Immunomodulatory Effect of Human Lactoferrin on Toll-like Receptors 2 Expression as Therapeutic Approach for Keratoconus
Abstract
1. Introduction
2. Results
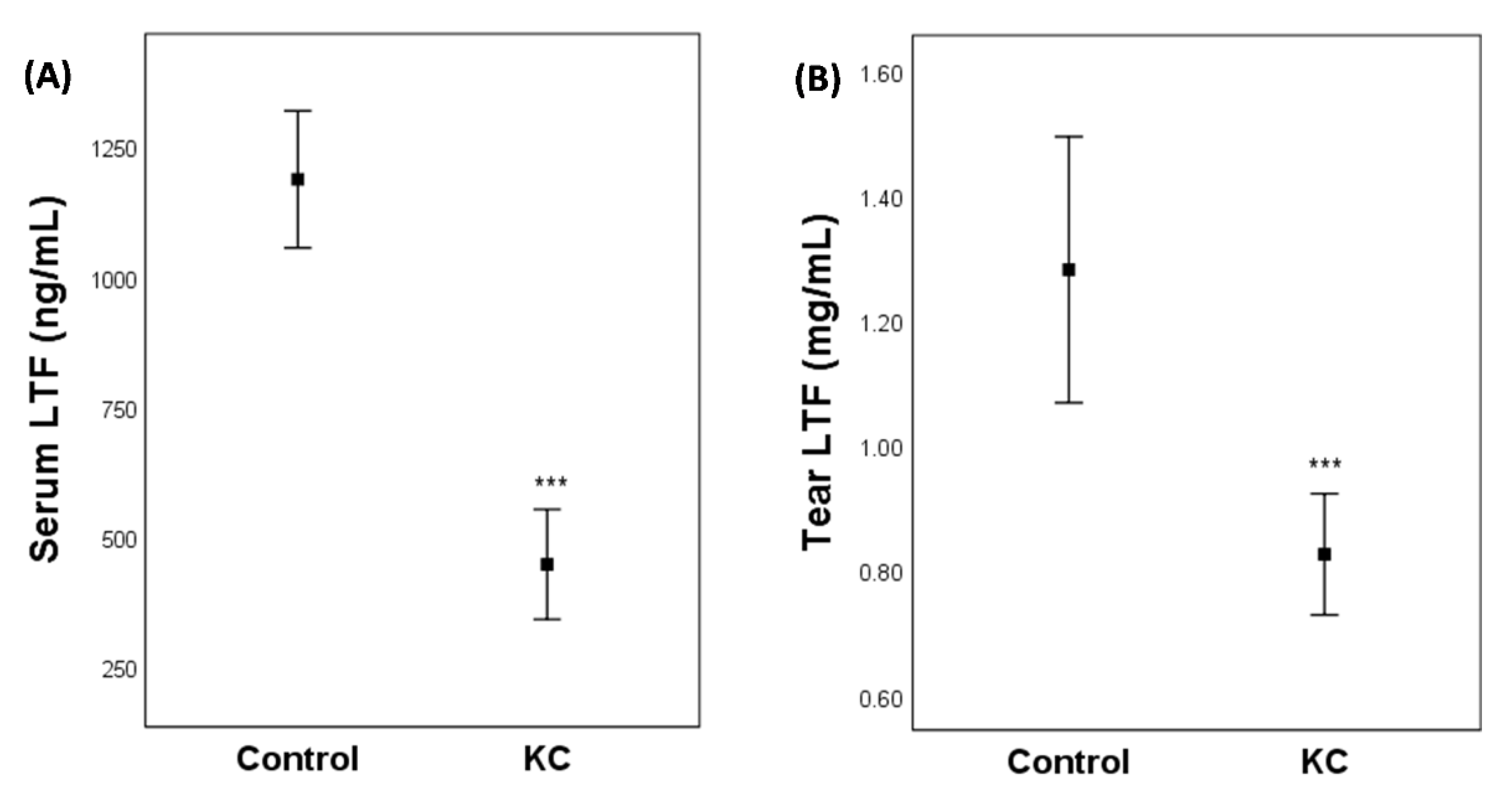
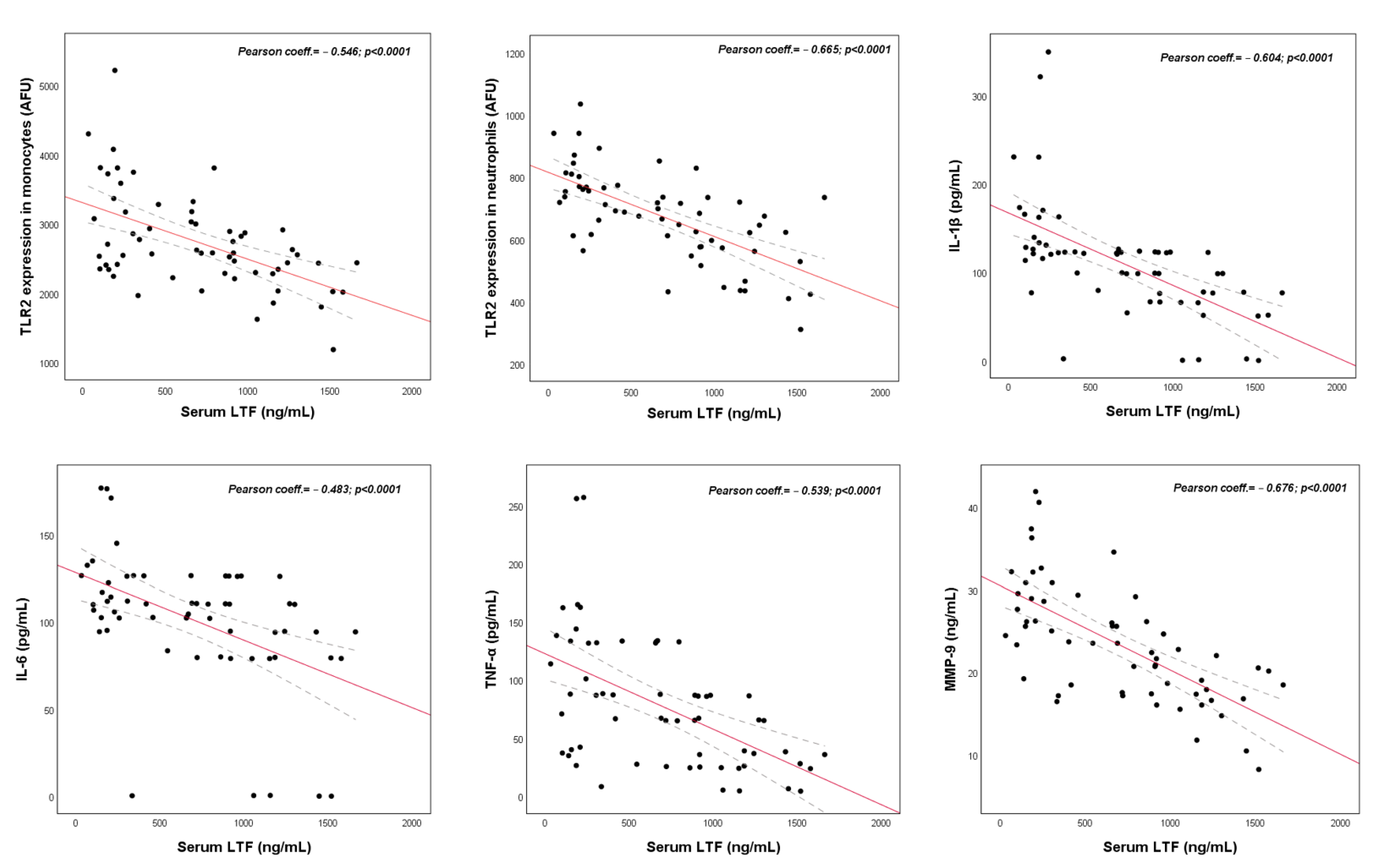
2.1. Clinical Study
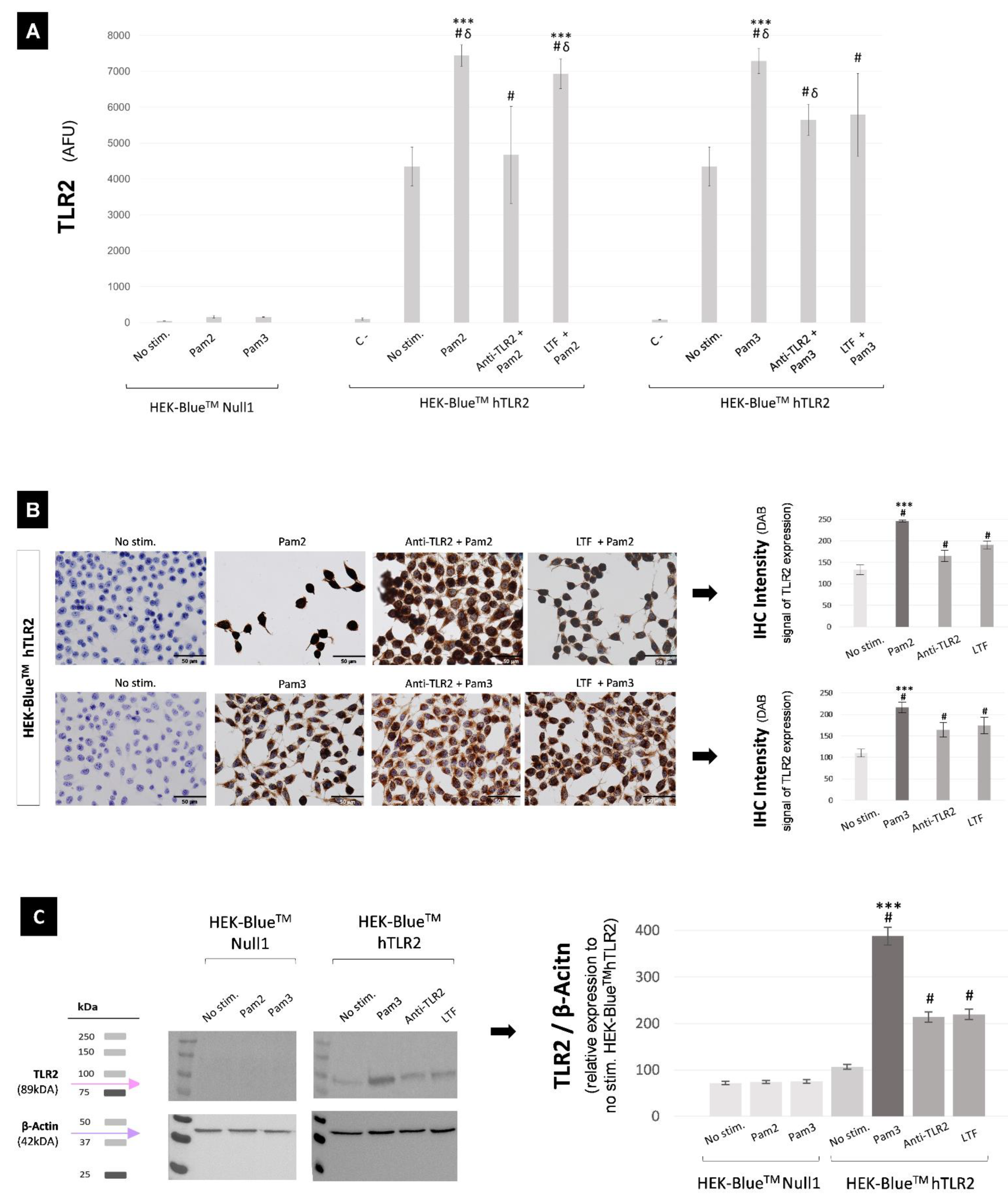
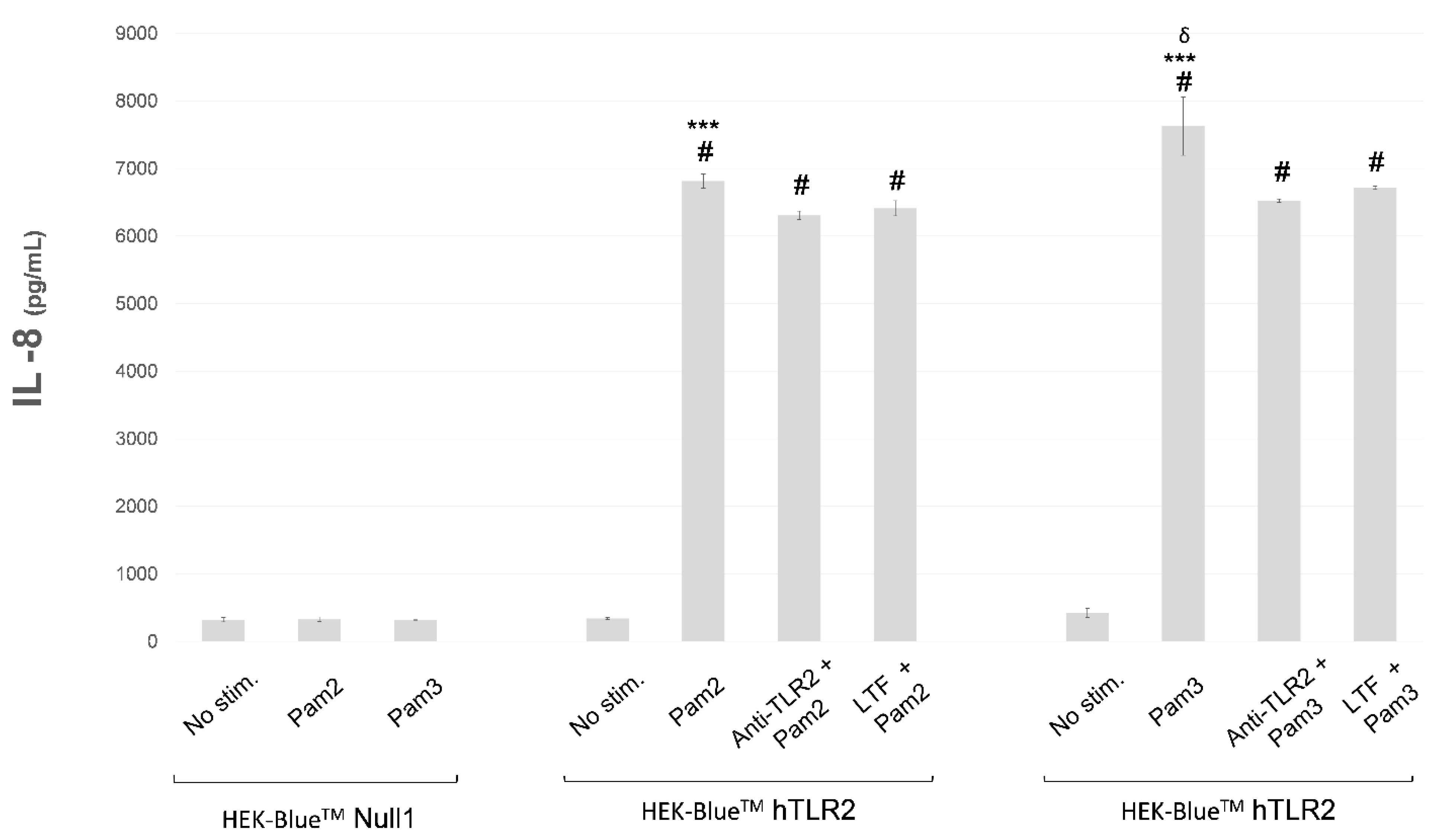
2.2. In Vitro Study
3. Discussion
4. Materials and Methods
4.1. Clinical Study
4.1.1. Blood Sample Extraction and Analysis
4.1.2. Tear Sample Collection and Analysis
4.1.3. LTF Correlation Study with Immune, Inflammatory and Clinical Variables
4.2. In Vitro Study
4.2.1. Experimental Design
4.2.2. QUANTI-BlueTM
4.2.3. Flow Cytometry
4.2.4. Immunohistochemistry
4.2.5. Western Blot
4.2.6. ELISA
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301.e1–301.e8. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, L.; Calvo, M.; Brock, J.H. Biological role of lactoferrin. Arch. Dis. Child. 1992, 67, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Vorland, L.H. Lactoferrin: A multifunctional glycoprotein. APMIS 1999, 107, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, C.; Zhang, J. Lactoferrin and Its Detection Methods: A Review. Nutrients 2021, 13, 2492. [Google Scholar] [CrossRef] [PubMed]
- Steijns, J.M.; van Hooijdonk, A.C. Occurrence, structure, biochemical properties and technological characteristics of lactoferrin. Br. J. Nutr. 2000, 84, 11–17. [Google Scholar] [CrossRef]
- Santagati, M.G.; La Terra Mulè, S.; Amico, C.; Pistone, M.; Rusciano, D.; Enea, V. Lactoferrin expression by bovine ocular surface epithelia: A primary cell culture model to study lactoferrin gene promoter activity. Ophthalmic. Res. 2005, 37, 270–278. [Google Scholar] [CrossRef]
- Kijlstra, A.; Jeurissen, S.H.; Koning, K.M. Lactoferrin levels in normal human tears. Br. J. Ophthalmol. 1983, 67, 199–202. [Google Scholar] [CrossRef]
- Wang, J.; Tian, Z. Recent advances in lactoferrin research and development during the past two years (2007–2009): In lieu of a preface of the Special Issue Lactoferrin. Biometals 2010, 23, 355–357. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Willcox, M.D. Role of lactoferrin in the tear film. Biochimie 2009, 91, 35–43. [Google Scholar] [CrossRef]
- Lema, I.; Brea, D.; Rodríguez-González, R.; Díez-Feijoo, E.; Sobrino, T. Proteomic analysis of the tear film in patients with keratoconus. Mol. Vis. 2010, 16, 2055–2061. [Google Scholar]
- Balasubramanian, S.A.; Pye, D.C.; Willcox, M.D. Levels of lactoferrin, secretory IgA and serum albumin in the tear film of people with keratoconus. Exp. Eye Res. 2012, 96, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Dierick, M.; Vanrompay, D.; Devriendt, B.; Cox, E. Lactoferrin, a versatile natural antimicrobial glycoprotein that modulates the host’s innate immunity. Biochem. Cell Biol. 2021, 99, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Agwa, M.M.; Sabra, S. Lactoferrin coated or conjugated nanomaterials as an active targeting approach in nanomedicine. Int. J. Biol. Macromol. 2021, 167, 1527–1543. [Google Scholar] [CrossRef] [PubMed]
- Belin, M.W.; Asota, I.M.; Ambrosio, R., Jr.; Khachikian, S.S. What’s in a name: Keratoconus, pellucid marginal degeneration, and related thinning disorders. Am. J. Ophthalmol. 2011, 152, 157–162.e1. [Google Scholar] [CrossRef]
- Wagner, H.; Barr, J.T.; Zadnik, K. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: Methods and findings to date. Cont. Lens Anterior Eye 2007, 30, 223–232. [Google Scholar] [CrossRef]
- Sobrino, T.; Regueiro, U.; Malfeito, M.; Vieites-Prado, A.; Pérez-Mato, M.; Campos, F.; Lema, I. Higher Expression of Toll-Like Receptors 2 and 4 in Blood Cells of Keratoconus Patiens. Sci. Rep. 2017, 7, 12975. [Google Scholar] [CrossRef]
- Malfeito, M.; Regueiro, U.; Pérez-Mato, M.; Campos, F.; Sobrino, T.; Lema, I. Innate Immunity Biomarkers for Early Detection of Keratoconus. Ocul. Immunol. Inflamm. 2019, 27, 942–948. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef]
- Hanstock, H.G.; Edwards, J.P.; Walsh, N.P. Tear Lactoferrin and Lysozyme as Clinically Relevant Biomarkers of Mucosal Immune Competence. Front. Immunol. 2019, 10, 1178. [Google Scholar] [CrossRef]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 2005, 202, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.D.M. The Iron Lines of the Superficial Cornea: Hudson-Stahli Line, Stocker’s Line and Fleischer’s Ring. Arch. Ophthalmol. 1964, 71, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Barraquer-Somers, E.; Chan, C.C.; Green, W.R. Corneal epithelial iron deposition. Ophthalmology 1983, 90, 729–734. [Google Scholar] [CrossRef]
- Volatier, T.L.A.; Figueiredo, F.C.; Connon, C.J. Keratoconus at a Molecular Level: A Review. Anat. Rec. 2020, 303, 1680–1688. [Google Scholar] [CrossRef]
- López-López, M.; Regueiro, U.; Bravo, S.B.; Chantada-Vázquez, M.D.P.; Varela-Fernández, R.; Ávila-Gómez, P.; Hervella, P.; Lema, I. Tear Proteomics in Keratoconus: A Quantitative SWATH-MS Analysis. Investig. Ophthalmol. Vis. Sci. 2021, 62, 30. [Google Scholar] [CrossRef]
- Loh, A.; Hadziahmetovic, M.; Dunaief, J.L. Iron homeostasis and eye disease. Biochim. Biophys. Acta 2009, 1790, 637–649. [Google Scholar] [CrossRef]
- Regueiro, U.; López-López, M.; Hervella, P.; Sobrino, T.; Lema, I. Corneal and conjunctival alteration of innate immune expression in first-degree relatives of keratoconus patients. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 459–467. [Google Scholar] [CrossRef]
- Regueiro, U.; Pérez-Mato, M.; Hervella, P.; Campos, F.; Sobrino, T.; Lema, I. Toll-like receptors as diagnostic targets in pellucid marginal degeneration. Exp. Eye Res. 2020, 200, 108211. [Google Scholar] [CrossRef]
- Telang, S. Lactoferrin: A Critical Player in Neonatal Host Defense. Nutrients 2018, 10, 1228. [Google Scholar] [CrossRef]
- Elass, E.; Masson, M.; Mazurier, J.; Legrand, D. Lactoferrin inhibits the lipopolysaccharide-induced expression and proteoglycan-binding ability of interleukin-8 in human endothelial cells. Infect. Immun. 2002, 70, 1860–1866. [Google Scholar] [CrossRef]
- Legrand, D.; Mazurier, J. A critical review of the roles of host lactoferrin in immunity. Biometals 2010, 23, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Ando, K.; Hasegawa, K.; Shindo, K.; Furusawa, T.; Fujino, T.; Kikugawa, K.; Nakano, H.; Takeuchi, O.; Akira, S.; Akiyama, T.; et al. Human lactoferrin activates NF-kappaB through the Toll-like receptor 4 pathway while it interferes with the lipopolysaccharide-stimulated TLR4 signaling. FEBS J. 2010, 277, 2051–2066. [Google Scholar] [CrossRef] [PubMed]
- Varela-Fernández, R.; García-Otero, X.; Díaz-Tomé, V.; Regueiro, U.; López-López, M.; González-Barcia, M.; Lema, M.I.; Otero-Espinar, F.J. Design, Optimization, and Characterization of Lactoferrin-Loaded Chitosan/TPP and Chitosan/Sulfobutylether-β-cyclodextrin Nanoparticles as a Pharmacological Alternative for Keratoconus Treatment. ACS Appl. Mater. Interfaces 2021, 13, 3559–3575. [Google Scholar] [CrossRef]
- Pastori, V.; Tavazzi, S.; Lecchi, M. Lactoferrin-loaded contact lenses counteract cytotoxicity caused in vitro by keratoconic tears. Cont. Lens. Anterior Eye. 2019, 42, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Fam, H.B.; Lim, K.L. Corneal elevation indices in normal and keratoconic eyes. J. Cataract. Refract. Surg. 2006, 32, 1281–1287. [Google Scholar] [CrossRef]
- Alió, J.L.; Shabayek, M.H. Corneal higher order aberrations: A method to grade keratoconus. J. Refract. Surg. 2006, 22, 539–545. [Google Scholar] [CrossRef]
- Rao, S.N.; Raviv, T.; Majmudar, P.A.; Epstein, R.J. Role of Orbscan II in screening keratoconus suspects before refractive corneal surgery. Ophthalmology 2002, 109, 1642–1646. [Google Scholar] [CrossRef]
- Krachmer, J.H.; Feder, R.S.; Belin, M.W. Keratoconus and related noninflammatory corneal thinning disorders. Surv. Ophthalmol. 1984, 28, 293–322. [Google Scholar] [CrossRef]
- Efron, N. Contact lens wear is intrinsically inflammatory. Clin. Exp. Optom. 2017, 100, 3–19. [Google Scholar] [CrossRef]

| Tear LTF/Ocular Markers | Pearson’s Coefficient, p Value |
|---|---|
| Tear LTF/TLR2 cornea | r = −0.289, =0.007 |
| Tear LTF/TLR2 conjunctiva | r = −0.266, =0.01 |
| Tear LTF/I-S asymmetry | r = −0.282, =0.008 |
| Tear LTF/Coma | r = −0.314, =0.003 |
| Tear LTF/Coma-like | r = −0.330, =0.002 |
| Tear LTF/Post. elevation | r = −0.227, =0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regueiro, U.; López-López, M.; Varela-Fernández, R.; Sobrino, T.; Diez-Feijoo, E.; Lema, I. Immunomodulatory Effect of Human Lactoferrin on Toll-like Receptors 2 Expression as Therapeutic Approach for Keratoconus. Int. J. Mol. Sci. 2022, 23, 12350. https://doi.org/10.3390/ijms232012350
Regueiro U, López-López M, Varela-Fernández R, Sobrino T, Diez-Feijoo E, Lema I. Immunomodulatory Effect of Human Lactoferrin on Toll-like Receptors 2 Expression as Therapeutic Approach for Keratoconus. International Journal of Molecular Sciences. 2022; 23(20):12350. https://doi.org/10.3390/ijms232012350
Chicago/Turabian StyleRegueiro, Uxía, Maite López-López, Rubén Varela-Fernández, Tomás Sobrino, Elio Diez-Feijoo, and Isabel Lema. 2022. "Immunomodulatory Effect of Human Lactoferrin on Toll-like Receptors 2 Expression as Therapeutic Approach for Keratoconus" International Journal of Molecular Sciences 23, no. 20: 12350. https://doi.org/10.3390/ijms232012350
APA StyleRegueiro, U., López-López, M., Varela-Fernández, R., Sobrino, T., Diez-Feijoo, E., & Lema, I. (2022). Immunomodulatory Effect of Human Lactoferrin on Toll-like Receptors 2 Expression as Therapeutic Approach for Keratoconus. International Journal of Molecular Sciences, 23(20), 12350. https://doi.org/10.3390/ijms232012350







