Modification of Regulatory T Cell Epitopes Promotes Effector T Cell Responses to Aspartyl/Asparaginyl β-Hydroxylase
Abstract
1. Introduction
2. Results
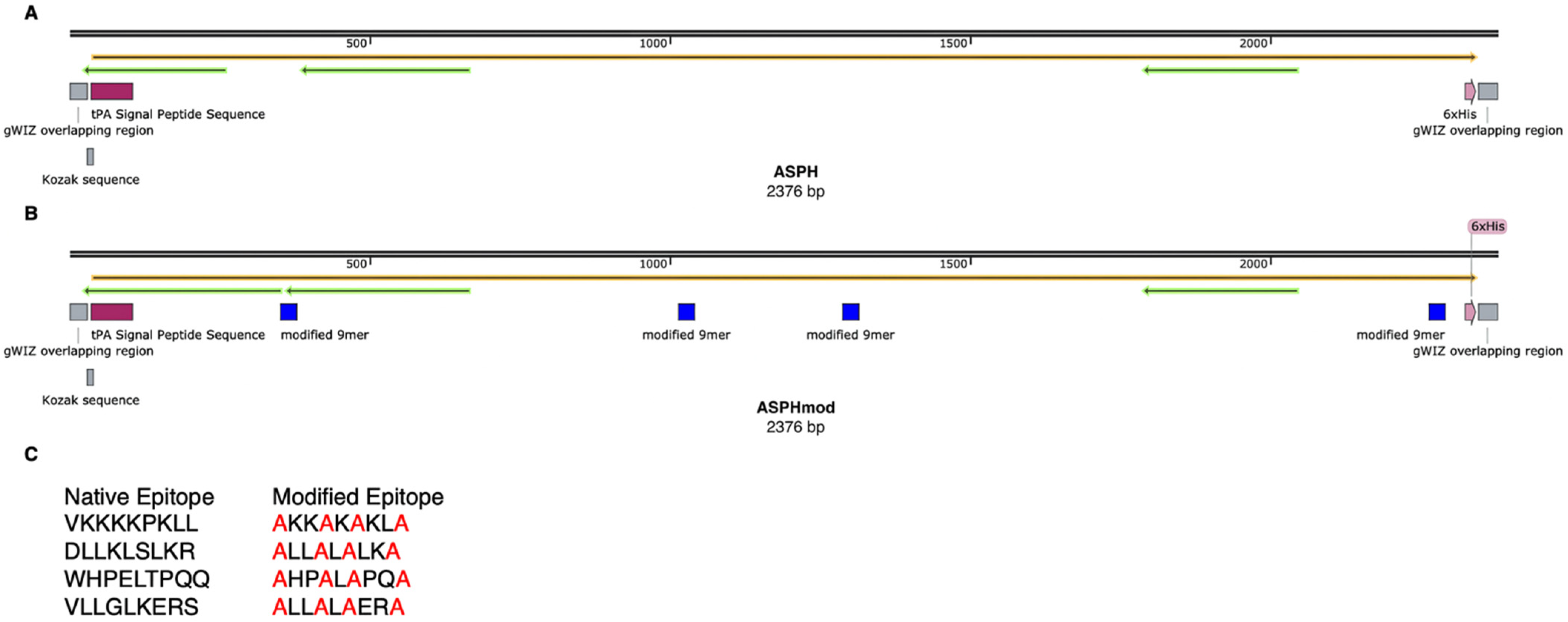
2.1. Recombinant ASPH and ASPHmod
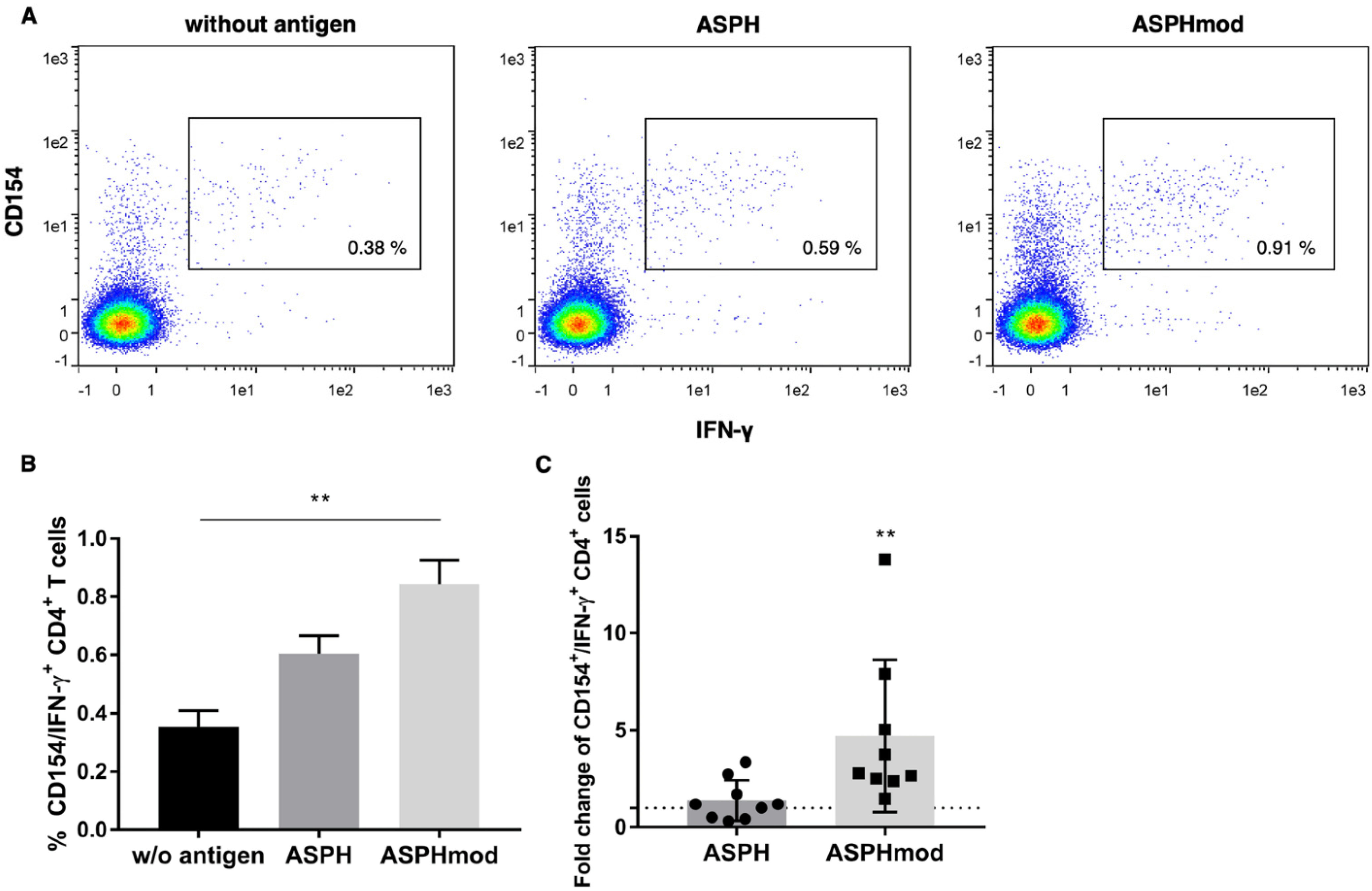
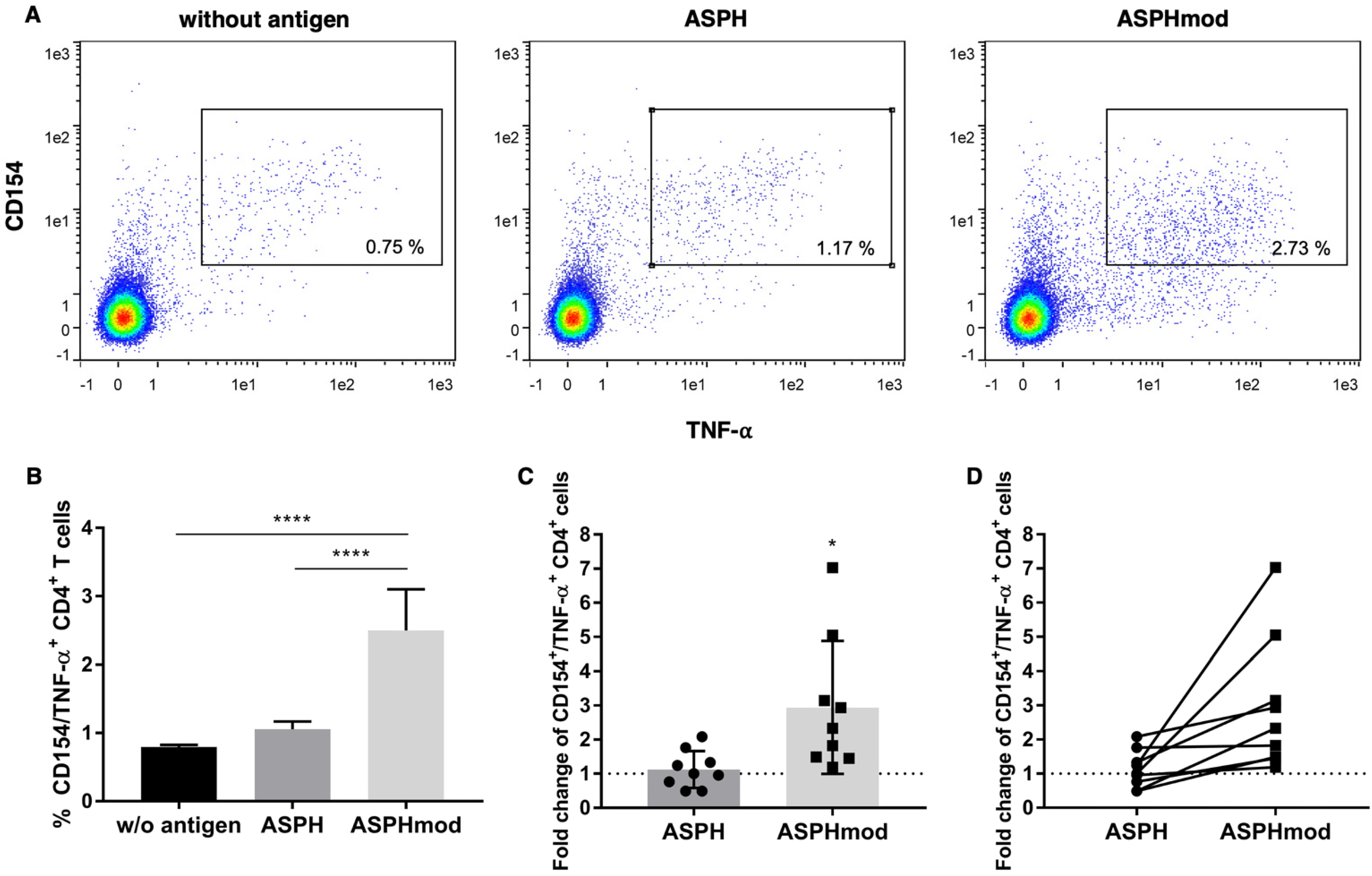
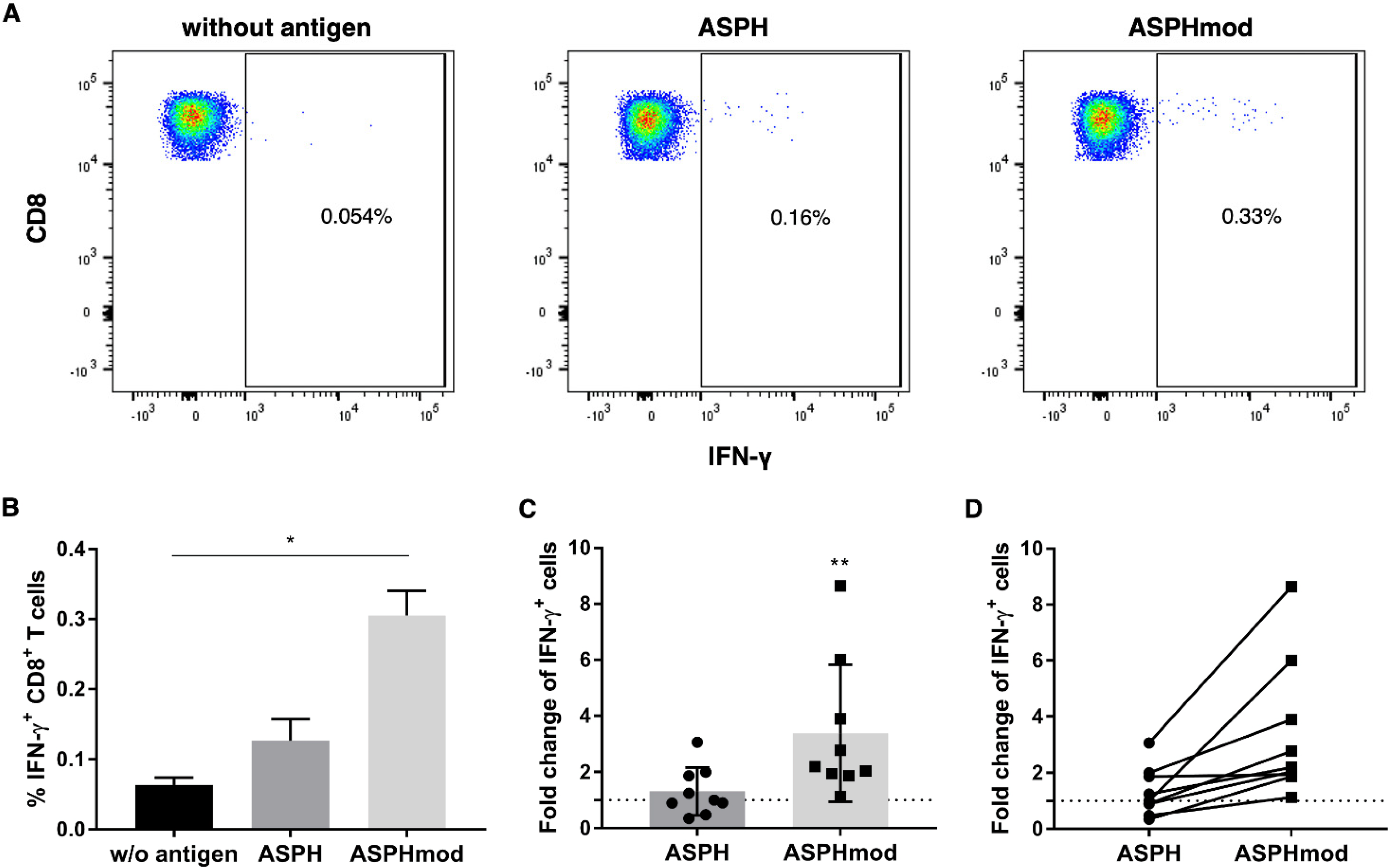
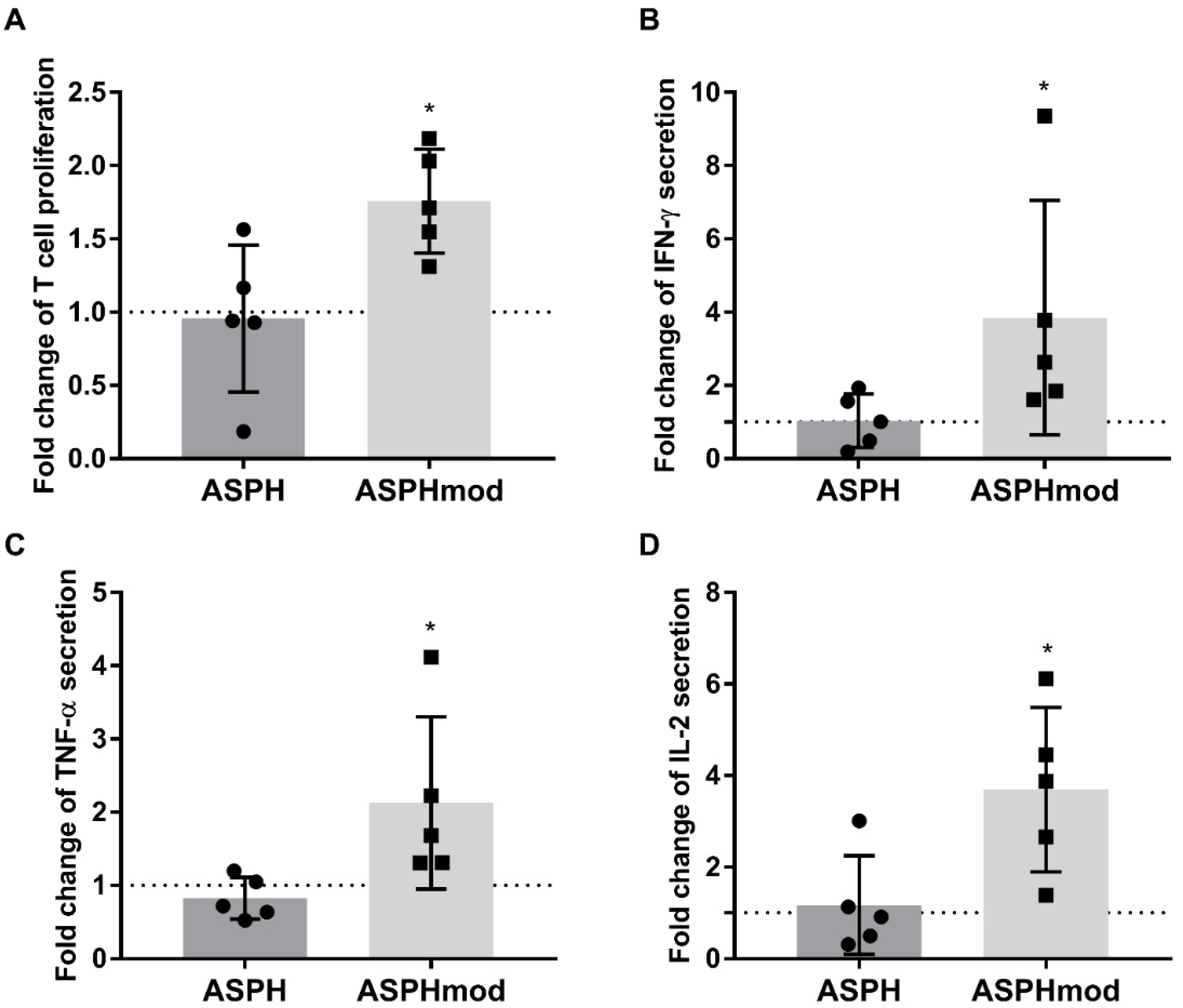
2.2. Elevated T Cell Responses to ASPHmod
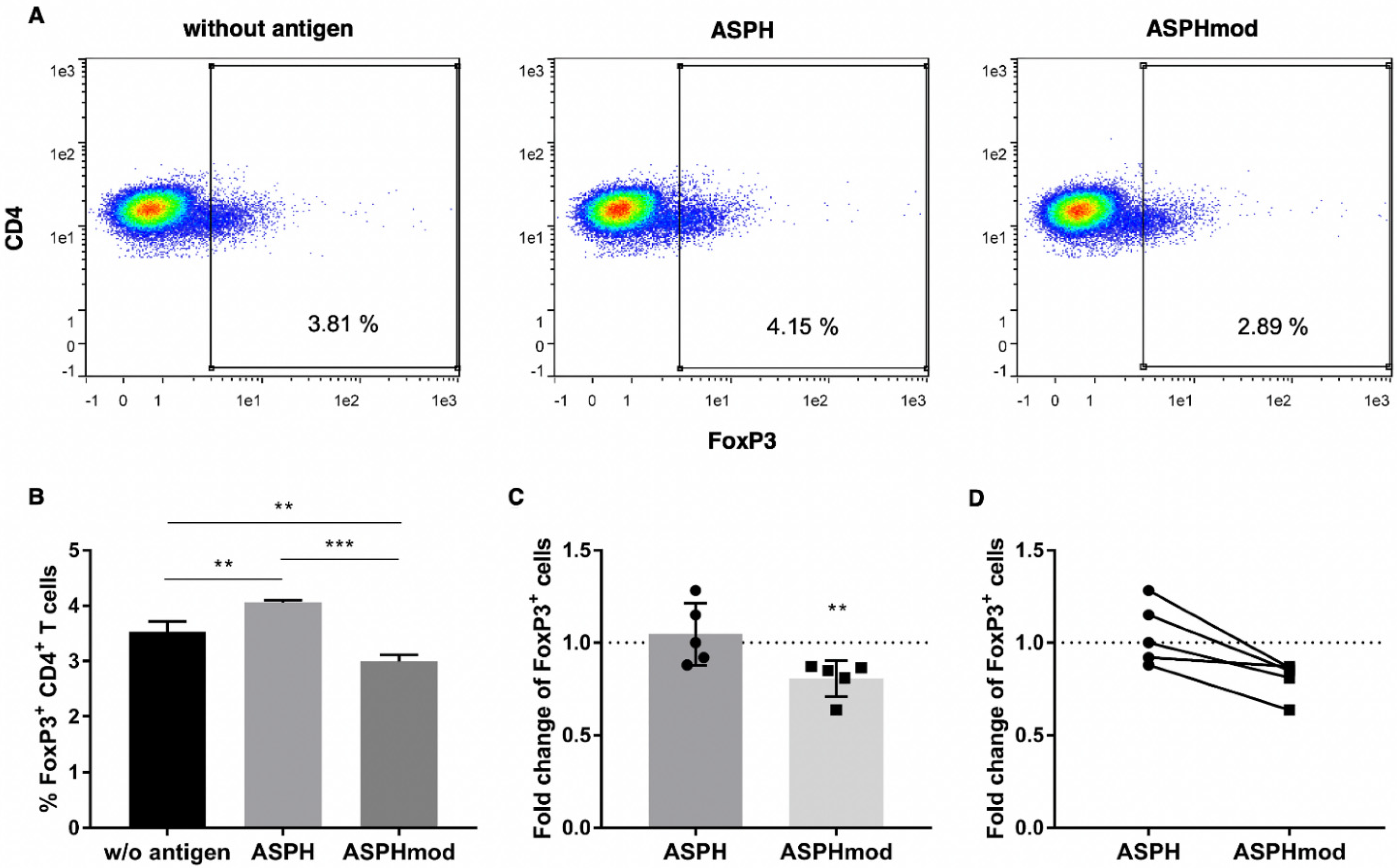
2.3. Increased ASPHmod Immunogenicity Correlates Inversely with Treg Cell Number
3. Discussion
4. Materials and Methods
4.1. Recombinant ASPH and Modified ASPH (ASPHmod)
4.2. Ex Vivo, Human Lymphoid Tissue-Equivalent Culture System
4.3. Intracellular Cytokine Staining (ICCS) and Flow Cytometric Analysis
4.4. T Cell Proliferation
4.5. Cytometric Bead Array
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Jia, S.; VanDusen, W.; Diehl, R.; Kohl, N.; Dixon, R.; Elliston, K.; Stern, A.; Friedman, P. cDNA cloning and expression of bovine aspartyl (asparaginyl) beta-hydroxylase. J. Biol. Chem. 1992, 267, 14322–14327. [Google Scholar] [CrossRef]
- Lavaissiere, L.; Jia, S.; Nishiyama, M.; De La Monte, S.; Stern, A.; Wands, J.R.; A Friedman, P. Overexpression of human aspartyl(asparaginyl)beta-hydroxylase in hepatocellular carcinoma and cholangiocarcinoma. J. Clin. Investig. 1996, 98, 1313–1323. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, X.; Hu, J.; Bai, B.; Zhu, H. Diverse molecular functions of aspartate β-hydroxylase in cancer (Review). Oncol. Rep. 2020, 44, 2364–2372. [Google Scholar] [CrossRef]
- Yang, H.; Song, K.; Xue, T.; Xue, X.-P.; Huyan, T.; Wang, W.; Wang, H. The distribution and expression profiles of human Aspartyl/Asparaginyl beta-hydroxylase in tumor cell lines and human tissues. Oncol. Rep. 2010, 24, 1257–1264. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Tamaki, S.; Cantarini, M.C.; Ince, N.; Wiedmann, M.; Carter, J.J.; Lahousse, S.A.; Califano, S.; Maeda, T.; Ueno, T.; et al. Aspartyl-(asparaginyl)-β-hydroxylase regulates hepatocellular carcinoma invasiveness. J. Hepatol. 2006, 44, 971–983. [Google Scholar] [CrossRef]
- Wang, K.; Liu, J.; Yan, Z.-L.; Li, J.; Shi, L.-H.; Cong, W.-M.; Xia, Y.; Zou, Q.-F.; Xi, T.; Shen, F.; et al. Overexpression of aspartyl-(asparaginyl)-β-hydroxylase in hepatocellular carcinoma is associated with worse surgical outcome. Hepatology 2010, 52, 164–173. [Google Scholar] [CrossRef]
- Aihara, A.; Huang, C.-K.; Olsen, M.J.; Lin, Q.; Chung, W.; Tang, Q.; Dong, X.; Wands, J.R. A cell-surface β-hydroxylase is a biomarker and therapeutic target for hepatocellular carcinoma. Hepatology 2014, 60, 1302–1313. [Google Scholar] [CrossRef]
- Shimoda, M.; Tomimaru, Y.; Charpentier, K.P.; Safran, H.; Carlson, R.I.; Wands, J. Tumor progression-related transmembrane protein aspartate-β-hydroxylase is a target for immunotherapy of hepatocellular carcinoma. J. Hepatol. 2012, 56, 1129–1135. [Google Scholar] [CrossRef]
- Iwagami, Y.; Casulli, S.; Nagaoka, K.; Kim, M.; Carlson, R.I.; Ogawa, K.; Lebowitz, M.S.; Fuller, S.; Biswas, B.; Stewart, S.; et al. Lambda phage-based vaccine induces antitumor immunity in hepatocellular carcinoma. Heliyon 2017, 3, e00407. [Google Scholar] [CrossRef]
- A Lugade, A.; Kalathil, S.; Miller, A.; Iyer, R.; Thanavala, Y. High immunosuppressive burden in advanced hepatocellular carcinoma patients. OncoImmunology 2013, 2, e24679. [Google Scholar] [CrossRef]
- Ruf, B.; Heinrich, B.; Greten, T.F. Immunobiology and immunotherapy of HCC: Spotlight on innate and innate-like immune cells. Cell Mol. Immunol. 2020, 18, 112–127. [Google Scholar] [CrossRef]
- Kalathil, S.; Lugade, A.A.; Miller, A.; Iyer, R.; Thanavala, Y. Higher Frequencies of GARP+CTLA-4+Foxp3+ T Regulatory Cells and Myeloid-Derived Suppressor Cells in Hepatocellular Carcinoma Patients Are Associated with Impaired T-Cell Functionality. Cancer Res. 2013, 73, 2435–2444. [Google Scholar] [CrossRef]
- Self, A.A.; Losikoff, P.T.; Gregory, S.H. Divergent contributions of regulatory T cells to the pathogenesis of chronic hepatitis C. Hum. Vaccines Immunother. 2013, 9, 1569–1576. [Google Scholar] [CrossRef][Green Version]
- Tomimaru, Y.; Mishra, S.; Safran, H.; Charpentier, K.P.; Martin, W.; De Groot, A.S.; Gregory, S.H.; Wands, J.R. Aspartate-β-hydroxylase induces epitope-specific T cell responses in hepatocellular carcinoma. Vaccine 2015, 33, 1256–1266. [Google Scholar] [CrossRef]
- Moise, L.; Gutierrez, A.H.; Bailey-Kellogg, C.; Terry, F.; Leng, Q.; Hady, K.M.A.; VerBerkmoes, N.C.; Sztein, M.B.; Losikoff, P.T.; Martin, W.D.; et al. The two-faced T cell epitope: Examining the host-microbe interface with JanusMatrix. Hum. Vaccines Immunother. 2013, 9, 1577–1586. [Google Scholar] [CrossRef]
- Moser, J.M.; Sassano, E.R.; Leistritz, D.C.; Eatrides, J.M.; Phogat, S.; Koff, W.; Drake, D.R. Optimization of a dendritic cell-based assay for the in vitro priming of naïve human CD4+ T cells. J. Immunol. Methods 2010, 353, 8–19. [Google Scholar] [CrossRef]
- Unitt, E.; Marshall, A.; Gelson, W.; Rushbrook, S.M.; Davies, S.; Vowler, S.L.; Morris, L.S.; Coleman, N.; Alexander, G.J. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J. Hepatol. 2006, 45, 246–253. [Google Scholar] [CrossRef]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Gravante, G.; Sconocchia, G.; Ong, S.L.; Dennison, A.; Lloyd, D.M. Immunoregulatory effects of liver ablation therapies for the treatment of primary and metastatic liver malignancies. Liver Int. 2009, 29, 18–24. [Google Scholar] [CrossRef]
- Guo, C.; Manjili, M.H.; Subjeck, J.R.; Sarkar, D.; Fisher, P.B.; Wang, X.-Y. Therapeutic cancer vaccines: Past, present, and future. Adv. Cancer Res. 2013, 119, 421–475. [Google Scholar] [CrossRef]
- Melief, C.J.; van Hall, T.; Arens, R.; Ossendorp, F.; Van Der Burg, S.H. Therapeutic cancer vaccines. J. Clin. Investig. 2015, 125, 3401–3412. [Google Scholar] [CrossRef]
- Baxevanis, C.N.; Perez, S.A. Cancer vaccines: Limited success but the research should remain viable. Expert Rev. Vaccines 2016, 15, 677–680. [Google Scholar] [CrossRef][Green Version]
- Buonaguro, L.; Petrizzo, A.; Tagliamonte, M.; Tornesello, M.L.; Buonaguro, F.M. Challenges in cancer vaccine development for hepatocellular carcinoma. J. Hepatol. 2013, 59, 897–903. [Google Scholar] [CrossRef]
- Zarour, H.M. Reversing T-cell Dysfunction and Exhaustion in Cancer. Clin. Cancer Res. 2016, 22, 1856–1864. [Google Scholar] [CrossRef]
- De Groot, A.S.; Moise, L.; Terry, F.; Gutierrez, A.; Hindocha, P.; Richard, G.; Hoft, D.F.; Ross, T.M.; Noe, A.R.; Takahashi, Y.; et al. Better Epitope Discovery, Precision Immune Engineering, and Accelerated Vaccine Design Using Immunoinformatics Tools. Front. Immunol. 2020, 11, 442. [Google Scholar] [CrossRef]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef]
- Nordquist, L.T.; Shore, N.D.; Elist, J.J.; Oliver, J.C.; Gannon, W.; Shahlaee, A.H.; Fuller, S.A.; Ghanbari, H.A. Phase 1 open-label trial to evaluate the safety and immunogenicity of PAN-301-1, a novel nanoparticle therapeutic vaccine, in patients with biochemically relapsed prostate cancer. J. Clin. Oncol. 2018, 36, e15166. [Google Scholar] [CrossRef]
- Algazi, A.P.; Shah, D.; Smith, W.; Panella, T.J.; Shin, D.M.; Bruce, J.Y.; Melhem, R.; Campbell, J.S.; Abell, L.; Fjaellskog, M.-L.; et al. Update on safety and efficacy of a phase 1/2 of SNS-301 added to pembrolizumab in patients with advanced squamous cell carcinoma of the head and neck (SCCHN). J. Clin. Oncol. 2021, 39, 6029. [Google Scholar] [CrossRef]
- Kanwal, M.; Smahel, M.; Olsen, M.; Smahelova, J.; Tachezy, R. Aspartate β-hydroxylase as a target for cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 163. [Google Scholar] [CrossRef]
- Gehring, S.; Gregory, S.H.; Wintermeyer, P.; Martin, M.S.; Aloman, C.; Wands, J.R. Generation and characterization of an immunogenic dendritic cell population. J. Immunol. Methods 2008, 332, 18–30. [Google Scholar] [CrossRef]
- Fichter, M.; Baier, G.; Dedters, M.; Pretsch, L.; Pietrzak-Nguyen, A.; Landfester, K.; Gehring, S. Nanocapsules generated out of a polymeric dexamethasone shell suppress the inflammatory response of liver macrophages. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1223–1234. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wirsching, S.; Fichter, M.; Cacicedo, M.L.; Landfester, K.; Gehring, S. Modification of Regulatory T Cell Epitopes Promotes Effector T Cell Responses to Aspartyl/Asparaginyl β-Hydroxylase. Int. J. Mol. Sci. 2022, 23, 12444. https://doi.org/10.3390/ijms232012444
Wirsching S, Fichter M, Cacicedo ML, Landfester K, Gehring S. Modification of Regulatory T Cell Epitopes Promotes Effector T Cell Responses to Aspartyl/Asparaginyl β-Hydroxylase. International Journal of Molecular Sciences. 2022; 23(20):12444. https://doi.org/10.3390/ijms232012444
Chicago/Turabian StyleWirsching, Sebastian, Michael Fichter, Maximiliano L. Cacicedo, Katharina Landfester, and Stephan Gehring. 2022. "Modification of Regulatory T Cell Epitopes Promotes Effector T Cell Responses to Aspartyl/Asparaginyl β-Hydroxylase" International Journal of Molecular Sciences 23, no. 20: 12444. https://doi.org/10.3390/ijms232012444
APA StyleWirsching, S., Fichter, M., Cacicedo, M. L., Landfester, K., & Gehring, S. (2022). Modification of Regulatory T Cell Epitopes Promotes Effector T Cell Responses to Aspartyl/Asparaginyl β-Hydroxylase. International Journal of Molecular Sciences, 23(20), 12444. https://doi.org/10.3390/ijms232012444





