Placenta-Specific miR-125b Overexpression Leads to Increased Rates of Pregnancy Loss in Mice
Abstract
1. Introduction
2. Results
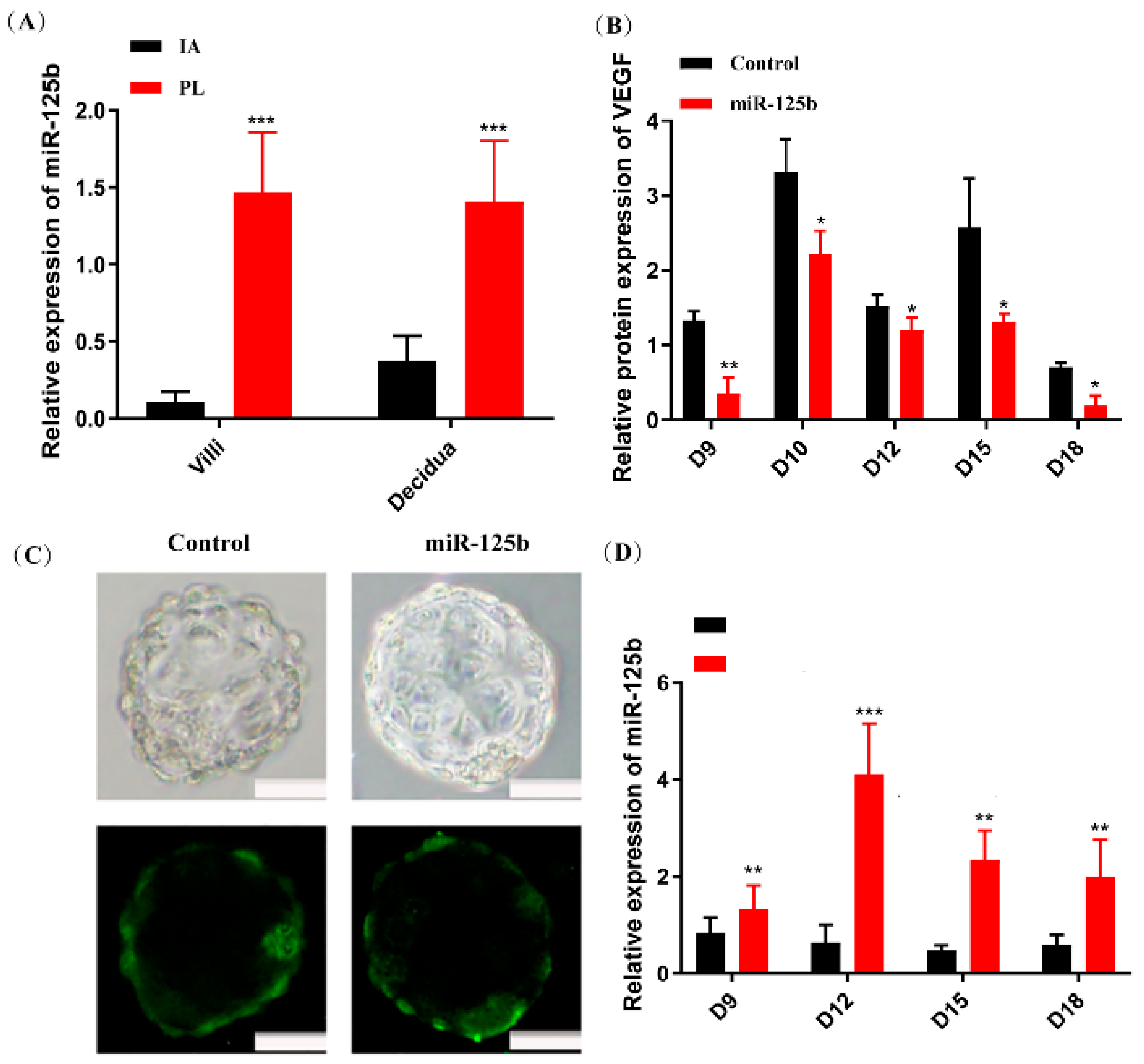
2.1. The Expression of miR-125b Was Upregulated in Samples from PL Patients
2.2. Placenta-Specific miR-125b Overexpression in a Mouse Model
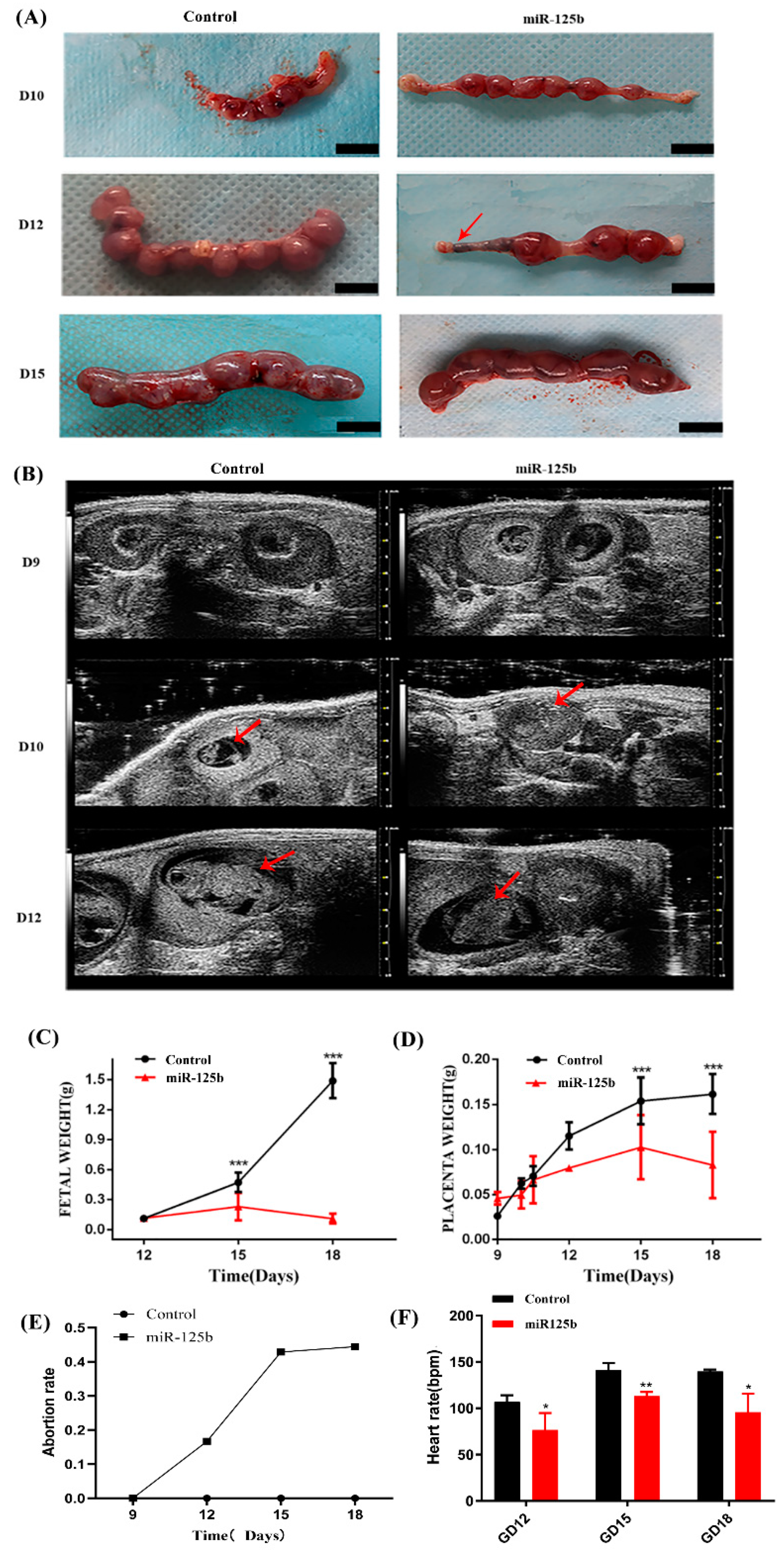
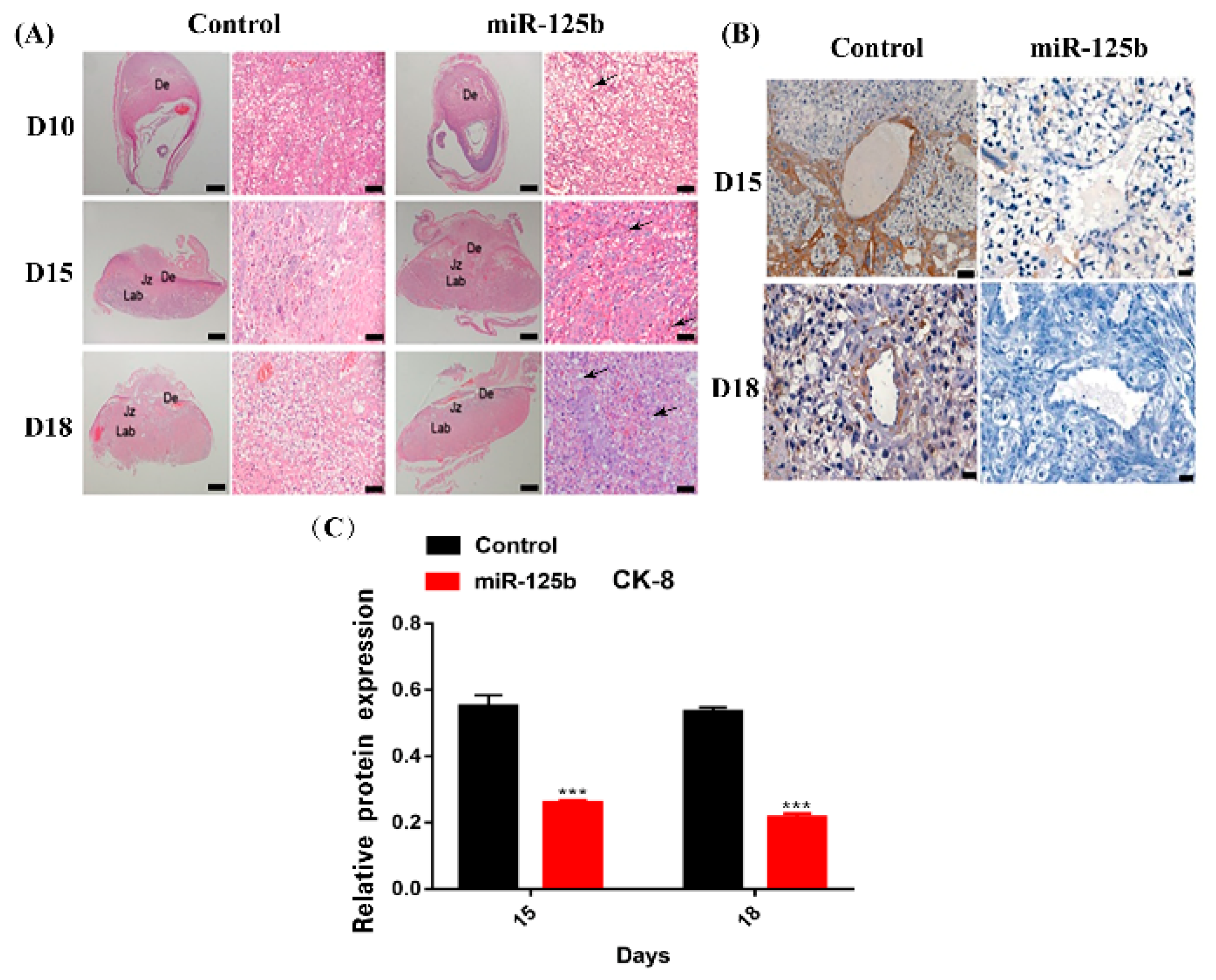
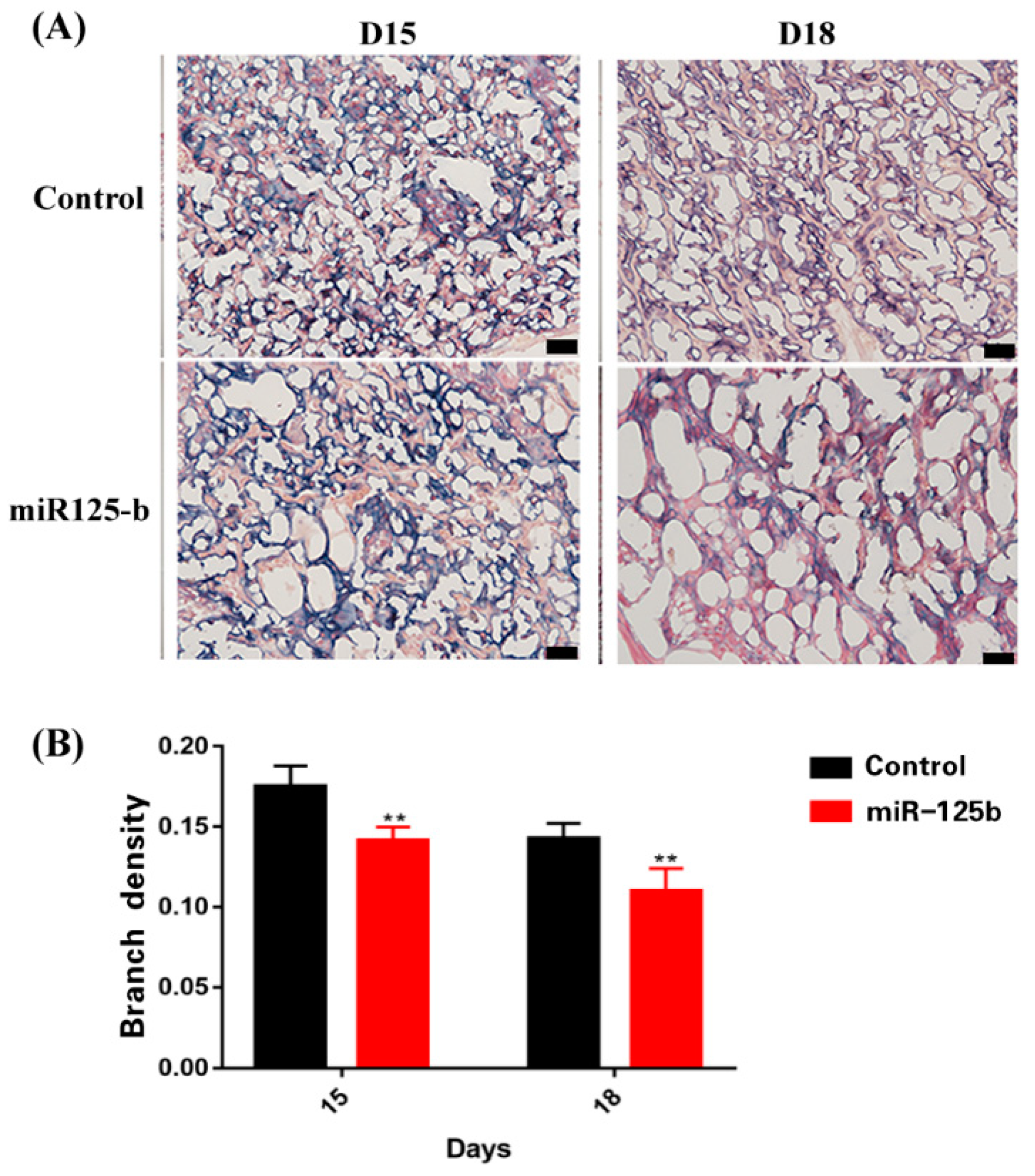
2.3. Placenta-Specific miR-125b Overexpression Affected the Growth of Fetus and Placenta in Mouse
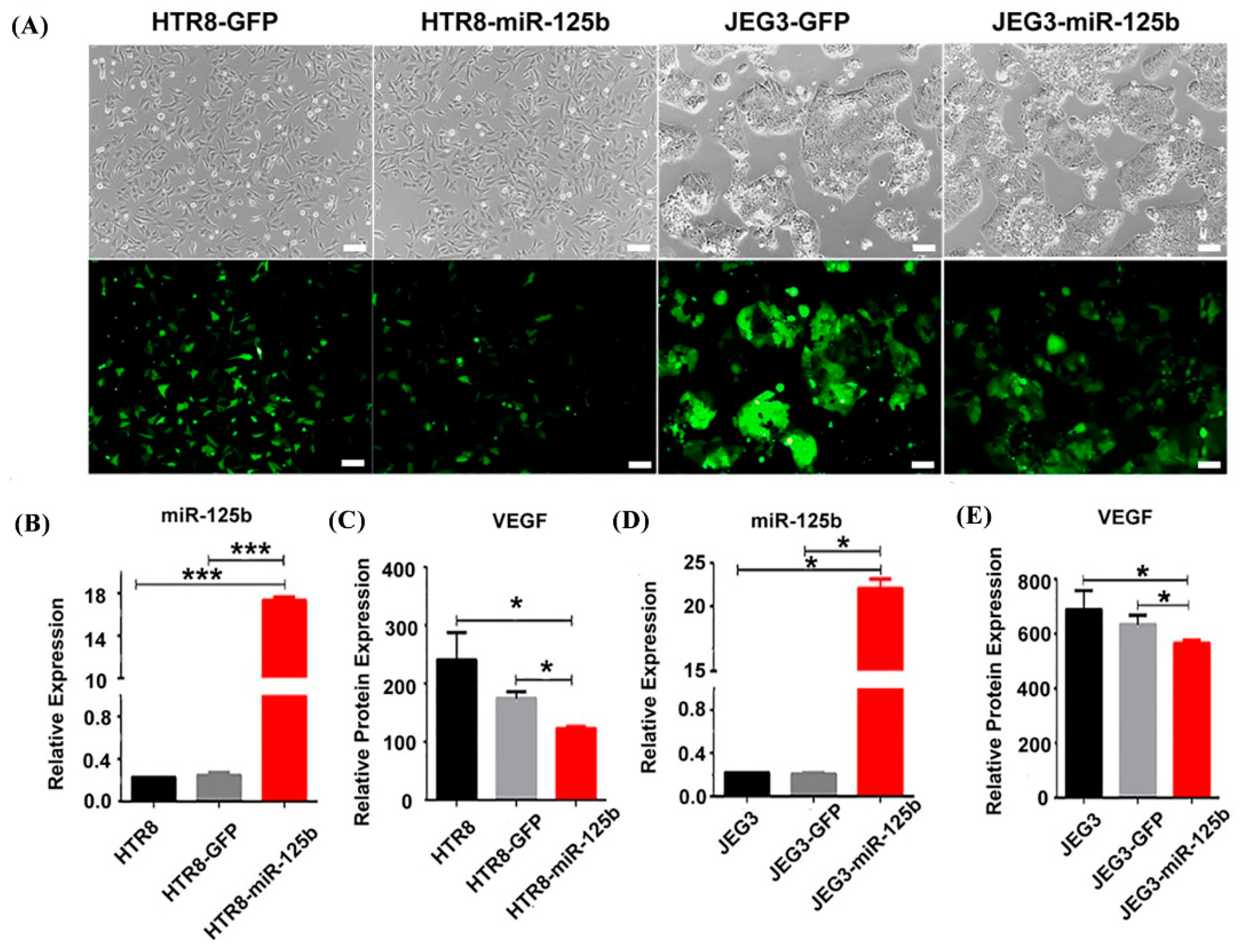
2.4. MiR-125b Overexpression Affected the Growth of HUVECs
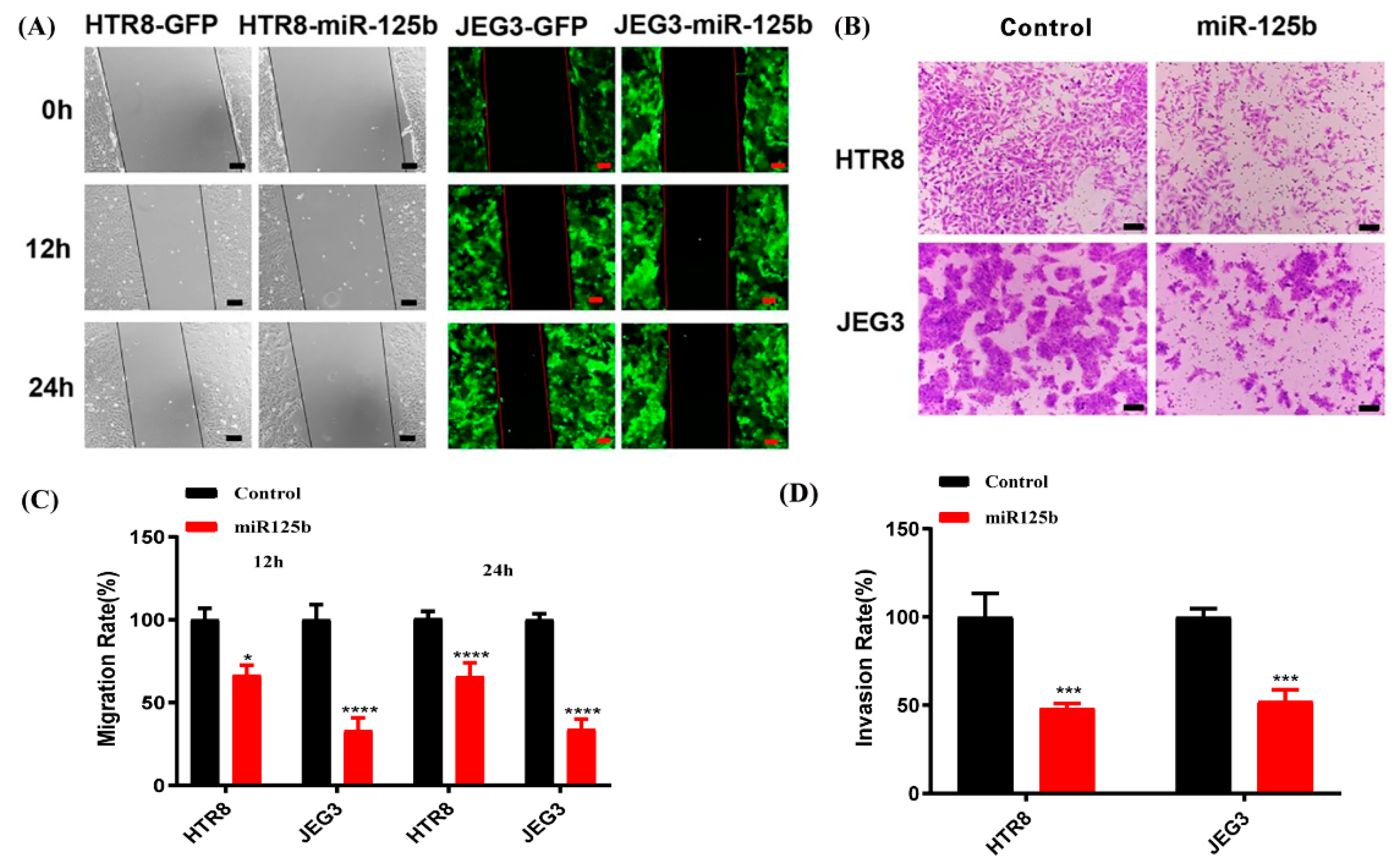
2.5. Overexpression of miR-125b Can Alter Cell Migration and Invasion by Regulating the Expression of VEGF
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Production and Purification of Lentiviral Vector
4.3. Mouse Model and In Vivo Study Protocol
4.4. Real-Time PCR
4.5. Immunocytochemistry (IHC) of CK-8
4.6. Cell Culture and Transfection
4.7. AP Staining
4.8. ELISA
4.9. The Type-B ultrasonic Assay
4.10. Cell Migration and Invasion Assays
4.11. Western Blot
4.12. Colony Formation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Savitz, D.A.; Hertz-Picciotto, I.; Poole, C.; Olshan, A.F. Epidemiologic measures of the course and outcome of pregnancy. Epidemiol. Rev. 2002, 24, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Med, A.S.R. Definitions of infertility and recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2020, 113, 533–535. [Google Scholar] [CrossRef]
- Pandey, M.K.; Rani, R.; Agrawal, S. An update in recurrent spontaneous abortion. Arch. Gynecol. Obs. 2005, 272, 95–108. [Google Scholar] [CrossRef]
- Cui, Q.; Yu, Z.; Purisima, E.O.; Wang, E. MicroRNA regulation and interspecific variation of gene expression. Trends Genet. 2007, 23, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Doerks, T.; Copley, R.R.; Schultz, J.; Ponting, C.P.; Bork, P. Systematic identification of novel protein domain families associated with nuclear functions. Genome Res. 2002, 12, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Incoronato, M.; Grimaldi, A.M.; Mirabelli, P.; Cavaliere, C.; Parente, C.A.; Franzese, M.; Staibano, S.; Ilardi, G.; Russo, D.; Soricelli, A.; et al. Circulating miRNAs in Untreated Breast Cancer: An Exploratory Multimodality Morpho-Functional Study. Cancers 2019, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Dunford, J.V.; Aguilar, S.; Castillo, E.; Patel, E.; Fisher, R.; Ochs, G.; Mahmud, E. Pre-hospital electrocardiography by emergency medical personnel: Effects on scene and transport times for chest pain and ST-segment elevation myocardial infarction patients. J. Am. Coll. Cardiol. 2012, 60, 806–811. [Google Scholar] [CrossRef][Green Version]
- Hua, S.; Quan, Y.; Zhan, M.; Liao, H.; Li, Y.; Lu, L. miR-125b-5p inhibits cell proliferation, migration, and invasion in hepatocellular carcinoma via targeting. Cancer Cell Int. 2019, 19, 203. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wei, Y.-Y.; Yang, C.-C.; Liu, C.-J.; Yeh, L.-Y.; Chou, C.-H.; Chang, K.-W.; Lin, S.-C. miR-125b suppresses oral oncogenicity by targeting the anti-oxidative gene PRXL2A. Redox Biol. 2019, 22, 101140. [Google Scholar] [CrossRef]
- Bousquet, M.; Nguyen, D.; Chen, C.; Shields, L.; Lodish, H.F. MicroRNA-125b transforms myeloid cell lines by repressing multiple mRNA. Haematologica 2012, 97, 1713–1721. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, Y.; Xia, F.; Yang, Y.; Xiong, S.; Jin, L.; Zhang, J. Genome-wide miRNA profiling of villus and decidua of recurrent spontaneous abortion patients. Reproduction 2014, 148, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Brkić, J.; Hayder, H.; Peng, C. MicroRNAs in Human Placental Development and Pregnancy Complications. Int. J. Mol. Sci. 2013, 14, 5519–5544. [Google Scholar] [CrossRef]
- Ventura, W.; Koide, K.; Hori, K.; Yotsumoto, J.; Sekizawa, A.; Saito, H.; Okai, T. Placental expression of microRNA-17 and -19b is down-regulated in early pregnancy loss. Eur. J. Obs. Gynecol. Reprod. Biol. 2013, 169, 28–32. [Google Scholar] [CrossRef]
- Vrijens, K.; Bollati, V.; Nawrot, T.S. MicroRNAs as potential signatures of environmental exposure or effect: A systematic review. Environ. Health Perspect. 2015, 123, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Qiao, J.; Wang, L.; Li, L.; Zhen, X.; Liu, P.; Zheng, X. MicroRNA array and microarray evaluation of endometrial receptivity in patients with high serum progesterone levels on the day of hCG administration. Reprod. Biol. Endocrinol. 2011, 9, 29. [Google Scholar] [CrossRef]
- Su, L.; Zhao, S.; Zhu, M.; Yu, M. Differential expression of microRNAs in porcine placentas on days 30 and 90 of gestation. Reprod. Fertil. Dev. 2010, 22, 1175–1182. [Google Scholar] [CrossRef]
- Gusar, V.; Ganichkina, M.; Chagovets, V.; Kan, N.; Sukhikh, G. MiRNAs Regulating Oxidative Stress: A Correlation with Doppler Sonography of Uteroplacental Complex and Clinical State Assessments of Newborns in Fetal Growth Restriction. J. Clin. Med. 2020, 9, 3227. [Google Scholar] [CrossRef]
- Su, M.-T.; Lin, S.-H.; Lee, I.W.; Chen, Y.-C.; Kuo, P.-L. Association of polymorphisms/haplotypes of the genes encoding vascular endothelial growth factor and its KDR receptor with recurrent pregnancy loss. Hum. Reprod. 2011, 26, 758–764. [Google Scholar] [CrossRef]
- Keskin, U.; Ulubay, M.; Dede, M.; Ozgurtas, T.; Koçyiğit, Y.K.; Aydin, F.N.; Ergün, A. The relationship between the VEGF/sVEGFR-1 ratio and threatened abortion. Arch. Gynecol. Obstet. 2015, 291, 557–561. [Google Scholar] [CrossRef]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eberhardt, C.; et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef]
- Fabjan-Vodusek, V.; Kumer, K.; Osredkar, J.; Verdenik, I.; Gersak, K.; Premru-Srsen, T. Correlation between uterine artery Doppler and the sFlt-1/PlGF ratio in different phenotypes of placental dysfunction. Hypertens Pregnancy 2019, 38, 32–40. [Google Scholar] [CrossRef]
- Ren, Z.; Cui, N.; Zhu, M.; Khalil, R.A. Placental growth factor reverses decreased vascular and uteroplacental MMP-2 and MMP-9 and increased MMP-1 and MMP-7 and collagen types I and IV in hypertensive pregnancy. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H33–H47. [Google Scholar] [CrossRef]
- Gysler, S.M.; Mulla, M.J.; Stuhlman, M.; Sfakianaki, A.K.; Paidas, M.J.; Stanwood, N.L.; Gariepy, A.; Brosens, J.J.; Chamley, L.W.; Abrahams, V.M. Vitamin D reverses aPL-induced inflammation and LMWH-induced sFlt-1 release by human trophoblast. Am. J. Reprod. Immunol. 2015, 73, 242–250. [Google Scholar] [CrossRef]
- Pang, L.H.; Li, M.J.; Li, M.Q.; Yang, D.M.; Shi, L. Vascular endothelial growth factor (VEGF) and the VEGF soluble receptor-1 (sFlt-1) in chorionic villus tissue from Chinese women with early recurrent spontaneous abortion. J. Int. Med. Res. 2011, 39, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Amirchaghmaghi, E.; Rezaei, A.; Moini, A.; Roghaei, M.A.; Hafezi, M.; Aflatoonian, R. Gene expression analysis of VEGF and its receptors and assessment of its serum level in unexplained recurrent spontaneous abortion. Cell J. 2015, 16, 538–545. [Google Scholar]
- Xia, X.; Wang, X.; Cheng, Z.; Qin, W.; Lei, L.; Jiang, J.; Hu, J. The role of pyroptosis in cancer: Pro-cancer or pro-“host”? Cell Death Dis. 2019, 10, 650. [Google Scholar] [CrossRef]
- Fan, X.; Ren, P.; Dhal, S.; Bejerano, G.; Goodman, S.B.; Druzin, M.L.; Gambhir, S.S.; Nayak, N.R. Noninvasive monitoring of placenta-specific transgene expression by bioluminescence imaging. PLoS ONE 2011, 6, e16348. [Google Scholar] [CrossRef]
- Kim, S.T.; Adair-Kirk, T.L.; Senior, R.M.; Miner, J.H. Functional consequences of cell type-restricted expression of laminin α5 in mouse placental labyrinth and kidney glomerular capillaries. PLoS ONE 2012, 7, e41348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pardi, G.; Marconi, A.M.; Cetin, I. Placental-fetal interrelationship in IUGR fetuses—A review. Placenta 2002, 23 (Suppl. A), S136–S141. [Google Scholar] [CrossRef]
- Watson, E.D.; Cross, J.C. Development of structures and transport functions in the mouse placenta. Physiology 2005, 20, 180–193. [Google Scholar] [CrossRef]
- Krüssel, J.S.; Behr, B.; Milki, A.A.; Hirchenhain, J.; Wen, Y.; Bielfeld, P.; Lake Polan, M. Vascular endothelial growth factor (VEGF) mRNA splice variants are differentially expressed in human blastocysts. Mol. Hum. Reprod. 2001, 7, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Schiessl, B.; Innes, B.A.; Bulmer, J.N.; Otun, H.A.; Chadwick, T.J.; Robson, S.C.; Lash, G.E. Localization of angiogenic growth factors and their receptors in the human placental bed throughout normal human pregnancy. Placenta 2009, 30, 79–87. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Li, Q.; Pan, Z.; Wang, X.; Gao, Z.; Ren, C.; Yang, W. miR-125b-1-3p inhibits trophoblast cell invasion by targeting sphingosine-1-phosphate receptor 1 in preeclampsia. Biochem Biophys. Res. Commun. 2014, 453, 57–63. [Google Scholar] [CrossRef]
- Kohan-Ghadr, H.-R.; Kadam, L.; Jain, C.; Armant, D.R.; Drewlo, S. Potential role of epigenetic mechanisms in regulation of trophoblast differentiation, migration, and invasion in the human placenta. Cell Adhes. Migr. 2016, 10, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Tsai, H.-L.; Syu, J.-S.; Chen, T.-Y.; Su, M.-T. Primary Cilium-Regulated EG-VEGF Signaling Facilitates Trophoblast Invasion. J. Cell. Physiol. 2017, 232, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Su, M.-T.; Tsai, P.-Y.; Tsai, H.-L.; Chen, Y.-C.; Kuo, P.-L. miR-346 and miR-582-3p-regulated EG-VEGF expression and trophoblast invasion via matrix metalloproteinases 2 and 9. Biofactors 2017, 43, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Abou-Kheir, W.; Eid, A.; El-Merahbi, R.; Assaf, R.; Daoud, G. A Unique Expression of Keratin 14 in a Subset of Trophoblast Cells. PLoS ONE 2015, 10, e0139939. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, C.; Dong, W.B.; Zhao, S.; Li, Q.P.; Kang, L.; Lei, X.P.; Guo, L.; Zhai, X.S. Construction of p66Shc gene interfering lentivirus vectors and its effects on alveolar epithelial cells apoptosis induced by hyperoxia. Drug Des. Dev. Ther. 2016, 10, 2611–2622. [Google Scholar] [CrossRef][Green Version]
- Renaud, S.J.; Sullivan, R.; Graham, C.H. Tumour necrosis factor alpha stimulates the production of monocyte chemoattractants by extravillous trophoblast cells via differential activation of MAPK pathways. Placenta 2009, 30, 313–319. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Forward | Reverse |
|---|---|---|
| hsa-pri-miR-125b | CGGAATTCCTCGTGATCGTATGTGTATGTGCG | TTGCGGCCGCCATACACATAGCAGCC AACACGC |
| mmu-pri-miR-125b | CCGGAATTCTCCTTTGATACAACAGAC | TGCGGCCGCCTAACATACTATGCCTACTT |
| Chr9 | CCGGAATTCCGGTCAAAGCATAAAC | TTGCGGCCGcCAGCAAACAGGTGGTC |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| miR-125b-5pRT | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACTCACAAG | |
| hsa-actin | AAGGAAGGCTGGAAGAGTGC | CTACAATGTGCTGCGTGTGG |
| mmu-actin | GATTCTGGCGATGGTGTAACTCA | AGATTCCATACCAATGAAAGAGGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, F.; Cai, H.; Tan, L.; Qin, D.; Zhang, J.; Hua, J.; Fan, X.; Peng, S. Placenta-Specific miR-125b Overexpression Leads to Increased Rates of Pregnancy Loss in Mice. Int. J. Mol. Sci. 2022, 23, 943. https://doi.org/10.3390/ijms23020943
Sun F, Cai H, Tan L, Qin D, Zhang J, Hua J, Fan X, Peng S. Placenta-Specific miR-125b Overexpression Leads to Increased Rates of Pregnancy Loss in Mice. International Journal of Molecular Sciences. 2022; 23(2):943. https://doi.org/10.3390/ijms23020943
Chicago/Turabian StyleSun, Fen, Hui Cai, Lunbo Tan, Dezhe Qin, Jian Zhang, Jinlian Hua, Xiujun Fan, and Sha Peng. 2022. "Placenta-Specific miR-125b Overexpression Leads to Increased Rates of Pregnancy Loss in Mice" International Journal of Molecular Sciences 23, no. 2: 943. https://doi.org/10.3390/ijms23020943
APA StyleSun, F., Cai, H., Tan, L., Qin, D., Zhang, J., Hua, J., Fan, X., & Peng, S. (2022). Placenta-Specific miR-125b Overexpression Leads to Increased Rates of Pregnancy Loss in Mice. International Journal of Molecular Sciences, 23(2), 943. https://doi.org/10.3390/ijms23020943







