Etiology of IBD—Is It Still a Mystery?
Abstract
1. Introduction
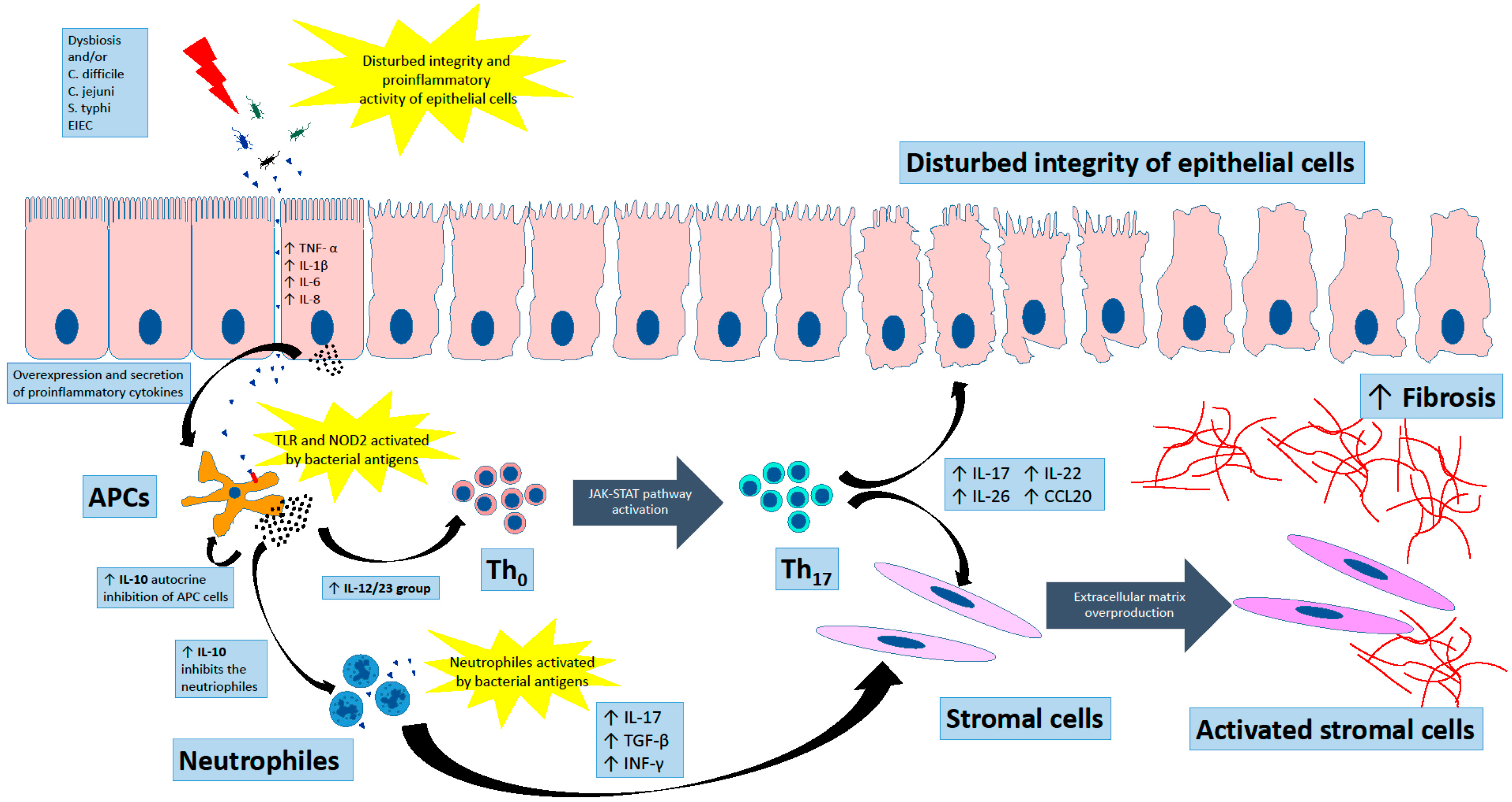
2. Primary Defence Line in IBD
3. Adaptive Immunity in Chronic Inflammation
4. The Group of 12/23 Cytokines
5. Interleukin 10
6. Fibrosis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Peña-Sánchez, J.N.; Osei, J.A.; Marques Santos, J.D.; Jennings, D.; Andkhoie, M.; Brass, C.; Bukassa-Kazadi, G.; Lu, X.; Johnson-Jennings, M.; Porter, L.; et al. Increasing Prevalence and Stable Incidence Rates of Inflammatory Bowel Disease Among First Nations: Population-Based Evidence from a Western Canadian Province. Inflamm. Bowel Dis. 2022, 28, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- M’Koma, A.E. Inflammatory bowel disease: An expanding global health problem. Clin. Med. Insights Gastroenterol. 2013, 6, 33–47. [Google Scholar] [CrossRef]
- Krzesiek, E.; Kofla-Dlubacz, A.; Akutko, K.; Stawarski, A. The Incidence of Inflammatory Bowel Disease in the Paediatric Population in the District of Lower Silesia, Poland. J. Clin. Med. 2021, 10, 3994. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Kaplan, G.G.; Ng, S.C. Changing Global Epidemiology of Inflammatory Bowel Diseases: Sustaining Health Care Delivery Into the 21st Century. Clin. Gastroenterol. Hepatol. 2020, 18, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Binion, D.G. Clostridium difficile Infection in Patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2012, 8, 615–617. [Google Scholar]
- Rocha, M.F.; Maia, M.E.; Bezerra, L.R.; Lyerly, D.M.; Guerrant, R.L.; Ribeiro, R.A.; Lima, A.A. Clostridium difficile toxin A induces the release of neutrophil chemotactic factors from rat peritoneal macrophages: Role of interleukin-1beta, tumor necrosis factor alpha, and leukotrienes. Infect. Immun. 1997, 65, 2740–2746. [Google Scholar] [CrossRef]
- Burnham, W.R.; Lennard-Jones, J.E.; Stanford, J.L.; Bird, R.G. Mycobacteria as a possible cause of inflammatory bowel disease. Lancet 1978, 312, 693–696. [Google Scholar] [CrossRef]
- Lidar, M.; Langevitz, P.; Shoenfeld, Y. The role of infection in inflammatory bowel disease: Initiation, exacerbation and protection. Isr. Med. Assoc. J. 2009, 11, 558–563. [Google Scholar]
- Kalischuk, L.D.; Buret, A.G. A role for Campylobacter jejuni-induced enteritis in inflammatory bowel disease? Am. J. Physiol. Gastrointest Liver Physiol. 2010, 298, G1–G9. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Bishai, J.; Reshef, L.; Abitbol, G.; Focht, G.; Marcus, D.; Ledder, O.; Lev-Tzion, R.; Orlanski-Meyer, E.; Yerushalmi, B.; et al. Antibiotic Cocktail for Pediatric Acute Severe Colitis and the Microbiome: The PRASCO Randomized Controlled Trial. Inflamm. Bowel. Dis. 2020, 26, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Pigneur, B.; Lepage, P.; Mondot, S.; Schmitz, J.; Goulet, O.; Doré, J.; Ruemmele, F.M. Mucosal Healing and Bacterial Composition in Response to Enteral Nutrition Vs Steroid-based Induction Therapy-A Randomised Prospective Clinical Trial in Children with Crohn’s Disease. J. Crohns Colitis 2019, 13, 846–855. [Google Scholar] [CrossRef]
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of Low FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients with Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterology 2020, 158, 176–188.e177. [Google Scholar] [CrossRef]
- Milajerdi, A.; Sadeghi, O.; Siadat, S.D.; Keshavarz, S.A.; Sima, A.; Vahedi, H.; Adibi, P.; Esmaillzadeh, A. A randomized controlled trial investigating the effect of a diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols on the intestinal microbiome and inflammation in patients with ulcerative colitis: Study protocol for a randomized controlled trial. Trials 2020, 21, 201. [Google Scholar] [CrossRef]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e106. [Google Scholar] [CrossRef]
- Sokol, H.; Landman, C.; Seksik, P.; Berard, L.; Montil, M.; Nion-Larmurier, I.; Bourrier, A.; Le Gall, G.; Lalande, V.; De Rougemont, A.; et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: A pilot randomized controlled study. Microbiome 2020, 8, 12. [Google Scholar] [CrossRef]
- Nissilä, E.; Korpela, K.; Lokki, A.I.; Paakkanen, R.; Jokiranta, S.; de Vos, W.M.; Lokki, M.L.; Kolho, K.L.; Meri, S. C4B gene influences intestinal microbiota through complement activation in patients with paediatric-onset inflammatory bowel disease. Clin. Exp. Immunol. 2017, 190, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Schwerd, T.; Bryant, R.V.; Pandey, S.; Capitani, M.; Meran, L.; Cazier, J.B.; Jung, J.; Mondal, K.; Parkes, M.; Mathew, C.G.; et al. NOX1 loss-of-function genetic variants in patients with inflammatory bowel disease. Mucosal Immunol. 2018, 11, 562–574. [Google Scholar] [CrossRef]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Meir, M.; Burkard, N.; Ungewiß, H.; Diefenbacher, M.; Flemming, S.; Kannapin, F.; Germer, C.T.; Schweinlin, M.; Metzger, M.; Waschke, J.; et al. Neurotrophic factor GDNF regulates intestinal barrier function in inflammatory bowel disease. J. Clin. Investig. 2019, 129, 2824–2840. [Google Scholar] [CrossRef]
- Meir, M.; Flemming, S.; Burkard, N.; Bergauer, L.; Metzger, M.; Germer, C.T.; Schlegel, N. Glial cell line-derived neurotrophic factor promotes barrier maturation and wound healing in intestinal epithelial cells in vitro. Am. J. Physiol. Gastrointest Liver Physiol. 2015, 309, G613–G624. [Google Scholar] [CrossRef] [PubMed]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef]
- Engevik, M.A.; Luk, B.; Chang-Graham, A.L.; Hall, A.; Herrmann, B.; Ruan, W.; Endres, B.T.; Shi, Z.; Garey, K.W.; Hyser, J.M.; et al. Bifidobacterium dentium Fortifies the Intestinal Mucus Layer via Autophagy and Calcium Signaling Pathways. mBio 2019, 10, e01087-19. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Larsson, J.M.; Hansson, G.C. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4659–4665. [Google Scholar] [CrossRef]
- Leon-Coria, A.; Kumar, M.; Workentine, M.; Moreau, F.; Surette, M.; Chadee, K. Muc2 Mucin and Nonmucin Microbiota Confer Distinct Innate Host Defense in Disease Susceptibility and Colonic Injury. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 77–98. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Stange, E.F.; Schroeder, B.O. Microbiota and mucosal defense in IBD: An update. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Seol, S.Y.; Yang, G.E.; Cho, Y.; Kim, M.C.; Choi, H.J.; Choi, Y.H.; Leem, S.H. Rare minisatellite alleles of MUC2-MS8 influence susceptibility to rectal carcinoma. Genes Genom. 2021, 43, 1381–1388. [Google Scholar] [CrossRef]
- Hugot, J.P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cézard, J.P.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.A.; Gassull, M.; et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Bonen, D.K.; Inohara, N.; Nicolae, D.L.; Chen, F.F.; Ramos, R.; Britton, H.; Moran, T.; Karaliuskas, R.; Duerr, R.H.; et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001, 411, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Warner, N.; Inohara, N.; Núñez, G. NOD1 and NOD2: Signaling, host defense, and inflammatory disease. Immunity 2014, 41, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T.; Hovingh, E.S.; Foerster, E.G.; Abdel-Nour, M.; Philpott, D.J.; Girardin, S.E. NOD1 and NOD2 in inflammation, immunity and disease. Arch. Biochem. Biophys. 2019, 670, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Inohara, N.; Ogura, Y.; Fontalba, A.; Gutierrez, O.; Pons, F.; Crespo, J.; Fukase, K.; Inamura, S.; Kusumoto, S.; Hashimoto, M.; et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J. Biol. Chem. 2003, 278, 5509–5512. [Google Scholar] [CrossRef]
- van Heel, D.A.; Ghosh, S.; Butler, M.; Hunt, K.A.; Lundberg, A.M.; Ahmad, T.; McGovern, D.P.; Onnie, C.; Negoro, K.; Goldthorpe, S.; et al. Muramyl dipeptide and toll-like receptor sensitivity in NOD2-associated Crohn’s disease. Lancet 2005, 365, 1794–1796. [Google Scholar] [CrossRef]
- Wehkamp, J.; Salzman, N.H.; Porter, E.; Nuding, S.; Weichenthal, M.; Petras, R.E.; Shen, B.; Schaeffeler, E.; Schwab, M.; Linzmeier, R.; et al. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 18129–18134. [Google Scholar] [CrossRef]
- Homer, C.R.; Richmond, A.L.; Rebert, N.A.; Achkar, J.P.; McDonald, C. ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn’s disease pathogenesis. Gastroenterology 2010, 139, 1630–1641.e2. [Google Scholar] [CrossRef]
- Wardle, E.N. Th-17 lymphocytes. Saudi J. Kidney Dis. Transpl. 2010, 21, 954–956. [Google Scholar]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef]
- Wilson, N.J.; Boniface, K.; Chan, J.R.; McKenzie, B.S.; Blumenschein, W.M.; Mattson, J.D.; Basham, B.; Smith, K.; Chen, T.; Morel, F.; et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 2007, 8, 950–957. [Google Scholar] [CrossRef]
- Abraham, C.; Cho, J.H. IL-23 and autoimmunity: New insights into the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 2009, 60, 97–110. [Google Scholar] [CrossRef]
- Vignali, D.A.; Kuchroo, V.K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef]
- Kobayashi, T.; Okamoto, S.; Hisamatsu, T.; Kamada, N.; Chinen, H.; Saito, R.; Kitazume, M.T.; Nakazawa, A.; Sugita, A.; Koganei, K.; et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut 2008, 57, 1682–1689. [Google Scholar] [CrossRef]
- Brand, S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: New immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut 2009, 58, 1152–1167. [Google Scholar] [CrossRef]
- Neumann, C.; Scheffold, A.; Rutz, S. Functions and regulation of T cell-derived interleukin-10. Semin. Immunol. 2019, 44, 101344. [Google Scholar] [CrossRef]
- Morita, M.; Takeuchi, I.; Kato, M.; Migita, O.; Jimbo, K.; Shimizu, H.; Yoshimura, S.; Tomizawa, D.; Shimizu, T.; Hata, K.; et al. Intestinal outcome of bone marrow transplantation for monogenic inflammatory bowel disease. Pediatr. Int. 2022, 64, e14750. [Google Scholar] [CrossRef]
- Huang, Z.; Peng, K.; Li, X.; Zhao, R.; You, J.; Cheng, X.; Wang, Z.; Wang, Y.; Wu, B.; Wang, H.; et al. Mutations in Interleukin-10 Receptor and Clinical Phenotypes in Patients with Very Early Onset Inflammatory Bowel Disease: A Chinese VEO-IBD Collaboration Group Survey. Inflamm. Bowel Dis. 2017, 23, 578–590. [Google Scholar] [CrossRef]
- Shim, J.O.; Seo, J.K. Very early-onset inflammatory bowel disease (IBD) in infancy is a different disease entity from adult-onset IBD; one form of interleukin-10 receptor mutations. J. Hum. Genet. 2014, 59, 337–341. [Google Scholar] [CrossRef]
- Glocker, E.O.; Kotlarz, D.; Klein, C.; Shah, N.; Grimbacher, B. IL-10 and IL-10 receptor defects in humans. Ann. N. Y. Acad. Sci. 2011, 1246, 102–107. [Google Scholar] [CrossRef]
- Rogy, M.A.; Beinhauer, B.G.; Reinisch, W.; Huang, L.; Pokieser, P. Transfer of interleukin-4 and interleukin-10 in patients with severe inflammatory bowel disease of the rectum. Hum. Gene Ther. 2000, 11, 1731–1741. [Google Scholar] [CrossRef]
- Dejaco, C.; Reinisch, W.; Lichtenberger, C.; Waldhoer, T.; Kuhn, I.; Tilg, H.; Gasche, C. In vivo effects of recombinant human interleukin-10 on lymphocyte phenotypes and leukocyte activation markers in inflammatory bowel disease. J. Investig. Med. 2000, 48, 449–456. [Google Scholar]
- Liu, Z.; Feng, B.S.; Yang, S.B.; Chen, X.; Su, J.; Yang, P.C. Interleukin (IL)-23 suppresses IL-10 in inflammatory bowel disease. J. Biol. Chem. 2012, 287, 3591–3597. [Google Scholar] [CrossRef] [PubMed]
- Alipour, S.; Nouri, M.; Khabbazi, A.; Samadi, N.; Babaloo, Z.; Abolhasani, S.; Farhadi, J.; Roshanravan, N.; Jadideslam, G.; Sakhinia, E. Hypermethylation of IL-10 gene is responsible for its low mRNA expression in Behçet’s disease. J. Cell Biochem. 2018, 119, 6614–6622. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.P.; Mulsow, J.J.; O’Keane, C.; Docherty, N.G.; Watson, R.W.; O’Connell, P.R. Fibrogenesis in Crohn’s disease. Am. J. Gastroenterol. 2007, 102, 439–448. [Google Scholar] [CrossRef]
- Li, C.; Kuemmerle, J.F. Mechanisms that mediate the development of fibrosis in patients with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1250–1258. [Google Scholar] [CrossRef]
- McKaig, B.C.; McWilliams, D.; Watson, S.A.; Mahida, Y.R. Expression and regulation of tissue inhibitor of metalloproteinase-1 and matrix metalloproteinases by intestinal myofibroblasts in inflammatory bowel disease. Am. J. Pathol. 2003, 162, 1355–1360. [Google Scholar] [CrossRef]
- Warnaar, N.; Hofker, H.S.; Maathuis, M.H.; Niesing, J.; Bruggink, A.H.; Dijkstra, G.; Ploeg, R.J.; Schuurs, T.A. Matrix metalloproteinases as profibrotic factors in terminal ileum in Crohn’s disease. Inflamm. Bowel Dis. 2006, 12, 863–869. [Google Scholar] [CrossRef]
- Kofla-Dlubacz, A.; Matusiewicz, M.; Krzystek-Korpacka, M.; Iwanczak, B. Correlation of MMP-3 and MMP-9 with Crohn’s disease activity in children. Dig. Dis. Sci. 2012, 57, 706–712. [Google Scholar] [CrossRef][Green Version]
- Medina, C.; Radomski, M.W. Role of matrix metalloproteinases in intestinal inflammation. J. Pharmacol. Exp. Ther. 2006, 318, 933–938. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef]
- O’Sullivan, S.; Gilmer, J.F.; Medina, C. Matrix Metalloproteinases in Inflammatory Bowel Disease: An Update. Mediat. Inflamm. 2015, 2015, 964131. [Google Scholar] [CrossRef]
- Yang, L.; Tang, S.; Baker, S.S.; Arijs, I.; Liu, W.; Alkhouri, R.; Lan, P.; Baker, R.D.; Tang, Z.; Ji, G.; et al. Corrigendum: Difference in Pathomechanism Between Crohn’s Disease and Ulcerative Colitis Revealed by Colon Transcriptome. Inflamm. Bowel Dis. 2019, 25, e154. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, J.S.; Attumi, T.; Opekun, A.R.; Abraham, B.; Hou, J.; Shelby, H.; Graham, D.Y.; Streckfus, C.; Klein, J.R. MicroRNA signatures differentiate Crohn’s disease from ulcerative colitis. BMC Immunol. 2015, 16, 5. [Google Scholar] [CrossRef]
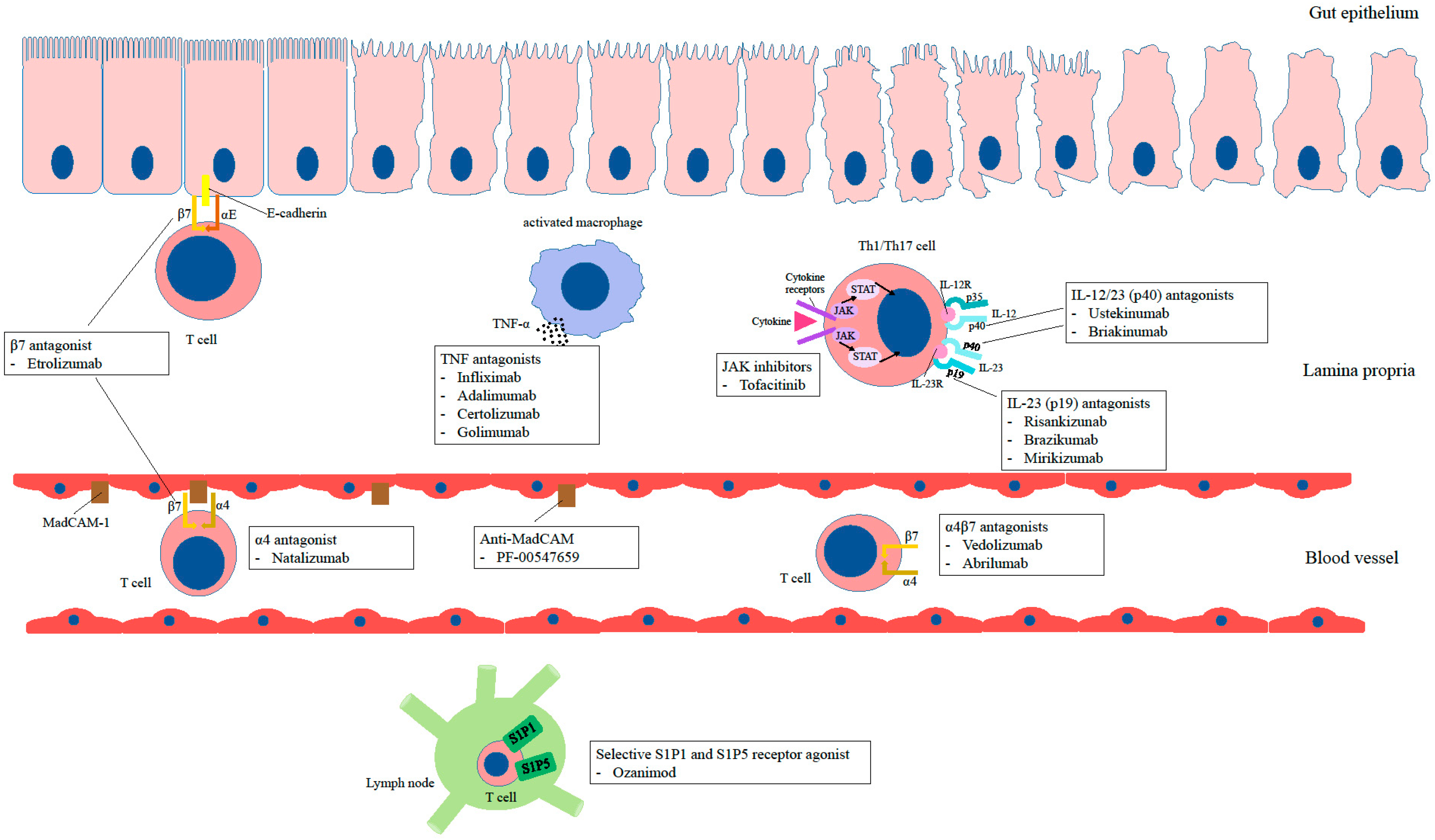
- Kofla-Dłubacz, A.; Akutko, K.; Krzesiek, E.; Jamer, T.; Braksator, J.; Grębska, P.; Pytrus, T.; Stawarski, A. Selective Forms of Therapy in the Treatment of Inflammatory Bowel Diseases. J. Clin. Med. 2022, 11, 994. [Google Scholar] [CrossRef]
- Billmeier, U.; Dieterich, W.; Neurath, M.F.; Atreya, R. Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 9300–9313. [Google Scholar] [CrossRef]
- Cholapranee, A.; Hazlewood, G.S.; Kaplan, G.G.; Peyrin-Biroulet, L.; Ananthakrishnan, A.N. Systematic review with meta-analysis: Comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment. Pharmacol. Ther. 2017, 45, 1291–1302. [Google Scholar] [CrossRef]
- Breton, J.; Kastl, A.; Conrad, M.A.; Baldassano, R.N. Positioning Biologic Therapies in the Management of Pediatric Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2020, 16, 400–414. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kofla-Dłubacz, A.; Pytrus, T.; Akutko, K.; Sputa-Grzegrzółka, P.; Piotrowska, A.; Dzięgiel, P. Etiology of IBD—Is It Still a Mystery? Int. J. Mol. Sci. 2022, 23, 12445. https://doi.org/10.3390/ijms232012445
Kofla-Dłubacz A, Pytrus T, Akutko K, Sputa-Grzegrzółka P, Piotrowska A, Dzięgiel P. Etiology of IBD—Is It Still a Mystery? International Journal of Molecular Sciences. 2022; 23(20):12445. https://doi.org/10.3390/ijms232012445
Chicago/Turabian StyleKofla-Dłubacz, Anna, Tomasz Pytrus, Katarzyna Akutko, Patrycja Sputa-Grzegrzółka, Aleksandra Piotrowska, and Piotr Dzięgiel. 2022. "Etiology of IBD—Is It Still a Mystery?" International Journal of Molecular Sciences 23, no. 20: 12445. https://doi.org/10.3390/ijms232012445
APA StyleKofla-Dłubacz, A., Pytrus, T., Akutko, K., Sputa-Grzegrzółka, P., Piotrowska, A., & Dzięgiel, P. (2022). Etiology of IBD—Is It Still a Mystery? International Journal of Molecular Sciences, 23(20), 12445. https://doi.org/10.3390/ijms232012445







