott, D.; et al. Osteocalcin and its forms across the lifespan in adult men. Bone 2020, 130, 115085. [Google Scholar] [CrossRef]
- Zhang, X.L.; Shen, Y.; Ma, X.J.; Lu, Z.G.; Xu, Y.T.; Xiong, Q.; Bao, Y.Q. Low serum osteocalcin levels are correlated with left ventricular systolic dysfunction and cardiac death in Chinese men. Acta Pharmacol. Sin. 2019, 40, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Kabir, M.H.; Park, J.H.; Park, J.I.; Kang, J.S.; Ju, S.; Shin, Y.J.; Lee, S.M.; Lee, J.; Kim, S.; et al. Plasma proteomic profiling of young and old mice reveals cadherin-13 prevents age-related bone loss. Aging 2020, 12, 8652–8668. [Google Scholar] [CrossRef] [PubMed]
- Yousef, H.; Czupalla, C.J.; Lee, D.; Chen, M.B.; Burke, A.N.; Zera, K.A.; Zandstra, J.; Berber, E.; Lehallier, B.; Mathur, V.; et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat. Med. 2019, 25, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, E.; Haarmann-Stemmann, T.; Kapr, J.; Galanjuk, S.; Hartmann, J.; Mertens, P.R.; Kampfer, A.A.M.; Schins, R.P.F.; Tigges, J.; Koch, K. Stem Cells for Next Level Toxicity Testing in the 21st Century. Small 2021, 17, e2006252. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, B.; Buono, M.F.; von Boehmer, L.; Hoerstrup, S.P.; Emmert, M.Y. Human Cardiac Organoids for Disease Modeling. Clin. Pharmacol. Ther. 2019, 105, 79–85. [Google Scholar] [CrossRef]
- Walenda, T.; Diener, Y.; Jost, E.; Morin-Kensicki, E.; Goecke, T.W.; Bosio, A.; Rath, B.; Brummendorf, T.H.; Bissels, U.; Wagner, W. MicroRNAs and Metabolites in Serum Change after Chemotherapy: Impact on Hematopoietic Stem and Progenitor Cells. PLoS ONE 2015, 10, e0128231. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Gnanasampanthapandian, D.; Rajasingh, J.; Palaniyandi, K. Stem Cell-Derived Exosomes Potential Therapeutic Roles in Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 8, 723236. [Google Scholar] [CrossRef]
- Sun, S.J.; Wei, R.; Li, F.; Liao, S.Y.; Tse, H.F. Mesenchymal stromal cell-derived exosomes in cardiac regeneration and repair. Stem Cell Rep. 2021, 16, 1662–1673. [Google Scholar] [CrossRef]
- Ju, C.; Shen, Y.; Ma, G.; Liu, Y.; Cai, J.; Kim, I.M.; Weintraub, N.L.; Liu, N.; Tang, Y. Transplantation of Cardiac Mesenchymal Stem Cell-Derived Exosomes Promotes Repair in Ischemic Myocardium. J. Cardiovasc. Transl. Res. 2018, 11, 420–428. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Wang, Z.; Liu, L.; Zhang, B.; Li, B. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J. Cell. Biochem. 2020, 121, 2089–2102. [Google Scholar] [CrossRef] [PubMed]
- Bobis-Wozowicz, S.; Kmiotek, K.; Sekula, M.; Kedracka-Krok, S.; Kamycka, E.; Adamiak, M.; Jankowska, U.; Madetko-Talowska, A.; Sarna, M.; Bik-Multanowski, M.; et al. Human Induced Pluripotent Stem Cell-Derived Microvesicles Transmit RNAs and Proteins to Recipient Mature Heart Cells Modulating Cell Fate and Behavior. Stem Cells 2015, 33, 2748–2761. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Im, W.; Kim, M. Exosomes as a potential messenger unit during heterochronic parabiosis for amelioration of Huntington’s disease. Neurobiol. Dis. 2021, 155, 105374. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.W. Review article: Albumin as a drug--biological effects of albumin unrelated to oncotic pressure. Aliment. Pharmacol. Ther. 2002, 16 (Suppl. S5), 6–11. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Y.; Liang, H. Interactive association of drugs binding to human serum albumin. Int. J. Mol. Sci. 2014, 15, 3580–3595. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Weaving, G.; Batstone, G.F.; Jones, R.G. Age and sex variation in serum albumin concentration: An observational study. Ann. Clin. Biochem. 2016, 53, 106–111. [Google Scholar] [CrossRef]
- Greenblatt, D.J. Reduced serum albumin concentration in the elderly: A report from the Boston Collaborative Drug Surveillance Program. J. Am. Geriatr. Soc. 1979, 27, 20–22. [Google Scholar] [CrossRef]
- Wang, Y.; Du, P.; Xiao, Q.; Li, J.; Liu, X.; Tan, J.; Zhang, X. Relationship Between Serum Albumin and Risk of Atrial Fibrillation: A Dose-Response Meta-Analysis. Front. Nutr. 2021, 8, 728353. [Google Scholar] [CrossRef]
- Costa, M.; Paez, A. Emerging insights into the role of albumin with plasma exchange in Alzheimer’s disease management. Transfus. Apher. Sci. 2021, 60, 103164. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Perera, L.M.; Hoving, A.L.; Schmidt, K.E.; Cenan, S.; Wohllebe, M.; Greiner, J.F.W.; Kaltschmidt, C.; Simon, M.; Knabbe, C.; Kaltschmidt, B. Neuroprotection Mediated by Human Blood Plasma in Mouse Hippocampal Slice Cultures and in Oxidatively Stressed Human Neurons. Int. J. Mol. Sci. 2021, 22, 9567. [Google Scholar] [CrossRef]
- Höving, A.L.; Schmitz, J.; Schmidt, K.E.; Greiner, J.F.W.; Knabbe, C.; Kaltschmidt, B.; Grünberger, A.; Kaltschmidt, C. Human Blood Serum Induces p38-MAPK- and Hsp27-Dependent Migration Dynamics of Adult Human Cardiac Stem Cells: Single-Cell Analysis via a Microfluidic-Based Cultivation Platform. Biology 2021, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Palovics, R.; Keller, A.; Schaum, N.; Tan, W.; Fehlmann, T.; Borja, M.; Kern, F.; Bonanno, L.; Calcuttawala, K.; Webber, J.; et al. Molecular hallmarks of heterochronic parabiosis at single-cell resolution. Nature 2022, 603, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Edgren, G.; Ullum, H.; Rostgaard, K.; Erikstrup, C.; Sartipy, U.; Holzmann, M.J.; Nyren, O.; Hjalgrim, H. Association of Donor Age and Sex with Survival of Patients Receiving Transfusions. JAMA Intern. Med. 2017, 177, 854–860. [Google Scholar] [CrossRef]
- Sha, S.J.; Deutsch, G.K.; Tian, L.; Richardson, K.; Coburn, M.; Gaudioso, J.L.; Marcal, T.; Solomon, E.; Boumis, A.; Bet, A.; et al. Safety, Tolerability, and Feasibility of Young Plasma Infusion in the Plasma for Alzheimer Symptom Amelioration Study: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 35–40. [Google Scholar] [CrossRef]
- Hannestad, J.; Koborsi, K.; Klutzaritz, V.; Chao, W.; Ray, R.; Paez, A.; Jackson, S.; Lohr, S.; Cummings, J.L.; Kay, G.; et al. Safety and tolerability of GRF6019 in mild-to-moderate Alzheimer’s disease dementia. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2020, 6, e12115. [Google Scholar] [CrossRef]
- Parker, J.E.; Martinez, A.; Deutsch, G.K.; Prabhakar, V.; Lising, M.; Kapphahn, K.I.; Anidi, C.M.; Neuville, R.; Coburn, M.; Shah, N.; et al. Safety of Plasma Infusions in Parkinson’s Disease. Mov. Disord. 2020, 35, 1905–1913. [Google Scholar] [CrossRef]
- NCT03229785, U.S.National Library of Medicine. Quality of Life Improvements with Cord Blood Plasma. Available online: https://clinicaltrials.gov/ct2/show/NCT03229785 (accessed on 4 February 2022).
- NCT02418013, U.S.National Library of Medicine. Clinical Trial to Evaluate the Potential Efficacy and Safety of Human Umbilical Cord Blood and Plasma. Available online: https://clinicaltrials.gov/ct2/show/NCT02418013 (accessed on 15 August 2022).
- NCT04566757, U.S.National Library of Medicine. Safety and Efficacy of Human Umbilical Cord Blood Plasma Infusion for Age-Related Cognitive Decline. Available online: https://clinicaltrials.gov/ct2/show/NCT04566757 (accessed on 18 February 2022).
- NCT05054894, U.S.National Library of Medicine. Plasmapheresis for Treatment of Age-Related Frailty. Available online: https://clinicaltrials.gov/ct2/show/NCT05054894 (accessed on 18 February 2022).
- NCT02913183, U.S.National Library of Medicine. Efficacy and Safety of Young Health Plasma on Acute Stroke. Available online: https://clinicaltrials.gov/ct2/show/NCT02913183 (accessed on 4 February 2022).
- NCT02803554, U.S.National Library of Medicine. Young Donor Plasma Transfusion and Age-Related Biomarkers. Available online: https://clinicaltrials.gov/ct2/show/NCT02803554 (accessed on 4 February 2022).
- FDA. Statement from FDA Commissioner Scott Gottlieb, M.D. and Director of FDA’s Center for Biologics Evaluation and Research Peter Marks, M.D., Ph.D., Cautioning Consumers Against Receiving Young Donor Plasma Infusions that Are Promoted as Unproven Treatment for Varying Conditions. Available online: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-director-fdas-center-biologics-evaluation-and-0 (accessed on 4 February 2022).
- NCT04241159, U.S.National Library of Medicine. Study to Explore the Safety and Feasibility of Allogeneic Young Plasma Infusion in Older Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04241159 (accessed on 17 June 2022).
- NCT02256306, U.S.National Library of Medicine. The PLasma for Alzheimer SymptoM Amelioration (PLASMA) Study (PLASMA). Available online: https://clinicaltrials.gov/ct2/show/NCT02256306 (accessed on 17 June 2022).
- NCT03520998, U.S.National Library of Medicine. A Randomized Study to Assess the Safety of GRF6019 Infusions in Subjects with Mild to Moderate Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT03520998 (accessed on 17 June 2022).
- NCT02968433, U.S.National Library of Medicine. The Stanford Parkinson’s Disease Plasma Study (SPDP). Available online: https://clinicaltrials.gov/ct2/show/NCT02968433 (accessed on 17 June 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höving, A.L.; Schmidt, K.E.; Kaltschmidt, B.; Kaltschmidt, C.; Knabbe, C. The Role of Blood-Derived Factors in Protection and Regeneration of Aged Tissues. Int. J. Mol. Sci. 2022, 23, 9626. https://doi.org/10.3390/ijms23179626
Höving AL, Schmidt KE, Kaltschmidt B, Kaltschmidt C, Knabbe C. The Role of Blood-Derived Factors in Protection and Regeneration of Aged Tissues. International Journal of Molecular Sciences. 2022; 23(17):9626. https://doi.org/10.3390/ijms23179626
Chicago/Turabian StyleHöving, Anna L., Kazuko E. Schmidt, Barbara Kaltschmidt, Christian Kaltschmidt, and Cornelius Knabbe. 2022. "The Role of Blood-Derived Factors in Protection and Regeneration of Aged Tissues" International Journal of Molecular Sciences 23, no. 17: 9626. https://doi.org/10.3390/ijms23179626
APA StyleHöving, A. L., Schmidt, K. E., Kaltschmidt, B., Kaltschmidt, C., & Knabbe, C. (2022). The Role of Blood-Derived Factors in Protection and Regeneration of Aged Tissues. International Journal of Molecular Sciences, 23(17), 9626. https://doi.org/10.3390/ijms23179626







