Association of Circulating Platelet Extracellular Vesicles and Pulse Wave Velocity with Cardiovascular Risk Estimation
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics
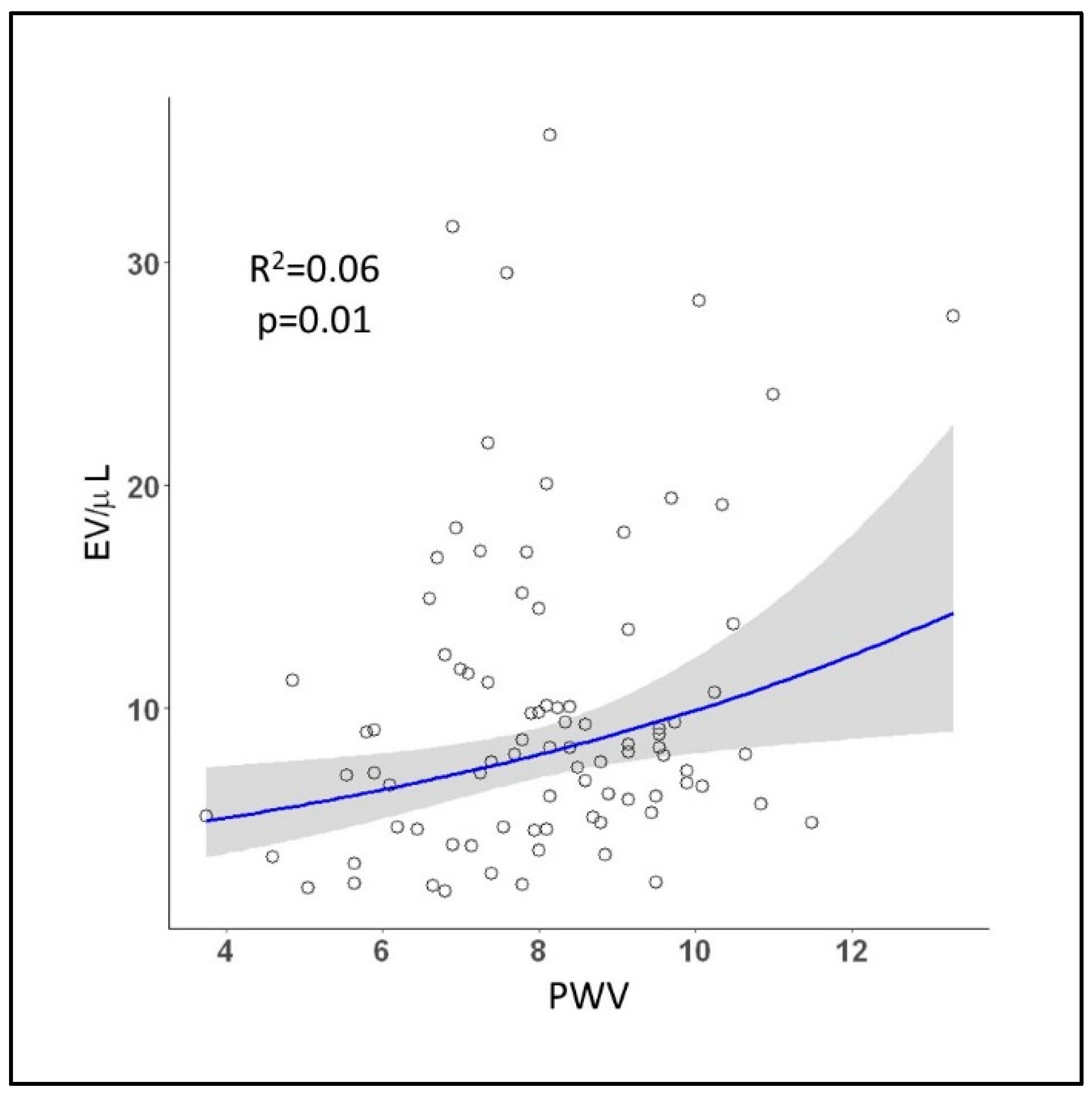
2.2. Assessment of Extracelular Vesicles and Cardiovascular Risk
2.3. Target Organ Damage Evaluation
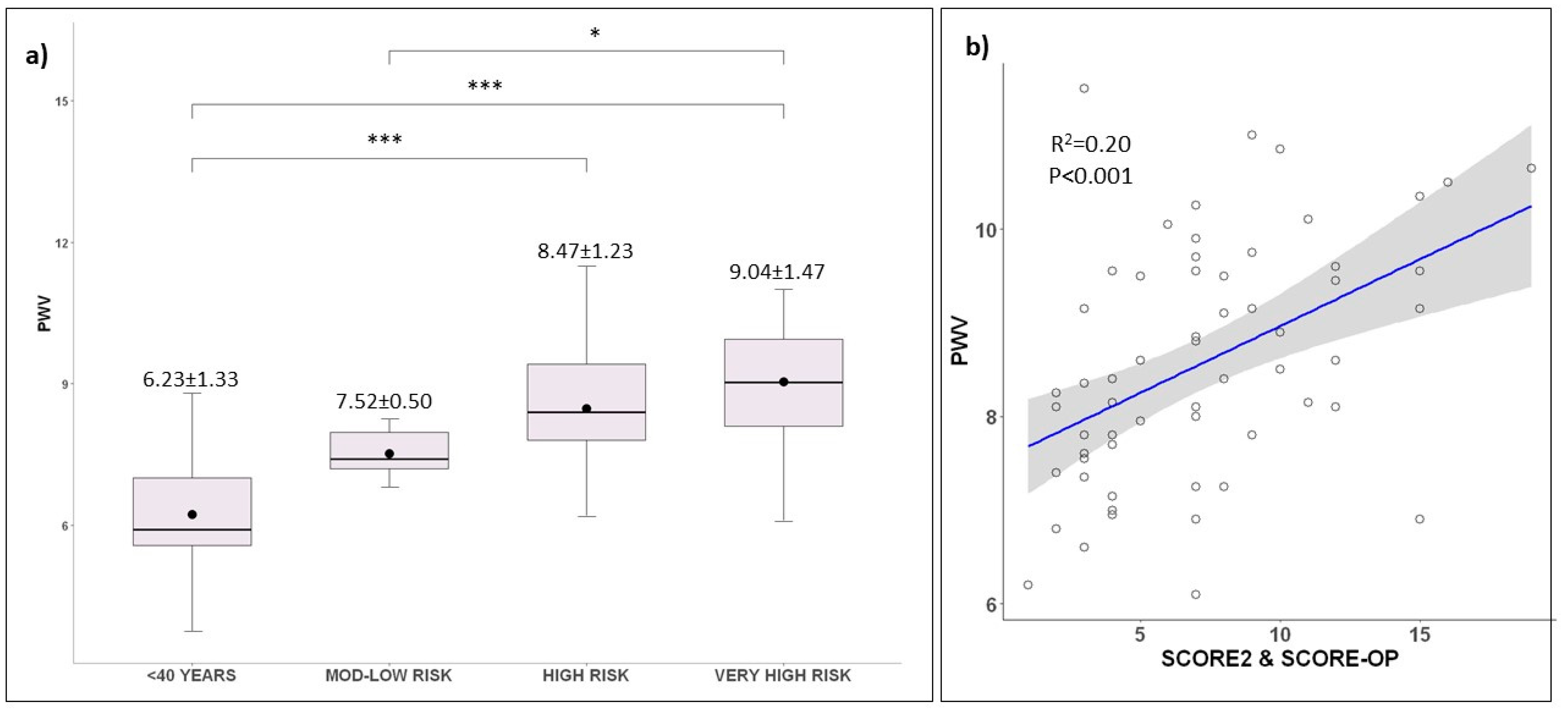
2.4. Cardiovascular Risk Groups’ Sub-Analysis
3. Discussion
4. Materials and Methods
4.1. Subject Population and Study Design
4.2. Platelet EV Characterization
4.3. Markers of Macrovascular Organ Damage
4.4. Statistical Analysis
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Tokgozoglu, L.; Torp-Pedersen, C. Redefining cardiovascular risk prediction: Is the crystal ball clearer now? Eur. Heart J. 2021, 42, 2468–2471. [Google Scholar] [CrossRef]
- Davidson, S.M.; Boulanger, C.M.; Aikawa, E.; Badimon, L.; Barile, L.; Binder, C.J.; Brisson, A.; Buzas, E.; Emanueli, C.; Jansen, F.; et al. Methods for the identification and characterization of extracellular vesicles in cardiovascular studies: From exosomes to microvesicles. Cardiovasc. Res. 2022, cvac031. [Google Scholar] [CrossRef] [PubMed]
- Preston, R.A.; Jy, W.; Jimenez, J.J.; Mauro, L.M.; Horstman, L.L.; Valle, M.; Aime, G.; Ahn, Y.S. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 2003, 41, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Turner, M.; Xiao, F.; Munkonda, M.N.; Akbari, S.; Burns, K.D. High glucose increases the formation and pro-oxidative activity of endothelial microparticles. Diabetologia 2017, 60, 1791–1800. [Google Scholar] [CrossRef]
- Macchi, C.; Greco, M.F.; Favero, C.; Dioni, L.; Cantone, L.; Hoxha, M.; Vigna, L.; Solazzo, G.; Corsini, A.; Banach, M.; et al. Associations Among PCSK9 Levels, Atherosclerosis-Derived Extracellular Vesicles, and Their miRNA Content in Adults with Obesity. Front. Cardiovasc. Med. 2022, 8, 785250. [Google Scholar] [CrossRef]
- Camera, M.; Brambilla, M.; Canzano, P.; Cavallotti, L.; Parolari, A.; Tedesco, C.C.; Zara, C.; Rossetti, L.; Tremoli, E. Association of Microvesicles with Graft Patency in Patients Undergoing CABG Surgery. J. Am. Coll. Cardiol. 2020, 75, 2819–2832. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Gavidia, L.M.; Burger, D.; Matthews, V.B.; Nolde, J.M.; Galindo Kiuchi, M.; Carnagarin, R.; Kannenkeril, D.; Chan, J.; Joyson, A.; Herat, L.Y.; et al. Role of Microparticles in Cardiovascular Disease: Implications for Endothelial Dysfunction, Thrombosis, and Inflammation. Hypertension 2021, 77, 1825–1844. [Google Scholar] [CrossRef] [PubMed]
- França, C.N.; Izar, M.C.; Amaral, J.B.; Tegani, D.M.; Fonseca, F.A. Microparticles as potential biomarkers of cardiovascular disease. Arq. Bras. Cardiol. 2015, 104, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Schock, S.; Thompson, C.S.; Montezano, A.C.; Hakim, A.M.; Touyz, R.M. Microparticles: Biomarkers and beyond. Clin. Sci. 2013, 124, 423–441. [Google Scholar] [CrossRef]
- Ridger, V.C.; Boulanger, C.M.; Angelillo-Scherrer, A.; Badimon, L.; Blanc-Brude, O.; Bochaton-Piallat, M.L.; Boilard, E.; Buzas, E.I.; Caporali, A.; Dignat-George, F.; et al. Microvesicles in vascular homeostasis and diseases. Position Paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb. Haemost. 2017, 117, 1296–1316. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Park, C.G.; Park, J.S.; Suh, S.Y.; Choi, C.U.; Kim, J.W.; Kim, S.H.; Lim, H.E.; Rha, S.W.; Seo, H.S.; et al. Relationship between blood pressure parameters and pulse wave velocity in normotensive and hypertensive subjects: Invasive study. J. Hum. Hypertens. 2007, 21, 141–148. [Google Scholar] [CrossRef]
- O’Rourke, M.F.; Pauca, A.; Jiang, X.J. Pulse wave analysis. Br. J. Clin. Pharm. 2001, 51, 507–522. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Figtree, G.A.; Vernon, S.T. Coronary artery disease patients without standard modifiable risk factors (SMuRFs)—A forgotten group calling out for new discoveries. Cardiovasc. Res. 2021, 117, e76–e78. [Google Scholar] [CrossRef]
- Figtree, G.A.; Vernon, S.T.; Hadziosmanovic, N.; Sundström, J.; Alfredsson, J.; Arnott, C.; Delatour, V.; Leósdóttir, M.; Hagström, E. Mortality in STEMI patients without standard modifiable risk factors: A sex-disaggregated analysis of SWEDEHEART registry data. Lancet 2021, 397, 1085–1094. [Google Scholar] [CrossRef]
- Vernon, S.T.; Coffey, S.; Bhindi, R.; Soo Hoo, S.Y.; Nelson, G.I.; Ward, M.R.; Hansen, P.S.; Asrress, K.N.; Chow, C.K.; Celermajer, D.S.; et al. Increasing proportion of ST elevation myocardial infarction patients with coronary atherosclerosis poorly explained by standard modifiable risk factors. Eur. J. Prev. Cardiol. 2017, 24, 1824–1830. [Google Scholar] [CrossRef] [PubMed]
- Vernon, S.T.; Coffey, S.; D’Souza, M.; Chow, C.K.; Kilian, J.; Hyun, K.; Shaw, J.A.; Adams, M.; Roberts-Thomson, P.; Brieger, D.; et al. ST-Segment-Elevation Myocardial Infarction (STEMI) Patients without Standard Modifiable Cardiovascular Risk Factors-How Common Are They, and What Are Their Outcomes? J. Am. Heart Assoc. 2019, 8, e013296. [Google Scholar] [CrossRef]
- Yang, J.; Biery, D.W.; Singh, A.; Divakaran, S.; DeFilippis, E.M.; Wu, W.Y.; Klein, J.; Hainer, J.; Ramsis, M.; Natarajan, P.; et al. Risk Factors and Outcomes of Very Young Adults Who Experience Myocardial Infarction: The Partners YOUNG-MI Registry. Am. J. Med. 2020, 133, 605–612.e601. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Collins, B.L.; Gupta, A.; Fatima, A.; Qamar, A.; Biery, D.; Baez, J.; Cawley, M.; Klein, J.; Hainer, J.; et al. Cardiovascular Risk and Statin Eligibility of Young Adults After an MI: Partners YOUNG-MI Registry. J. Am. Coll. Cardiol. 2018, 71, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Maldonado, J.; Polónia, J.; Silva, J.A.; Morais, J.; Rodrigues, T.; Marques, M. Aortic pulse wave velocity and HeartSCORE: Improving cardiovascular risk stratification. a sub-analysis of the EDIVA (Estudo de DIstensibilidade VAscular) project. Blood Press. 2014, 23, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Boutouyrie, P.; Chowienczyk, P.; Humphrey, J.D.; Mitchell, G.F. Arterial Stiffness and Cardiovascular Risk in Hypertension. Circ. Res. 2021, 128, 864–886. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Ohkuma, T.; Ninomiya, T.; Tomiyama, H.; Kario, K.; Hoshide, S.; Kita, Y.; Inoguchi, T.; Maeda, Y.; Kohara, K.; Tabara, Y.; et al. Brachial-Ankle Pulse Wave Velocity and the Risk Prediction of Cardiovascular Disease: An Individual Participant Data Meta-Analysis. Hypertension 2017, 69, 1045–1052. [Google Scholar] [CrossRef]
- Badimon, L.; Suades, R.; Fuentes, E.; Palomo, I.; Padró, T. Role of Platelet-Derived Microvesicles as Crosstalk Mediators in Atherothrombosis and Future Pharmacology Targets: A Link between Inflammation, Atherosclerosis, and Thrombosis. Front. Pharmacol. 2016, 7, 293. [Google Scholar] [CrossRef] [Green Version]
- Zarà, M.; Guidetti, G.F.; Camera, M.; Canobbio, I.; Amadio, P.; Torti, M.; Tremoli, E.; Barbieri, S.S. Biology and Role of Extracellular Vesicles (EVs) in the Pathogenesis of Thrombosis. Int. J. Mol. Sci. 2019, 20, 2840. [Google Scholar] [CrossRef] [PubMed]
- Suades, R.; Padró, T.; Vilahur, G.; Badimon, L. Platelet-released extracellular vesicles: The effects of thrombin activation. Cell. Mol. Life Sci. 2022, 79, 190. [Google Scholar] [CrossRef] [PubMed]
- Amabile, N.; Cheng, S.; Renard, J.M.; Larson, M.G.; Ghorbani, A.; McCabe, E.; Griffin, G.; Guerin, C.; Ho, J.E.; Shaw, S.Y.; et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur. Heart J. 2014, 35, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Gavidia, L.M.; Burger, D.; Bosio, E.; Robinson, S.; Nolde, J.; Carnagarin, R.; Matthews, V.B.; Schlaich, M.P. A standarized protocol for evaluation of large extracellular vesicles using the attuneTM NXT system. J. Immunol. Methods 2021, 499, 113170. [Google Scholar] [CrossRef]

| Overall | <40 Years | Mod-Low Risk | High Risk | Very High Risk | p-Value | |
|---|---|---|---|---|---|---|
| (N = 92) | (N = 18) | (N = 11) | (N = 26) | (N = 37) | ||
| Male | 54 (58.7%) | 9 (50.0%) | 2 (18.2%) | 19 (73.1%) | 24 (64.9%) | 0.01 |
| Age | 55.5 ± 15.1 | 32.4 ± 5.75 | 54.8 ± 5.09 | 54.9 ± 10.1 | 67.4 ± 8.13 | <0.001 |
| BMI (Kg/m2) | 30.1 ± 5.97 | 25.3 ± 5.89 | 31.3 ± 7.37 | 32.0 ± 5.18 | 30.6 ± 4.98 | 0.001 |
| Diabetes | 25 (27.2%) | - | - | 8 (30.8%) | 17 (45.9%) | <0.001 |
| Hypertension | 81 (88.0%) | 8 (44.4%) | 11 (100%) | 25 (96.2%) | 37 (100%) | <0.001 |
| Dyslipidemia | 58 (63.0%) | 2 (11.1%) | 7 (63.6%) | 16 (61.5%) | 33 (89.2%) | <0.001 |
| Coronary artery disease | 13 (14.1%) | - | - | - | 13 (35.1%) | - |
| Stroke/TIA | 4 (4.3%) | - | - | - | 4 (10.8%) | - |
| Peripheral artery disease | 4 (4.3%) | - | - | - | 4 (10.8%) | - |
| Glucose (µmmol/L) | 5.98 ± 1.67 | 5.01 ± 0.448 | 5.20 ± 0.673 | 6.04 ± 1.60 | 6.48 ± 1.98 | 0.03 |
| HbA1c (%) | 6.18 ± 1.42 | 5.19 ± 0.285 | 5.55 ± 0.459 | 6.33 ± 1.27 | 6.63 ± 1.70 | 0.03 |
| Total cholesterol (mmol/L) | 4.89 ± 1.13 | 4.64 ± 1.13 | 5.47 ± 0.822 | 5.24 ± 0.972 | 4.55 ± 1.22 | 0.02 |
| Triglyceride (mmol/L) | 1.60 ± 1.04 | 1.29 ± 1.38 | 1.44 ± 0.741 | 1.82 ± 1.19 | 1.61 ± 0.868 | 0.47 |
| HDL cholesterol (mmol/L) | 1.30 ± 0.391 | 1.30 ± 0.333 | 1.58 ± 0.610 | 1.24 ± 0.318 | 1.26 ± 0.352 | 0.08 |
| LDL cholesterol (mmol/L) | 2.87 ± 0.921 | 2.79 ± 0.697 | 3.24 ± 0.661 | 3.20 ± 0.906 | 2.56 ± 0.973 | 0.02 |
| Creatinine (µmol/L) | 77.0 ± 27.6 | 78.8 ± 18.2 | 65.6 ± 7.26 | 71.1 ± 14.9 | 83.8 ± 37.7 | 0.14 |
| eGFR (mL/min/1.73 m2) | 84.4 ± 11.2 | 88.5 ± 4.50 | 89.0 ± 1.76 | 87.8 ± 5.66 | 77.9 ± 15.4 | 0.001 |
| UACR (mg/mmol) | 2.07 ± 3.97 | 1.66 ± 3.21 | 0.88 ± 0.504 | 1.10 ± 1.07 | 3.50 ± 5.86 | 0.23 |
| Sys AOBP (mmHg) | 132 ± 18.6 | 119 ± 13.1 | 127 ± 18.2 | 134 ± 13.8 | 139 ± 20.6 | 0.001 |
| Dia AOBP (mmHg) | 79.1 ± 13.6 | 74.6 ± 11.3 | 83.0 ± 15.3 | 84.2 ± 10.9 | 76.5 ± 14.7 | 0.04 |
| PWV (mmHg) | 8.11 ± 1.67 | 6.23 ± 1.33 | 7.52 ± 0.501 | 8.47 ± 1.23 | 9.04 ± 1.47 | <0.001 |
| Central MAP (mmHg) | 96.3 ± 12.7 | 87.4 ± 10.3 | 101 ± 19.6 | 100 ± 8.72 | 96.7 ± 12.1 | 0.004 |
| Augmentation index (mmHg) | 19.0 ± 13.6 | 3.88 ± 11.7 | 25.9 ± 10.6 | 22.1 ± 11.7 | 22.7 ± 10.9 | <0.001 |
| T1 | T2 | T3 | p-Value * | |
|---|---|---|---|---|
| (N = 10) | (N = 10) | (N = 9) | ||
| Dyslipidemia | 2 (20.0%) | 2 (20.0%) | 5 (55.6%) | 0.18 |
| Hypertension | 5 (50.0%) | 6 (60.0%) | 8 (88.8%) | 0.23 |
| Age | 39.3 ± 12.2 | 36.7 ± 11.7 | 47.3 ± 11.8 | 0.16 |
| BMI (kg/m2) | 27.0 ± 8.24 | 27.5 ± 7.16 | 28.2 ± 6.16 | 0.94 |
| White cell count (109/L) | 5.26 ± 0.973 | 5.69 ± 1.87 | 5.68 ± 1.43 | 0.83 |
| Red cell count (109/L) | 4.59 ± 0.386 | 4.78 ± 0.407 | 4.55 ± 0.272 | 0.51 |
| Haematocrit (L/L) | 0.410 ± 0.0245 | 0.423 ± 0.0207 | 0.413 ± 0.0287 | 0.62 |
| Haemoglobin (g/L) | 137 ± 14.5 | 141 ± 7.37 | 138 ± 9.97 | 0.86 |
| Platelet count (10*9/L) | 244 ± 80.6 | 249 ± 49.7 | 220 ± 23.5 | 0.62 |
| Glucose (mmol/L) | 5.10 ± 0.454 | 4.93 ± 0.486 | 5.30 ± 0.772 | 0.52 |
| HOMA-IR | 1.66 ± 1.16 | 1.87 ± 1.38 | 2.43 ± 1.15 | 0.61 |
| HbA1c (%) | 5.40 ± 0.381 | 5.07 ± 0.327 | 5.56 ± 0.336 | 0.09 ¥ |
| Total cholesterol (mmol/L) | 4.83 ± 0.814 | 4.71 ± 0.866 | 5.53 ± 1.38 | 0.27 |
| Triglycerides (mmol/L) | 1.04 ± 0.431 | 1.46 ± 0.956 | 1.58 ± 1.67 | 0.62 |
| HDL cholesterol (mmol/L) | 1.53 ± 0.427 | 1.34 ± 0.355 | 1.43 ± 0.680 | 0.79 |
| LDL cholesterol (mmol/L) | 2.83 ± 0.483 | 2.74 ± 0.586 | 3.41 ± 0.853 | 0.12 |
| eGFR (mL/min/1.73 m2) | 87.1 ± 5.67 | 89.2 ± 1.33 | 89.9 ± 0.7 | 0.3 |
| Homocysteine (µmol/L) | 9.50 ± 2.42 | 8.30 ± 0.548 | 10.1 ± 2.67 | 0.52 |
| Systolic AOBP (mmHg) | 123 ± 19.0 | 124 ± 10.3 | 120 ± 17.4 | 0.88 |
| Diastolic AOBP (mmHg) | 81.9 ± 18.0 | 73.8 ± 11.0 | 77.8 ± 9.00 | 0.41 |
| ABPM 24h—SBP (mmHg) | 119 ± 11.8 | 125 ± 12.5 | 124 ± 15.5 | 0.62 |
| ABPM 24h—DBP (mmHg) | 75.6 ± 11.6 | 71.1 ± 9.97 | 78.8 ± 9.57 | 0.39 |
| ABPM Day—SBP (mmHg) | 121 ± 12.3 | 128 ± 13.3 | 126 ± 15.3 | 0.57 |
| ABPM Day—DBP (mmHg) | 77.4 ± 11.9 | 73.6 ± 10.1 | 80.8 ± 10.7 | 0.46 |
| ABPM Night—SBP (mmHg) | 111 ± 14.6 | 113 ± 12.5 | 119 ± 20.6 | 0.60 |
| ABPM Night—DBP (mmHg) | 69.0 ± 16.3 | 61.0 ± 9.81 | 72.4 ± 11.0 | 0.23 |
| Nocturnal systolic dipping (%) | 7.83 ± 9.28 | 11.7 ± 5.98 | 5.39 ± 11.3 | 0.43 |
| Nocturnal diastolic dipping (%) | 10.9 ± 15.2 | 17.1 ± 6.21 | 9.93 ± 11.4 | 0.45 |
| White coat hypertension phenotype | 2 (20.0%) | 1 (10.0%) | 0 (0.0%) | <0.001 |
| Masked hypertension phenotype | 0 (0.0%) | 3 (30.0%) | 2 (22.2%) | 0.23 |
| PWV (m/s) | 6.42 ± 1.24 | 6.66 ± 1.50 | 7.07 ± 1.01 | 0.57 |
| Mean arterial pressure (mmHg) | 94.6 ± 21.9 | 91.0 ± 13.0 | 90.9 ± 8.51 | 0.85 |
| Augmentation index (%) | 11.8 ± 17.6 | 3.67 ± 14.1 | 20.0 ± 10.3 | 0.09 ¥ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugo-Gavidia, L.M.; Nolde, J.M.; Carnagarin, R.; Burger, D.; Chan, J.; Robinson, S.; Bosio, E.; Matthews, V.B.; Schlaich, M.P. Association of Circulating Platelet Extracellular Vesicles and Pulse Wave Velocity with Cardiovascular Risk Estimation. Int. J. Mol. Sci. 2022, 23, 10524. https://doi.org/10.3390/ijms231810524
Lugo-Gavidia LM, Nolde JM, Carnagarin R, Burger D, Chan J, Robinson S, Bosio E, Matthews VB, Schlaich MP. Association of Circulating Platelet Extracellular Vesicles and Pulse Wave Velocity with Cardiovascular Risk Estimation. International Journal of Molecular Sciences. 2022; 23(18):10524. https://doi.org/10.3390/ijms231810524
Chicago/Turabian StyleLugo-Gavidia, Leslie Marisol, Janis M. Nolde, Revathy Carnagarin, Dylan Burger, Justine Chan, Sandi Robinson, Erika Bosio, Vance B. Matthews, and Markus P. Schlaich. 2022. "Association of Circulating Platelet Extracellular Vesicles and Pulse Wave Velocity with Cardiovascular Risk Estimation" International Journal of Molecular Sciences 23, no. 18: 10524. https://doi.org/10.3390/ijms231810524
APA StyleLugo-Gavidia, L. M., Nolde, J. M., Carnagarin, R., Burger, D., Chan, J., Robinson, S., Bosio, E., Matthews, V. B., & Schlaich, M. P. (2022). Association of Circulating Platelet Extracellular Vesicles and Pulse Wave Velocity with Cardiovascular Risk Estimation. International Journal of Molecular Sciences, 23(18), 10524. https://doi.org/10.3390/ijms231810524






