The Impacts of Cholesterol, Oxysterols, and Cholesterol Lowering Dietary Compounds on the Immune System
Abstract
1. Introduction
2. Intake and De Novo Synthesis of Cholesterol and Oxysterols
2.1. Dietary and De Novo Synthesized Cholesterol
2.1.1. Dietary Cholesterol
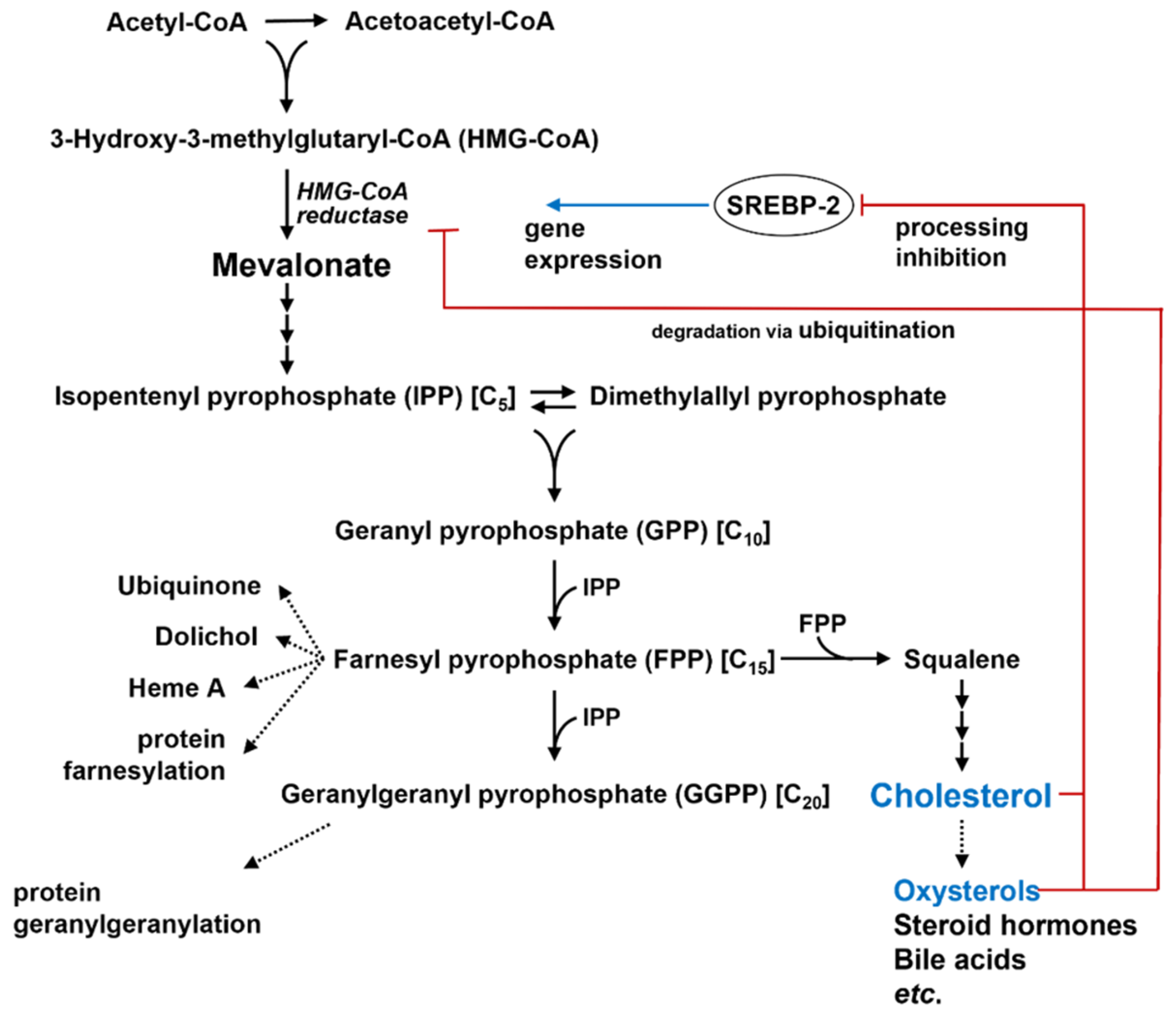
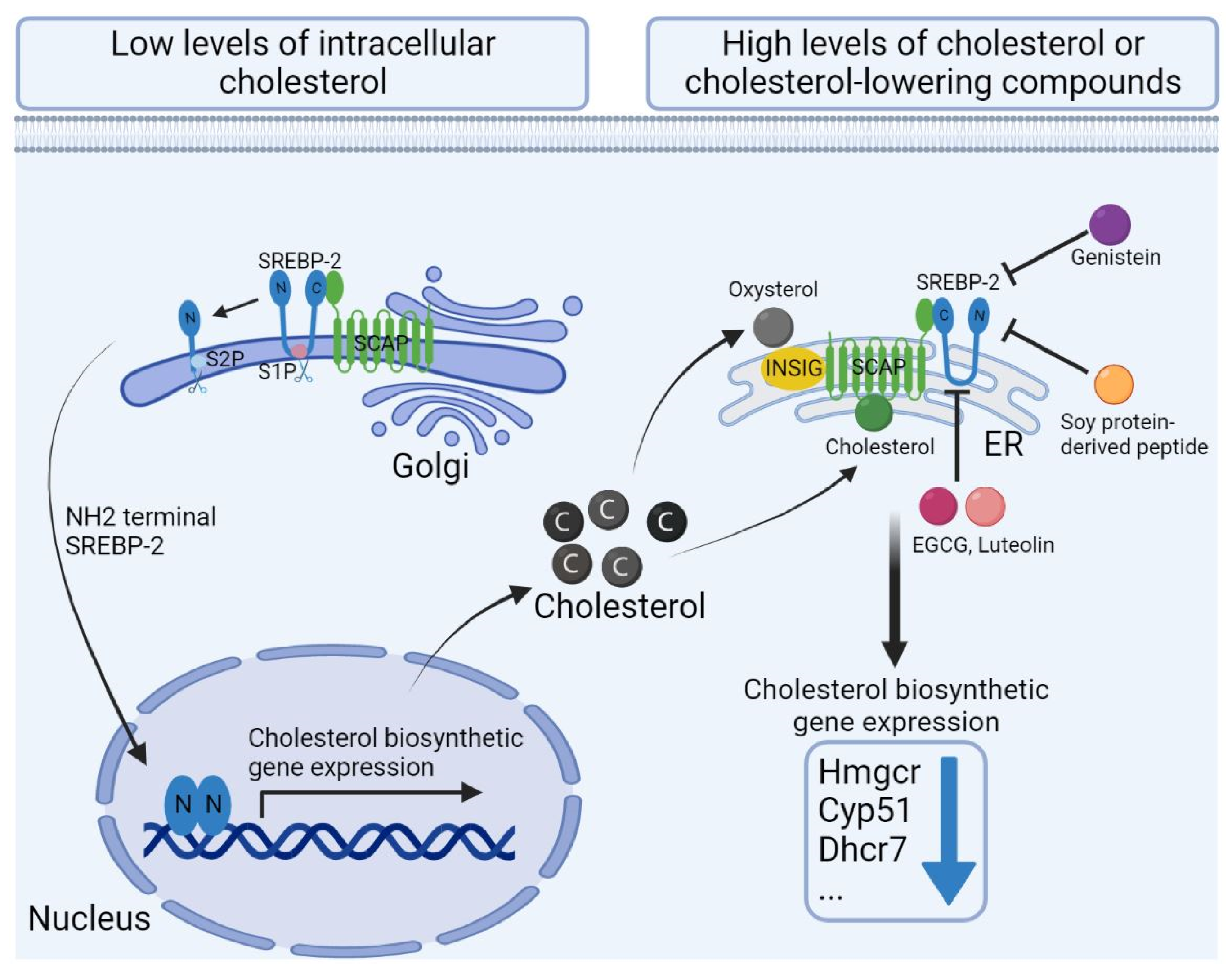
2.1.2. De Novo Synthesis of Cholesterol
2.2. Dietary and De Novo Synthesized Oxysterols
2.2.1. Dietary Oxysterols
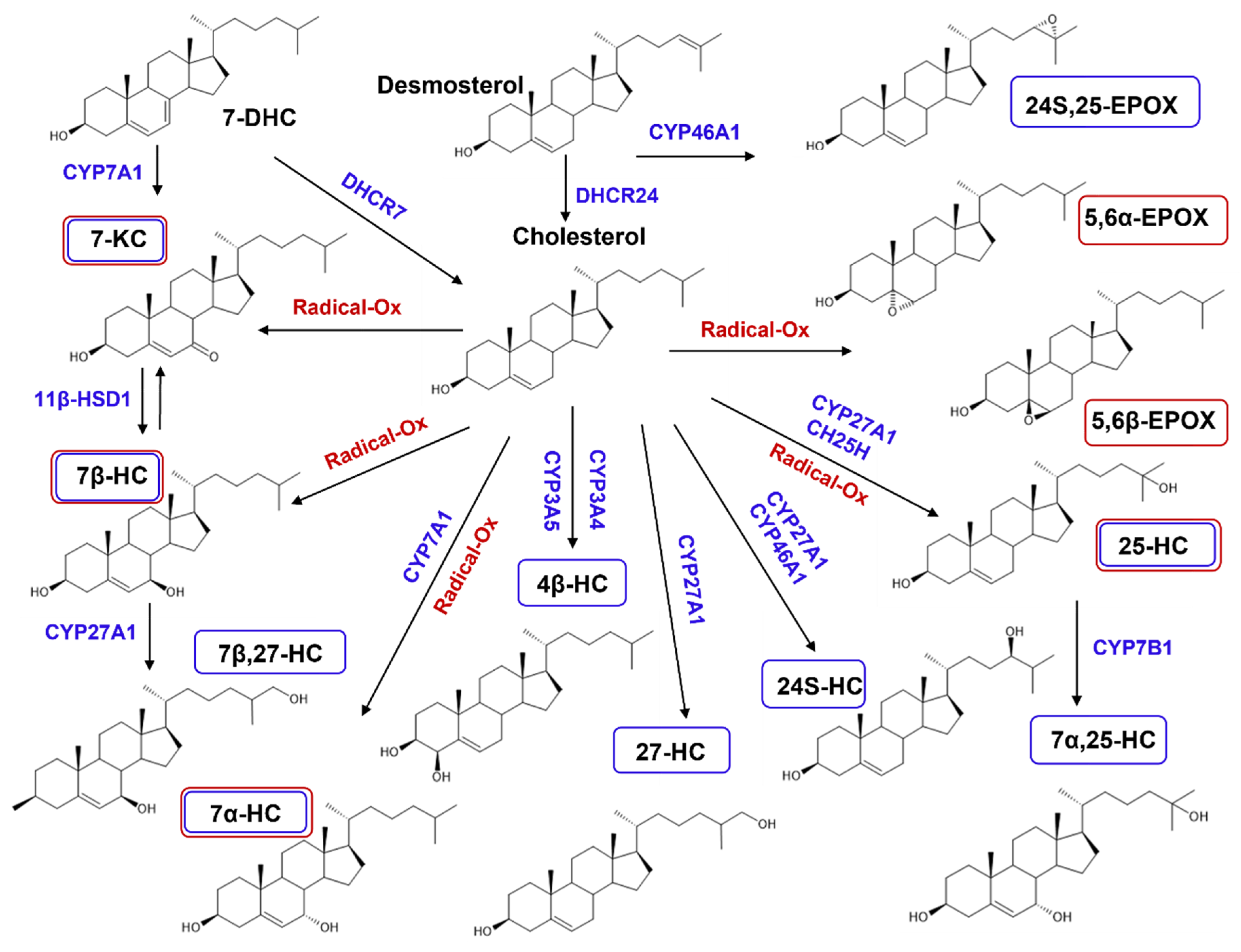
2.2.2. De Novo Synthesized Oxysterols
3. The Impact of Cholesterol and Oxysterols on Immune Cells
3.1. Macrophages
3.1.1. Activation and Induction of Inflammation
3.1.2. Induction of Anti-Inflammation
3.1.3. Differentiation
3.1.4. Migration
3.2. Dendritic Cells
3.2.1. Activation
3.2.2. Migration
3.2.3. Differentiation
3.3. T Cells
3.3.1. Activation
3.3.2. Exhaustion
3.3.3. Migration
3.3.4. Differentiation
3.4. B Cells
3.4.1. Activation
3.4.2. Migration
3.4.3. Differentiation
3.5. Innate Lymphoid Cells
3.5.1. Migration and Inflammation
3.5.2. Differentiation
3.6. Impact of Cholesterol and Oxysterol on Protection from Pathogen Infection
3.6.1. In Protection against Viral Infection
3.6.2. In Protection against Bacterial Infection
4. Cholesterol lowering Dietary Compounds
4.1. β-Glucans
4.1.1. Cholesterol Lowering Effect
4.1.2. Immunomodulatory Effects
4.2. Plant Sterols and Stanols
4.2.1. Cholesterol Lowering Effects
4.2.2. Immunomodulatory Effects
4.3. Omega-3 Fatty Acids
4.3.1. Cholesterol Lowering Effects
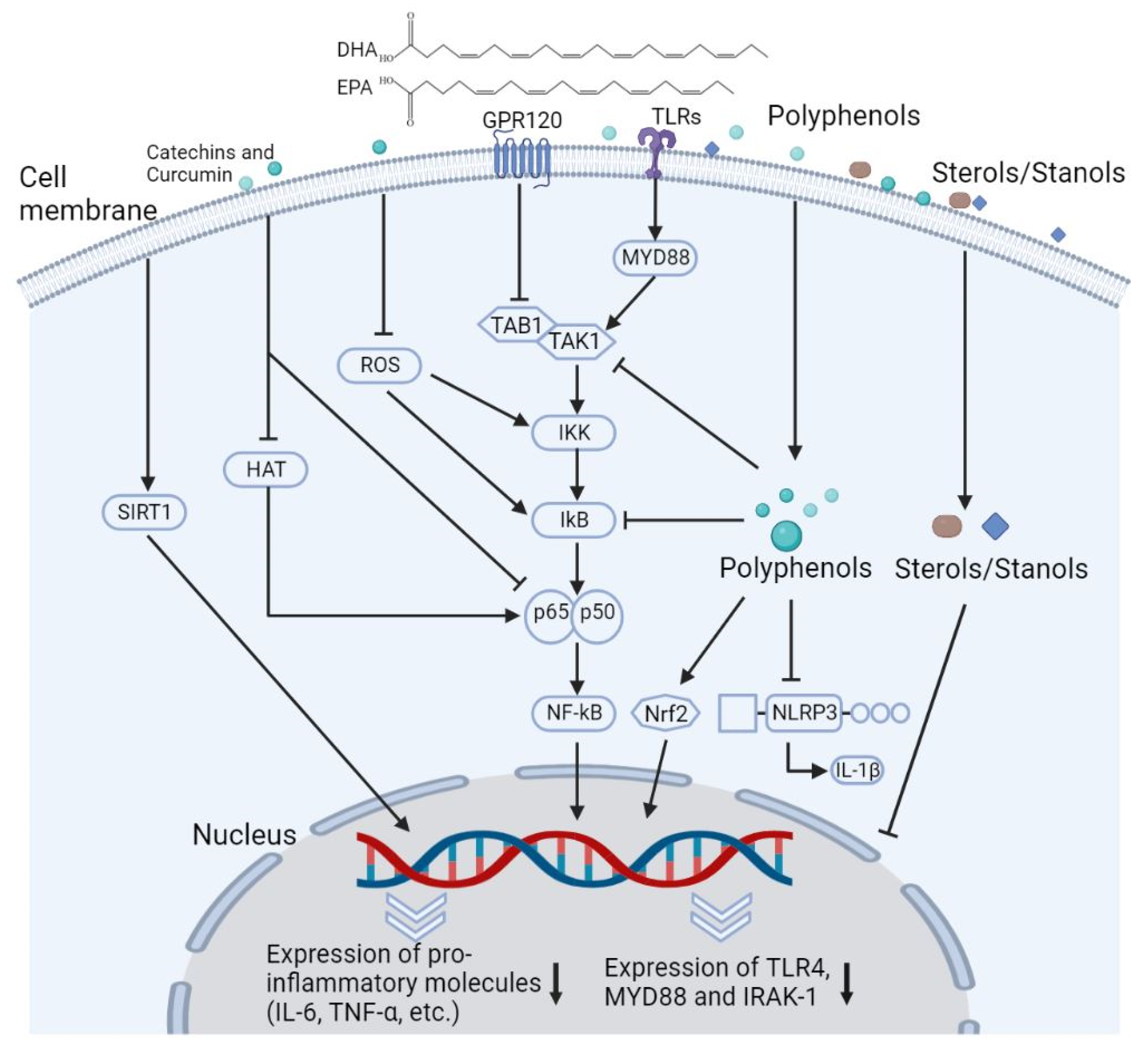
4.3.2. Immunomodulatory Effects
4.4. Polyphenols
4.4.1. Cholesterol Lowering Effects
4.4.2. Immunomodulatory Effects
4.5. Soy Proteins
4.5.1. Cholesterol Lowering Effects
4.5.2. Immunomodulatory Effects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, J.; Yang, H.; Song, B.-L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Shimano, H.; Sato, R. SREBP-regulated lipid metabolism: Convergent physiology—Divergent pathophysiology. Nat. Rev. Endocrinol 2017, 13, 710–730. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, F.A.; Levy, D.; Zarrouk, A.; Lizard, G.; Bydlowski, S.P. Impact of oxysterols on cell death, proliferation, and differentiation induction: Current status. Cells 2021, 10, 2301. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Wang, Y. Oxysterols as lipid mediators: Their biosynthetic genes, enzymes and metabolites. Prostaglandins Other Lipid Mediat. 2020, 147, 106381. [Google Scholar] [CrossRef]
- Ma, L.; Nelson, E.R. Oxysterols and nuclear receptors. Mol. Cell Endocrinol. 2019, 484, 42–51. [Google Scholar] [CrossRef] [PubMed]
- King, R.J.; Singh, P.K.; Mehla, K. The cholesterol pathway: Impact on immunity and cancer. Trends Immunol. 2022, 43, 78–92. [Google Scholar] [CrossRef]
- Spann, N.J.; Glass, C.K. Sterols and oxysterols in immune cell function. Nat. Immunol. 2013, 14, 893–900. [Google Scholar] [CrossRef]
- Zmysłowski, A.; Szterk, A. Oxysterols as a biomarker in diseases. Clin. Chim. Acta 2019, 491, 103–113. [Google Scholar] [CrossRef]
- FDA final rule for federal labeling: Health claims; oats and coronary heart disease. Fed. Regist. 1997, 62, 3584–3681.
- Food and Drug Administration, HHS. Food labeling: Health claims; soluble dietary fiber from certain foods and coronary heart disease. Final rule. Fed. Regist. 2006, 71, 29248–29250. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of a health claim related to oat beta glucan and lowering blood cholesterol and reduced risk of (coronary) heart disease pursuant to article 14 of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1885. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of a health claim related to barley beta-glucans and lowering of blood cholesterol and reduced risk of (coronary) heart disease pursuant to article 14 of regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2470. [Google Scholar] [CrossRef]
- Food and Drug Administration. Food labeling: Health claims; Plant sterol/stanol esters and coronary heart disease. Food and Drug Administration, HHS. Interim Final Rule. Fed. Regist. 2000, 65, 54686–54739. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of a health claim related to 3 g/day plant sterols/stanols and lowering blood LDL-cholesterol and reduced risk of (coronary) heart disease pursuant to article 19 of regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2693. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. β-glucan metabolic and immunomodulatory properties and potential for clinical application. J. Fungi 2020, 6, E356. [Google Scholar] [CrossRef]
- Vilahur, G.; Ben-Aicha, S.; Diaz-Riera, E.; Badimon, L.; Padró, T. Phytosterols and inflammation. Curr. Med. Chem. 2019, 26, 6724–6734. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, E1618. [Google Scholar] [CrossRef]
- Focaccetti, C.; Izzi, V.; Benvenuto, M.; Fazi, S.; Ciuffa, S.; Giganti, M.G.; Potenza, V.; Manzari, V.; Modesti, A.; Bei, R. Polyphenols as immunomodulatory compounds in the tumor microenvironment: Friends or foes? Int. J. Mol. Sci. 2019, 20, E1714. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean bioactive peptides and their functional properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of omega-3 fatty acids on immune cells. Int. J. Mol. Sci. 2019, 20, E5028. [Google Scholar] [CrossRef]
- Kumar, N.G.; Contaifer, D.; Madurantakam, P.; Carbone, S.; Price, E.T.; Van Tassell, B.; Brophy, D.F.; Wijesinghe, D.S. Dietary bioactive fatty acids as modulators of immune function: Implications on human health. Nutrients 2019, 11, E2974. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; McClure, S.T.; Appel, L.J. Dietary cholesterol intake and sources among U.S adults: Results from National Health and Nutrition Examination Surveys (NHANES), 2001−2014. Nutrients 2018, 10, E771. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.M.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L.; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Dietary Cholesterol and Cardiovascular Risk: A Science Advisory from the American Heart Association. Circulation 2020, 141, e39–e53. [Google Scholar] [CrossRef] [PubMed]
- Sehayek, E. Genetic regulation of cholesterol absorption and plasma plant sterol levels: Commonalities and differences. J. Lipid Res. 2003, 44, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Luu, W.; Sharpe, L.J.; Capell-Hattam, I.; Gelissen, I.C.; Brown, A.J. Oxysterols: Old tale, new twists. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 447–467. [Google Scholar] [CrossRef]
- Echarte, M.; Ansorena, D.; Astiasarán, I. Consequences of microwave heating and frying on the lipid fraction of chicken and beef patties. J. Agric. Food Chem. 2003, 51, 5941–5945. [Google Scholar] [CrossRef]
- Chudy, S.; Teichert, J. Oxysterols in stored powders as potential health hazards. Sci. Rep. 2021, 11, 21192. [Google Scholar] [CrossRef]
- Risso, D.; Leoni, V.; Fania, C.; Arveda, M.; Falchero, L.; Barattero, M.; Civra, A.; Lembo, D.; Poli, G.; Menta, R. Effect of industrial processing and storage procedures on oxysterols in milk and milk products. Food Funct. 2021, 12, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Xiao, F.; Jie, F.; Zhang, Q.; Liu, Y.; Xiao, H.; Lu, B. Dietary cholesterol oxidation products: Perspectives linking food processing and storage with health implications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 738–779. [Google Scholar] [CrossRef] [PubMed]
- Brzeska, M.; Szymczyk, K.; Szterk, A. Current knowledge about oxysterols: A review. J. Food Sci 2016, 81, R2299–R2308. [Google Scholar] [CrossRef] [PubMed]
- Hajeyah, A.A.; Griffiths, W.J.; Wang, Y.; Finch, A.J.; O’Donnell, V.B. The biosynthesis of enzymatically oxidized lipids. Front. Endocrinol. 2020, 11, 591819. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Sharpe, L.J.; Rogers, M.J. Oxysterols: From physiological tuners to pharmacological opportunities. Br. J. Pharmacol. 2021, 178, 3089–3103. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, K.M.; Lee, C.; Jo, Y.S.; Muradillaevna, M.S.; Kim, J.H.; Yoon, J.H.; Song, P. Pathophysiological role of 27-hydroxycholesterol in human diseases. Adv. Biol. Regul. 2022, 83, 100837. [Google Scholar] [CrossRef]
- Pataj, Z.; Liebisch, G.; Schmitz, G.; Matysik, S. Quantification of oxysterols in human plasma and red blood cells by liquid chromatography high-resolution tandem mass spectrometry. J. Chromatogr. A 2016, 1439, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Borah, K.; Rickman, O.J.; Voutsina, N.; Ampong, I.; Gao, D.; Baple, E.L.; Dias, I.H.K.; Crosby, A.H.; Griffiths, H.R. A Quantitative LC-MS/MS method for analysis of mitochondrial-specific oxysterol metabolism. Redox Biol. 2020, 36, 101595. [Google Scholar] [CrossRef] [PubMed]
- Borah, K.; Rickman, O.J.; Voutsina, N.; Baple, E.L.; Dias, I.H.; Crosby, A.H.; Griffiths, H.R. Datasets of whole cell and mitochondrial oxysterols derived from THP-1, SH-SY5Y and human peripheral blood mononuclear cells using targeted Metabolomics. Data Brief 2020, 33, 106382. [Google Scholar] [CrossRef] [PubMed]
- Helmschrodt, C.; Becker, S.; Schröter, J.; Hecht, M.; Aust, G.; Thiery, J.; Ceglarek, U. Fast LC–MS/MS analysis of free oxysterols derived from reactive oxygen species in human plasma and carotid plaque. Clinica. Chimica. Acta 2013, 425, 3–8. [Google Scholar] [CrossRef]
- Liu, Q.; An, Y.; Ma, W.; Feng, L.; Wang, C.; Lu, Y.; Xiao, R. High-cholesterol diet results in elevated amyloid-β and oxysterols in rats. Mol. Med. Rep. 2018, 17, 1235–1240. [Google Scholar] [CrossRef]
- Karu, K.; Hornshaw, M.; Woffendin, G.; Bodin, K.; Hamberg, M.; Alvelius, G.; Sjövall, J.; Turton, J.; Wang, Y.; Griffiths, W.J. Liquid chromatography-mass spectrometry utilizing multi-stage fragmentation for the identification of oxysterols. J. Lipid Res. 2007, 48, 976–987. [Google Scholar] [CrossRef]
- Testa, G.; Staurenghi, E.; Zerbinati, C.; Gargiulo, S.; Iuliano, L.; Giaccone, G.; Fantò, F.; Poli, G.; Leonarduzzi, G.; Gamba, P. Changes in brain oxysterols at different stages of alzheimer’s disease: Their involvement in neuroinflammation. Redox Biol. 2016, 10, 24–33. [Google Scholar] [CrossRef]
- Loera-Valencia, R.; Goikolea, J.; Parrado-Fernandez, C.; Merino-Serrais, P.; Maioli, S. Alterations in cholesterol metabolism as a risk factor for developing alzheimer’s disease: Potential novel targets for treatment. J. Steroid. Biochem. Mol. Biol. 2019, 190, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Gurlek, A.; Sendur, S.N.; Karahan, S.; Akbiyik, F.; Lay, I. Oxysterol species: Reliable markers of oxidative stress in diabetes mellitus. J. Endocrinol. Investig. 2019, 42, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Ménégaut, L.; Jalil, A.; Pilot, T.; van Dongen, K.; Crespy, V.; Steinmetz, E.; Pais de Barros, J.P.; Geissler, A.; Le Goff, W.; Venteclef, N.; et al. Regulation of glycolytic genes in human macrophages by oxysterols: A potential role for Liver X receptors. Br. J. Pharmacol. 2021, 178, 3124–3139. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.-J.; Zhang, C.; LeMaster, E.; Adamos, C.; Berdyshev, E.; Bogachkov, Y.; Kohler, E.E.; Baruah, J.; Fang, Y.; Schraufnagel, D.E.; et al. Oxidized LDL signals through Rho-GTPase to induce endothelial cell stiffening and promote capillary formation[S]. J. Lipid Res. 2016, 57, 791–808. [Google Scholar] [CrossRef]
- Foo, C.X.; Bartlett, S.; Ronacher, K. Oxysterols in the immune response to bacterial and viral infections. Cells 2022, 11, 201. [Google Scholar] [CrossRef]
- Cyster, J.C.; Dang, V.E.; Reboldi, A.; Yi, T. 25-Hydroxycholesterols in innate and adaptive immunity. Nat. Rev. Immunol. 2014, 14, 731–743. [Google Scholar] [CrossRef]
- Martin, L.A.; Kennedy, B.E.; Karten, B. Mitochondrial cholesterol: Mechanisms of import and effects on mitochondrial function. J. Bioenerg. Biomembr. 2016, 48, 137–151. [Google Scholar] [CrossRef]
- Garcia-Ruiz, C.; Conde de la Rosa, L.; Ribas, V.; Fernandez-Checa, J.C. Mitochondrial cholesterol and cancer. Semin Cancer Biol. 2021, 73, 76–85. [Google Scholar] [CrossRef]
- Pandak, M.W.; Kakiyama, G. The acidic pathway of bile acid synthesis: Not just an alternative pathway. Liver Res. 2019, 3, 88–98. [Google Scholar] [CrossRef]
- Gisterå, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef]
- Anand, P.K. Lipids, Inflammasomes, metabolism, and disease. Immunol. Rev. 2020, 297, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Campo, A.; Fulton, E.; Corwin, A.; Jerome, W.G.; O’Connor, M.S. 7-Ketocholesterol in disease and aging. Redox Biol. 2020, 29, 101380. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Wang, Y. Cholesterol metabolism: From lipidomics to immunology. J. Lipid Res. 2021, 100165. [Google Scholar] [CrossRef] [PubMed]
- Reinmuth, L.; Hsiao, C.-C.; Hamann, J.; Rosenkilde, M.; Mackrill, J. Multiple targets for oxysterols in their regulation of the immune system. Cells 2021, 10, 2078. [Google Scholar] [CrossRef]
- Samadi, A.; Sabuncuoglu, S.; Samadi, M.; Isikhan, S.Y.; Chirumbolo, S.; Peana, M.; Lay, I.; Yalcinkaya, A.; Bjørklund, G. A comprehensive review on oxysterols and related diseases. Curr. Med. Chem. 2021, 28, 110–136. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, C. 7a, 25-Dihydroxycholesterol-mediated activation of EBI2 in immune regulation and diseases. Front. Pharmacol. 2015, 6, 60. [Google Scholar] [CrossRef]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European atherosclerosis society consensus panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Rhoads, J.P.; Major, A.S. How oxidized low-density lipoprotein activates inflammatory responses. Crit. Rev. Immunol. 2018, 38, 333–342. [Google Scholar] [CrossRef]
- Truong, R.; Thankam, F.G.; Agrawal, D.G. Immunological mechanisms underlying sterile inflammation in the pathogenesis of atherosclerosis: Potential sites for intervention. Expert. Rev. Clin. Immunol. 2021, 17, 37–50. [Google Scholar] [CrossRef]
- Goulopoulou, S.; McCarthy, C.G.; Webb, R.C. Toll-like receptors in the vascular system: Sensing the dangers within. Pharmacol. Rev. 2016, 68, 142–167. [Google Scholar] [CrossRef] [PubMed]
- Zingg, J.-M.; Vlad, A.; Ricciarelli, R. Oxidized LDLs as signaling molecules. Antioxidants 2021, 10, 1184. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Melnichenko, A.A.; Wetzker, R.; Gerasimova, E.V.; Orekhov, A.N. NLPR3 inflammasomes and their significance for atherosclerosis. Biomedicines 2020, 8, 205. [Google Scholar] [CrossRef]
- Umetani, M.; Ghosh, P.; Ishikawa, T.; Umetani, J.; Ahmed, M.; Mineo, C.; Shaul, P.W. The cholesterol metabolite 27-hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor alpha. Cell Metabolism. 2014, 20, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Koseki, M.; Saga, A.; Kanno, K.; Higo, T.; Okuzaki, D.; Okada, T.; Inui, H.; Tanaka, K.; Asaji, M.; et al. Dietary Oxysterol, 7-Ketocholesterol accelerates hepatic lipid accumulation and macrophage infiltration in obese mice. Front. Endocrinol. 2021, 11, 614692. [Google Scholar] [CrossRef]
- Leonarduzzi, G.; Vizio, B.; Sottero, B.; Verde, V.; Gamba, P.; Mascia, C.; Chiarpotto, E.; Poli, G.; Biasi, F. Early involvement of ROS overproduction in apoptosis induced by 7-ketocholesterol. Antioxid. Redox Signal. 2006, 8, 375–380. [Google Scholar] [CrossRef]
- Pedruzzi, E.; Guichard, C.; Ollivier, V.; Driss, F.; Fay, M.; Prunet, C.; Marie, J.-C.; Pouzet, C.; Samadi, M.; Elbim, C.; et al. NAD(P)H oxidase nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell Biol. 2004, 24, 10703–10717. [Google Scholar] [CrossRef]
- Umetani, M.; Shaul, P.W. 27-Hydroxycholesterol: The first identified endogenous SERM. Trends Endocrinol. Metab. 2011, 22, 130–135. [Google Scholar] [CrossRef]
- Bilotta, M.T.; Petillo, S.; Santoni, A.; Cippitelli, M. Liver X receptors: Regulators of cholesterol metabolism, inflammation, autoimmunity, and cancer. Front. Immunol. 2020, 11, 584303. [Google Scholar] [CrossRef]
- Dang, E.V.; McDonald, J.G.; Russell, D.W.; Cyster, J.G. Oxysterol restraint of cholesterol synthesis prevents AIM2 inflammasome activation. Cell 2017, 171, 1057–1071.e11. [Google Scholar] [CrossRef]
- Gold, E.S.; Diercks, A.H.; Podolsky, I.; Podyminogin, R.L.; Askovich, P.S.; Treuting, P.M.; Aderem, A. 25-Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 10666–10671. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Park, S.; Jin Hur, H.; Cho, H.-J.; Hwang, I.; Pyo Kang, Y.; Im, I.; Lee, H.; Lee, E.; Yang, W.; et al. 25-Hydroxycholesterol contributes to cerebral inflammation of X-linked adrenoleukodystrophy through activation of the NLRP3 inflammasome. Nat. Commun. 2016, 7, 13129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Ren, S. Cholesterol metabolites 25-hydroxycholesterol and 25-hydroxycholesterol 3-sulfate are potent paired regulators: From discovery to clinical usage. Metabolites 2021, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Pandak, W.M.; Heuman, D.; Hylemon, P.B.; Ren, S. High glucose induces lipid accumulation via 25-hydroxycholesterol DNA-CpG methylation. iScience 2020, 23, 101102. [Google Scholar] [CrossRef]
- Testa, G.; Staurenghi, E.; Giannelli, S.; Gargiulo, S.; Guglielmotto, M.; Tabaton, M.; Tamagno, E.; Gamba, P.; Leonarduzzi, G. A Silver Lining for 24-Hydroxycholesterol in Alzheimer’s Disease: The involvement of the neuroprotective enzyme Sirtuin 1. Redox Biol. 2018, 17, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.Y.; Linsenbardt, A.; Emnett, C.; Eisenman, L.N.; Izumi, Y.; Zorumski, C.; Mennerick, S. 24(S)-hydroxycholesterol as a modulator of neuronal signaling and survival. Neuroscientist 2016, 22, 132–144. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and its roles in inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Buttari, B.; Profumo, E.; Segoni, L.; D’Arcangelo, D.; Rossi, S.; Facchiano, F.; Saso, L.; Businaro, R.; Iuliano, L.; Riganò, R. Resveratrol counteracts inflammation in human M1 and M2 macrophages upon challenge with 7-oxo-cholesterol: Potential therapeutic implications in atherosclerosis. Oxid. Med. Cell Longev. 2014, 2014, 257543. [Google Scholar] [CrossRef]
- Preuss, I.; Ludwig, M.-G.; Baumgarten, B.; Bassilana, F.; Gessier, F.; Seuwen, K.; Sailer, A.W. Transcriptional regulation and functional characterization of the oxysterol/EBI2 system in primary human macrophages. Biochem. Biophys. Res. Commun. 2014, 446, 663–668. [Google Scholar] [CrossRef]
- Hannedouche, S.; Zhang, J.; Yi, T.; Shen, W.; Nguyen, D.; Pereira, J.P.; Guerini, D.; Baumgarten, B.U.; Roggo, S.; Wen, B.; et al. Oxysterols direct immune cell migration via EBI2. Nature 2011, 475, 524–527. [Google Scholar] [CrossRef]
- Frostegård, J.; Zhang, Y.; Sun, J.; Yan, K.; Liu, A. Oxidized low-density lipoprotein (OxLDL)-treated dendritic cells promote activation of T cells in human atherosclerotic plaque and blood, which is repressed by statins: MicroRNA let-7c is integral to the effect. J. Am. Heart Assoc. 2016, 5, e003976. [Google Scholar] [CrossRef] [PubMed]
- Lembo, D.; Cagno, V.; Civra, A.; Poli, G. Oxysterols: An emerging class of broad spectrum antiviral effectors. Mol. Aspects. Med. 2016, 49, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.; Aliyari, R.; Chikere, K.; Li, G.; Marsden, M.D.; Smith, J.K.; Pernet, O.; Guo, H.; Nusbaum, R.; Zack, J.A.; et al. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity 2013, 38, 92–105. [Google Scholar] [CrossRef]
- Li, C.; Deng, Y.-Q.; Wang, S.; Ma, F.; Aliyari, R.; Huang, X.-Y.; Zhang, N.-N.; Watanabe, M.; Dong, H.-L.; Liu, P.; et al. 25-Hydroxycholesterol protects host against zika virus infection and its associated microcephaly in a mouse model. Immunity 2017, 46, 446–456. [Google Scholar] [CrossRef]
- Romero-Brey, I.; Berger, C.; Colpitts, C.C.; Boldanova, T.; Engelmann, M.; Todt, D.; Perin, P.M.; Behrendt, P.; Vondran, F.W.R.; Xu, S.; et al. Interferon-inducible cholesterol-25-hydroxylase restricts hepatitis C virus replication through blockage of membranous web formation. Hepatology 2015, 62, 702–714. [Google Scholar]
- Gatto, D.; Wood, K.; Caminschi, I.; Murphy-Durland, D.; Schofield, P.; Christ, D.; Karupiah, G.; Brink, R. The chemotactic receptor EBI2 regulates the homeostasis, localization and immunological function of splenic dendritic cells. Nat. Immunol. 2013, 14, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Cyster, J.G. EBI2-mediated bridging channel positioning supports splenic dendritic cell homeostasis and particulate antigen capture. eLife 2013, 2, e00757. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Yang, Q.; Xie, W.; Zhou, J. Liver X receptor regulates mouse GM-CSF-derived dendritic cell differentiation in vitro. Mol. Immunol. 2014, 60, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Kim, B.-Y.; Eo, S.-K.; Park, Y.C.; Kim, K. Dexamethasone suppresses oxysterol-induced differentiation of monocytic cells. Oxid. Med. Cell Longev. 2016, 2016, 2915382. [Google Scholar] [CrossRef]
- Pathan-Chhatbar, S.; Drechsler, C.; Richter, K.; Morath, A.; Wu, W.; OuYang, B.; Xu, C.; Schamel, W.W. Direct regulation of the T cell antigen receptor’s activity by cholesterol. Front. Cell Dev. Biol. 2021, 8, 615996. [Google Scholar] [CrossRef]
- Yang, W.; Bai, Y.; Xiong, Y.; Zhang, J.; Chen, S.; Zheng, X.; Meng, X.; Li, L.; Wang, J.; Xu, C.; et al. Potentiating the antitumor response of CD8(+) T cells by modulating cholesterol metabolism. Nature 2016, 531, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Kidani, Y.; Elsaesser, H.; Hock, M.B.; Vergnes, L.; Williams, K.J.; Argus, J.P.; Marbois, B.N.; Komisopoulou, E.; Wilson, E.B.; Osborne, T.F.; et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat. Immunol. 2013, 14, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Bensinger, S.J.; Bradley, M.N.; Joseph, S.B.; Zelcer, N.; Janssen, E.M.; Hausner, M.A.; Shih, R.; Parks, J.S.; Edwards, P.A.; Jamieson, B.D.; et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 2008, 134, 97–111. [Google Scholar] [CrossRef]
- Kuzu, O.F.; Noory, M.A.; Robertson, G.P. The role of cholesterol in cancer. Cancer Res. 2016, 76, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Bi, E.; Lu, Y.; Su, P.; Huang, C.; Liu, L.; Wang, Q.; Yang, M.; Kalady, M.F.; Qian, J.; et al. Cholesterol induces CD8+ T cell exhaustion in the tumor microenvironment. Cell Metab. 2019, 30, 143–156.e5. [Google Scholar] [CrossRef]
- Huang, B.; Song, B.-L.; Xu, C. Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2020, 2, 132–141. [Google Scholar] [CrossRef]
- Lu, E.; Dang, E.V.; McDonald, J.G.; Cyster, J.G. Distinct oxysterol requirements for positioning naïve and activated dendritic cells in the spleen. Sci. Immunol. 2017, 2, eaal5237. [Google Scholar] [CrossRef]
- Chalmin, F.; Rochemont, V.; Lippens, C.; Clottu, A.; Sailer, A.W.; Merkler, D.; Hugues, S.; Pot, C. Oxysterols regulate encephalitogenic CD4(+) T cell trafficking during central nervous system autoimmunity. J. Autoimmun 2015, 56, 45–55. [Google Scholar] [CrossRef]
- Soroosh, P.; Wu, J.; Xue, X.; Song, J.; Sutton, S.W.; Sablad, M.; Yu, J.; Nelen, M.I.; Liu, X.; Castro, G.; et al. Oxysterols are agonist ligands of RORγt and drive Th17 cell differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 12163–12168. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Hao, L.-Y.; Liu, X.; Lesch, C.A.; Sanchez, B.M.; Wendling, J.M.; Morgan, R.W.; Aicher, T.D.; Carter, L.L.; et al. Sterol metabolism controls Th17 differentiation by generating endogenous RORγ agonists. Nat. Chem. Biol. 2015, 11, 141–147. [Google Scholar] [CrossRef]
- Cai, F.; Jin, S.; Chen, G. The effect of lipid metabolism on CD4+ T cells. Mediat. Inflamm. 2021, 2021, e6634532. [Google Scholar] [CrossRef] [PubMed]
- Vigne, S.; Chalmin, F.; Duc, D.; Clottu, A.S.; Apetoh, L.; Lobaccaro, J.-M.A.; Christen, I.; Zhang, J.; Pot, C. IL-27-induced type 1 regulatory T-cells produce oxysterols that constrain IL-10 production. Front. Immunol. 2017, 8, 1184. [Google Scholar] [CrossRef] [PubMed]
- Perucha, E.; Melchiotti, R.; Bibby, J.A.; Wu, W.; Frederiksen, K.S.; Roberts, C.A.; Hall, Z.; LeFriec, G.; Robertson, K.A.; Lavender, P.; et al. The cholesterol biosynthesis pathway regulates IL-10 expression in human Th1 cells. Nat. Commun. 2019, 10, 498. [Google Scholar] [CrossRef]
- Mahlangu, T.J.; Dludla, P.V.; Mxinwa, V.; Mkandla, Z.; Tiano, L.; Louw, J.; Mutize, T.; Nyambuya, T.M.; Nkambule, B.B. Elevated T-helper 2 cytokine levels in high fat diet-fed C57BL/6 mice are attenuated by short-term 6-week treatment with a combination of low-dose aspirin and metformin. Cytokine 2020, 128, 154999. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J. Impact of dietary cholesterol on the pathophysiology of infectious and autoimmune disease. Nutrients 2018, 10, E764. [Google Scholar] [CrossRef]
- Al-Shawwa, B.; Al-Huniti, N.; Titus, G.; Abu-Hasan, M. Hypercholesterolemia is a potential risk factor for asthma. J. Asthma 2006, 43, 231–233. [Google Scholar] [CrossRef]
- Fessler, M.B.; Massing, M.W.; Spruell, B.; Jaramillo, R.; Draper, D.W.; Madenspacher, J.H.; Arbes, S.J.; Calatroni, A.; Zeldin, D.C. Novel relationship of serum cholesterol with asthma and wheeze in the United States. J. Allergy Clin. Immunol. 2009, 124, 967–974.e15. [Google Scholar] [CrossRef]
- Verstegen, N.J.M.; Ubels, V.; Westerhoff, H.V.; van Ham, S.M.; Barberis, M. System-level scenarios for the elucidation of T cell-mediated germinal center B cell differentiation. Front. Immunol. 2021, 12, 734282. [Google Scholar] [CrossRef]
- Ricker, E.; Chinenov, Y.; Pannellini, T.; Flores-Castro, D.; Ye, C.; Gupta, S.; Manni, M.; Liao, J.K.; Pernis, A.B. Serine-threonine kinase ROCK2 regulates germinal center B cell positioning and cholesterol biosynthesis. J. Clin. Investig. 2020, 130, 3654–3670. [Google Scholar] [CrossRef]
- Liu, C.; Yang, X.V.; Wu, J.; Kuei, C.; Mani, N.S.; Zhang, L.; Yu, J.; Sutton, S.W.; Qin, N.; Banie, H.; et al. Oxysterols direct B-cell migration through EBI2. Nature 2011, 475, 519–523. [Google Scholar] [CrossRef]
- Gatto, D.; Brink, R. B cell localization: Regulation by EBI2 and its oxysterol ligand. Trends Immunol. 2013, 34, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Wang, X.; Kelly, L.M.; An, J.; Xu, Y.; Sailer, A.W.; Gustafsson, J.-A.; Russell, D.W.; Cyster, J.G. Oxysterol gradient generation by lymphoid stromal cells guides activated B cell movement during humoral responses. Immunity 2012, 37, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Trindade, B.C.; Ceglia, S.; Berthelette, A.; Raso, F.; Howley, K.; Muppidi, J.R.; Reboldi, A. The cholesterol metabolite 25-hydroxycholesterol restrains the transcriptional regulator SREBP2 and limits intestinal IgA plasma cell differentiation. Immunity 2021, 54, 2273–2287.e6. [Google Scholar] [CrossRef] [PubMed]
- Emgård, J.; Kammoun, H.; García-Cassani, B.; Chesné, J.; Parigi, S.M.; Jacob, J.-M.; Cheng, H.-W.; Evren, E.; Das, S.; Czarnewski, P.; et al. Oxysterol sensing through the receptor GPR183 promotes the lymphoid-tissue-inducing function of innate lymphoid cells and colonic inflammation. Immunity 2018, 48, 120–132.e8. [Google Scholar] [CrossRef] [PubMed]
- Wyss, A.; Raselli, T.; Perkins, N.; Ruiz, F.; Schmelczer, G.; Klinke, G.; Moncsek, A.; Roth, R.; Spalinger, M.R.; Hering, L.; et al. The EBI2-oxysterol axis promotes the development of intestinal lymphoid structures and colitis. Mucosal. Immunol. 2019, 12, 733–745. [Google Scholar]
- Cui, Y.; Lu, S.; Xu, J.; Peng, Y.; Miao, Q.; Wang, X.; Chen, X.; Ran, Z. Microscopic features of small bowel mucosa of patients with Crohn’s disease. BMC Gastroenterol. 2019, 19, 232. [Google Scholar] [CrossRef]
- Willinger, T. Metabolic control of innate lymphoid cell migration. Front. Immunol. 2019, 10, 2010. [Google Scholar] [CrossRef]
- Ruiz, F.; Wyss, A.; Rossel, J.-B.; Sulz, M.C.; Brand, S.; Moncsek, A.; Mertens, J.C.; Roth, R.; Clottu, A.S.; Burri, E.; et al. A single nucleotide polymorphism in the gene for GPR183 increases its surface expression on blood lymphocytes of patients with inflammatory bowel disease. Br. J. Pharmacol. 2021, 178, 3157–3175. [Google Scholar] [CrossRef]
- Misselwitz, B.; Wyss, A.; Raselli, T.; Cerovic, V.; Sailer, A.W.; Krupka, N.; Ruiz, F.; Pot, C.; Pabst, O. The oxysterol receptor GPR183 in inflammatory bowel diseases. Br. J. Pharmacol. 2021, 178, 3140–3156. [Google Scholar]
- Majdoul, S.; Compton, A.A. Lessons in Self-defence: Inhibition of virus entry by intrinsic immunity. Nat. Rev. Immunol. 2022, 22, 339–352. [Google Scholar] [CrossRef]
- Rahman, K.; Datta, S.A.K.; Beaven, A.H.; Jolley, A.A.; Sodt, A.J.; Compton, A.A. Cholesterol binds the amphipathic helix of IFITM3 and regulates antiviral activity. J. Mol. Biol. 2022, 434, 167759. [Google Scholar] [CrossRef] [PubMed]
- Abrams, M.E.; Johnson, K.A.; Perelman, S.S.; Zhang, L.; Endapally, S.; Mar, K.B.; Thompson, B.M.; McDonald, J.G.; Schoggins, J.W.; Radhakrishnan, A.; et al. Oxysterols provide innate immunity to bacterial infection by mobilizing cell surface accessible cholesterol. Nat. Microbiol. 2020, 5, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Chandramohan, D. Risk of active tuberculosis among people with diabetes mellitus: Systematic review and meta-analysis. Trop. Med. Int. Health. 2018, 23, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Vrieling, F.; Wilson, L.; Rensen, P.C.N.; Walzl, G.; Ottenhoff, T.H.M.; Joosten, S.A. Oxidized low-density lipoprotein (OxLDL) aupports mycobacterium tuberculosis survival in macrophages by inducing lysosomal dysfunction. PLoS Pathog. 2019, 15, e1007724. [Google Scholar] [CrossRef]
- Schoeneck, M.; Iggman, D. The Effects of Foods on LDL Cholesterol Levels: A systematic review of the accumulated evidence from systematic reviews and meta-analyses of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1325–1338. [Google Scholar] [CrossRef]
- Malhotra, A.; Shafiq, N.; Arora, A.; Singh, M.; Kumar, R.; Malhotra, S. Dietary interventions (plant sterols, stanols, omega-3 fatty acids, soy protein and dietary fibers) for familial hypercholesterolaemia. Cochrane Database Syst. Rev. 2014, 6, CD001918. [Google Scholar] [CrossRef]
- Hills, R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut microbiome: Profound implications for diet and disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Joyce, S.A.; Kamil, A.; Fleige, L.; Gahan, C.G.M. The cholesterol-lowering effect of oats and oat beta glucan: Modes of action and potential role of bile acids and the microbiome. Front. Nutr. 2019, 6, 171. [Google Scholar] [CrossRef]
- He, W.-S.; Zhu, H.; Chen, Z.-Y. Plant sterols: Chemical and enzymatic structural modifications and effects on their cholesterol-lowering activity. J. Agric. Food Chem. 2018, 66, 3047–3062. [Google Scholar] [CrossRef]
- Trautwein, E.A.; Vermeer, M.A.; Hiemstra, H.; Ras, R.T. LDL-cholesterol lowering of plant sterols and stanols-which factors influence their efficacy? Nutrients 2018, 10, E1262. [Google Scholar] [CrossRef]
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Pizzini, A.; Lunger, L.; Demetz, E.; Hilbe, R.; Weiss, G.; Ebenbichler, C.; Tancevski, I. The role of omega-3 fatty acids in reverse cholesterol transport: A review. Nutrients 2017, 9, E1099. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhao, L.; Zhang, N.; Zhou, J.; Zhang, L.; Wu, W.; Ji, B.; Zhou, F. Bioactivity of dietary polyphenols: The role in LDL-C lowering. Foods 2021, 10, 2666. [Google Scholar] [CrossRef] [PubMed]
- Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of polyphenols in the management of dyslipidemia: A focus on clinical studies. Nutrients 2021, 13, 672. [Google Scholar] [CrossRef] [PubMed]
- Naghshi, S.; Sadeghi, O.; Willett, W.C.; Esmaillzadeh, A. Dietary intake of total, animal, and plant proteins and risk of all cause, cardiovascular, and cancer mortality: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2020, 370, m2412. [Google Scholar] [CrossRef]
- Chalvon-Demersay, T.; Azzout-Marniche, D.; Arfsten, J.; Egli, L.; Gaudichon, C.; Karagounis, L.G.; Tomé, D. A systematic review of the effects of plant compared with animal protein sources on features of metabolic syndrome. J. Nutr. 2017, 147, 281–292. [Google Scholar] [CrossRef]
- Nagaoka, S.; Takeuchi, A.; Banno, A. Plant-derived peptides improving lipid and glucose metabolism. Peptides 2021, 142, 170577. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, E1738. [Google Scholar] [CrossRef]
- Andersson, K.E.; Hellstrand, P. Dietary oats and modulation of atherogenic pathways. Mol. Nutr. Food Res. 2012, 56, 1003–1013. [Google Scholar] [CrossRef]
- Pifferi, C.; Fuentes, R.; Fernández-Tejada, A. Natural and synthetic carbohydrate-based vaccine adjuvants and their mechanisms of action. Nat. Rev. Chem. 2021, 5, 197–216. [Google Scholar] [CrossRef]
- Pan, W.; Hao, S.; Zheng, M.; Lin, D.; Jiang, P.; Zhao, J.; Shi, H.; Yang, X.; Li, X.; Yu, Y. Oat-derived β-glucans induced trained immunity through metabolic reprogramming. Inflammation 2020, 43, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- van der Hee, B.; Wells, J.M. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut microbial metabolites fuel host antibody responses. Cell Host. Microbe. 2016, 20, 202–214. [Google Scholar] [CrossRef]
- Kawamoto, S.; Maruya, M.; Kato, L.M.; Suda, W.; Atarashi, K.; Doi, Y.; Tsutsui, Y.; Qin, H.; Honda, K.; Okada, T.; et al. Foxp3+ T cells regulate immunoglobulin A selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity 2014, 41, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; She, Y.; Kaur, R.; Guo, N.; Zhang, X.; Zhang, R.; Gou, X. Is plant sterols a good strategy to lower cholesterol? J. Oleo Sci. 2019, 68, 811–816. [Google Scholar] [CrossRef]
- Nattagh-Eshtivani, E.; Barghchi, H.; Pahlavani, N.; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; Ziaei, R.; et al. Biological and pharmacological effects and nutritional impact of phytosterols: A comprehensive review. Phytother. Res. 2022, 36, 299–322. [Google Scholar] [CrossRef]
- Marahatha, R.; Gyawali, K.; Sharma, K.; Gyawali, N.; Tandan, P.; Adhikari, A.; Timilsina, G.; Bhattarai, S.; Lamichhane, G.; Acharya, A.; et al. Pharmacologic activities of phytosteroids in inflammatory diseases: Mechanism of action and therapeutic potentials. Phytother. Res. 2021, 35, 5103–5124. [Google Scholar] [CrossRef]
- Valerio, M.S.; Minderman, H.; Mace, T.; Awad, A.B. β-Sitosterol modulates TLR4 receptor expression and intracellular MyD88-dependent pathway activation in J774A.1 murine macrophages. Cell Immunol. 2013, 285, 76–83. [Google Scholar] [CrossRef]
- Brüll, F.; De Smet, E.; Mensink, R.P.; Vreugdenhil, A.; Kerksiek, A.; Lütjohann, D.; Wesseling, G.; Plat, J. Dietary plant stanol ester consumption improves immune function in asthma patients: Results of a randomized, double-blind clinical trial1. Am. J. Clin. Nutr. 2016, 103, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Stanasila, L.; Marques-Vidal, P. Serum phytosterols are not associated with inflammatory markers in two cross-sectional, Swiss population-based studies (the CoLaus|PsyCoLaus study). Nutrients 2022, 14, 2500. [Google Scholar] [CrossRef]
- Mohammad Shahi, M.; Javanmardi, M.A.; Seyedian, S.S.; Haghighizadeh, M.H. Effects of phytosterol supplementation on serum levels of lipid profiles, liver enzymes, inflammatory markers, adiponectin, and leptin in patients affected by nonalcoholic fatty liver disease: A double-blind, placebo-controlled, randomized clinical trial. J. Am. Coll. Nutr. 2018, 37, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Varikuti, S.; Shelton, A.B.; Kotha, S.R.; Gurney, T.; Gupta, G.; Hund, T.J.; Fuchs, J.R.; Kinghorn, A.D.; Srivastava, N.; Satoskar, A.R.; et al. Pentalinonsterol, a phytosterol from Pentalinon Andrieuxii, is immunomodulatory through phospholipase A2 in macrophages toward its antileishmanial action. Cell Biochem. Biophys. 2022, 80, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.M.; Cheng, A.Y.Y.; Gilbert, J.D.; Lonn, E.M.; Pedersen, S.D.; Verma, S. The role of icosapent ethyl in cardiovascular risk reduction. Curr. Opin. Cardiol. 2021, 36, 661–671. [Google Scholar] [CrossRef]
- Balentine, D. RE: Petition for a Health Claim for Eicosapentaenoic Acid and Docosahexaenoic Acid and Reduction of Blood Pressure in the General Population (Docket no. FDA-2014-Q-1146). US Food and Drug Administration Website. 2019. Available online: https://www.fda.gov/media/128043/ (accessed on 15 August 2019).
- US Food and Drug Administration. Label Claims for Conventional Foods and Dietary Supplements. 2022. Available online: https://www.fda.gov/food/food-labeling-nutrition/label-claims-conventional-foods-and-dietary-supplements (accessed on 7 March 2022).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), docosapentaenoic acid (DPA) and maintenance of normal cardiac function (ID 504, 506, 516, 527, 538, 703, 1128, 1317, 1324, 1325), maintenance of normal blood glucose concentrations (ID 566), maintenance of normal blood pressure (ID 506, 516, 703, 1317, 1324), maintenance of normal blood HDL-cholesterol concentrations (ID 506), maintenance of normal (Fasting) blood concentrations of triglycerides (ID 506, 527, 538, 1317, 1324, 1325), maintenance of normal blood LDL-cholesterol concentrations (ID 527, 538, 1317, 1325, 4689), protection of the skin from photo-oxidative (UV-Induced) damage (ID 530), improved absorption of EPA and DHA (ID 522, 523), contribution to the normal function of the immune system by decreasing the levels of eicosanoids, arachidonic acid-derived mediators and pro-inflammatory cytokines (ID 520, 2914), and “immunomodulating agent” (4690) pursuant to article 13(1) of regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1796. [Google Scholar]
- Zaloga, G.P. Narrative review of N-3 polyunsaturated fatty acid supplementation upon immune functions, resolution molecules and lipid peroxidation. Nutrients 2021, 13, 662. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Rodríguez-Cruz, M.; Serna, D.S. Nutrigenomics of ω-3 fatty acids: Regulators of the master transcription factors. Nutrition 2017, 41, 90–96. [Google Scholar] [CrossRef]
- Li, C.; Wu, X.; Liu, S.; Shen, D.; Zhu, J.; Liu, K. Role of resolvins in the inflammatory resolution of neurological diseases. Front. Pharmacol. 2020, 11, 612. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, F.; Kovanen, P.T.; Mardani, R.; Gheibi-Hayat, S.M.; Bo, S.; Sahebkar, A. Resolvins: Emerging players in autoimmune and inflammatory diseases. Clin. Rev. Allergy Immunol 2020, 58, 82–91. [Google Scholar] [CrossRef]
- Chiang, N.; Libreros, S.; Norris, P.C.; de la Rosa, X.; Serhan, C.N. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J. Clin. Investig. 2019, 129, 5294–5311. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009, 206, 15–23. [Google Scholar] [CrossRef] [PubMed]
- El Kebir, D.; Gjorstrup, P.; Filep, J.G. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc. Natl. Acad. Sci. USA 2012, 109, 14983–14988. [Google Scholar] [CrossRef]
- Krishnamoorthy, N.; Burkett, P.R.; Dalli, J.; Abdulnour, R.-E.E.; Colas, R.; Ramon, S.; Phipps, R.P.; Petasis, N.A.; Kuchroo, V.K.; Serhan, C.N.; et al. Maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J. Immunol. 2015, 194, 863–867. [Google Scholar] [CrossRef]
- Perez-Hernandez, J.; Chiurchiù, V.; Perruche, S.; You, S. Regulation of T-cell immune responses by pro-resolving lipid mediators. Front. Immunol. 2021, 12, 768133. [Google Scholar] [CrossRef]
- Fadiyah, N.N.; Megawati, G.; Luftimas, D.E. Potential of omega 3 supplementation for coronavirus disease 2019 (COVID-19): A scoping review. IJGM 2022, 15, 3915–3922. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nishizawa, M.; Inoue, N.; Hosoya, T.; Yoshida, M.; Ukawa, Y.; Sagesaka, Y.M.; Doi, T.; Nakayama, T.; Kumazawa, S.; et al. Epigallocatechin gallate decreases the micellar solubility of cholesterol via specific interaction with phosphatidylcholine. J. Agric. Food Chem. 2014, 62, 2881–2890. [Google Scholar] [CrossRef]
- Koo, S.I.; Noh, S.K. Green tea as inhibitor of the intestinal absorption of lipids: Potential mechanism for its lipid-lowering effect. J. Nutr. Biochem. 2007, 18, 179–183. [Google Scholar] [CrossRef]
- Kobayashi, S. The effect of polyphenols on hypercholesterolemia through inhibiting the transport and expression of Niemann-Pick C1-Like 1. Int. J. Mol. Sci. 2019, 20, E4939. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Zou, J.; Jiang, X.; Yang, J.; Cao, Z.; He, Y.; Feng, D. Curcumin supplementation ameliorates bile cholesterol supersaturation in hamsters by modulating gut microbiota and cholesterol absorption. Nutrients 2022, 14, 1828. [Google Scholar] [CrossRef]
- Melendez, Q.M.; Krishnaji, S.T.; Wooten, C.J.; Lopez, D. Hypercholesterolemia: The role of PCSK9. Arch. Biochem. Biophys. 2017, 625–626, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Adorni, M.P.; Zimetti, F.; Lupo, M.G.; Ruscica, M.; Ferri, N. Naturally occurring PCSK9 inhibitors. Nutrients 2020, 12, 1440. [Google Scholar] [CrossRef] [PubMed]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol effects on cholesterol metabolism via bile acid biosynthesis, CYP7A1: A review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Hariri, M. Effect of resveratrol on lipid profile: An updated systematic review and meta-analysis on randomized clinical trials. Pharmacol. Res. 2018, 129, 141–150. [Google Scholar] [CrossRef]
- Sahebkar, A. A systematic review and meta-analysis of randomized controlled trials investigating the effects of curcumin on blood lipid levels. Clin. Nutr. 2014, 33, 406–414. [Google Scholar] [CrossRef]
- Xu, R.; Yang, K.; Li, S.; Dai, M.; Chen, G. Effect of green tea consumption on blood lipids: A systematic review and meta-analysis of randomized controlled trials. Nutr. J. 2020, 19, 48. [Google Scholar] [CrossRef]
- del Cornò, M.; Scazzocchio, B.; Masella, R.; Gessani, S. Regulation of dendritic cell function by dietary polyphenols. Crit. Rev. Food Sci. Nutr. 2016, 56, 737–747. [Google Scholar] [CrossRef]
- Behl, T.; Rana, T.; Alotaibi, G.H.; Shamsuzzaman, M.; Naqvi, M.; Sehgal, A.; Singh, S.; Sharma, N.; Almoshari, Y.; Abdellatif, A.A.H.; et al. Polyphenols Inhibiting MAPK signaling pathway mediated oxidative stress and inflammation in depression. Biomed. Pharmacother. 2022, 146, 112545. [Google Scholar] [CrossRef] [PubMed]
- Borra, M.T.; Smith, B.C.; Denu, J.M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005, 280, 17187–17195. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-C.; Jung, M.G.; Lee, Y.-H.; Yoon, J.C.; Kwon, S.H.; Kang, H.-B.; Kim, M.-J.; Cha, J.-H.; Kim, Y.J.; Jun, W.J.; et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2009, 69, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Chung, S. Dietary polyphenols, deacetylases and chromatin remodeling in inflammation. J. Nutrigenet. Nutr. 2011, 3, 220–230. [Google Scholar] [CrossRef]
- Shim, J.-H.; Choi, H.S.; Pugliese, A.; Lee, S.-Y.; Chae, J.-I.; Choi, B.Y.; Bode, A.M.; Dong, Z. (-)-Epigallocatechin gallate regulates CD3-mediated T cell receptor signaling in leukemia through the inhibition of ZAP-70 kinase. J. Biol. Chem. 2008, 283, 28370–28379. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.P.; Nguyen, L.P.; Noh, S.K.; Bray, T.M.; Bruno, R.S.; Ho, E. Induction of regulatory T cells by green tea polyphenol EGCG. Immunol. Lett. 2011, 139, 7–13. [Google Scholar] [CrossRef]
- Chang, Y.; Zhai, L.; Peng, J.; Wu, H.; Bian, Z.; Xiao, H. Phytochemicals as regulators of Th17/Treg balance in inflammatory bowel diseases. Biomed. Pharmacother. 2021, 141, 111931. [Google Scholar] [CrossRef]
- Wang, H.-K.; Yeh, C.-H.; Iwamoto, T.; Satsu, H.; Shimizu, M.; Totsuka, M. Dietary flavonoid naringenin induces regulatory T cells via an aryl hydrocarbon receptor mediated pathway. J. Agric. Food Chem. 2012, 60, 2171–2178. [Google Scholar] [CrossRef]
- Zhong, C.; Zhu, J. Transcriptional regulators dictate innate lymphoid cell fates. Protein Cell. 2017, 8, 242–254. [Google Scholar] [CrossRef]
- Mittal, M.; Mehta, P.; Rajput, S.; Rajender, S.; Chattopadhyay, N. The pharmacological assessment of resveratrol on preclinical models of rheumatoid arthritis through a systematic review and meta-analysis. Eur. J. Pharmacol. 2021, 910, 174504. [Google Scholar] [CrossRef]
- Zeng, B.; Jiang, T.; Xiong, W.; Che, H.; Sun, S. Protective Properties of Polyphenols in Food Allergy: A Review. Allergy 2022. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory effects of dietary polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Omraninava, M.; Razi, B.; Aslani, S.; Imani, D.; Jamialahmadi, T.; Sahebkar, A. Effect of resveratrol on inflammatory cytokines: A meta-analysis of randomized controlled trials. Eur. J. Pharmacol. 2021, 908, 174380. [Google Scholar] [CrossRef] [PubMed]
- Giang, J.; Lan, X.; Crichton, M.; Marx, W.; Marshall, S. Efficacy and safety of biophenol-rich nutraceuticals in adults with inflammatory gastrointestinal diseases or irritable bowel syndrome: A systematic literature review and meta-analysis. Nutr. Diet. 2022, 79, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wei, L.; Wang, N.; Li, X.; Miao, M. Efficacy and safety of adjuvant curcumin therapy in ulcerative colitis: A systematic review and meta-analysis. J. Ethnopharmacol. 2022, 289, 115041. [Google Scholar] [CrossRef]
- Food Labeling: Health Claims; Soy Protein and Coronary Heart Disease. Food and Drug Administration, HHS. Final Rule. Fed. Regist. 1999, 64, 57700–57733. [Google Scholar]
- Anna, K.A. Food Labeling: Health Claims; Soy Protein and Coronary Heart Disease. 2017. Available online: https://www.federalregister.gov/documents/2017/10/31/2017-23629/food-labeling-health-claims-soy-protein-and-coronary-heart-disease (accessed on 31 October 2017).
- Blanco Mejia, S.; Messina, M.; Li, S.S.; Viguiliouk, E.; Chiavaroli, L.; Khan, T.A.; Srichaikul, K.; Mirrahimi, A.; Sievenpiper, J.L.; Kris-Etherton, P.; et al. A meta-analysis of 46 studies identified by the FDA demonstrates that soy protein decreases circulating LDL and total cholesterol concentrations in adults. J. Nutr. 2019, 149, 968–981. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of a health claim related to isolated soy protein and reduction of blood LDL-cholesterol concentrations pursuant to article 14 of regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2555. [Google Scholar] [CrossRef]
- Hashidume, T.; Kato, A.; Tanaka, T.; Miyoshi, S.; Itoh, N.; Nakata, R.; Inoue, H.; Oikawa, A.; Nakai, Y.; Shimizu, M.; et al. Single ingestion of soy β-conglycinin induces increased postprandial circulating FGF21 levels exerting beneficial health effects. Sci. Rep. 2016, 6, 28183. [Google Scholar] [CrossRef]
- Arellano-Martínez, G.L.; Granados, O.; Palacios-González, B.; Torres, N.; Medina-Vera, I.; Tovar, A.R. Soya protein stimulates bile acid excretion by the liver and intestine through direct and indirect pathways influenced by the presence of dietary cholesterol. Br. J. Nutr. 2014, 111, 2059–2066. [Google Scholar] [CrossRef]
- Busnelli, M.; Manzini, S.; Sirtori, C.R.; Chiesa, G.; Parolini, C. Effects of vegetable proteins on hypercholesterolemia and gut microbiota modulation. Nutrients 2018, 10, 1249. [Google Scholar] [CrossRef] [PubMed]
- Katz, Y.; Gutierrez-Castrellon, P.; González, M.G.; Rivas, R.; Lee, B.W.; Alarcon, P. A comprehensive review of sensitization and allergy to soy-based products. Clin. Rev. Allergy Immunol. 2014, 46, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.L.; Eigenmann, P.A.; Sicherer, S.H. Clinical relevance of cross-reactivity in food allergy. J. Allergy Clin. Immunol. Pract. 2021, 9, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Kolarich, D.; Altmann, F. N-glycan analysis by matrix-assisted laser desorption/ionization mass spectrometry of electrophoretically separated nonmammalian proteins: Application to peanut allergen Ara h 1 and olive pollen allergen Ole e 1. Anal. Biochem. 2000, 285, 64–75. [Google Scholar] [CrossRef]
- Krause, M.; Crauwels, P.; Blanco-Pérez, F.; Globisch, M.; Wangorsch, A.; Henle, T.; Lidholm, J.; van Zandbergen, G.; Vieths, S.; Scheurer, S.; et al. Human monocyte-derived type 1 and 2 macrophages recognize Ara h 1, a major peanut allergen, by different mechanisms. Sci. Rep. 2021, 11, 10141. [Google Scholar] [CrossRef]
- Shreffler, W.G.; Castro, R.R.; Kucuk, Z.Y.; Charlop-Powers, Z.; Grishina, G.; Yoo, S.; Burks, A.W.; Sampson, H.A. The major glycoprotein allergen from arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J. Immunol. 2006, 177, 3677–3685. [Google Scholar] [CrossRef]
- Picariello, G.; Amigo-Benavent, M.; del Castillo, M.D.; Ferranti, P. Structural characterization of the N-glycosylation of individual soybean β-conglycinin subunits. J.Chromatogr. A 2013, 1313, 96–102. [Google Scholar] [CrossRef]
- Yi, G.; Li, H.; Liu, M.; Ying, Z.; Zhang, J.; Liu, X. Soybean protein-derived peptides inhibit inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4-mediated MAPK-JNK and NF-Kappa B activation. J. Food Biochem. 2020, 44, e13289. [Google Scholar] [CrossRef]
- Rein, D.; Ternes, P.; Demin, R.; Gierke, J.; Helgason, T.; Schön, C. Artificial intelligence identified peptides modulate inflammation in healthy adults. Food Funct. 2019, 10, 6030–6041. [Google Scholar] [CrossRef]
- Kamgang Nzekoue, F.; Henle, T.; Caprioli, G.; Sagratini, G.; Hellwig, M. Food protein sterylation: Chemical reactions between reactive amino acids and sterol oxidation products under food processing conditions. Foods 2020, 9, 1882. [Google Scholar] [CrossRef]
- Feng, S.; Wang, L.; Shao, P.; Sun, P.; Yang, C.S. A review on chemical and physical modifications of phytosterols and their influence on bioavailability and safety. Crit. Rev. Food Sci. Nutr. 2022, 62, 5638–5657. [Google Scholar] [CrossRef] [PubMed]
- van Daal, M.T.; Folkerts, G.; Garssen, J.; Braber, S. Pharmacological modulation of immune responses by nutritional components. Pharmacol. Rev. 2021, 73, 198–232. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanagisawa, R.; He, C.; Asai, A.; Hellwig, M.; Henle, T.; Toda, M. The Impacts of Cholesterol, Oxysterols, and Cholesterol Lowering Dietary Compounds on the Immune System. Int. J. Mol. Sci. 2022, 23, 12236. https://doi.org/10.3390/ijms232012236
Yanagisawa R, He C, Asai A, Hellwig M, Henle T, Toda M. The Impacts of Cholesterol, Oxysterols, and Cholesterol Lowering Dietary Compounds on the Immune System. International Journal of Molecular Sciences. 2022; 23(20):12236. https://doi.org/10.3390/ijms232012236
Chicago/Turabian StyleYanagisawa, Rintaro, Chaoqi He, Akira Asai, Michael Hellwig, Thomas Henle, and Masako Toda. 2022. "The Impacts of Cholesterol, Oxysterols, and Cholesterol Lowering Dietary Compounds on the Immune System" International Journal of Molecular Sciences 23, no. 20: 12236. https://doi.org/10.3390/ijms232012236
APA StyleYanagisawa, R., He, C., Asai, A., Hellwig, M., Henle, T., & Toda, M. (2022). The Impacts of Cholesterol, Oxysterols, and Cholesterol Lowering Dietary Compounds on the Immune System. International Journal of Molecular Sciences, 23(20), 12236. https://doi.org/10.3390/ijms232012236





