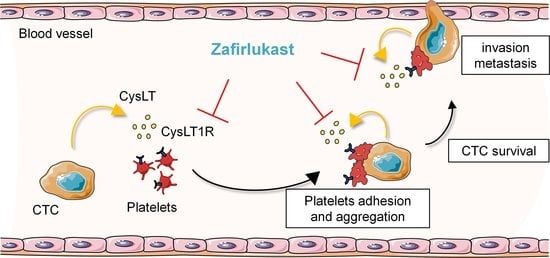
Blockade of Platelet CysLT1R Receptor with Zafirlukast Counteracts Platelet Protumoral Action and Prevents Breast Cancer Metastasis to Bone and Lung
Abstract
1. Introduction
2. Results
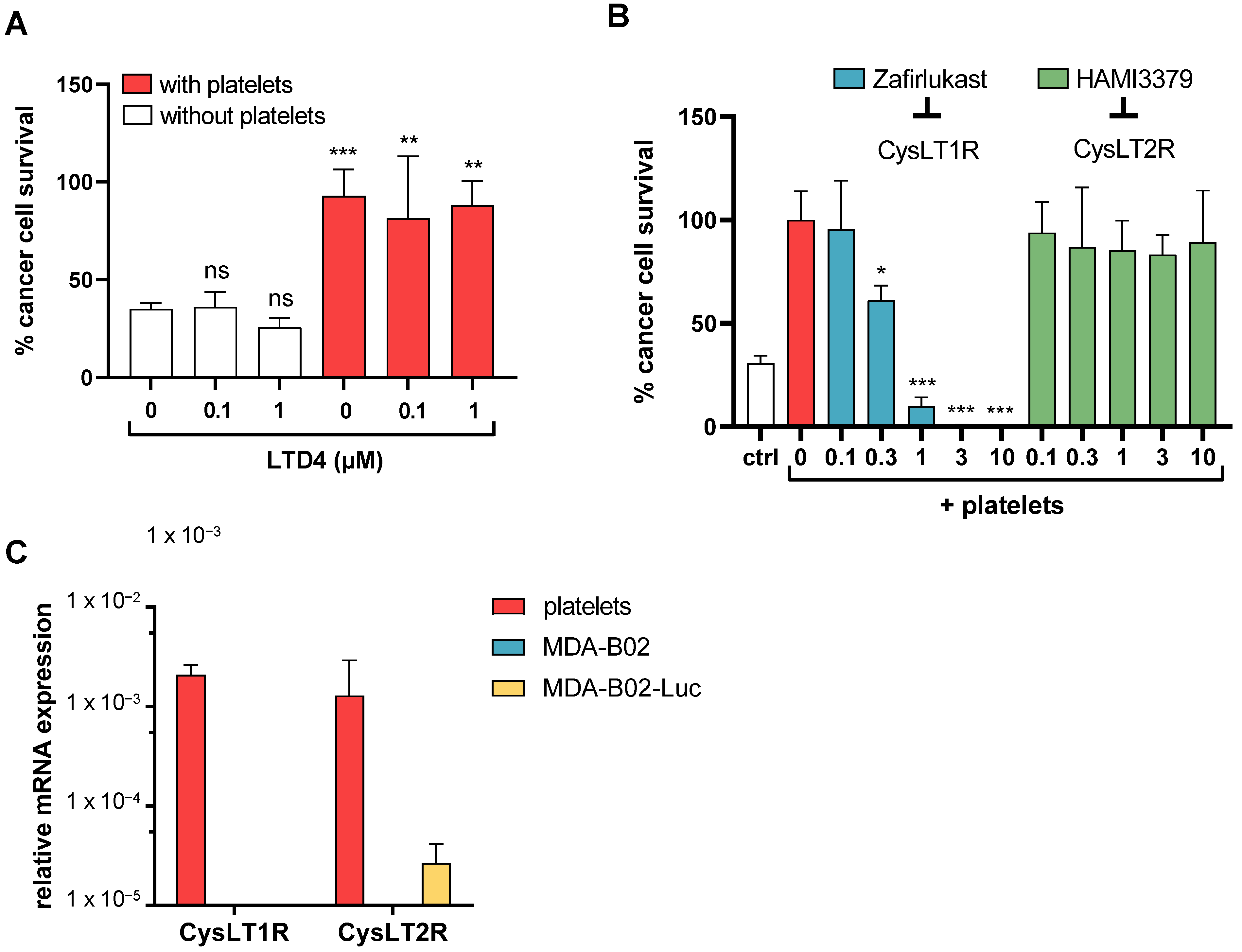
2.1. High-Throughput Screening Identifies CysLT1R Antagonists as Specific Inhibitors of Human Platelet-Induced Human Breast Tumor Cell Survival (PITCS)
2.2. Cysteinyl Leukotrienes Promote Platelet-Induced Survival of Human Breast Cancer Cells via Platelet–CysLT1 Receptor
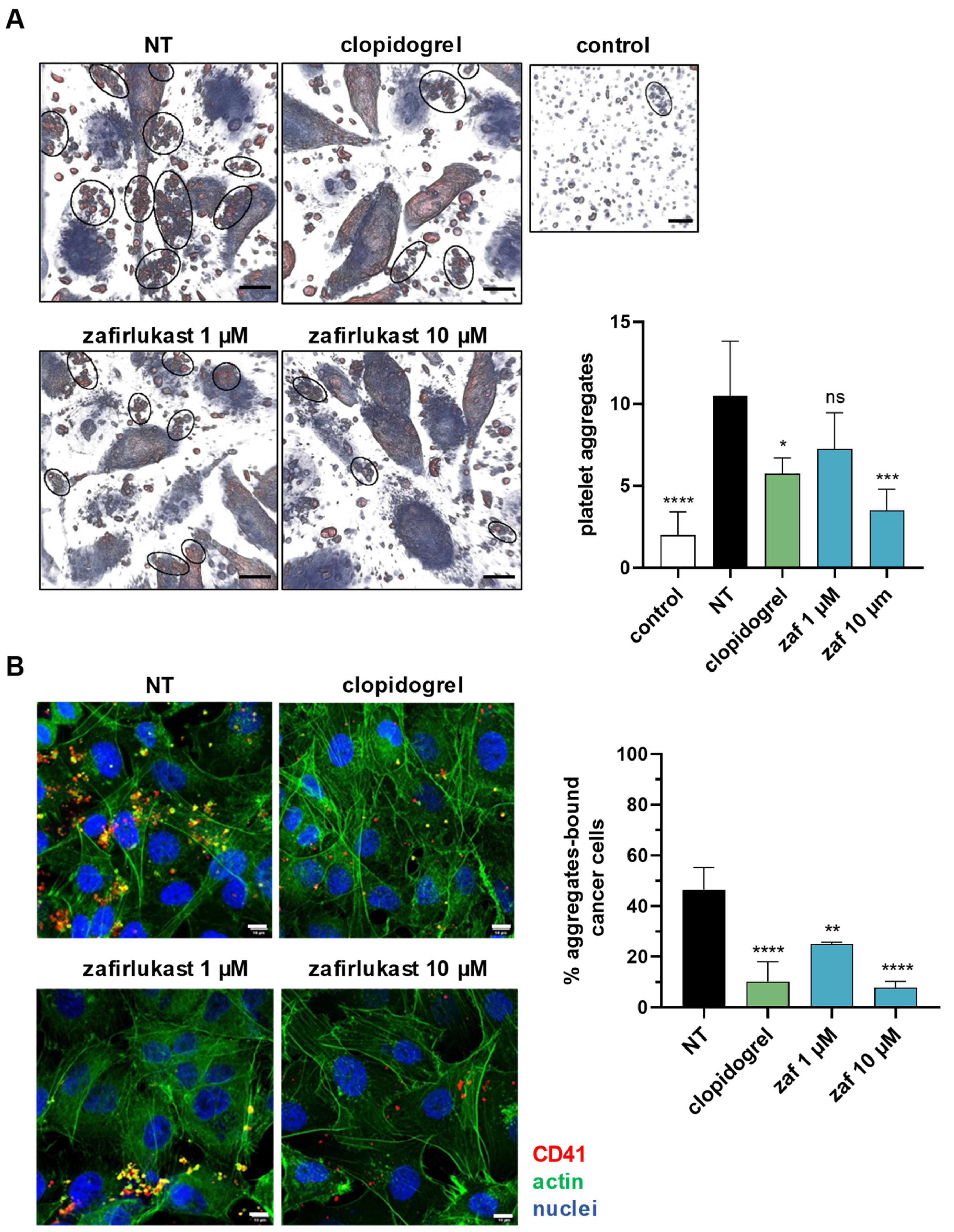
2.3. Cysteinyl Leukotrienes Mediate Tumor-Cell-Induced Platelet Aggregation
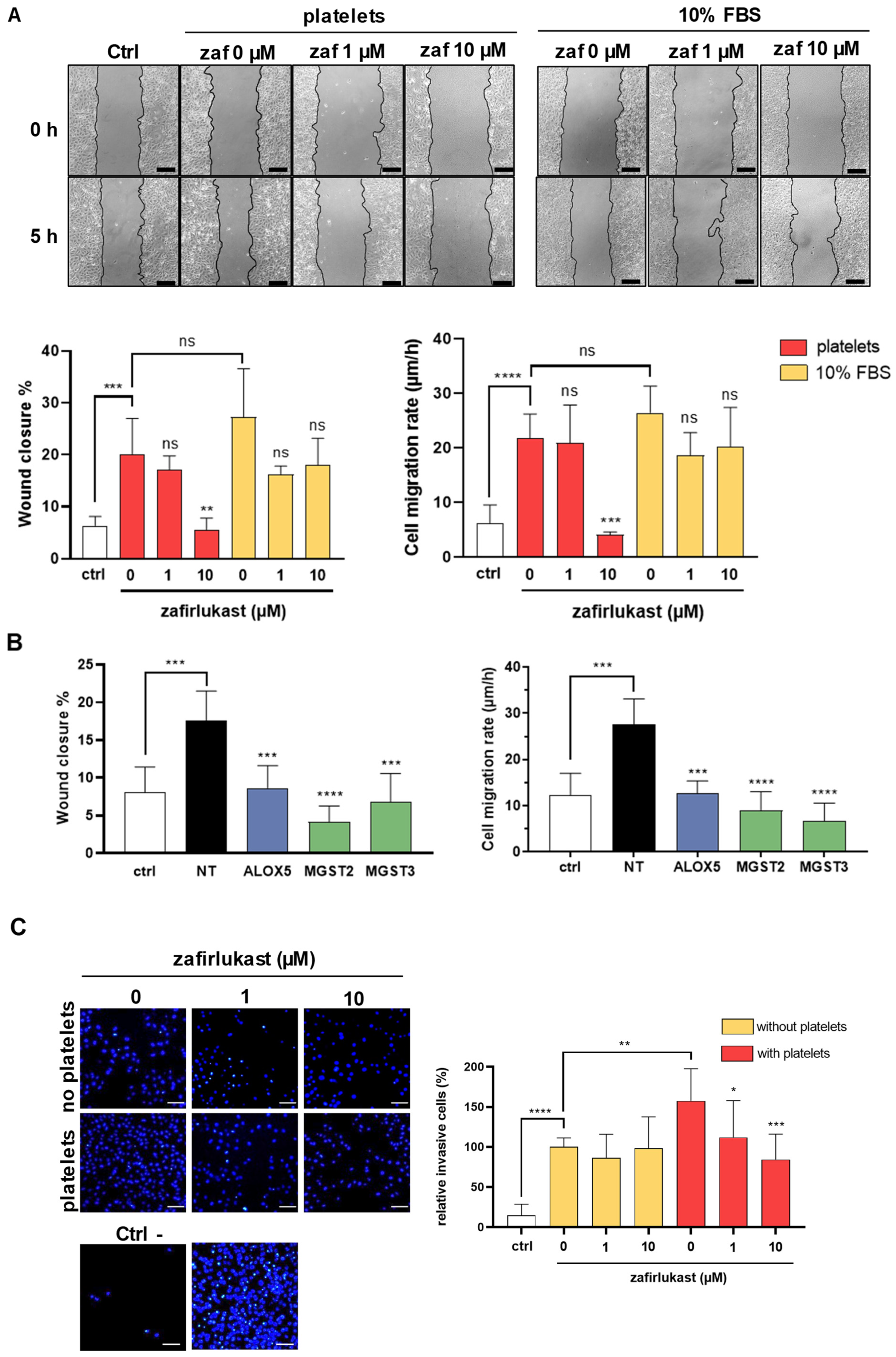
2.4. Cysteinyl Leukotrienes Promote Platelet-Induced Breast Cancer Cells Migration and Invasion
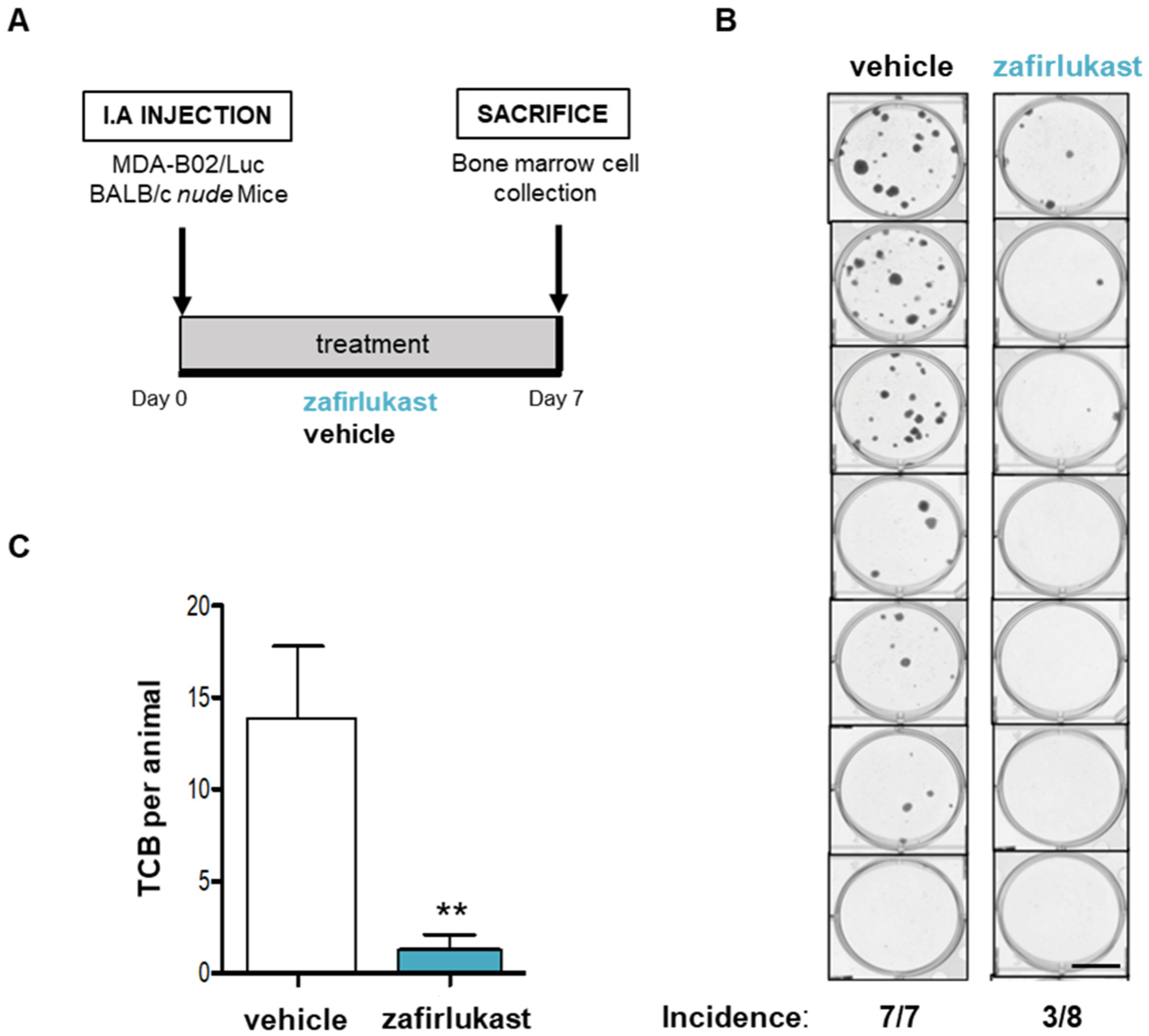
2.5. Blockade of CysLT1R with Zafirlukast Prevents Early Bone Colonization of Human Breast Cancer Cells in BALB/c Nude Mice
2.6. Blockade of CysLT1R with Zafirlukast Prevents Spontaneous Lung Colonization by 4T1 Cells without Affecting Orthotopic Tumor Growth in a Syngeneic Mouse Model
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Platelet Preparation
4.3. CysLT Enzyme Immunoassay
4.4. Western Blot
4.5. Quantitative RT-PCR
4.6. siRNA Transfection
4.7. Cell Survival Assay
4.8. Live-Cell Imaging of Tumor-Cell-Induced Platelet Aggregation
4.9. Platelet Adhesion Assay
4.10. Scratch Assay
4.11. Invasion Assay
4.12. Early Bone Colonization of Breast Cancer Cells in Mice
4.13. Syngeneic Model of Lung Metastasis
4.14. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Anders, H.-J.; Gudermann, T.; Mammadova-Bach, E. Platelet-Cancer Interplay: Molecular Mechanisms and New Therapeutic Avenues. Front. Oncol. 2021, 11, 665534. [Google Scholar] [CrossRef] [PubMed]
- Boucharaba, A.; Serre, C.-M.; Grès, S.; Saulnier-Blache, J.S.; Bordet, J.-C.; Guglielmi, J.; Clézardin, P.; Peyruchaud, O. Platelet-Derived Lysophosphatidic Acid Supports the Progression of Osteolytic Bone Metastases in Breast Cancer. J. Clin. Investig. 2004, 114, 1714–1725. [Google Scholar] [CrossRef]
- Leblanc, R.; Lee, S.-C.; David, M.; Bordet, J.-C.; Norman, D.D.; Patil, R.; Miller, D.; Sahay, D.; Ribeiro, J.; Clézardin, P.; et al. Interaction of Platelet-Derived Autotaxin with Tumor Integrin AVβ3 Controls Metastasis of Breast Cancer Cells to Bone. Blood 2014, 124, 3141–3150. [Google Scholar] [CrossRef]
- Kim, H.K.; Song, K.S.; Park, Y.S.; Kang, Y.H.; Lee, Y.J.; Lee, K.R.; Kim, H.K.; Ryu, K.W.; Bae, J.M.; Kim, S. Elevated Levels of Circulating Platelet Microparticles, VEGF, IL-6 and RANTES in Patients with Gastric Cancer: Possible Role of a Metastasis Predictor. Eur. J. Cancer 2003, 39, 184–191. [Google Scholar] [CrossRef]
- Bakewell, S.J.; Nestor, P.; Prasad, S.; Tomasson, M.H.; Dowland, N.; Mehrotra, M.; Scarborough, R.; Kanter, J.; Abe, K.; Phillips, D.; et al. Platelet and Osteoclast Β3 Integrins Are Critical for Bone Metastasis. Proc. Natl. Acad. Sci. USA 2003, 100, 14205–14210. [Google Scholar] [CrossRef] [PubMed]
- Camerer, E.; Qazi, A.A.; Duong, D.N.; Cornelissen, I.; Advincula, R.; Coughlin, S.R. Platelets, Protease-Activated Receptors, and Fibrinogen in Hematogenous Metastasis. Blood 2004, 104, 397–401. [Google Scholar] [CrossRef]
- Lam, B.K.; Austen, K.F. Leukotriene C4 Synthase: A Pivotal Enzyme in Cellular Biosynthesis of the Cysteinyl Leukotrienes. Prostaglandins Other Lipid Mediat. 2002, 68–69, 511–520. [Google Scholar] [CrossRef]
- Han, B.; Luo, G.; Shi, Z.-Z.; Barrios, R.; Atwood, D.; Liu, W.; Habib, G.M.; Sifers, R.N.; Corry, D.B.; Lieberman, M.W. Gamma-Glutamyl Leukotrienase, a Novel Endothelial Membrane Protein, Is Specifically Responsible for Leukotriene D(4) Formation in Vivo. Am. J. Pathol. 2002, 161, 481–490. [Google Scholar] [CrossRef]
- Lee, C.W.; Lewis, R.A.; Corey, E.J.; Austen, K.F. Conversion of Leukotriene D4 to Leukotriene E4 by a Dipeptidase Released from the Specific Granule of Human Polymorphonuclear Leucocytes. Immunology 1983, 48, 27–35. [Google Scholar]
- Lynch, K.R.; O’Neill, G.P.; Liu, Q.; Im, D.S.; Sawyer, N.; Metters, K.M.; Coulombe, N.; Abramovitz, M.; Figueroa, D.J.; Zeng, Z.; et al. Characterization of the Human Cysteinyl Leukotriene CysLT1 Receptor. Nature 1999, 399, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Heise, C.E.; O’Dowd, B.F.; Figueroa, D.J.; Sawyer, N.; Nguyen, T.; Im, D.S.; Stocco, R.; Bellefeuille, J.N.; Abramovitz, M.; Cheng, R.; et al. Characterization of the Human Cysteinyl Leukotriene 2 Receptor. J. Biol. Chem. 2000, 275, 30531–30536. [Google Scholar] [CrossRef]
- Kanaoka, Y.; Maekawa, A.; Austen, K.F. Identification of GPR99 Protein as a Potential Third Cysteinyl Leukotriene Receptor with a Preference for Leukotriene E4 Ligand. J. Biol. Chem. 2013, 288, 10967–10972. [Google Scholar] [CrossRef]
- Tornhamre, S.; Stenke, L.; Granzelius, A.; Sjölinder, M.; Näsman-Glaser, B.; Roos, C.; Widell, S.; Lindgren, J.A. Inverse Relationship between Myeloid Maturation and Leukotriene C4 Synthase Expression in Normal and Leukemic Myelopoiesis-Consistent Overexpression of the Enzyme in Myeloid Cells from Patients with Chronic Myeloid Leukemia. Exp. Hematol. 2003, 31, 122–130. [Google Scholar] [CrossRef]
- Ali, H.E.A.; Lung, P.-Y.; Sholl, A.B.; Gad, S.A.; Bustamante, J.J.; Ali, H.I.; Rhim, J.S.; Deep, G.; Zhang, J.; Abd Elmageed, Z.Y. Dysregulated Gene Expression Predicts Tumor Aggressiveness in African-American Prostate Cancer Patients. Sci. Rep. 2018, 8, 16335. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, C.; Liu, J.; Ehrnström, R.; Manjer, J.; Jirström, K.; Andersson, T.; Sjölander, A. Cysteinyl Leukotriene Receptor Expression Pattern Affects Migration of Breast Cancer Cells and Survival of Breast Cancer Patients. Int. J. Cancer 2011, 129, 9–22. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Wu, P.-H.; Sheu, C.-C.; Hsu, Y.-L.; Chang, W.-A.; Hung, J.-Y.; Yang, C.-J.; Yang, Y.-H.; Kuo, P.-L.; Huang, M.-S. Cysteinyl Leukotriene Receptor Antagonists Decrease Cancer Risk in Asthma Patients. Sci. Rep. 2016, 6, 23979–23991. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.Y.; Kim, I.-W.; Oh, J.M. Cysteinyl Leukotriene Receptor Antagonists Associated With a Decreased Incidence of Cancer: A Retrospective Cohort Study. Front. Oncol. 2022, 12, 858855. [Google Scholar] [CrossRef] [PubMed]
- Savari, S.; Liu, M.; Zhang, Y.; Sime, W.; Sjölander, A. CysLT(1)R Antagonists Inhibit Tumor Growth in a Xenograft Model of Colon Cancer. PLoS ONE 2013, 8, e73466. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Yoshikawa, M.; Ishitani, K.; Kobayashi, H.; Houkin, K.; Imai, K.; Ito, Y.; Muraki, T. Cysteinyl Leukotriene Receptor Antagonists Inhibit Tumor Metastasis by Inhibiting Capillary Permeability. Keio J. Med. 2010, 59, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Gunning, W.T.; Kramer, P.M.; Steele, V.E.; Pereira, M.A. Chemoprevention by Lipoxygenase and Leukotriene Pathway Inhibitors of Vinyl Carbamate-Induced Lung Tumors in Mice. Cancer Res. 2002, 62, 4199–4201. [Google Scholar] [PubMed]
- Satapathy, S.R.; Sjölander, A. Cysteinyl Leukotriene Receptor 1 Promotes 5-Fluorouracil Resistance and Resistance-Derived Stemness in Colon Cancer Cells. Cancer Lett. 2020, 488, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Saier, L.; Peyruchaud, O. Emerging Role of Cysteinyl LTs in Cancer. Br. J. Pharm. 2021, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Ding, Y.; Zhuang, R. Platelet-Mediated Tumor Metastasis Mechanism and the Role of Cell Adhesion Molecules. Crit. Rev. Oncol. Hematol. 2021, 167, 103502. [Google Scholar] [CrossRef] [PubMed]
- Söderström, M.; Mannervik, B.; Garkov, V.; Hammarström, S. On the Nature of Leukotriene C4 Synthase in Human Platelets. Arch. Biochem. Biophys. 1992, 294, 70–74. [Google Scholar] [CrossRef]
- Jakobsson, P.J.; Mancini, J.A.; Riendeau, D.; Ford-Hutchinson, A.W. Identification and Characterization of a Novel Microsomal Enzyme with Glutathione-Dependent Transferase and Peroxidase Activities. J. Biol. Chem. 1997, 272, 22934–22939. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, P.J.; Mancini, J.A.; Ford-Hutchinson, A.W. Identification and Characterization of a Novel Human Microsomal Glutathione S-Transferase with Leukotriene C4 Synthase Activity and Significant Sequence Identity to 5-Lipoxygenase-Activating Protein and Leukotriene C4 Synthase. J. Biol. Chem. 1996, 271, 22203–22210. [Google Scholar] [CrossRef]
- Penno, C.A.; Wack, N.; Laguerre, C.; Hasler, F.; Numao, S.; Röhn, T.A. Comment on “An Extracellular Matrix Fragment Drives Epithelial Remodeling and Airway Hyperresponsiveness”. Sci. Transl. Med. 2019, 11, eaav4538. [Google Scholar] [CrossRef]
- Hasegawa, S.; Ichiyama, T.; Hashimoto, K.; Suzuki, Y.; Hirano, R.; Fukano, R.; Furukawa, S. Functional Expression of Cysteinyl Leukotriene Receptors on Human Platelets. Platelets 2010, 21, 253–259. [Google Scholar] [CrossRef]
- Herbert, J.-M.; Savi, P. P2Y12, a New Platelet ADP Receptor, Target of Clopidogrel. Semin. Vasc. Med. 2003, 3, 113–122. [Google Scholar] [CrossRef]
- Paruchuri, S.; Tashimo, H.; Feng, C.; Maekawa, A.; Xing, W.; Jiang, Y.; Kanaoka, Y.; Conley, P.; Boyce, J.A. Leukotriene E4-Induced Pulmonary Inflammation Is Mediated by the P2Y12 Receptor. J. Exp. Med. 2009, 206, 2543–2555. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood, A.K. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell 2018, 33, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Gasic, G.J.; Gasic, T.B.; Stewart, C.C. Antimetastatic Effects Associated with Platelet Reduction. Proc. Natl. Acad. Sci. USA 1968, 61, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Gasic, G.J.; Gasic, T.B.; Galanti, N.; Johnson, T.; Murphy, S. Platelet-Tumor-Cell Interactions in Mice. The Role of Platelets in the Spread of Malignant Disease. Int. J. Cancer 1973, 11, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Pacchiarini, L.; Zucchella, M.; Milanesi, G.; Tacconi, F.; Bonomi, E.; Canevari, A.; Grignani, G. Thromboxane Production by Platelets during Tumor Cell-Induced Platelet Activation. Invasion Metastasis 1991, 11, 102–109. [Google Scholar]
- Alonso-Escolano, D.; Strongin, A.Y.; Chung, A.W.; Deryugina, E.I.; Radomski, M.W. Membrane Type-1 Matrix Metalloproteinase Stimulates Tumour Cell-Induced Platelet Aggregation: Role of Receptor Glycoproteins. Br. J. Pharm. 2004, 141, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Mitrugno, A.; Williams, D.; Kerrigan, S.W.; Moran, N. A Novel and Essential Role for FcγRIIa in Cancer Cell–Induced Platelet Activation. Blood 2014, 123, 249–260. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Wilson, M.; Price, J.F.; Belch, J.F.F.; Meade, T.W.; Mehta, Z. Effect of Daily Aspirin on Risk of Cancer Metastasis: A Study of Incident Cancers during Randomised Controlled Trials. Lancet 2012, 379, 1591–1601. [Google Scholar] [CrossRef]
- Dovizio, M.; Tacconelli, S.; Sostres, C.; Ricciotti, E.; Patrignani, P. Mechanistic and Pharmacological Issues of Aspirin as an Anticancer Agent. Pharmaceuticals 2012, 5, 1346–1371. [Google Scholar] [CrossRef] [PubMed]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct Signaling between Platelets and Cancer Cells Induces an Epithelial-Mesenchymal-like Transition and Promotes Metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef]
- Gareau, A.J.; Brien, C.; Gebremeskel, S.; Liwski, R.S.; Johnston, B.; Bezuhly, M. Ticagrelor Inhibits Platelet-Tumor Cell Interactions and Metastasis in Human and Murine Breast Cancer. Clin. Exp. Metastasis 2018, 35, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Boukerche, H.; Berthier-Vergnes, O.; Penin, F.; Tabone, E.; Lizard, G.; Bailly, M.; McGregor, J.L. Human Melanoma Cell Lines Differ in Their Capacity to Release ADP and Aggregate Platelets. Br. J. Haematol. 1994, 87, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Cummings, H.E.; Liu, T.; Feng, C.; Laidlaw, T.M.; Conley, P.B.; Kanaoka, Y.; Boyce, J.A. Cutting Edge: Leukotriene C4 Activates Mouse Platelets in Plasma Exclusively through the Type 2 Cysteinyl Leukotriene Receptor. J. Immunol. 2013, 191, 5807–5810. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Mehta, J.; Lawson, D.; Krop, I.; Letts, L.G. Leukotrienes Potentiate the Effects of Epinephrine and Thrombin on Human Platelet Aggregation. Thromb. Res. 1986, 41, 731–738. [Google Scholar] [CrossRef]
- Yokomizo, T.; Nakamura, M.; Shimizu, T. Leukotriene Receptors as Potential Therapeutic Targets. J. Clin. Investig. 2018, 128, 2691–2701. [Google Scholar] [CrossRef] [PubMed]
- Peyruchaud, O.; Serre, C.-M.; NicAmhlaoibh, R.; Fournier, P.; Clezardin, P. Angiostatin Inhibits Bone Metastasis Formation in Nude Mice through a Direct Anti-Osteoclastic Activity. J. Biol. Chem. 2003, 278, 45826–45832. [Google Scholar] [CrossRef]
- Aslakson, C.J.; Miller, F.R. Selective Events in the Metastatic Process Defined by Analysis of the Sequential Dissemination of Subpopulations of a Mouse Mammary Tumor. Cancer Res. 1992, 52, 1399–1405. [Google Scholar]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- David, M.; Ribeiro, J.; Descotes, F.; Serre, C.-M.; Barbier, M.; MURONE, M.; Clézardin, P.; Peyruchaud, O. Targeting Lysophosphatidic Acid Receptor Type 1 with Debio 0719 Inhibits Spontaneous Metastasis Dissemination of Breast Cancer Cells Independently of Cell Proliferation and Angiogenesis. Int. J. Oncol. 2011, 40, 1133–1141. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saier, L.; Ribeiro, J.; Daunizeau, T.; Houssin, A.; Ichim, G.; Barette, C.; Bouazza, L.; Peyruchaud, O. Blockade of Platelet CysLT1R Receptor with Zafirlukast Counteracts Platelet Protumoral Action and Prevents Breast Cancer Metastasis to Bone and Lung. Int. J. Mol. Sci. 2022, 23, 12221. https://doi.org/10.3390/ijms232012221
Saier L, Ribeiro J, Daunizeau T, Houssin A, Ichim G, Barette C, Bouazza L, Peyruchaud O. Blockade of Platelet CysLT1R Receptor with Zafirlukast Counteracts Platelet Protumoral Action and Prevents Breast Cancer Metastasis to Bone and Lung. International Journal of Molecular Sciences. 2022; 23(20):12221. https://doi.org/10.3390/ijms232012221
Chicago/Turabian StyleSaier, Lou, Johnny Ribeiro, Thomas Daunizeau, Audrey Houssin, Gabriel Ichim, Caroline Barette, Lamia Bouazza, and Olivier Peyruchaud. 2022. "Blockade of Platelet CysLT1R Receptor with Zafirlukast Counteracts Platelet Protumoral Action and Prevents Breast Cancer Metastasis to Bone and Lung" International Journal of Molecular Sciences 23, no. 20: 12221. https://doi.org/10.3390/ijms232012221
APA StyleSaier, L., Ribeiro, J., Daunizeau, T., Houssin, A., Ichim, G., Barette, C., Bouazza, L., & Peyruchaud, O. (2022). Blockade of Platelet CysLT1R Receptor with Zafirlukast Counteracts Platelet Protumoral Action and Prevents Breast Cancer Metastasis to Bone and Lung. International Journal of Molecular Sciences, 23(20), 12221. https://doi.org/10.3390/ijms232012221