PUF-Immobilized Bjerkandera adusta DSM 3375 as a Tool for Bioremediation of Creosote Oil Contaminated Soil
Abstract
1. Introduction
2. Results
2.1. Immobilization of the Mycelium of B. adusta DSM 3375 in a Polyurethane Carrier
2.2. Creosote Removal Using PUF-Immobilized B. adusta DSM 3375 Cells
3. Discussion
4. Materials and Methods
4.1. Microorganism
4.2. Materials
4.3. Immobilization of B. adusta DSM 3375 Mycelium in a Polyurethane Carrier
4.4. Bioremediation of Soil Contaminated with Creosote Oil
4.5. UV-VIS Spectroscopy
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jurys, A.; Gailiūtė, I.; Aikaitė-Stanaitienė, J.; Grigiškis, S.; Maruška, A.; Stankevičius, M.; Levišauskas, D. Review of Creosote Pollution Toxicity and Possibilities of Bioremediation. Environ. Technol. Resour. Proc. Int. Sci. Pract. Conf. 2013, 1, 33–38. [Google Scholar] [CrossRef][Green Version]
- Smułek, W.; Sydow, W.; Zabielska-Matejuk, J.; Kaczorek, E. Bacteria involved in biodegradation of creosote PAH—A case study of long-term contaminated industrial area. Ecotoxicol. Environ. Saf. 2020, 187, 109843. [Google Scholar] [CrossRef] [PubMed]
- Khademibami, L.; Bobadilha, G.S. Recent Developments Studies on Wood Protection Research in Academia: A Review. Front. For. Glob. Chang. 2022, 5, 793177. [Google Scholar] [CrossRef]
- Commission Directive 2011/71/EU of 26 July 2011, Amending Directive 98/8/EC of the European Parliament and of the Council to Include Creosote as an Active Substance in Annex I Thereto, OJ L195/46. 27 July 2011. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX:32011L0071 (accessed on 3 October 2022).
- Website of the European Chemicals Agency. ECHA/NR/20/39. Available online: https://echa.europa.eu/-/creosote-approval-with-more-stringent-conditions-proposed (accessed on 3 October 2022).
- Aranha, R.M.; Magalhães, V.M.A.; Mendes, G.P.; Soares, L.C.R.; Muselli Barbosa, A.; Nascimento, C.A.O.; Vianna, M.M.G.R.; Chiavone-Filho, O. Characterization and Partitioning Behavior of Creosote in Different Matrices: Soil, Water, and Air. Water Air Soil Pollut. 2020, 231, 402. [Google Scholar] [CrossRef]
- Atagana, H.I. Bioremediation of creosote-contaminated soil in South Africa by landfarming. J. Appl. Microbiol. 2004, 96, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Marchut-Mikołajczyk, O.; Kwapisz, E.; Wieczorek, D.; Antczak, T. Biodegradation of diesel oil hydrocarbons enhanced with Mucor circinelloides enzyme preparation. Int. Biodeterior. Biodegrad. 2015, 104, 142–148. [Google Scholar] [CrossRef]
- Adams, G.O.; Fufeyin, P.T.; Okoro, S.E.; Ehinomen, I. Bioremediation, Biostimulation and Bioaugumention: A Review. Int. J. Environ. Bioremediat. Biodegrad. 2015, 3, 28–39. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Chaudhary, D.K. Molecular tools in petroleum hydrocarbon degradation: An overview. BAOJ Biotechnol. 2016, 2, 018. [Google Scholar]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Reyes-César, A.L.; Absalón, Á.E.; Fernández, F.J.; González, J.M.; Cortés-Espinosa, D.V. Biodegradation of a mixture of PAHs by non-ligninolytic fungal strains isolated from crude oil-contaminated soil. World. J. Microbiol. Biotechnol. 2014, 30, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Robak, M.; Boruczkowski, T.; Drozdz, W.; Lazar, Z.; Baranowska, M.; Przado, D. Application of the yeasts Yarrowia lipolytica for in-situ bioremediation of soil contaminated with creosote oil—A case study. Ochr. Srodowiska 2011, 33, 27–33. (In Polish) [Google Scholar]
- Kołwzan, B.; Adamiak, W.; Dziubek, A.M. Możliwości zastosowania grzybów w technologiach oczyszczania i remediacji wybranych elementów środowiska. Ochr. Srodowiska 2018, 40, 3–20. (In Polish) [Google Scholar]
- Valentín, L.; Lu-Chau, T.A.; López, C.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Biodegradation of dibenzothiophene, fluoranthene, pyrene and chrysene in a soil slurry reactor by the white-rot fungus Bjerkandera sp. BOS55. Process Biochem. 2007, 42, 641–648. [Google Scholar] [CrossRef]
- Di Gregorio, S.; Becarelli, S.; Siracusa, G.; Ruffini Castiglione, M.; Petroni, G.; Masini, G.; Gentini, A.; De Lima e Silva, M.R.; Lorenzi, R. Pleurotus ostreatus spent mushroom substrate for the degradation of polycyclic aromatic hydrocarbons: The case study of a pilot dynamic biopile for the decontamination of a historically contaminated soil. J. Chem. Technol. Biotechnol. 2016, 91, 1654–1664. [Google Scholar] [CrossRef]
- Lee, A.H.; Lee, H.; Heo, Y.M.; Lim, Y.W.; Kim, C.-M.; Kim, G.-H.; Chang, W.; Kim, J.-J. A proposed stepwise screening framework for the selection of polycyclic aromatic hydrocarbon (PAH)-degrading white rot fungi. Bioprocess Biosyst. Eng. 2020, 43, 767–783. [Google Scholar] [CrossRef] [PubMed]
- Vipotnik, Z.; Michelin, M.; Tavares, T. Development of a packed bed reactor for the removal of aromatic hydrocarbons from soil using laccase/mediator feeding system. Microbiol. Res. 2021, 245, 126687. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-S.; Seo, J.-Y.; Lee, H.; Yoo, J.; Jung, J.; Kim, J.-J.; Kim, G.-H. Decolorization and Detoxification of Wastewater Containing Industrial Dyes by Bjerkandera adusta KUC9065. Water Air Soil Pollut. 2014, 225, 1801. [Google Scholar] [CrossRef]
- Zhang, J.; Chi, Y.; Feng, L. The mechanism of degradation of alizarin red by a white-rot fungus Trametes gibbosa. BMC Biotechnol. 2021, 21, 64. [Google Scholar] [CrossRef]
- Mayans, B.; Camacho-Arévalo, R.; García-Delgado, C.; Alcántara, C.; Nägele, N.; Antón-Herrero, R.; Escolástico, C.; Eymar, E. Mycoremediation of Soils Polluted with Trichloroethylene: First Evidence of Pleurotus Genus Effectiveness. Appl. Sci. 2021, 11, 1354. [Google Scholar] [CrossRef]
- Gouma, S.; Papadaki, A.A.; Markakis, G.; Magan, N.; Goumas, D. Studies on Pesticides Mixture Degradation by White Rot Fungi. Ecol. Eng. 2019, 20, 16–26. [Google Scholar] [CrossRef]
- Koroleva, O.V.; Zherdev, A.V.; Kulikova, N.A. The Role of White-rot Fungi in Herbicide Transformation. In Herbicides, Physiology of Action, and Safety; Price, A., Kelton, J., Sarunaite, L., Eds.; IntechOpen: London, UK, 2015; pp. 187–221. [Google Scholar] [CrossRef]
- Yadav, A.; Rene, E.R.; Kanti Mandal, M.; Kumar Dubey, K. Biodegradation of cyclophosphamide and etoposide by white rot fungi and their degradation kinetics. Bioresour. Technol. 2022, 346, 126355. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yun, S.Y.; Jang, S.; Kim, G.-H.; Kim, J.-J. Bioremediation of Polycyclic Aromatic Hydrocarbons in Creosote-Contaminated Soil by Peniophora incarnata KUC8836. Bioremediat. J. 2015, 19, 1–8. [Google Scholar] [CrossRef]
- Atagana, H.I.; Haynes, R.J.; Wallis, M. Fungal Bioremediation of Creosote-Contaminated Soil: A Laboratory Scale Bioremediation Study Using Indigenous Soil Fungi. Water Air Soil Pollut. 2006, 172, 201–219. [Google Scholar] [CrossRef]
- Eggen, T.; Majcherczyk, A. Removal of polycyclic aromatic hydrocarbons (PAH) in contaminated soil by white rot fungus Pleurotus ostreatus. Int. Biodeterior. Biodegrad. 1998, 41, 111–117. [Google Scholar] [CrossRef]
- Eggen, T. Application of fungal substrate from commercial mushroom production—Pleuorotus ostreatus—For bioremediation of creosote contaminated soil. Int. Biodeterior. Biodegrad. 1999, 44, 117–126. [Google Scholar] [CrossRef]
- Lamar, R.T.; White, R.B. Evaluation of white-rot fungi for the remediation of creosote-contaminated soil. Remediation 2002, 12, 97–106. [Google Scholar] [CrossRef]
- Zabielska-Matejuk, J.; Stangierska, A.; Kropacz, A. Research on the effectiveness of selected species of white-rot fungi and bacteria in the biodegradation of creosote oil. Ann. WULS-SGGW For. Wood Technol. 2017, 100, 48–53. [Google Scholar]
- Nakamura, Y.; Sungusia, M.G.; Sawada, T.; Kuwahara, M. Lignin-degrading enzyme production by Bjerkandera adusta immobilized on polyurethane foam. J. Biosci. Bioeng. 1999, 88, 41–47. [Google Scholar] [CrossRef]
- Mtui, G.; Nakamura, Y. Continuous production of lignin-degrading enzymes by Bjerkandera adusta immobilized on polyurethane foam. Biotechnol. Lett. 2002, 24, 1743–1747. [Google Scholar] [CrossRef]
- Bardi, A.; Yuan, Q.; Tigini, V.; Spina, F.; Varese, G.C.; Spennati, F.; Becarelli, S.; Di Gregorio, S.; Petroni, G.; Munz, G. Recalcitrant Compounds Removal in Raw Leachate and Synthetic Effluents Using the White-Rot Fungus Bjerkandera adusta. Water 2017, 9, 824. [Google Scholar] [CrossRef]
- Lapadatescu, C.; Feron, G.; Vergoignan, C.; Djian, A.; Durand, A.; Bonnarme, P. Influence of cell immobilization on the production of benzaldehyde and benzyl alcohol by the white-rot fungi Bjerkandera adusta, Ischnoderma benzoinum and Dichomitus squalens. Appl. Microbiol. Biotechnol. 1997, 47, 708–714. [Google Scholar] [CrossRef]
- Tripathi, A.; Upadhyay, R.C.; Singh, S. Extracellular Ligninolytic Enzymes in Bjerkandera adusta and Lentinus squarrosulus. Indian J. Microbiol. 2012, 52, 381–387. [Google Scholar] [CrossRef]
- Andriani, A.; Tachibana, S. Lignocellulosic materials as solid support agents for Bjerkandera adusta SM46 to enhance polycyclic aromatic hydrocarbon degradation on sea sand and sea water media. Biocatal. Agric. Biotechnol. 2016, 8, 310–320. [Google Scholar] [CrossRef]
- Marchut-Mikołajczyk, O.; Drożdżyński, P.; Struszczyk-Świta, K. Biodegradation of slop oil by endophytic Bacillus cereus EN18 coupled with lipase from Rhizomucor miehei (Palatase®). Chemosphere 2020, 250, 126203. [Google Scholar] [CrossRef] [PubMed]
- Polewczyk, A.; Marchut-Mikołajczyk, O.; Drożdżyński, P.; Domański, J.; Śmigielski, K. Effects of ozonized rapeseed oil on bioremediation of diesel oil contaminated soil by Bacillus mycoides NS1020. Bioremediat. J. 2020, 24, 204–213. [Google Scholar] [CrossRef]
- Cavalcanti, T.G.; de Souza, A.F.; Ferreira, G.F.; Dias, D.S.B.; Severino, L.S.; Morais, J.P.S.; de Sousa, K.A.; Vasconcelos, U. Use of Agro-Industrial Waste in the Removal of Phenanthrene and Pyrene by Microbial Consortia in Soil. Waste Biomass Valor 2019, 10, 205–214. [Google Scholar] [CrossRef]
- Okere, U.V.; Semple, K.T. Biodegradation of PAHs in ‘Pristine’ Soils from Different Climatic Regions. J. Bioremed. Biodegrad. 2012, S1, 006. [Google Scholar] [CrossRef]
- Verdin, A.; Sahraoui, A.L.-H.; Durand, R. Degradation of benzo[a]pyrene by mitosporic fungi and extracellular oxidative enzymes. Int. Biodeterior. Biodegrad. 2004, 53, 65–70. [Google Scholar] [CrossRef]
- Ghosal, D.; Ghosh, D.; Dutta, T.K.; Ahn, Y. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 31, 1369. [Google Scholar] [CrossRef] [PubMed]
- Abo-State, M.A.M.; Osman, M.E.; Khattabe, O.H.; El-Kelani, T.A.; Abdel-Rahman, Z.M. Degradative pathways of polycyclic aromatic hydrocarbons (PAHs) by Phanerochaete chrysosporium under optimum conditions. JRRAS 2021, 14, 507–520. [Google Scholar] [CrossRef]
- Lamichhane, S.; Krishna, K.C.B.; Sarukkalige, R. Surfactant-enhanced remediation of polycyclic aromatic hydrocarbons: A review. J. Environ. Manag. 2017, 199, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Marchut-Mikołajczyk, O.; Drożdżyński, P.; Pietrzyk, D.; Antczak, T. Biosurfactant production and hydrocarbon degradation activity of endophytic bacteria isolated from Chelidonium majus L. Microb. Cell Fact. 2018, 17, 171. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-G. Comparison of pyrene biodegradation by white rot fungi. World J. Microbiol. Biotechnol. 1999, 15, 669–672. [Google Scholar] [CrossRef]
- Byss, M.; Elhottová, D.; Tlíska, J.; Baldrian, P. Fungal Bioremediation of the Creosote-Contaminated Soil: Influence of Pleurotus ostreatus and Irpex lacteus on Polycyclic Aromatic Hydrocarbons Removal and Soil Microbial Community Composition in the Laboratory-Scale Study. Chemosphere 2008, 73, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- PN-R-04032; Soil and Mineral Deposits—Sampling and Determination of Soil Grain Size Distribution. Polish Committee for Standardizations: Warsaw, Poland, 1998.
- PN-ISO 11465; Soil Quality—Determination of Soil Dry Matter and Soil Water Based on Soil Dry Matter—Weight Method. Polish Committee for Standardizations: Warsaw, Poland, 1999.
- PN-EN ISO 15309; Characterization of Waste and Soil—Determination of Elemental Composition by X-ray Fluorescence. Polish Committee for Standardizations: Warsaw, Poland, 2010.
- PN-R-04028; Chemical-Agricultural Analysis of Soil. The Method of Sampling and Determination of the Content of Nitrate and Ammonium Ions in Mineral Soils. Polish Committee for Standardizations: Warsaw, Poland, 1997.
- PN-EN ISO 10390; Soil, Treated Biowaste and Sludge—Determination of pH. Polish Committee for Standardizations: Warsaw, Poland, 2022.
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Han, M.-J.; Chai, H.-T.; Song, H.-G. Purification and characterization of laccase from the white-rot fungus Trametes versicolour. J. Microbiol. 2005, 46, 555–560. [Google Scholar]
- Kaneko, R.; Iimori, T.; Yoshikawa, H.; Machioa, M.; Yoshioka, H.; Murakami, K. A Possible Role of Manganese Peroxidase during Biobleaching by the Pulp Bleaching Fungus SKB-1152. Biosci. Biotechnol. Biochem. 1994, 58, 1517–1518. [Google Scholar] [CrossRef][Green Version]
- Tien, M.; Kirk, T.K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc. Natl. Acad. Sci. USA. 1984, 81, 2280–2284. [Google Scholar] [CrossRef]
- Szczęsna-Antczak, M.; Kaczorowska, A.; Kaczorowski, W.; Antczak, T. Biomodification and biodeterioration of carbon coatings by fungal strains. Int. Biodeterior. Biodegrad. 2014, 88, 106–117. [Google Scholar] [CrossRef]

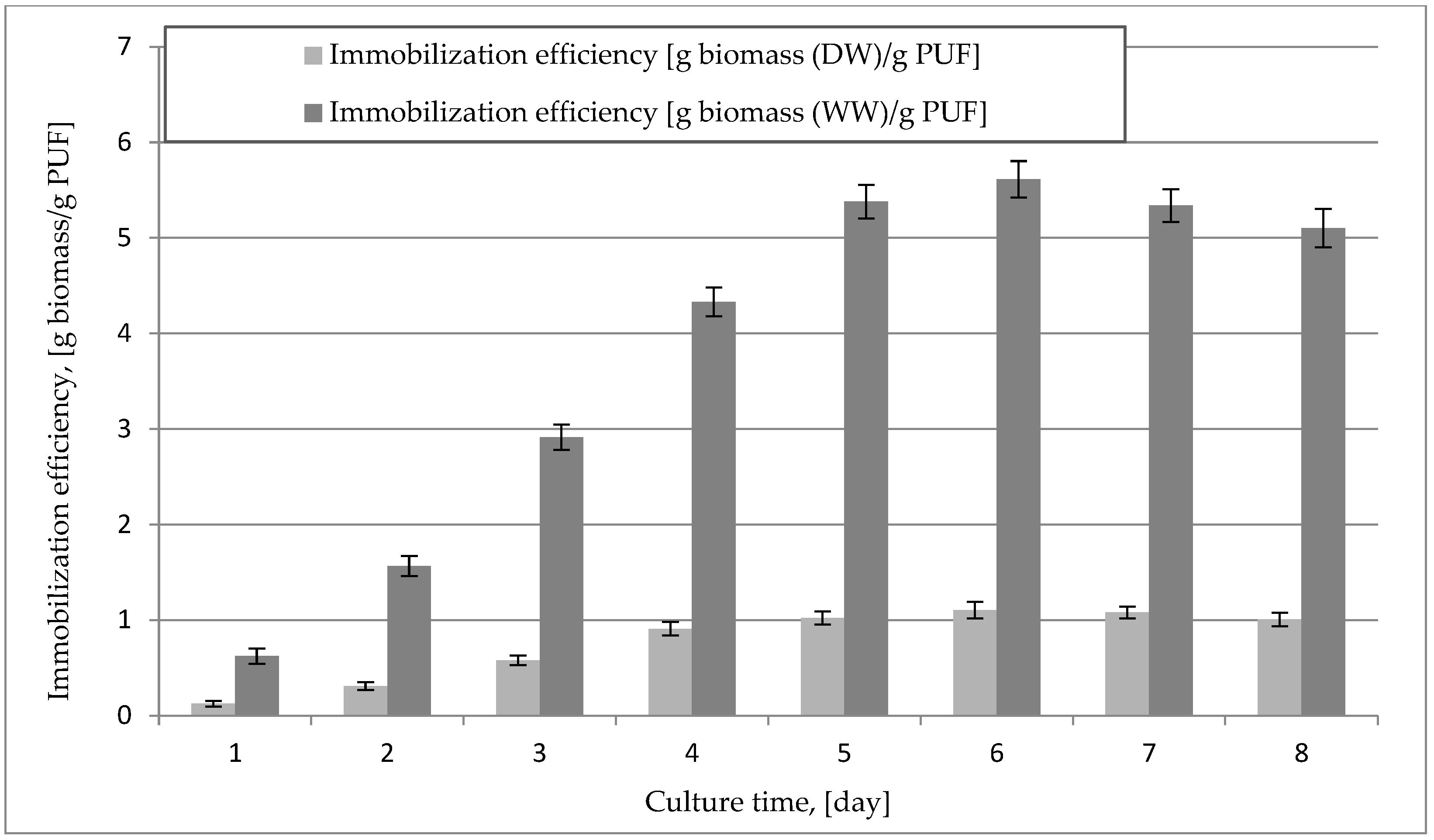
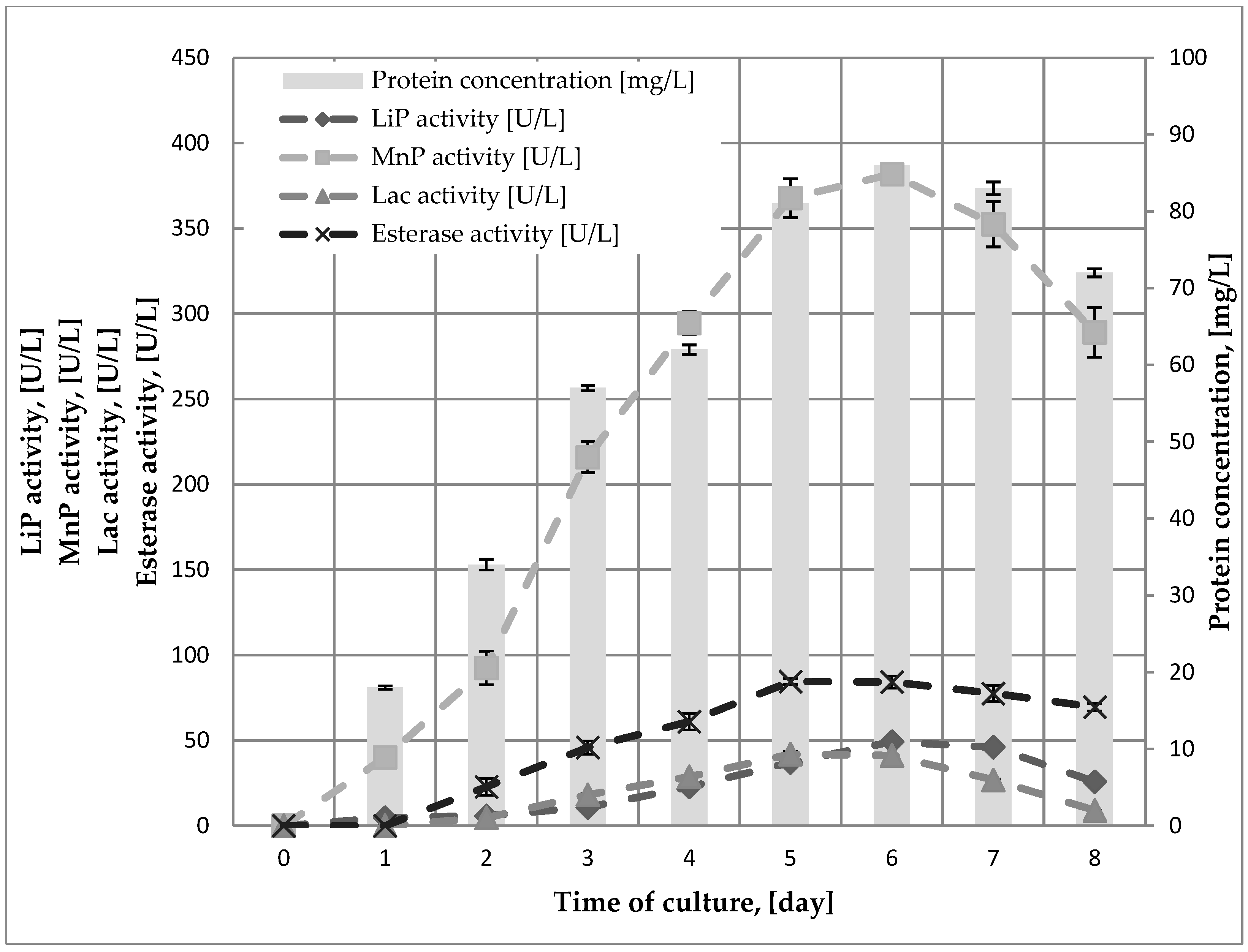
| Bioremediation Time [Week] | Mass of PUF-Immobilized Mycelium [g DW/kg of Soil] | Mass of Mycelium [g DW/kg of Soil] | Loss of Creosote Oil [%] * |
|---|---|---|---|
| 5 | 3.898 ± 0.101 | 1.997 ± 0.088 | 26.10 ± 1.50 |
| Control sample—PUF without mycelium (1.911 ± 0.03 g/kg of soil) | 6.70 ± 0.50 | ||
| 10 | 3.698 ± 0.104 | 1.922 ± 0.042 | 39.03 ± 0.98 |
| Control sample—PUF without mycelium (1.784 ± 0.07 g/kg of soil) | 8.78 ± 0.62 | ||
| 15 | 3.944 ± 0.080 | 2.036 ± 0.051 | 47.88 ± 1.27 |
| Control sample—PUF without mycelium (1.917 ± 0.04 g/kg of soil) | 12.53 ± 0.95 | ||
| PAH | Absorption Maximum [nm] | Bioremediation Time [Weeks] | |||
|---|---|---|---|---|---|
| Sample | 5 | 10 | 15 | ||
| Polyaromatic Hydrocarbons Loss [%] * | |||||
| Fluoranthene | PUF with mycelium | 302 | 65.628 ± 0.39 | 73.185 ± 2.10 | 79.309 ± 1.24 |
| PUF without mycelium | 2.730 ± 0.13 | 4.508 ± 0.18 | 7.807 ± 0.63 | ||
| Fluorene | PUF with mycelium | 299 | 58.268 ± 1.69 | 66.564 ± 2.18 | 71.738 ± 2.51 |
| PUF without mycelium | 3.175 ± 0.17 | 5.158 ± 0.23 | 6.685 ± 0.40 | ||
| Pyrene | PUF with mycelium | 297 | 58.297 ± 1.80 | 65.351 ± 1.92 | 72.745 ± 2.36 |
| PUF without mycelium | 2.968 ± 0.97 | 4.581 ± 0.65 | 8.589 ± 1.53 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Struszczyk-Świta, K.; Drożdżyński, P.; Murawska, K.; Marchut-Mikołajczyk, O. PUF-Immobilized Bjerkandera adusta DSM 3375 as a Tool for Bioremediation of Creosote Oil Contaminated Soil. Int. J. Mol. Sci. 2022, 23, 12441. https://doi.org/10.3390/ijms232012441
Struszczyk-Świta K, Drożdżyński P, Murawska K, Marchut-Mikołajczyk O. PUF-Immobilized Bjerkandera adusta DSM 3375 as a Tool for Bioremediation of Creosote Oil Contaminated Soil. International Journal of Molecular Sciences. 2022; 23(20):12441. https://doi.org/10.3390/ijms232012441
Chicago/Turabian StyleStruszczyk-Świta, Katarzyna, Piotr Drożdżyński, Karolina Murawska, and Olga Marchut-Mikołajczyk. 2022. "PUF-Immobilized Bjerkandera adusta DSM 3375 as a Tool for Bioremediation of Creosote Oil Contaminated Soil" International Journal of Molecular Sciences 23, no. 20: 12441. https://doi.org/10.3390/ijms232012441
APA StyleStruszczyk-Świta, K., Drożdżyński, P., Murawska, K., & Marchut-Mikołajczyk, O. (2022). PUF-Immobilized Bjerkandera adusta DSM 3375 as a Tool for Bioremediation of Creosote Oil Contaminated Soil. International Journal of Molecular Sciences, 23(20), 12441. https://doi.org/10.3390/ijms232012441






