The Effect of Ethanol on Disassembly of Amyloid-β1-42 Pentamer Revealed by Atomic Force Microscopy and Gel Electrophoresis
Abstract
:1. Introduction
2. Results
2.1. Electrophoresis
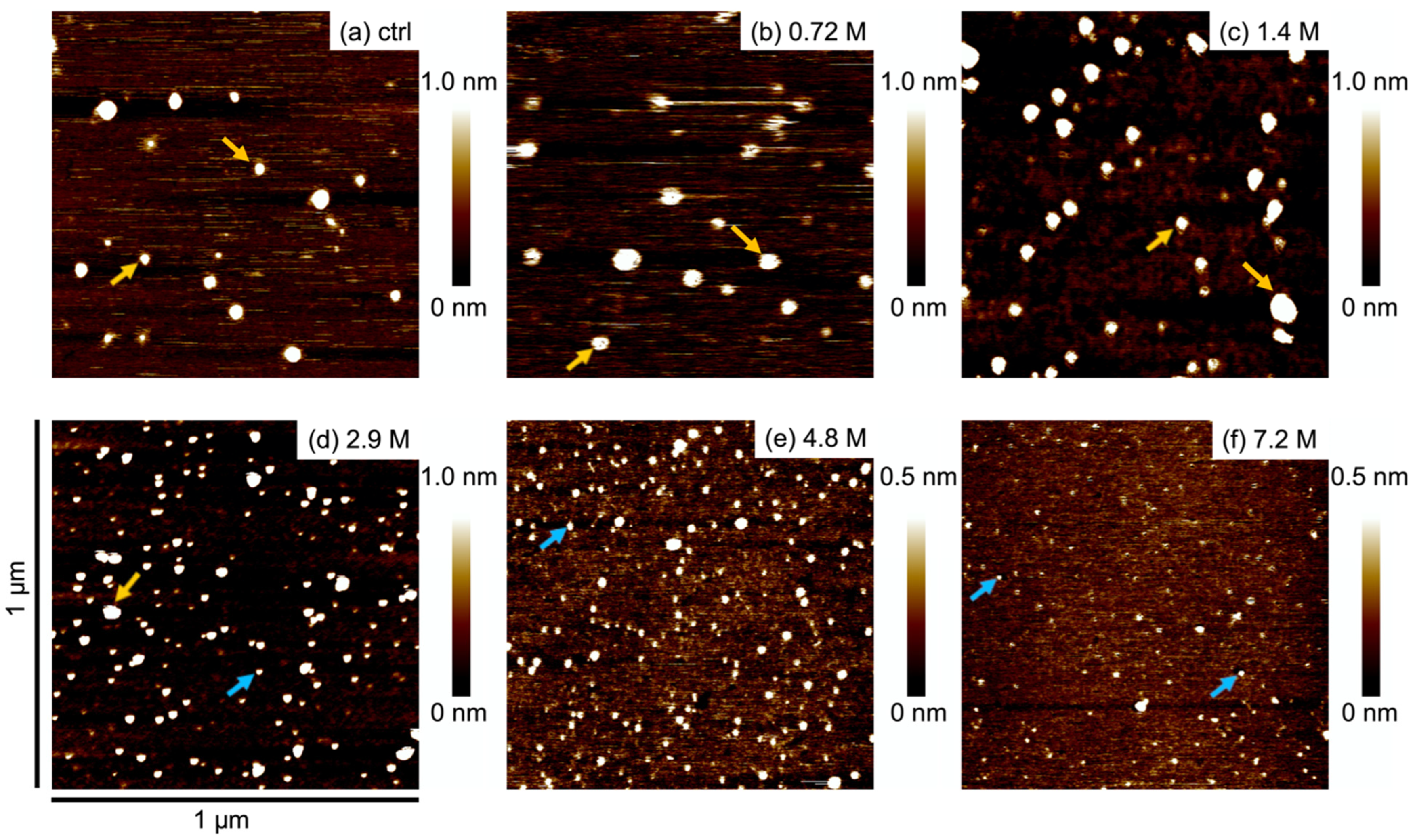
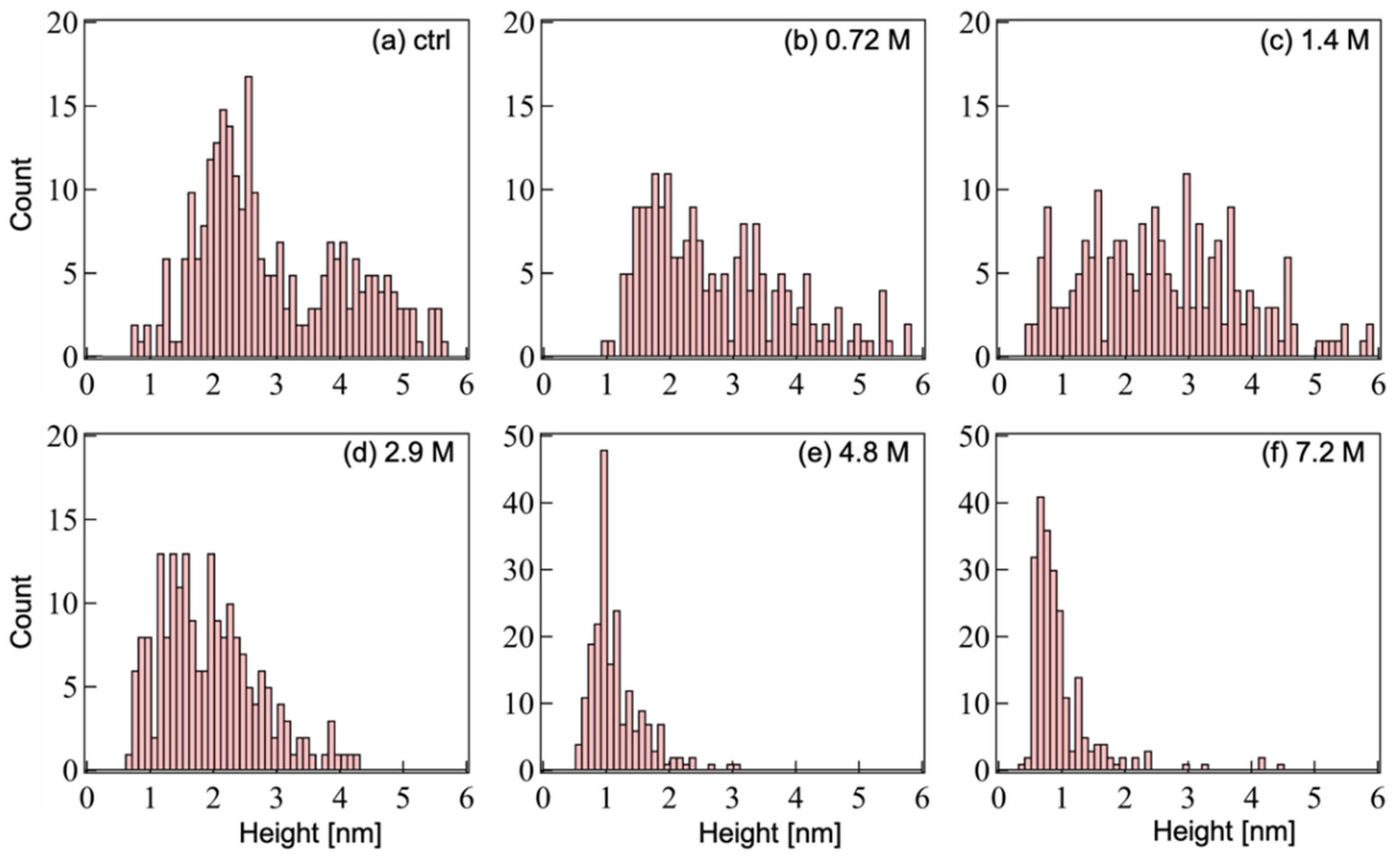
2.2. AFM Imaging and Height Distribution
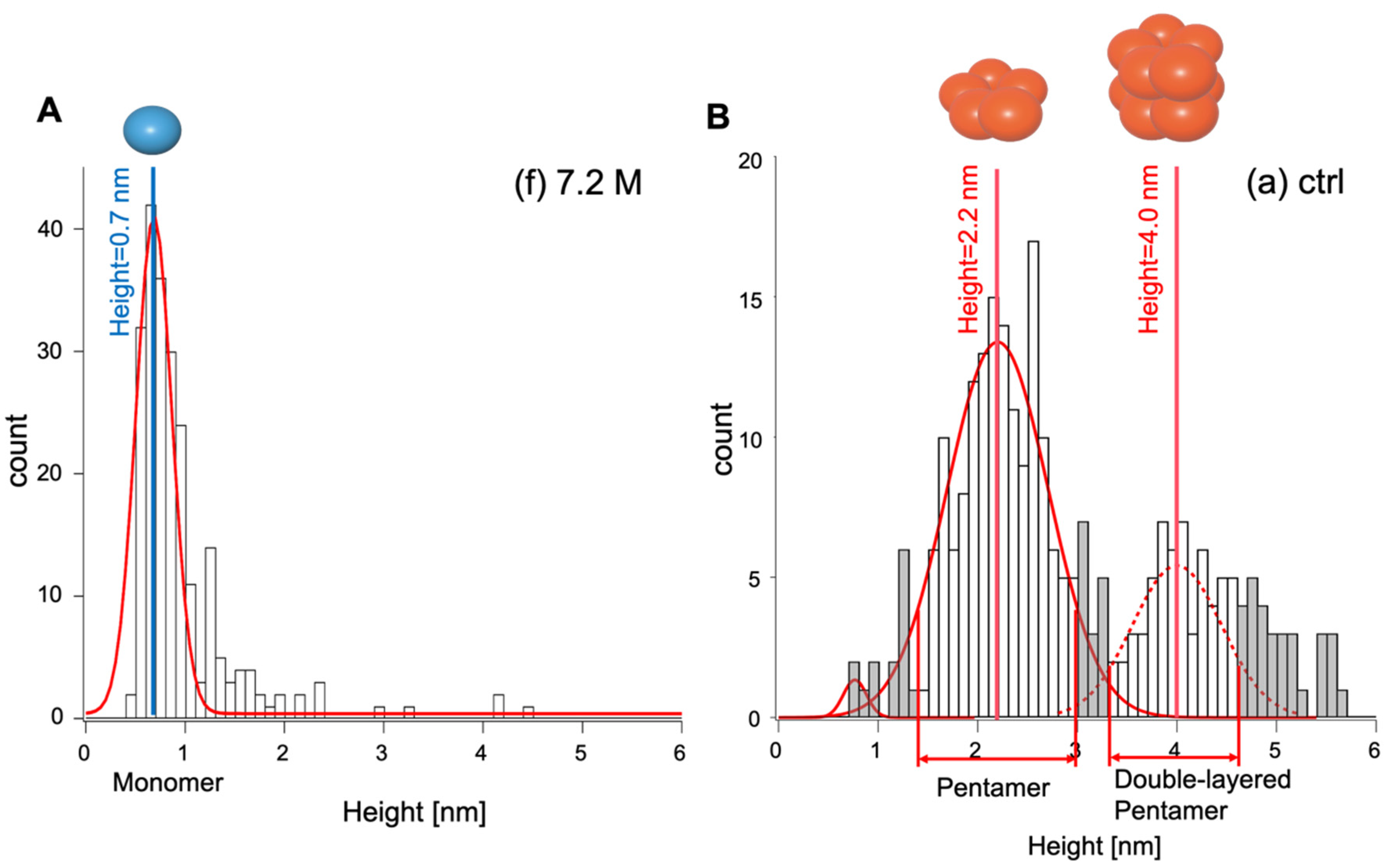
2.3. Distribution Height Analysis by Gaussian Fitting
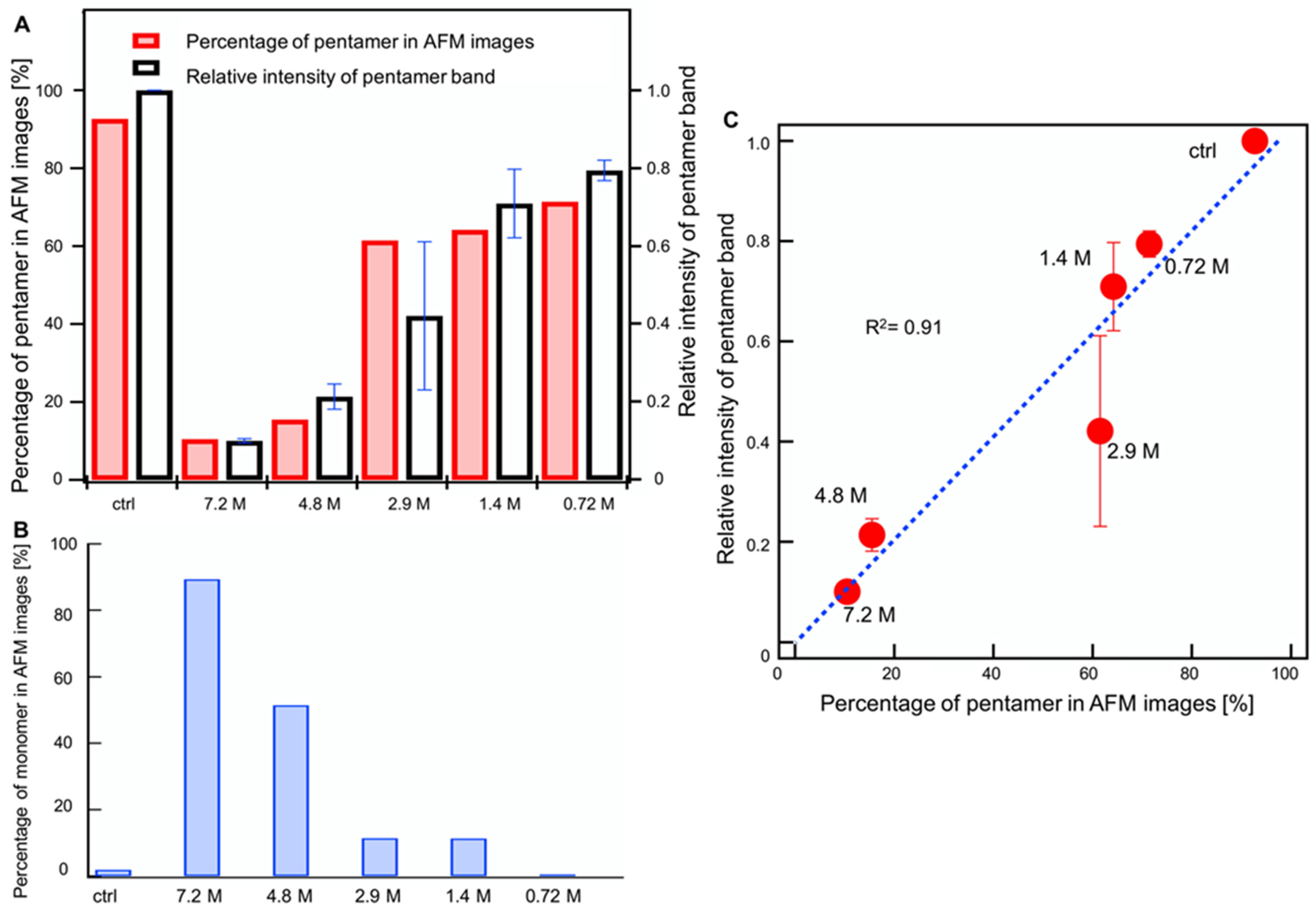
2.4. Combined AFM and Electrophoresis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Aβ Oligomer Sample Preparation
4.3. Electrophoresis
4.4. AFM Observations
4.5. Image and Histogram Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harper, J.D.; Wong, S.S.; Lieber, C.M.; Lansbury, P.T. Assembly of Aβ Amyloid Protofibrils: An in Vitro Model for a Possible Early Event in Alzheimer’s Disease. Biochemistry 1999, 38, 8972–8980. [Google Scholar] [CrossRef]
- Lacor, P.N.; Buniel, M.C.; Furlow, P.W.; Clemente, A.S.; Velasco, P.T.; Wood, M.; Viola, K.L.; Klein, W.L. Aβ Oligomer-Induced Aberrations in Synapse Composition, Shape, and Density Provide a Molecular Basis for Loss of Connectivity in Alzheimer’s Disease. J. Neurosci. 2007, 27, 796–807. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Lammich, S.; Kojro, E.; Postina, R.; Gilbert, S.; Pfeiffer, R.; Jasionowski, M.; Haass, C.; Fahrenholz, F. Constitutive and Regulated α-Secretase Cleavage of Alzheimer’s Amyloid Precursor Protein by a Disintegrin Metalloprotease. Proc. Natl. Acad. Sci. USA 1999, 96, 3922–3927. [Google Scholar] [CrossRef] [Green Version]
- Ye, I.Y.; Bassit, B.; Zhu, L.; Yang, X.; Wang, C.; Li, Y.M. γ-Secretase Substrate Concentration Modulates the Aβ42/Aβ40 Ratio: Implications for Alzheimer Disease. J. Biol. Chem. 2007, 282, 23639–23644. [Google Scholar] [CrossRef] [Green Version]
- Iwatsubo, T.; Odaka, A.; Suzuki, N.; Mizusawa, H.; Nukina, N.; Ihara, Y. Visualization of Aβ42(43) and Aβ40 in Senile Plaques with End-Specific Aβ Monoclonals: Evidence That an Initially Deposited Species Is Aβ42(43). Neuron 1994, 13, 45–53. [Google Scholar] [CrossRef]
- Viola, K.L.; Klein, W.L.; Klein, W.L. Amyloid β Oligomers in Alzheimer’s Disease Pathogenesis, Treatment, and Diagnosis. Acta Neuropathol. 2015, 3, 183–206. [Google Scholar] [CrossRef]
- Walsh, D.M.; Selkoe, D.J. Deciphering the Molecular Basis of Memory Failure in Alzheimer’s Disease. Neuron 2004, 44, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayre, L.M.; Perry, G.; Harris, P.L.R.; Liu, Y.; Schubert, K.A.; Smith, M.A. In Situ Oxidative Catalysis by Neurofibrillary Tangles and Senile Plaques in Alzheimer’s Disease: A Central Role for Bound Transition Metals. J. Neurochem. 2000, 74, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Campioni, S.; Mannini, B.; Zampagni, M.; Pensalfini, A.; Parrini, C.; Evangelisti, E.; Relini, A.; Stefani, M.; Dobson, C.M.; Cecchi, C.; et al. A Causative Link between the Structure of Aberrant Protein Oligomers and Their Toxicity. Nat. Chem. Biol. 2010, 6, 140–147. [Google Scholar] [CrossRef]
- Lambert, M.P.; Barlow, A.K.; Chromy, B.A.; Edwards, C.; Freed, R.; Liosatos, M.; Morgan, T.E.; Rozovsky, I.; Trommer, B.; Viola, K.L.; et al. Diffusible, Nonfibrillar Ligands Derived from Aβ1-42 Are Potent Central Nervous System Neurotoxins. Proc. Natl. Acad. Sci. USA 1998, 95, 6448–6453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De, S.; Wirthensohn, D.C.; Flagmeier, P.; Hughes, C.; Aprile, F.A.; Ruggeri, F.S.; Whiten, D.R.; Emin, D.; Xia, Z.; Varela, J.A.; et al. Different Soluble Aggregates of Aβ42 Can Give Rise to Cellular Toxicity through Different Mechanisms. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barz, B.; Liao, Q.; Strodel, B. Pathways of Amyloid-β Aggregation Depend on Oligomer Shape. J. Am. Chem. Soc. 2018, 140, 319–327. [Google Scholar] [CrossRef]
- Michaels, T.C.T.; Šarić, A.; Curk, S.; Bernfur, K.; Arosio, P.; Meisl, G.; Dear, A.J.; Cohen, S.I.A.; Dobson, C.M.; Vendruscolo, M.; et al. Dynamics of Oligomer Populations Formed during the Aggregation of Alzheimer’s Aβ42 Peptide. Nat. Chem. 2020, 12, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Jarvet, J.; Damberg, P.; Bodell, K.; Göran Eriksson, L.E.; Gräslund, A. Reversible Random Coil to β-Sheet Transition and the Early Stage of Aggregation of the Aβ(12–28) Fragment from the Alzheimer Peptide. J. Am. Chem. Soc. 2000, 122, 4261–4268. [Google Scholar] [CrossRef]
- Matsumura, S.; Shinoda, K.; Yamada, M.; Yokojima, S.; Inoue, M.; Ohnishi, T.; Shimada, T.; Kikuchi, K.; Masui, D.; Hashimoto, S.; et al. Two Distinct Amyloid β-Protein (Aβ) Assembly Pathways Leading to Oligomers and Fibrils Identified by Combined Fluorescence Correlation Spectroscopy, Morphology, and Toxicity Analyses. J. Biol. Chem. 2011, 286, 11555–11562. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Davis, J.; Aucoin, D.; Sato, T.; Ahuja, S.; Aimoto, S.; Elliott, J.I.; Van Nostrand, W.E.; Smith, S.O. Structural conversion of neurotoxic amyloid-β1–42 oligomers to fibrils. Nat. Struct. Mol. Biol. 2010, 17, 561–567. [Google Scholar] [CrossRef] [Green Version]
- Pagano, K.; Tomaselli, S.; Molinari, H.; Ragona, L. Natural Compounds as Inhibitors of Aβ Peptide Aggregation: Chemical Requirements and Molecular Mechanisms. Front Neurosci. 2020, 14, 619667. [Google Scholar] [CrossRef]
- Hong, D.P.; Hoshino, M.; Kuboi, R.; Goto, Y. Clustering of Fluorine-Substituted Alcohols as a Factor Responsible for Their Marked Effects on Proteins and Peptides. J. Am. Chem. Soc. 1999, 121, 8427–8433. [Google Scholar] [CrossRef]
- Stine, W.B.; Dahlgren, K.N.; Krafft, G.A.; LaDu, M.J. In Vitro Characterization of Conditions for Amyloid-β Peptide Oligomerization and Fibrillogenesis. J. Biol. Chem. 2003, 278, 11612–11622. [Google Scholar] [CrossRef] [Green Version]
- Laurents, D.V.; Pantoja-Uceda, D.; López, L.C.; Carrodeguas, J.A.; Mompeán, M.; Jiménez, M.; Sancho, J. DMSO Affects Aβ1-40’s Conformation and Interactions with Aggregation Inhibitors as Revealed by NMR. RSC Adv. 2015, 5, 69761–69764. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.L.; Murphy, R.M. Solvent Effects on Self-Assembly of Beta-Amyloid Peptide. Biophys. J. 1995, 69, 640–651. [Google Scholar] [CrossRef] [Green Version]
- Ormeño, D.; Romero, F.; López-Fenner, J.; Avila, Á.; Martínez-Torres, A.; Parodi, J. Ethanol Reduces Amyloid Aggregation In Vitro and Prevents Toxicity in Cell Lines. Arch. Med. Res. 2013, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bate, C.; Williams, A. Ethanol Protects Cultured Neurons against Amyloid-β and α-Synuclein-Induced Synapse Damage. Neuropharmacology 2011, 61, 1406–1412. [Google Scholar] [CrossRef]
- Simone Ruggeri, F.; Habchi, J.; Cerreta, A.; Dietler, G. AFM-Based Single Molecule Techniques: Unraveling the Amyloid Pathogenic Species. Curr. Pharm. Des. 2016, 22, 3950–3970. [Google Scholar] [CrossRef] [Green Version]
- Zorbas, V.; Ortiz-Acevedo, A.; Dalton, A.B.; Yoshida, M.M.; Dieckmann, G.R.; Draper, R.K.; Baughman, R.H.; Jose-Yacaman, M.; Musselman, I.H. Preparation and Characterization of Individual Peptide-Wrapped Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2004, 126, 7222–7227. [Google Scholar] [CrossRef] [PubMed]
- Naruse, N.; Satooka, H.; Todo, K.; Nakanishi, A.; Taguchi, H.; Mera, Y. Oligomers imaging of amyloid-β1-42 by scanning tunneling microscopy. Jpn. J. Appl. Phys. 2019, 58, SIIB30. [Google Scholar] [CrossRef]
- Lin, Y.C.; Komatsu, H.; Ma, J.; Axelsen, P.H.; Fakhraai, Z. Quantitative Analysis of Amyloid Polymorphism Using Height Histograms to Correct for Tip Convolution Effects in Atomic Force Microscopy Imaging. RSC Adv. 2016, 6, 114286–114295. [Google Scholar] [CrossRef]
- Losic, D.; Martin, L.L.; Mechler, A.; Aguilar, M.I.; Small, D.H. High Resolution Scanning Tunnelling Microscopy of the β-Amyloid Protein (Aβ1-40) of Alzheimer’s Disease Suggests a Novel Mechanism of Oligomer Assembly. J. Struct. Biol. 2006, 155, 104–110. [Google Scholar] [CrossRef]
- Mastrangelo, I.A.; Ahmed, M.; Sato, T.; Liu, W.; Wang, C.; Hough, P.; Smith, S.O. High-Resolution Atomic Force Microscopy of Soluble Aβ42 Oligomers. J. Mol. Biol. 2006, 358, 106–119. [Google Scholar] [CrossRef]
- Tran, L.; Basdevant, N.; Prévost, C.; Ha-Duong, T. Structure of Ring-Shaped Aβ42 Oligomers Determined by Conformational Selection. Sci. Rep. 2016, 6, 21429. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.; Xu, M.; Zhang, M.; Zhou, T.; Yang, Y.; Wang, C.; Ferapontova, E.E. Direct Electrochemical and AFM Detection of Amyloid-β Peptide Aggregation on Basal Plane HOPG. Nanoscale 2014, 6, 7853–7857. [Google Scholar] [CrossRef]
- Murphy, M.P.; Levine, H. Alzheimer’s Disease and the Amyloid-β Peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marina, G.B.; Kirkitadze, D.; Lomakin, A.; Vollers, S.S.; Benedek, G.B.; Teplow, D.B. Amyloid β-Protein (Aβ) Assembly: Aβ40 and Aβ42 Oligomerize through Distinct Pathways. Proc. Natl. Acad. Sci. USA 2003, 100, 330–335. [Google Scholar] [CrossRef] [Green Version]
- Ladiwala, A.R.A.; Litt, J.; Kane, R.S.; Aucoin, D.S.; Smith, S.O.; Ranjan, S.; Davis, J.; Van Nostrand, W.E.; Tessier, P.M. Conformational Differences between Two Amyloid β oligomers of Similar Size and Dissimilar Toxicity. J. Biol. Chem. 2012, 287, 24765–24773. [Google Scholar] [CrossRef] [Green Version]
- Jana, A.K.; Sengupta, N. Aβ Self-Association and Adsorption on a Hydrophobic Nanosurface: Competitive Effects and the Detection of Small Oligomers via Electrical Response. Soft Matter 2015, 11, 269–279. [Google Scholar] [CrossRef]
- Thomas, P.D.; Dill, K.A. Local and Nonlocal Interactions in Globular Proteins and Mechanisms of Alcohol Denaturation. Protein Sci. 1993, 2, 2050–2065. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Bolen, D.W. The Peptide Backbone Plays a Dominant Role in Protein Stabilization by Naturally Occurring Osmolytes. Biochemistry 1995, 34, 12884–12891. [Google Scholar] [CrossRef]
- Hirota, N.; Mizuno, K.; Goto, Y. Group Additive Contributions to the Alcohol-Induced α-Helix Formation of Melittin: Implication for the Mechanism of the Alcohol Effects on Proteins. J. Mol. Biol. 1998, 275, 365–378. [Google Scholar] [CrossRef]
- Bitan, G.; Tarus, B.; Vollers, S.S.; Lashuel, H.A.; Condron, M.M.; Straub, J.E.; Teplow, D.B. A Molecular Switch in Amyloid Assembly: Met 35 and Amyloid β-Protein Oligomerization. J. Am. Chem. Soc. 2003, 125, 15359–15365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovin, T.M. Multiphasic Zone Electrophoresis. II. Design of Integrated Discontinuous Buffer Systems for Analytical and Preparative Fractionation. Biochemistry 1973, 12, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Erickson, H.P. Size and Shape of Protein Molecules at the Nanometer Level Determined by Sedimentation, Gel Filtration, and Electron Microscopy. Biol. Proced. Online 2009, 11, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nirmalraj, P.N.; List, J.; Battacharya, S.; Howe, G.; Xu, L.; Thompson, D.; Mayer, M. Complete Aggregation Pathway of Amyloid β (1-40) and (1-42) Resolved on an Atomically Clean Interface. Sci. Adv. 2020, 6, eaaz6014. [Google Scholar] [CrossRef] [Green Version]
- Meisl, G.; Yang, X.; Hellstrand, E.; Frohm, B.; Kirkegaard, J.B.; Cohen, S.I.A.; Dobson, C.M.; Linse, S.; Knowles, T.P.J. Differences in Nucleation Behavior Underlie the Contrasting Aggregation Kinetics of the Aβ40 and Aβ42 Peptides. Proc. Natl. Acad. Sci. USA 2014, 111, 9384–9389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- How, S.-C.; Hsu, W.-T.; Tseng, C.-P.; Lo, C.-H.; Chou, W.-L.; Wang, S.S.-S. Brilliant Blue R Dye Is Capable of Suppressing Amyloid Fibril Formation of Lysozyme. J. Biomol. Struct. Dyn. 2018, 36, 3420–3433. [Google Scholar] [CrossRef]
- Schägger, H.; von Jagow, G. Blue Native Electrophoresis for Isolation of Membrane Protein Complexes in Enzymatically Active Form. Anal. Biochem. 1991, 199, 223–231. [Google Scholar] [CrossRef]
- Gassmann, M.; Grenacher, B.; Rohde, B.; Vogel, J. Quantifying Western Blots: Pitfalls of Densitometry. Electrophoresis 2009, 30, 1845–1855. [Google Scholar] [CrossRef]
- Gallo-Oller, G.; Ordoñez, R.; Dotor, J. A New Background Subtraction Method for Western Blot Densitometry Band Quantification through Image Analysis Software. J. Immunol. Methods 2018, 457, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Schägger, H. Tricine–SDS-PAGE. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef]
- Bellier, J.P.; Kimura, H. Acetylcholine Synthesis by Choline Acetyltransferase of a Peripheral Type as Demonstrated in Adult Rat Dorsal Root Ganglion. J. Neurochem. 2007, 101, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Ostendorf, F.; Schmitz, C.; Hirth, S.; Kühnle, A.; Kolodziej, J.J.; Reichling, M. Evidence for Potassium Carbonate Crystallites on Air-Cleaved Mica Surfaces. Langmuir 2009, 25, 10764–10767. [Google Scholar] [CrossRef] [Green Version]
- Trewby, W.; Livesey, D.; Voïtchovsky, K. Buffering Agents Modify the Hydration Landscape at Charged Interfaces. Soft Matter 2016, 12, 2642–2651. [Google Scholar] [CrossRef] [Green Version]
- Nečas, D.; Klapetek, P. Gwyddion: An Open-Source Software for SPM Data Analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Marek, J.; Demjénová, E.; Tomori, Z.; Janáček, J.; Zolotová, I.; Valle, F.; Favre, M.; Dietler, G. Interactive Measurement and Characterization of DNA Molecules by Analysis of AFM Images. Cytom. Part A J. Int. Soc. Anal. Cytol. 2005, 63, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Mou, J.; Czajkowsky, D.M.; Yang, J.; Yuan, J.Y. Biological Atomic Force Microscopy: What Is Achieved and What Is Needed. Adv. Phys. 1996, 45, 1–86. [Google Scholar] [CrossRef]
- Gan, Y. Atomic and Subnanometer Resolution in Ambient Conditions by Atomic Force Microscopy. Surf. Sci. Rep. 2009, 64, 99–121. [Google Scholar] [CrossRef]


| Aβ1-42 Peptide [nmol] | 21 | 21 | 21 | 21 | 21 | 21 |
| Ethanol [µL] | 0 (ctrl) | 120 | 80 | 48 | 24 | 12 |
| Ethanol [M] | 0 | 7.2 | 4.8 | 2.9 | 1.4 | 0.72 |
| Ethanol [%] | 0 | 41.4 | 27.6 | 16.6 | 8.3 | 4.1 |
| Molar Ratio (Aβ1-42/ethanol) | - | 1.0 × 10−5 | 1.5 × 10−5 | 2.5 × 10−5 | 5.0 × 10−5 | 1.0 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsui, A.; Bellier, J.-P.; Kanai, T.; Satooka, H.; Nakanishi, A.; Terada, T.; Ishibe, T.; Nakamura, Y.; Taguchi, H.; Naruse, N.; et al. The Effect of Ethanol on Disassembly of Amyloid-β1-42 Pentamer Revealed by Atomic Force Microscopy and Gel Electrophoresis. Int. J. Mol. Sci. 2022, 23, 889. https://doi.org/10.3390/ijms23020889
Matsui A, Bellier J-P, Kanai T, Satooka H, Nakanishi A, Terada T, Ishibe T, Nakamura Y, Taguchi H, Naruse N, et al. The Effect of Ethanol on Disassembly of Amyloid-β1-42 Pentamer Revealed by Atomic Force Microscopy and Gel Electrophoresis. International Journal of Molecular Sciences. 2022; 23(2):889. https://doi.org/10.3390/ijms23020889
Chicago/Turabian StyleMatsui, Atsuya, Jean-Pierre Bellier, Takeshi Kanai, Hiroki Satooka, Akio Nakanishi, Tsukasa Terada, Takafumi Ishibe, Yoshiaki Nakamura, Hiroyasu Taguchi, Nobuyasu Naruse, and et al. 2022. "The Effect of Ethanol on Disassembly of Amyloid-β1-42 Pentamer Revealed by Atomic Force Microscopy and Gel Electrophoresis" International Journal of Molecular Sciences 23, no. 2: 889. https://doi.org/10.3390/ijms23020889
APA StyleMatsui, A., Bellier, J.-P., Kanai, T., Satooka, H., Nakanishi, A., Terada, T., Ishibe, T., Nakamura, Y., Taguchi, H., Naruse, N., & Mera, Y. (2022). The Effect of Ethanol on Disassembly of Amyloid-β1-42 Pentamer Revealed by Atomic Force Microscopy and Gel Electrophoresis. International Journal of Molecular Sciences, 23(2), 889. https://doi.org/10.3390/ijms23020889






