Biodegradation and Metabolic Pathway of the Neonicotinoid Insecticide Thiamethoxam by Labrys portucalensis F11
Abstract
1. Introduction
2. Results
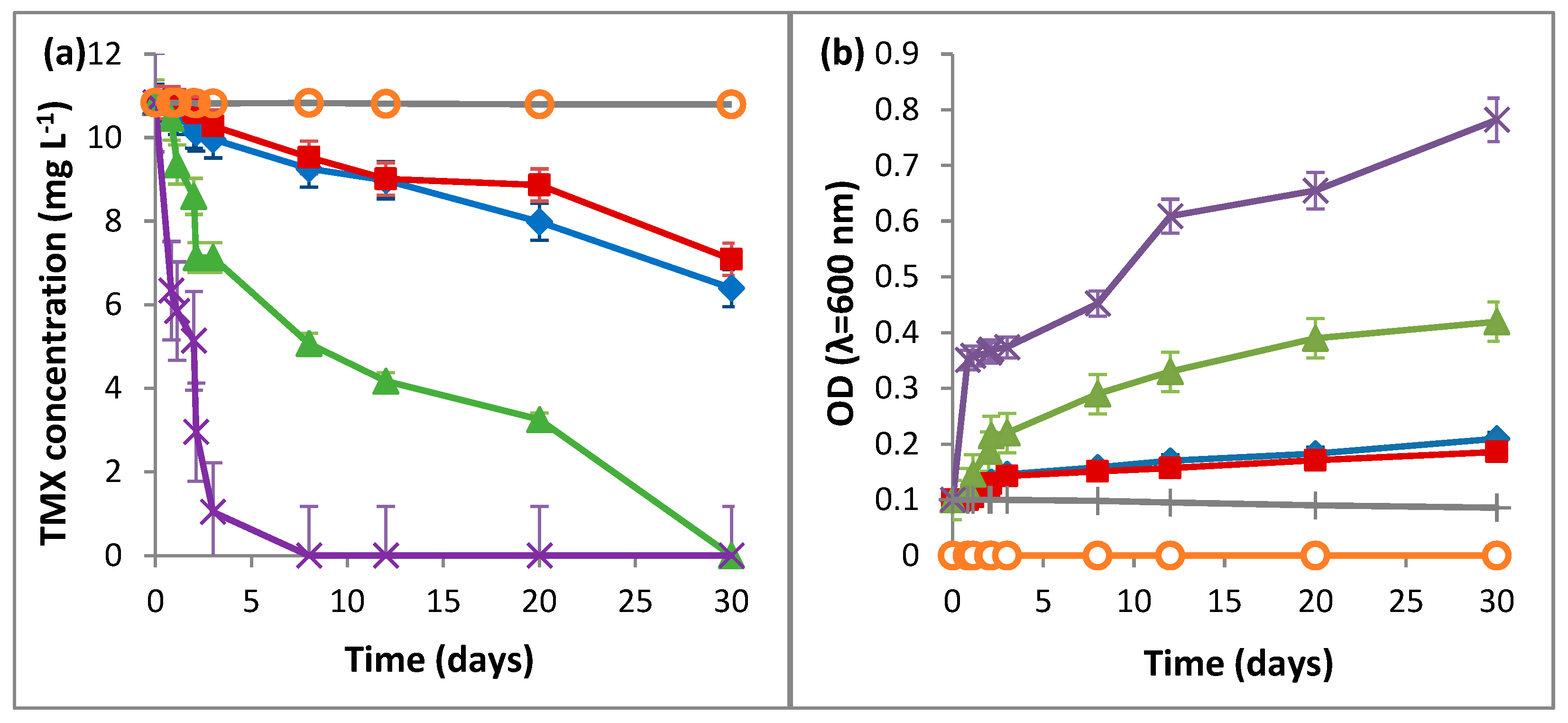
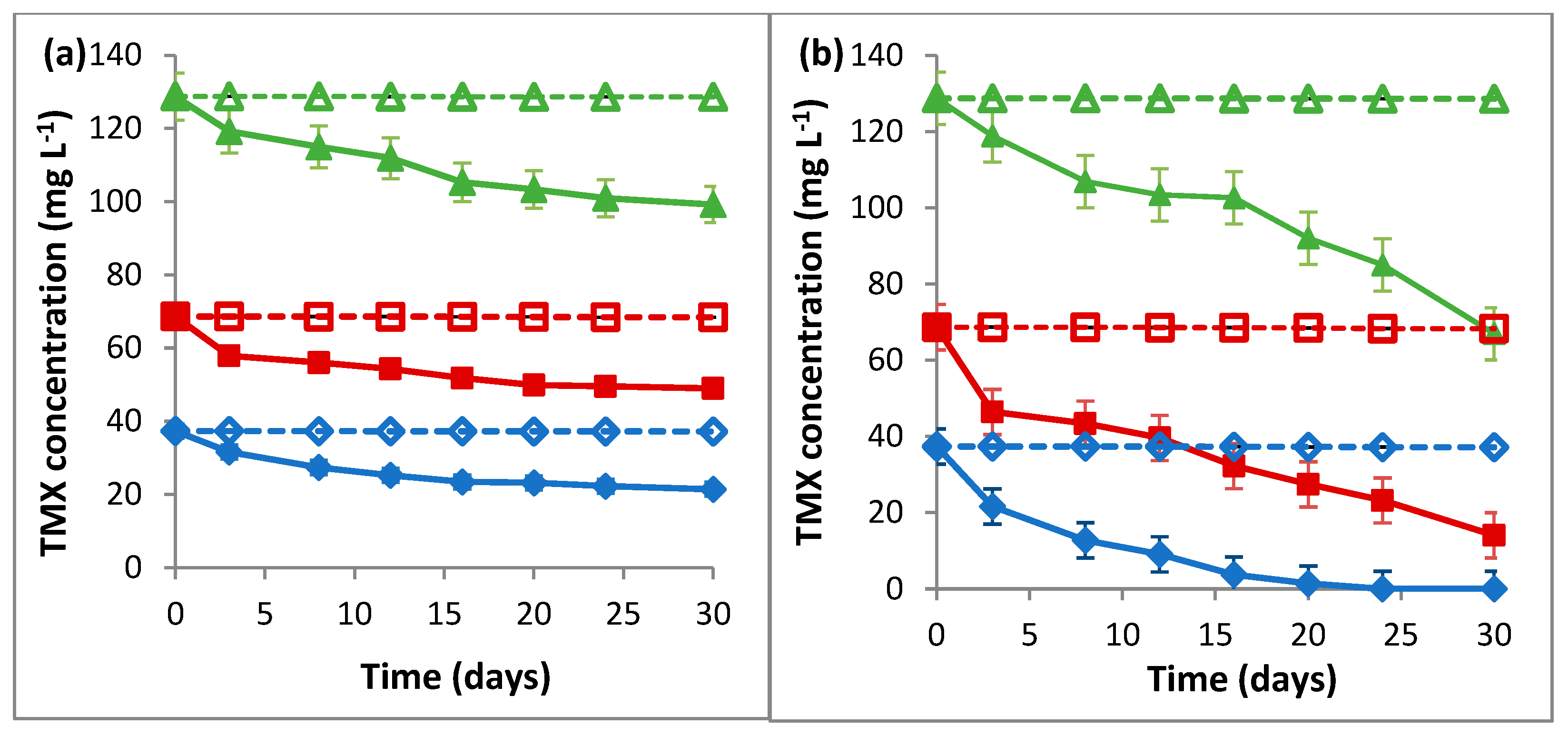
2.1. Biodegradation of TMX by L. portucalensis F11
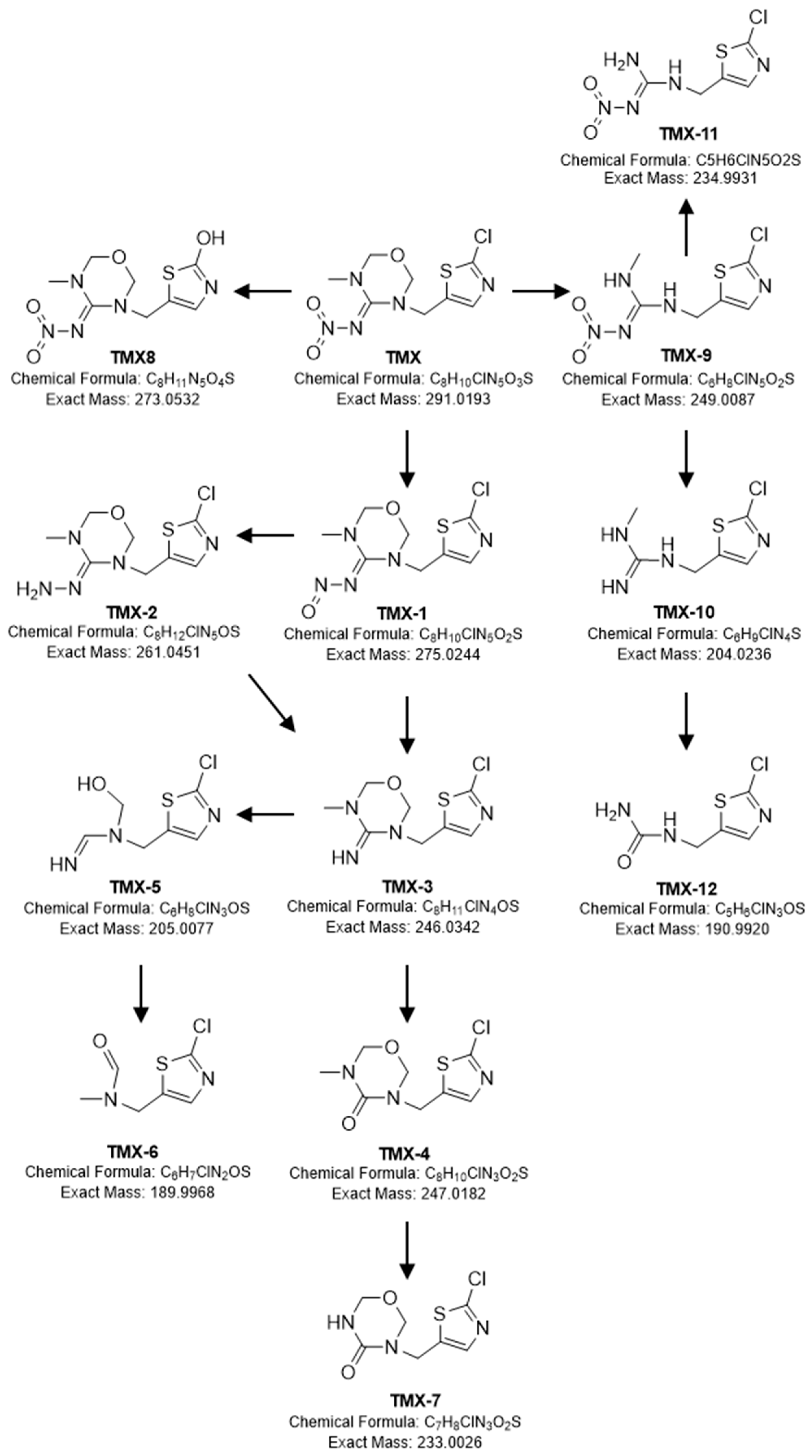
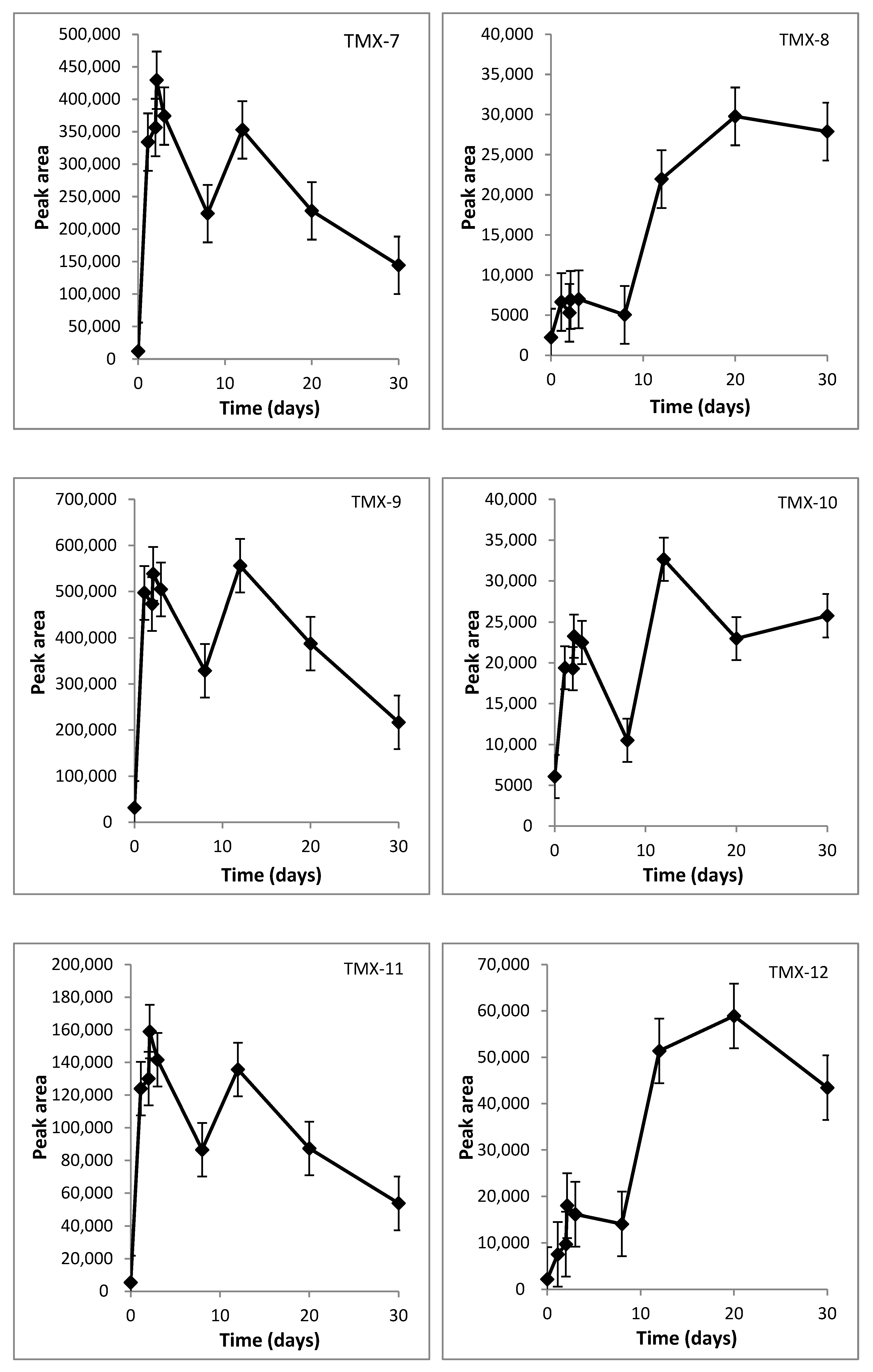
2.2. Identification of Transformation Products of TMX
2.3. Toxicity Tests
3. Discussion
3.1. Biodegradation of TMX by L. portucalensis F11
3.2. TMX Degradation Pathway
3.3. Toxicity Tests
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Culture Conditions
4.3. Biodegradation Experiments
4.4. Analytical Method
4.4.1. TOC-TN Analysis
4.4.2. TMX Analysis
4.4.3. Identification of Degradation Products
4.5. Toxicity Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A Review on Occurrence of Pesticides in Environment and Current Technologies for Their Remediation and Management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Stivaktakis, P.D.; Kavvalakis, M.P.; Tzatzarakis, M.N.; Alegakis, A.K.; Panagiotakis, M.N.; Fragkiadaki, P.; Vakonaki, E.; Ozcagli, E.; Hayes, W.A.; Rakitskii, V.N.; et al. Long-Term Exposure of Rabbits to Imidaclorpid as Quantified in Blood Induces Genotoxic Effect. Chemosphere 2016, 149, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Goka, K.; Hayasaka, D. Contamination of the Aquatic Environment with Neonicotinoids and Its Implication for Ecosystems. Front. Environ. Sci. 2016, 4, 71. [Google Scholar] [CrossRef]
- Buszewski, B.; Bukowska, M.; Ligor, M.; Staneczko-Baranowska, I. A Holistic Study of Neonicotinoids Neuroactive Insecticides—Properties, Applications, Occurrence, and Analysis. Environ. Sci. Pollut. Res. 2019, 26, 34723–34740. [Google Scholar] [CrossRef] [PubMed]
- Meijide, J.; Gómez, J.; Pazos, M.; Sanromán, M.A. Degradation of Thiamethoxam by the Synergetic Effect between Anodic Oxidation and Fenton Reactions. J. Hazard. Mater. 2016, 319, 43–50. [Google Scholar] [CrossRef]
- Sadaria, A.M.; Supowit, S.D.; Halden, R.U. Mass Balance Assessment for Six Neonicotinoid Insecticides During Conventional Wastewater and Wetland Treatment: Nationwide Reconnaissance in United States Wastewater. Environ. Sci. Technol. 2016, 50, 6199–6206. [Google Scholar] [CrossRef]
- Pang, S.; Lin, Z.; Zhang, W.; Mishra, S.; Bhatt, P.; Chen, S. Insights Into the Microbial Degradation and Biochemical Mechanisms of Neonicotinoids. Front. Microbiol. 2020, 11, 868. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Z.; Chang, C.-H.; Yu, C.; Wang, X.; Lu, C. Dietary Risk of Neonicotinoid Insecticides through Fruit and Vegetable Consumption in School-Age Children. Environ. Int. 2019, 126, 672–681. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Chang, C.H.; Lou, J.L.; Zhao, M.R.; Lu, C. Potential Human Exposures to Neonicotinoid Insecticides: A Review. Environ. Pollut. 2018, 236, 71–81. [Google Scholar] [CrossRef]
- Hussain, S.; Hartley, C.J.; Shettigar, M.; Pandey, G. Bacterial Biodegradation of Neonicotinoid Pesticides in Soil and Water Systems. FEMS Microbiol. Lett. 2016, 363, fnw252. [Google Scholar] [CrossRef]
- Wood, T.J.; Goulson, D. The Environmental Risks of Neonicotinoid Pesticides: A Review of the Evidence Post 2013. Environ. Sci. Pollut. Res. 2017, 24, 17285–17325. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Plant Protection Products and their Residues (PPR) Scientific Opinion on the Developmental Neurotoxicity Potential of Acetamiprid and Imidacloprid. EFS2 2013, 11, 3471. [CrossRef]
- Commission Implementing Regulation (EU) No 485/2013 of 24 May 2013 Amending Implementing Regulation (EU) No 540/2011, as Regards the Conditions of Approval of the Active Substances Clothianidin, Thiamethoxam and Imidacloprid, and Prohibiting the Use and Sale of Seeds Treated with Plant Protection Products Containing Those Active Substances Text with EEA Relevance. Available online: https://eur-lex.europa.eu/eli/reg_impl/2013/485/oj (accessed on 22 September 2022).
- Mitchell, E.A.D.; Mulhauser, B.; Mulot, M.; Mutabazi, A.; Glauser, G.; Aebi, A. A Worldwide Survey of Neonicotinoids in Honey. Science 2017, 358, 109–111. [Google Scholar] [CrossRef]
- Han, W.; Tian, Y.; Shen, X. Human Exposure to Neonicotinoid Insecticides and the Evaluation of Their Potential Toxicity: An Overview. Chemosphere 2018, 192, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Zeng, T.; Li, W.; Yang, L.; Guo, B. Accumulation and Toxicity of Thiamethoxam and Its Metabolite Clothianidin to the Gonads of Eremias Argus. Sci. Total Environ. 2019, 667, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, P.; Li, Y.; Wen, K.; Bi, G.; Cox, M. Adsorption-Desorption and Degradation of Insecticides Clothianidin and Thiamethoxam in Agricultural Soils. Chemosphere 2018, 207, 708–714. [Google Scholar] [CrossRef]
- Mir, N.A.; Khan, A.; Muneer, M.; Vijayalakhsmi, S. Photocatalytic Degradation of a Widely Used Insecticide Thiamethoxam in Aqueous Suspension of TiO2: Adsorption, Kinetics, Product Analysis and Toxicity Assessment. Sci. Total Environ. 2013, 458–460, 388–398. [Google Scholar] [CrossRef]
- Mörtl, M.; Vehovszky, Á.; Klátyik, S.; Takács, E.; Győri, J.; Székács, A. Neonicotinoids: Spreading, Translocation and Aquatic Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 2006. [Google Scholar] [CrossRef]
- Pietrzak, D.; Kania, J.; Kmiecik, E.; Malina, G.; Wątor, K. Fate of Selected Neonicotinoid Insecticides in Soil–Water Systems: Current State of the Art and Knowledge Gaps. Chemosphere 2020, 255, 126981. [Google Scholar] [CrossRef]
- Mei, J.; Ge, Q.; Han, L.; Zhang, H.; Long, Z.; Cui, Y.; Hua, R.; Yu, Y.; Fang, H. Deposition, Distribution, Metabolism, and Reduced Application Dose of Thiamethoxam in a Pepper-Planted Ecosystem. J. Agric. Food Chem. 2019, 67, 11848–11859. [Google Scholar] [CrossRef]
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental Risks and Challenges Associated with Neonicotinoid Insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Z.; Song, J.; Mei, J.; Fang, H.; Gui, W. Reduced Bacterial Network Complexity in Agricultural Soils after Application of the Neonicotinoid Insecticide Thiamethoxam. Environ. Pollut. 2021, 274, 116540. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.B.; Raut-Jadhav, S.; Pandit, A.B. Effect of Intensifying Additives on the Degradation of Thiamethoxam Using Ultrasound Cavitation. Ultrason. Sonochem. 2021, 70, 105310. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.Z.; Huseth, A.S.; Groves, R.L. Widespread Detections of Neonicotinoid Contaminants in Central Wisconsin Groundwater. PLoS ONE 2018, 13, e0201753. [Google Scholar] [CrossRef]
- Klarich, K.L.; Pflug, N.C.; DeWald, E.M.; Hladik, M.L.; Kolpin, D.W.; Cwiertny, D.M.; LeFevre, G.H. Occurrence of Neonicotinoid Insecticides in Finished Drinking Water and Fate during Drinking Water Treatment. Environ. Sci. Technol. Lett. 2017, 4, 168–173. [Google Scholar] [CrossRef]
- Struger, J.; Grabuski, J.; Cagampan, S.; Sverko, E.; McGoldrick, D.; Marvin, C.H. Factors Influencing the Occurrence and Distribution of Neonicotinoid Insecticides in Surface Waters of Southern Ontario, Canada. Chemosphere 2017, 169, 516–523. [Google Scholar] [CrossRef]
- Mahai, G.; Wan, Y.; Xia, W.; Yang, S.; He, Z.; Xu, S. Neonicotinoid Insecticides in Surface Water from the Central Yangtze River, China. Chemosphere 2019, 229, 452–460. [Google Scholar] [CrossRef]
- Iancu, V.-I.; Radu, G.-L. Occurrence of Neonicotinoids in Waste Water from the Bucharest Treatment Plant. Anal. Methods 2018, 10, 2691–2700. [Google Scholar] [CrossRef]
- Lebik-Elhadi, H.; Frontistis, Z.; Ait-Amar, H.; Amrani, S.; Mantzavinos, D. Electrochemical Oxidation of Pesticide Thiamethoxam on Boron Doped Diamond Anode: Role of Operating Parameters and Matrix Effect. Process Saf. Environ. Prot. 2018, 116, 535–541. [Google Scholar] [CrossRef]
- Šojić, D.; Despotović, V.; Orčić, D.; Szabó, E.; Arany, E.; Armaković, S.; Illés, E.; Gajda-Schrantz, K.; Dombi, A.; Alapi, T.; et al. Degradation of Thiamethoxam and Metoprolol by UV, O3 and UV/O3 Hybrid Processes: Kinetics, Degradation Intermediates and Toxicity. J. Hydrol. 2012, 472–473, 314–327. [Google Scholar] [CrossRef]
- Gomez-Herrero, E.; Lebik-ElHadi, H.; Ait-Amar, H.; Tobajas, M.; Rodriguez, J.J.; Mohedano, A.F. Thiamethoxam Removal by Fenton and Biological Oxidation: Fenton and Biological Oxidation of TMX. J. Chem. Technol. Biotechnol. 2020, 95, 913–921. [Google Scholar] [CrossRef]
- Pandey, G.; Dorrian, S.J.; Russell, R.J.; Oakeshott, J.G. Biotransformation of the Neonicotinoid Insecticides Imidacloprid and Thiamethoxam by Pseudomonas Sp. 1G. Biochem. Biophys. Res. Commun. 2009, 380, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhao, Y.; Zhang, W.; Yu, C.; Ji, W.; Xu, W.; Ni, J.; Yuan, S. Biotransformation of Thianicotinyl Neonicotinoid Insecticides: Diverse Molecular Substituents Response to Metabolism by Bacterium Stenotrophomonas maltophilia CGMCC 1.1788. Bioresour. Technol. 2010, 101, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Myresiotis, C.K.; Vryzas, Z.; Papadopoulou-Mourkidou, E. Biodegradation of Soil-Applied Pesticides by Selected Strains of Plant Growth-Promoting Rhizobacteria (PGPR) and Their Effects on Bacterial Growth. Biodegradation 2012, 23, 297–310. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Y.; Zhai, S.; Ge, F.; Liu, Z.; Dai, Y.; Yuan, S.; Hou, J. Biodegradation of the Neonicotinoid Insecticide Thiamethoxam by the Nitrogen-Fixing and Plant-Growth-Promoting Rhizobacterium Ensifer adhaerens Strain TMX-23. Appl. Microbiol. Biotechnol. 2013, 97, 4065–4074. [Google Scholar] [CrossRef]
- Rana, S.; Jindal, V.; Mandal, K.; Kaur, G.; Gupta, V.K. Thiamethoxam Degradation by Pseudomonas and Bacillus Strains Isolated from Agricultural Soils. Env. Monit Assess 2015, 187, 300. [Google Scholar] [CrossRef]
- Zamule, S.M.; Dupre, C.E.; Mendola, M.L.; Widmer, J.; Shebert, J.A.; Roote, C.E.; Das, P. Bioremediation Potential of Select Bacterial Species for the Neonicotinoid Insecticides, Thiamethoxam and Imidacloprid. Ecotoxicol. Environ. Saf. 2021, 209, 111814. [Google Scholar] [CrossRef]
- Chen, A.; Li, W.; Zhang, X.; Shang, C.; Luo, S.; Cao, R.; Jin, D. Biodegradation and Detoxification of Neonicotinoid Insecticide Thiamethoxam by White-Rot Fungus Phanerochaete chrysosporium. J. Hazard. Mater. 2021, 417, 126017. [Google Scholar] [CrossRef]
- Rodríguez-Castillo, G.; Molina-Rodríguez, M.; Cambronero-Heinrichs, J.C.; Quirós-Fournier, J.P.; Lizano-Fallas, V.; Jiménez-Rojas, C.; Masís-Mora, M.; Castro-Gutiérrez, V.; Mata-Araya, I.; Rodríguez-Rodríguez, C.E. Simultaneous Removal of Neonicotinoid Insecticides by a Microbial Degrading Consortium: Detoxification at Reactor Scale. Chemosphere 2019, 235, 1097–1106. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhatt, K.; Huang, Y.; Lin, Z.; Chen, S. Esterase Is a Powerful Tool for the Biodegradation of Pyrethroid Insecticides. Chemosphere 2020, 244, 125507. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Y.; Ma, Y.; Zhai, S.; Zhou, L.; Dai, Y.; Yuan, S. The Metabolism of Neonicotinoid Insecticide Thiamethoxam by Soil Enrichment Cultures, and the Bacterial Diversity and Plant Growth-promoting Properties of the Cultured Isolates. J. Environ. Sci. Health Part B 2014, 49, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, N.; Yang, J.; Jin, L.; Guo, H.; Shi, W.; Zhang, X.; Yang, L.; Yu, H.; Wei, S. Suspect and Non-Target Screening of Pesticides and Pharmaceuticals Transformation Products in Wastewater Using QTOF-MS. Environ. Int. 2020, 137, 105599. [Google Scholar] [CrossRef] [PubMed]
- Parte, S.G.; Kharat, A.S. Aerobic Degradation of Clothianidin to 2-Chloro-Methyl Thiazole and Methyl 3-(Thiazole-Yl) Methyl Guanidine Produced by Pseudomonas Stutzeri Smk. J. Environ. Public Health 2019, 2019, 4807913. [Google Scholar] [CrossRef] [PubMed]
- Commission Implementing Decision (EU) 2018/840—Of 5 June 2018—Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council and Repealing Commission Implementing Decision (EU) 2015/495—(Notified under Document C(2018) 3362). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018D0840 (accessed on 22 September 2022).
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy (OJ L 327 22.12.2000 p. 1). In Documents in European Community Environmental Law; Sands, P., Galizzi, P., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 879–969. ISBN 978-0-521-83303-5. [Google Scholar]
- Zhan, H.; Wan, Q.; Wang, Y.; Cheng, J.; Yu, X.; Ge, J. An Endophytic Bacterial Strain, Enterobacter Cloacae TMX-6, Enhances the Degradation of Thiamethoxam in Rice Plants. Chemosphere 2021, 269, 128751. [Google Scholar] [CrossRef] [PubMed]
- Boufercha, O.; Moreira, I.S.; Castro, P.M.L.; Boudemagh, A. Actinobacteria Isolated from Wastewater Treatment Plants Located in the East-North of Algeria Able to Degrade Pesticides. World J. Microbiol. Biotechnol. 2022, 38, 105. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, P.S.; Vo, D.-V.N.; Rajamohan, N.; Saravanan, R. Microbial Degradation of Recalcitrant Pesticides: A Review. Environ. Chem. Lett. 2021, 19, 3209–3228. [Google Scholar] [CrossRef]
- Wanguyun, A.P.; Geraldi, A. Understanding Pesticide Degrading-microbe Community Using Molecular Approaches. Pollut. Res. 2019, 38, 118–122. [Google Scholar]
- González, T.; Dominguez, J.R.; Correia, S. Neonicotinoids Removal by Associated Binary, Tertiary and Quaternary Advanced Oxidation Processes: Synergistic Effects, Kinetics and Mineralization. J. Environ. Manag. 2020, 261, 110156. [Google Scholar] [CrossRef]
- Cui, M.; Chen, F.; Fu, J.; Sheng, G.; Sun, G. Microbial Metabolism of Quinoline by Comamonas sp. World J. Microbiol. Biotechnol. 2004, 20, 539–543. [Google Scholar] [CrossRef]
- Žabar, R.; Komel, T.; Fabjan, J.; Kralj, M.B.; Trebše, P. Photocatalytic Degradation with Immobilised TiO2 of Three Selected Neonicotinoid Insecticides: Imidacloprid, Thiamethoxam and Clothianidin. Chemosphere 2012, 89, 293–301. [Google Scholar] [CrossRef]
- Mishra, S.; Lin, Z.; Pang, S.; Zhang, W.; Bhatt, P.; Chen, S. Recent Advanced Technologies for the Characterization of Xenobiotic-Degrading Microorganisms and Microbial Communities. Front. Bioeng. Biotechnol. 2021, 9, 632059. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.F.; De Marco, P.; Duque, A.F.; Pacheco, C.C.; Janssen, D.B.; Castro, P.M.L. Labrys Portucalensis Sp. Nov., a Fluorobenzene-Degrading Bacterium Isolated from an Industrially Contaminated Sediment in Northern Portugal. Int. J. Syst. Evol. Microbiol. 2008, 58, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.F.; Ferreira Jorge, R.; Pacheco, C.C.; De Marco, P.; Castro, P.M.L. Isolation and Properties of a Pure Bacterial Strain Capable of Fluorobenzene Degradation as Sole Carbon and Energy Source. Environ. Microbiol. 2005, 7, 294–298. [Google Scholar] [CrossRef]
- Amorim, C.L.; Moreira, I.S.; Maia, A.S.; Tiritan, M.E.; Castro, P.M.L. Biodegradation of Ofloxacin, Norfloxacin, and Ciprofloxacin as Single and Mixed Substrates by Labrys Portucalensis F11. Appl. Microbiol. Biotechnol. 2014, 98, 3181–3190. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.S.; Ribeiro, A.R.; Afonso, C.M.; Tiritan, M.E.; Castro, P.M.L. Enantioselective Biodegradation of Fluoxetine by the Bacterial Strain Labrys Portucalensis F11. Chemosphere 2014, 111, 103–111. [Google Scholar] [CrossRef]
- Moreira, I.S.; Bessa, V.S.; Murgolo, S.; Piccirillo, C.; Mascolo, G.; Castro, P.M.L. Biodegradation of Diclofenac by the Bacterial Strain Labrys Portucalensis F11. Ecotoxicol. Environ. Saf. 2018, 152, 104–113. [Google Scholar] [CrossRef]
- Bessa, V.S.; Moreira, I.S.; Murgolo, S.; Mascolo, G.; Castro, P.M.L. Carbamazepine Is Degraded by the Bacterial Strain Labrys Portucalensis F11. Sci. Total Environ. 2019, 690, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.S.; Amorim, C.L.; Carvalho, M.F.; Castro, P.M.L. Degradation of Difluorobenzenes by the Wild Strain Labrys portucalensis. Biodegradation 2012, 23, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.L.; Carvalho, M.F.; Afonso, C.M.M.; Castro, P.M.L. Biodegradation of Fluoroanilines by the Wild Strain Labrys portucalensis. Int. Biodeterior. Biodegrad. 2013, 80, 10–15. [Google Scholar] [CrossRef]
- Casida, J.E. Neonicotinoid Metabolism: Compounds, Substituents, Pathways, Enzymes, Organisms, and Relevance. J. Agric. Food Chem. 2011, 59, 2923–2931. [Google Scholar] [CrossRef]
- Wang, X.; Xue, L.; Chang, S.; He, X.; Fan, T.; Wu, J.; Niu, J.; Emaneghemi, B. Bioremediation and Metabolism of Clothianidin by Mixed Bacterial Consortia Enriched from Contaminated Soils in Chinese Greenhouse. Process Biochem. 2019, 78, 114–122. [Google Scholar] [CrossRef]
- Macedo, W.R.; e Castro, P.R.D.C. Thiamethoxam: Molecule Moderator of Growth, Metabolism and Production of Spring Wheat. Pestic. Biochem. Physiol. 2011, 100, 299–304. [Google Scholar] [CrossRef]
- Singh, B. Degradation of Clodinafop Propargyl by Pseudomonas sp. Strain B2. Bull Environ. Contam. Toxicol. 2013, 91, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Pileggi, M.; Pileggi, S.A.V.; Olchanheski, L.R.; da Silva, P.A.G.; Munoz Gonzalez, A.M.; Koskinen, W.C.; Barber, B.; Sadowsky, M.J. Isolation of Mesotrione-Degrading Bacteria from Aquatic Environments in Brazil. Chemosphere 2012, 86, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.S.; Amorim, C.L.; Carvalho, M.F.; Ferreira, A.C.; Afonso, C.M.; Castro, P.M.L. Effect of the Metals Iron, Copper and Silver on Fluorobenzene Biodegradation by Labrys portucalensis. Biodegradation 2013, 24, 245–255. [Google Scholar] [CrossRef]
- Carvalho, M.F.; Maia, A.S.; Tiritan, M.E.; Castro, P.M.L. Bacterial Degradation of Moxifloxacin in the Presence of Acetate as a Bulk Substrate. J. Environ. Manag. 2016, 168, 219–228. [Google Scholar] [CrossRef]
- Carvalho, M.F.; Ferreira, M.I.M.; Moreira, I.S.; Castro, P.M.L.; Janssen, D.B. Degradation of Fluorobenzene by Rhizobiales Strain F11 via Ortho Cleavage of 4-Fluorocatechol and Catechol. Appl. Environ. Microbiol. 2006, 72, 7413–7417. [Google Scholar] [CrossRef]
- de Kreuk, M.K.; Heijnen, J.J.; van Loosdrecht, M.C.M. Simultaneous COD, Nitrogen, and Phosphate Removal by Aerobic Granular Sludge. Biotechnol. Bioeng. 2005, 90, 761–769. [Google Scholar] [CrossRef]
- OECD Test Guideline 208: Terrestrial Plant Test—Seedling Emergence and Seedling Growth Test; Guidelines for the Testing of Chemicals; OECD: Paris, France, 2006; Volume 227, pp. 1–21.
- ISO 11348-2:2007; Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent Bacteria Test). International Organization for Standardization: Geneva, Switzerland, 2007.

| Biodegradation of TMX as: | k (d−1) | t1/2 (d) | R2 |
|---|---|---|---|
| Carbon and nitrogen source | 0.017 ± 0.002 | 40.77 ± 0.129 | 0.9904 |
| Carbon and sulfur source | 0.010 ± 0.002 | 69.31 ± 0.091 | 0.9836 |
| Carbon source | 0.077± 0.002 | 8.961 ± 0.015 | 0.9966 |
| Supplemented with acetate | 0.791 ± 0.002 | 0.872 ± 0.001 | 0.9974 |
| TMX Concentration (mg L−1) | TMX as Sole Carbon Source | TMX with Periodic Feeding with Acetate | ||||
|---|---|---|---|---|---|---|
| k (d−1) | t1/2 (d) | R2 | k (d−1) | t1/2 (d) | R2 | |
| 37.3 | 0.022 ± 0.0015 | 31.363 ± 0.005 | 0.9925 | 0.156 ± 0.0015 | 4.423 ± 0.0015 | 0.9895 |
| 68.6 | 0.016 ± 0.0009 | 43.125 ± 0.007 | 0.9933 | 0.053 ± 0.0004 | 13.018 ± 0.0054 | 0.9941 |
| 128.7 | 0.010 ± 0.0020 | 69.000 ± 0.150 | 0.9915 | 0.023 ± 0.0014 | 31.000 ± 0.0550 | 0.9994 |
| Metabolite | tr (min) | Measured m/z | Products MS2 | Empirical Formula | References |
|---|---|---|---|---|---|
| TMX-1 | 8.0 | 276.0144 | 131.9574, 181.0392, 100.0698, 69.0398 | C8H10ClN5O2S | [33,36,42] |
| TMX-2 | 8.1 | 262.0161 | 131.9667, 70.9950 | C8H12ClN5OS | [43] |
| TMX-3 | 4.4 | 247.0412 | 131.9665, 160.9940, 44.0488 | C8H11ClN4OS | [33,42,43] |
| TMX-4 | 11.2 | 248.0083 | 174.9607, 132.9650 | C8H10ClN3O2S | [33,36,42,43] |
| TMX-5 | 6.9 | 206.0156 | 174.9729, 113.0172, 86.0058, 58.9941 | C6H8N3OSCl | [43] |
| TMX-6 | 2.7 | 191.0032 | 131.9593, 162.8899, 70.9900 | C6H7ClN2OS | [43] |
| TMX-7 | 8.3 | 234.0102 | 174.9737, 131.9673 | C7H8ClN3O2S | [43] |
| TMX-8 | 8.3 | 274.0414 | 137.0723, 115.0664, 84.0490, 69.0398 | C8H11N5O4S | [39] |
| TMX-9 | 10.4 | 250.0171 | 131.9674, 169.0543, 70.9951 | C6H8ClN5O2S | [43] |
| TMX-10 | 21.8 | 205.0172 | 131.9587, 166.9478, 113.0102 | C6H9ClN4S | [43,44] |
| TMX-11 | 8.5 | 235.9951 | 131.9577, 174.9603, 125.0087 | C5H6ClN5O2S | This study |
| TMX-12 | 5.3 | 191.9868 | 131.9591, 174.9595, 70.9912 | C5H6ClN3OS | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boufercha, O.; Monforte, A.R.; Boudemagh, A.; Ferreira, A.C.; Castro, P.M.L.; Moreira, I.S. Biodegradation and Metabolic Pathway of the Neonicotinoid Insecticide Thiamethoxam by Labrys portucalensis F11. Int. J. Mol. Sci. 2022, 23, 14326. https://doi.org/10.3390/ijms232214326
Boufercha O, Monforte AR, Boudemagh A, Ferreira AC, Castro PML, Moreira IS. Biodegradation and Metabolic Pathway of the Neonicotinoid Insecticide Thiamethoxam by Labrys portucalensis F11. International Journal of Molecular Sciences. 2022; 23(22):14326. https://doi.org/10.3390/ijms232214326
Chicago/Turabian StyleBoufercha, Oumeima, Ana R. Monforte, Allaoueddine Boudemagh, António C. Ferreira, Paula M. L. Castro, and Irina S. Moreira. 2022. "Biodegradation and Metabolic Pathway of the Neonicotinoid Insecticide Thiamethoxam by Labrys portucalensis F11" International Journal of Molecular Sciences 23, no. 22: 14326. https://doi.org/10.3390/ijms232214326
APA StyleBoufercha, O., Monforte, A. R., Boudemagh, A., Ferreira, A. C., Castro, P. M. L., & Moreira, I. S. (2022). Biodegradation and Metabolic Pathway of the Neonicotinoid Insecticide Thiamethoxam by Labrys portucalensis F11. International Journal of Molecular Sciences, 23(22), 14326. https://doi.org/10.3390/ijms232214326






