Nutritional Approaches as a Treatment for Impaired Bone Growth and Quality Following the Consumption of Ultra-Processed Food
Abstract
1. Introduction
2. Results
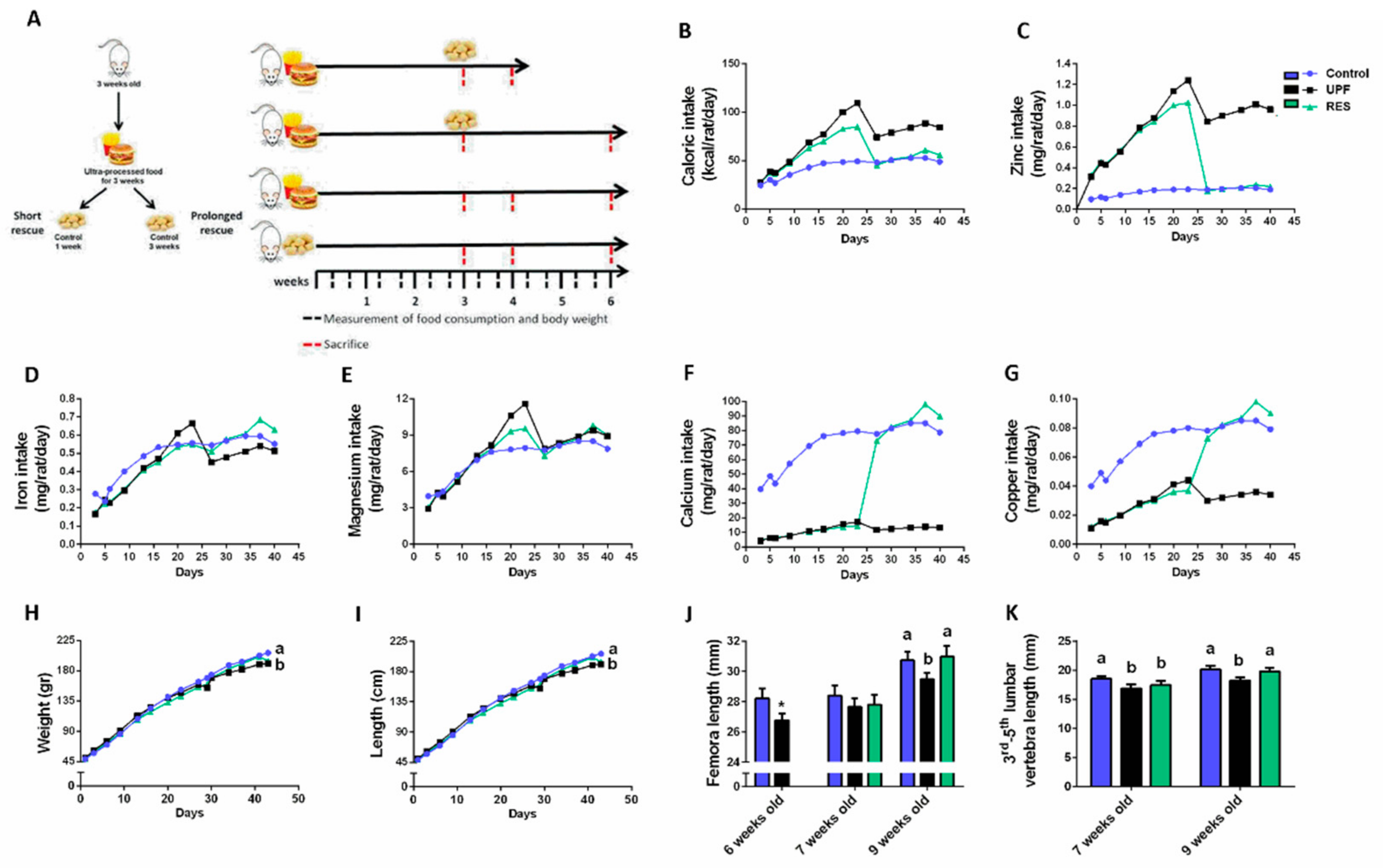
2.1. Analysis of the Macro and Micro-Nutrient Content of the Control and UPF
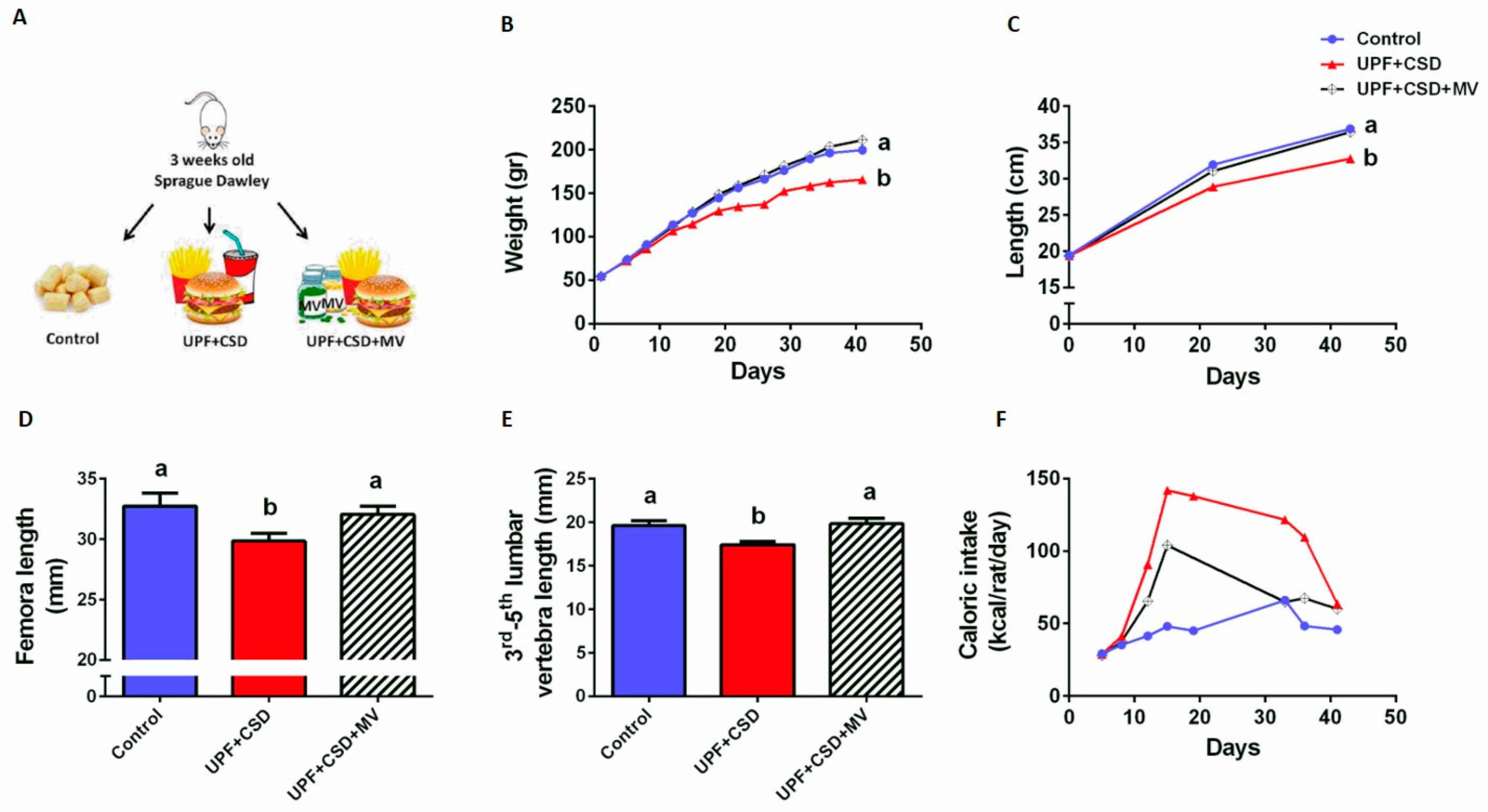
2.2. The Effect of Micro-Nutrient Composition on Growth and Physiological Parameters
2.2.1. The Effect of UPF and Supplementations on the Metabolic Profile of the Rats
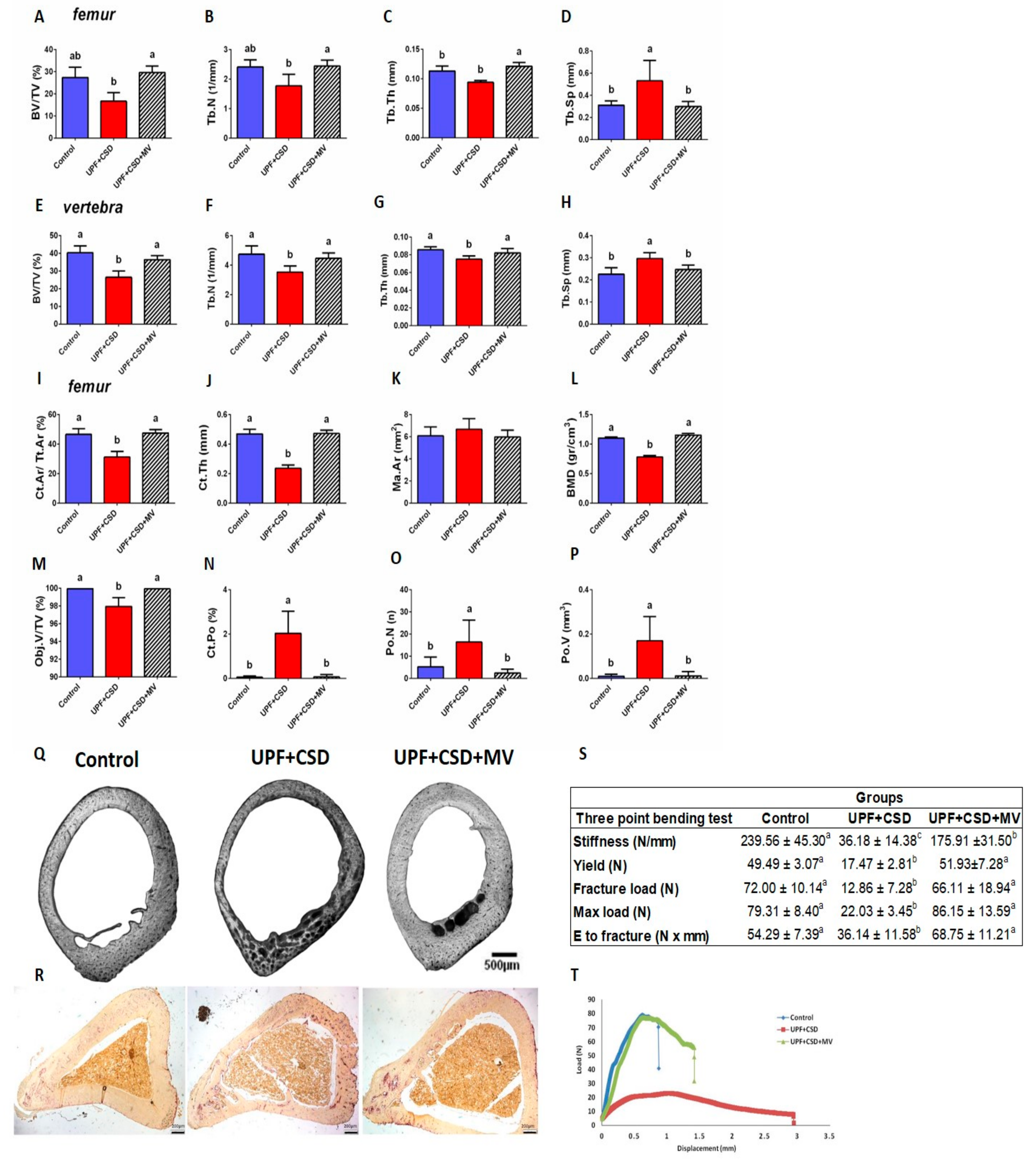
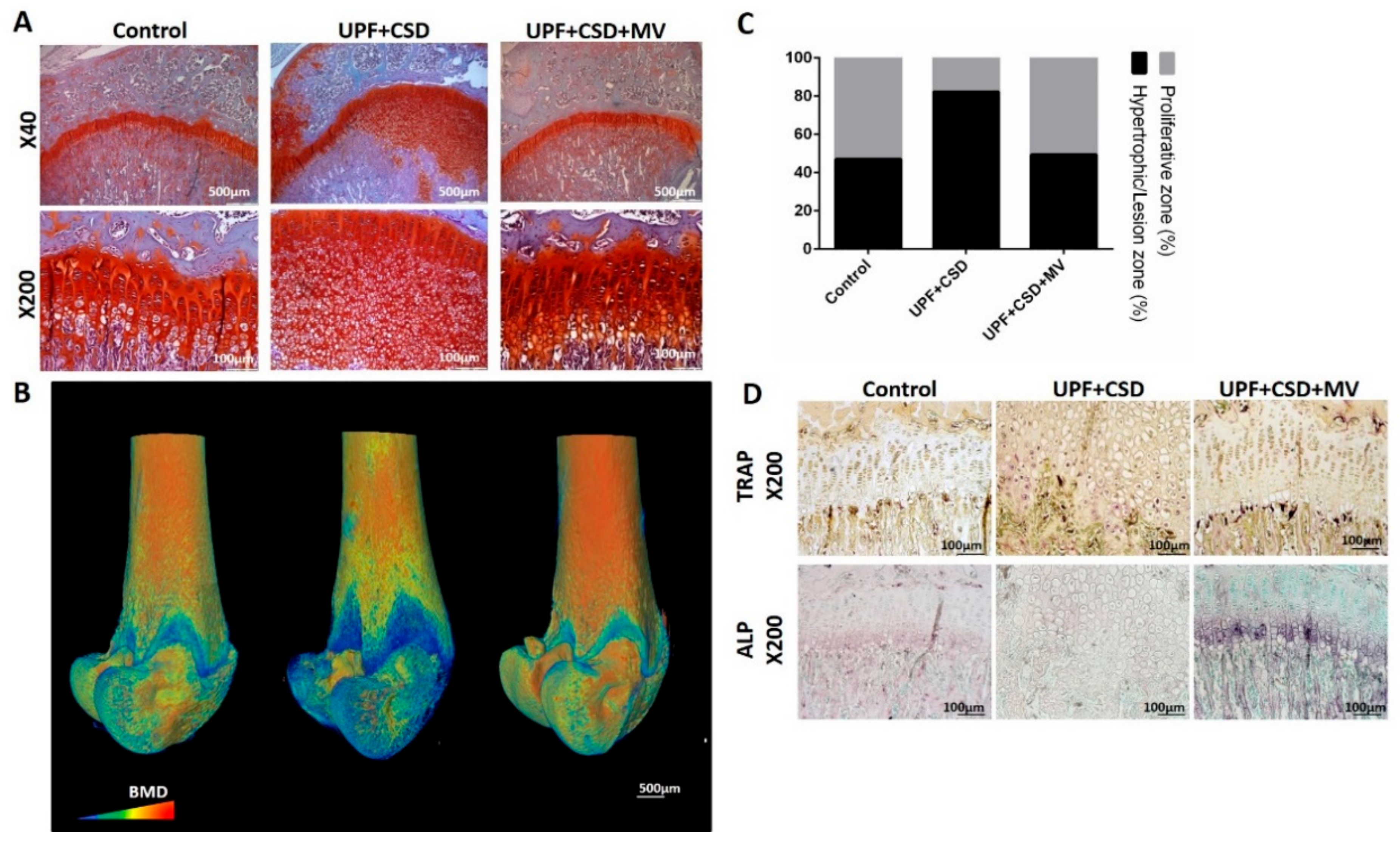
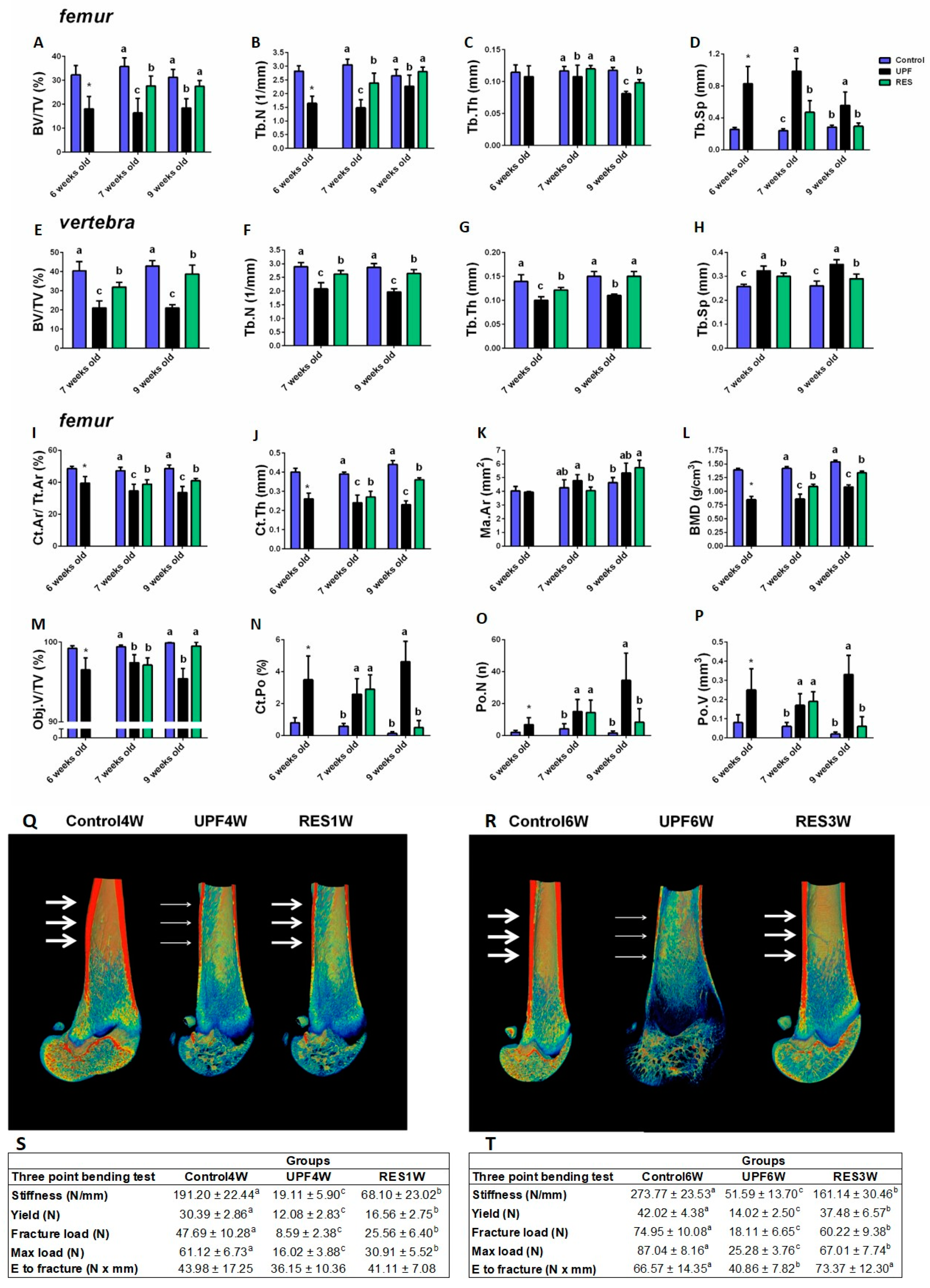
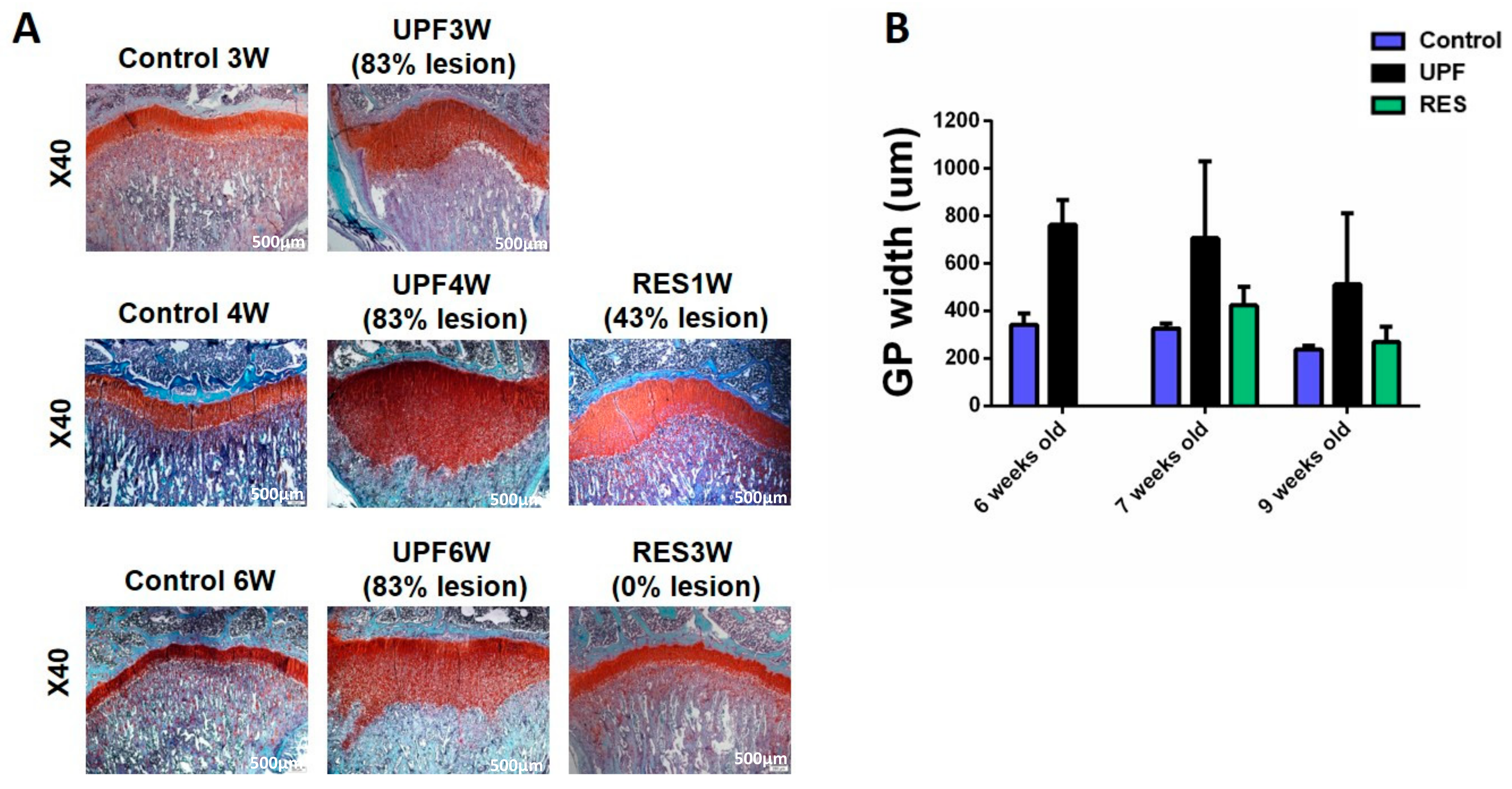
2.2.2. The Effect of UPF and Supplementations on the Rats’ Bone Properties
2.2.3. The Effect of Supplementations with Calcium or Copper on Growth and Metabolic Status
2.2.4. The Effect of Ca or Cu Supplementations on Rat Bone Properties
2.2.5. The Effect of the Different Diets and Supplementations on the Kidneys State
2.2.6. Rescue Experiment: Short or Prolonged Transition to Balanced Nutrition
3. Discussion
4. Materials and Methods
4.1. Experimental Design
- Control diet: 20% protein, 16% fat and 64% carbohydrate.
- Ultra-processed diet: 20% protein, 40% fat and 40% carbohydrate.
- Ultra-processed diet with multivitamin and mineral supplement: 20% protein, 40% fat and 40% carbohydrate; supplemented with multi vitamins and minerals (AIN-93-VX Vitamin Mix and AIN Mineral Mixture 76), while the Ca content was the guideline for supplementation and equal to the Control.
- Ultra-processed diet with Ca supplement: 20% protein, 40% fat and 40% carbohydrate; supplemented with Ca phosphate according to the calcium content of the Control chew diet.
- Ultra-processed diet with copper supplement: 20% protein, 40% fat and 40% carbohydrate; supplemented with copper carbonate basic according to Harlan’s copper content of the Control chew diet.
4.2. Diet Analysis
4.3. Blood Analysis
4.4. Histological Analyses of the Growth Plate and Kidneys
4.5. Skeleton Analyses
4.5.1. Micro Computed Tomography (µCT) Analysis
4.5.2. Light Microscopy Images of Cross-Sections of Rat Cortical Bone
4.5.3. Mechanical Testing
4.6. RNA Isolation, Reverse Transcription, and Real-Time PCR
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rauber, F.; da Costa Louzada, M.L.; Steele, E.M.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-processed food consumption and chronic non-communicable diseases-related dietary nutrient profile in the UK (2008–2014). Nutrients 2018, 10, 587. [Google Scholar] [CrossRef]
- Da Costa Louzada, M.L.; Martins, A.P.B.; Canella, D.S.; Baraldi, L.G.; Levy, R.B.; Claro, R.M.; Moubarac, J.C.; Cannon, G.; Monteiro, C.A. Impact of ultra-processed foods on micronutrient content in the Brazilian diet. Rev. Saude Publica 2015, 49. [Google Scholar] [CrossRef]
- Juul, F.; Hemmingsson, E. Trends in consumption of ultra-processed foods and obesity in Sweden between 1960 and 2010. Public Health Nutr. 2015, 18, 3096–3107. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; Louzada, C.M.L.; Pereira Machado, P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System; FAO: Rome, Italy, 2019; ISBN 978-92-5-131701-3. [Google Scholar]
- Moubarac, J.C.; Batal, M.; Louzada, M.L.; Martinez Steele, E.; Monteiro, C.A. Consumption of ultra-processed foods predicts diet quality in Canada. Appetite 2017, 108, 512–520. [Google Scholar] [CrossRef]
- Monteiro, C.; Levy, R.; Claro, R.; De Castro, I.; Cannon, G. A new classification of foods based on the extent and purpose of their processing. Cad. Saude Publica/Minist. da Saude, Fund. Oswaldo Cruz Esc. Nac. Saude Publica 2010, 26, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, L.; Kesse-Guyot, E.; Allès, B.; Touvier, M.; Srour, B.; Hercberg, S.; Buscail, C.; Julia, C. Association between Ultraprocessed Food Consumption and Risk of Mortality among Middle-aged Adults in France. JAMA Intern. Med. 2019, 179, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Moubarac, J.C.; Paula, A.; Martins, B.; Claro, R.M.; Levy, R.B.; Cannon, G.; Moubarac, J.; Monteiro, C.A. Consumption of ultra—Processed foods and likely impact on human health. Evidence from Canada. Public Health Nutr. 2012, 16, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; White, M. Characterisation of UK diets according to degree of food processing and associations with socio-demographics and obesity: Cross-sectional analysis of UK National Diet and Nutrition Survey (2008-12). Int. J. Behav. Nutr. Phys. Act. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Steele, E.M.; Baraldi, L.G.; Da Costa Louzada, M.L.; Moubarac, J.C.; Mozaffarian, D.; Monteiro, C.A. Ultra-processed foods and added sugars in the US diet: Evidence from a nationally representative cross-sectional study. BMJ Open 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Julia, C.; Martinez, L.; Allès, B.; Touvier, M.; Hercberg, S.; Méjean, C.; Kesse-Guyot, E. Contribution of ultra-processed foods in the diet of adults from the French NutriNet-Santé study. Public Health Nutr. 2018, 21, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Batalha, M.A.; França, A.K.T.D.C.; da Conceição, S.I.O.; dos Santos, A.M.; de Silva, F.S.; Padilha, L.L.; da Silva, A.A.M. Processed and ultra-processed food consumption among children aged 13 to 35 months and associated factors. Cad. Saude Publica 2017, 33, 1–16. [Google Scholar] [CrossRef]
- Mallarino, C.; Gómez, L.F.; González-Zapata, L.; Cadena, Y.; Parra, D.C. Advertising of ultra-processed foods and beverages: Children as a vulnerable population. Rev. Saude Publica 2013, 47, 1006–1010. [Google Scholar] [CrossRef]
- Gat-Yablonski, G.; Yackobovitch-Gavan, M.; Phillip, M. Nutrition and Bone Growth in Pediatrics. Endocrinol. Metab. Clin. N. Am. 2009, 38, 565–586. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H. Processed Food—An Experiment That Failed. JAMA Pediatr. 2017, 171, 212–214. [Google Scholar] [CrossRef]
- De Onis, M.; Monteiro, C.; Akre, J.; Clugston, G. The worldwide magnitude of protein-energy malnutrition: An overview from the WHO global database on child growth. Bull. World Health Organ. 1993, 71, 703–712. [Google Scholar]
- Gat-Yablonski, G.; De Luca, F. Effect of nutrition on statural growth. Horm. Res. Paediatr. 2017, 88, 46–62. [Google Scholar] [CrossRef]
- Erlebacher, A.; Filvaroff, E.H.; Gitelman, S.E.; Derynck, R. Toward a molecular understanding of skeletal development. Cell 1995, 80, 371–378. [Google Scholar] [CrossRef]
- Olsen, B.R.; Reginato, A.M.; Wang, W. Bone development. Annu. Rev. Cell Dev. Biol. 2000, 16, 191–220. [Google Scholar] [CrossRef] [PubMed]
- Matkovic, V.; Jelic, T.; Wardlaw, G.M.; Llich, J.Z.; Goel, P.K.; Wright, J.K.; Andon, M.B.; Smith, K.T.; Heaney, R.P. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis: Inference from a cross-sectional model. J. Clin. Investig. 1994, 93, 799–808. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Kerstetter, J.E. Nutrition in Bone Health Revisited: A Story Beyond Calcium. J. Am. Coll. Nutr. 2000, 19, 715–737. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.; Sävendahl, L.; De Luca, F.; Dauber, A.; Phillip, M.; Wit, J.M.; Nilsson, O. Short and tall stature: A new paradigm emerges. Nat. Rev. Endocrinol. 2015, 11, 735–746. [Google Scholar] [CrossRef]
- Zaretsky, J.; Griess-Fishheimer, S.; Carmi, A.; Travinsky Shmul, T.; Ofer, L.; Sinai, T.; Penn, S.; Shahar, R.; Monsonego-Ornan, E. Ultra-processed food targets bone quality via endochondral ossification. Bone Res. 2021, 9, 14. [Google Scholar] [CrossRef]
- Pichler, K.; Musumeci, G.; Vielgut, I.; Martinelli, E.; Sadoghi, P.; Loreto, C.; Weinberg, A.-M. Towards a better understanding of bone bridge formation in the growth plate—An immunohistochemical approach. Connect. Tissue Res. 2013, 54, 408–415. [Google Scholar] [CrossRef]
- Winkler, D.G.; Sutherland, M.K.; Geoghegan, J.C.; Yu, C.; Hayes, T.; Skonier, J.E.; Shpektor, D.; Jonas, M.; Kovacevich, B.R.; Staehling-Hampton, K.; et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003, 22, 6267–6276. [Google Scholar] [CrossRef]
- Travinsky-Shmul, T.; Beresh, O.; Zaretsky, J.; Griess-Fishheimer, S.; Rozner, R.; Kalev-Altman, R.; Penn, S.; Shahar, R.; Monsonego-Ornan, E. Ultra-Processed Food Impairs Bone Quality, Increases Marrow Adiposity and Alters Gut Microbiome in Mice. Foods 2021, 10, 3107. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.-C.; Jurutka, P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef]
- Omdahl, J.L.; Morris, H.A.; May, B.K. Hydroxlase Enzymes of the Vitamin D Pathway: Expression, Function, and Regulation. Annu. Rev. Nutr. 2002, 22, 139–166. [Google Scholar] [CrossRef] [PubMed]
- Martínez Steele, E.; Juul, F.; Neri, D.; Rauber, F.; Monteiro, C.A. Dietary share of ultra-processed foods and metabolic syndrome in the US adult population. Prev. Med. 2019, 125, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H. Bone strength: Current concepts. Ann. N. Y. Acad. Sci. 2006, 1068, 429–446. [Google Scholar] [CrossRef]
- Seeman, E.; Delmas, P.D. Bone quality--the material and structural basis of bone strength and fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef]
- Burstein, A.H.; Zika, J.M.; Heiple, K.G.; Klein, L. Contribution of Collagen and Mineral to the Elastic-Plastic Properties of Bone. JBJS 1975, 57, 956–961. [Google Scholar] [CrossRef]
- Bagi, C.M.; Berryman, E.; Moalli, M.R. Comparative bone anatomy of commonly used laboratory animals: Implications for drug discovery. Comp. Med. 2011, 61, 76–85. [Google Scholar]
- Duan, P.; Bonewald, L.F. The role of the wnt/β-catenin signaling pathway in formation and maintenance of bone and teeth. Int. J. Biochem. Cell Biol. 2016, 77, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.R.; Hock, J.M.; Burr, D.B. Periosteum: Biology, regulation, and response to osteoporosis therapies. Bone 2004, 35, 1003–1012. [Google Scholar] [CrossRef]
- Meakin, L.B.; Price, J.S.; Lanyon, L.E. The contribution of experimental in vivo models to understanding the mechanisms of adaptation to mechanical loading in bone. Front. Endocrinol. 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Odermatt, A. The western-style diet: A major risk factor for impaired kidney function and chronic kidney disease. Am. J. Physiol. - Ren. Physiol. 2011, 301. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Crystal-induced inflammation of the kidneys: Results from human studies, animal models, and tissue-culture studies. Clin. Exp. Nephrol. 2004, 8, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Calcium Supplementation and Incident Kidney Stone Risk: A Systematic Review. J. Am. Coll. Nutr. 2008, 27, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Bristow, S.M.; Bolland, M.J. Cardiovascular Complications of Calcium Supplements. J. Cell. Biochem. 2015, 116, 494–501. [Google Scholar] [CrossRef]
- Hulbert, M.; Turner, M.E.; Hopman, W.M.; Anastassiades, T.; Adams, M.A.; Holden, R.M. Changes in vascular calcification and bone mineral density in calcium supplement users from the Canadian Multi-center Osteoporosis Study (CaMOS). Atherosclerosis 2020, 296, 83–90. [Google Scholar] [CrossRef]
- Tuderman, L.; Myllyla, R.; Kivirikko, K.I. Mechanism of the Prolyl Hydroxylase Reaction. Nutr. Rev. 2009, 40, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Pando, R.; Masarwi, M.; Shtaif, B.; Idelevich, A.; Monsonego-Ornan, E.; Shahar, R.; Phillip, M.; Gat-Yablonski, G. Bone quality is affected by food restriction and by nutrition-induced catch-up growth. J. Endocrinol. 2014, 223, 227–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vogel, C.; Parsons, C.; Godfrey, K.; Robinson, S.; Harvey, N.C.; Inskip, H.; Cooper, C.; Baird, J. Greater access to fast-food outlets is associated with poorer bone health in young children. Osteoporos. Int. 2016, 27, 1011–1019. [Google Scholar] [CrossRef]
- Tucker, K.L.; Chen, H.; Hannan, M.T.; Adrienne Cupples, L.; Wilson, P.W.F.; Felson, D.; Kiel, D.P. Bone mineral density and dietary patterns in older adults: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2002, 76, 245–252. [Google Scholar] [CrossRef]
- Maynard, L.A. The Atwater System of Calculating the Caloric Value of Diets. J. Nutr. 1944, 443–452. [Google Scholar] [CrossRef]
- Simsa-Maziel, S.; Zaretsky, J.; Reich, A.; Koren, Y.; Shahar, R.; Monsonego-Ornan, E. IL-1RI participates in normal growth plate development and bone modeling. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E15–E21. [Google Scholar] [CrossRef]
- Idelevich, A.; Kerschnitzki, M.; Shahar, R.; Monsonego-ornan, E. 1,25(OH) 2D3 Alters Growth Plate Maturation and Bone Architecture in Young Rats with Normal Renal Function. PLoS ONE 2011, 6, e0020772. [Google Scholar] [CrossRef]
- Rungby, J.; Kassem, M.; Eriksen, E.F.; Danscher, G. The von Kossa reaction for calcium deposits: Silver lactate staining increases sensitivity and reduces background. Histochem. J. 1993, 25, 446–451. [Google Scholar] [CrossRef]
- Rozner, R.; Vernikov, J.; Griess-Fishheimer, S.; Travinsky, T.; Penn, S.; Schwartz, B.; Mesilati-Stahy, R.; Argov-Argaman, N.; Shahar, R.; Monsonego-Ornan, E. The role of omega-3 polyunsaturated fatty acids from different sources in bone development. Nutrients 2020, 12, 3494. [Google Scholar] [CrossRef]
- Reich, A.; Maziel, S.S.; Ashkenazi, Z.; Ornan, E.M. Involvement of matrix metalloproteinases in the growth plate response to physiological mechanical load. J. Appl. Physiol. 2010, 108, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; M??ller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Shipov, A.; Zaslansky, P.; Riesemeier, H.; Segev, G.; Atkins, A.; Shahar, R. Unremodeled endochondral bone is a major architectural component of the cortical bone of the rat (Rattus norvegicus). J. Struct. Biol. 2013, 183, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Reich, A.; Sharir, A.; Zelzer, E.; Hacker, L.; Monsonego-Ornan, E.; Shahar, R. The effect of weight loading and subsequent release from loading on the postnatal skeleton. Bone 2008, 43, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Koren, N.; Simsa-Maziel, S.; Shahar, R.; Schwartz, B.; Monsonego-Ornan, E. Exposure to omega-3 fatty acids at early age accelerate bone growth and improve bone quality. J. Nutr. Biochem. 2014, 25, 623–633. [Google Scholar] [CrossRef] [PubMed]
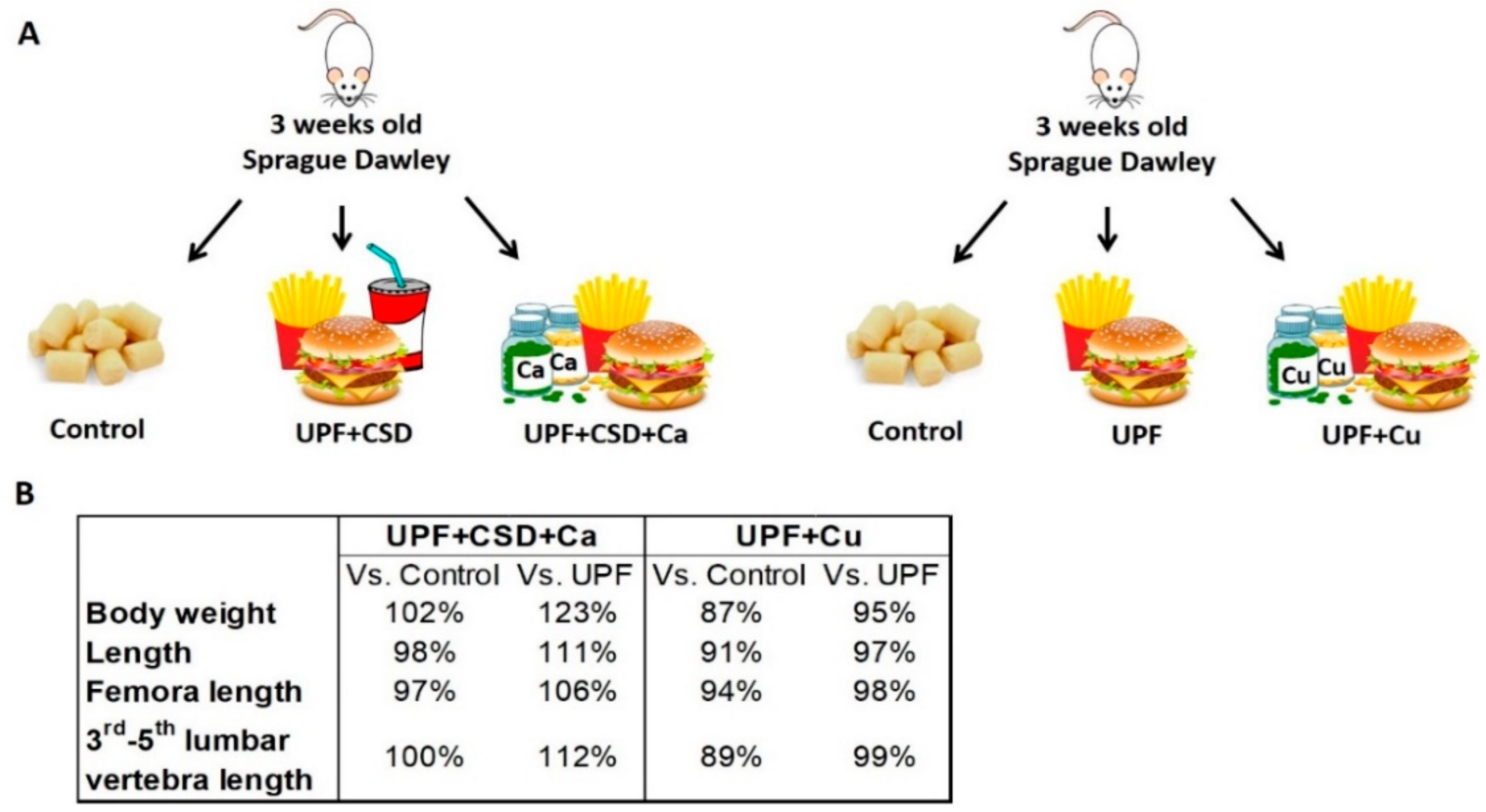


| The Tested Parameter | Calcium Supplementation Experiment | Copper Supplementation Experiment | ||||
|---|---|---|---|---|---|---|
| Trabeculacr analysis of the femur | Control | UPF + CSD | UPF + CSD + Ca | Control | UPF | UPF + Cu |
| BV/TV (%) | 27.37 ± 4.61 a | 16.61 ± 3.85 b | 24.86 ± 2.73 ab | 31.26 ± 3.33 a | 18.47 ± 3.82 b | 18.07 ± 2.54 b |
| Tb.N (1/mm) | 2.41 ± 0.25 a | 1.76 ± 0.40 b | 2.07 ± 0.19 ab | 2.65 ± 0.24 a | 2.27 ± 0.41 b | 2.23 ± 0.39 b |
| Tb.Th (mm) | 0.11 ± 0.008 ab | 0.09 ± 0.003 b | 0.12 ± 0.006 a | 0.12 ± 0.004 a | 0.08 ± 0.003 b | 0.08 ± 0.01 b |
| Tb.Sp (mm) | 0.31 ± 0.038 b | 0.53 ± 0.18 a | 0.42 ± 0.09 b | 0.28 ± 0.03 b | 0.56 ± 0.17 a | 0.43 ± 0.02 a |
| Trabeculacr analysis of the vertebra | Control | UPF + CSD | UPF + CSD + Ca | Control | UPF | UPF + Cu |
| BV/TV (%) | 40.37 ± 3.78 a | 26.50 ± 3.55 c | 34.05 ± 2.48 b | 42.9 ± 2.82 a | 21.06 ± 1.63 b | 22.62 ± 2.96 b |
| Tb.N (1/mm) | 4.73 ± 0.58 a | 3.53 ± 0.42 b | 4.03 ± 0.28 b | 2.87 ± 0.14 a | 1.97 ± 0.11 b | 2.00 ± 0.16 b |
| Tb.Th (mm) | 0.086 ± 0.003 a | 0.075 ± 0.004 b | 0.085 ± 0.006 a | 0.15 ± 0.01 a | 0.11 ± 0.003 b | 0.11 ± 0.01 b |
| Tb.Sp (mm) | 0.23 ± 0.03 b | 0.30 ± 0.03 a | 0.27 ± 0.01 a | 0.26 ± 0.02 b | 0.35 ± 0.02 a | 0.35 ± 0.02 a |
| Cortical analysis of the femur | Control | UPF + CSD | UPF + CSD + Ca | Control | UPF | UPF + Cu |
| Ct.Ar/Tt.Ar (%) | 46.62 ± 3.93 b | 31.34 ± 3.87 c | 51.29 ± 2.12 a | 48.61 ± 2.23 a | 33.59 ± 3.72 b | 36.23 ± 3.17 b |
| Ct.Th (mm) | 0.47 ± 0.03 a | 0.24 ± 0.02 b | 0.50 ± 0.02 a | 0.44 ± 0.02 a | 0.23 ± 0.02 c | 0.26 ± 0.02 b |
| Ma.Ar (mm2) | 6.07 ± 0.7 a | 6.67 ± 0.95 a | 4.91 ± 0.37 b | 4.63 ± 0.39 b | 5.34 ± 0.71 a | 5.12 ± 0.29 ab |
| BMD (g/cm3) | 1.10 ± 0.02 b | 0.81 ± 0.05 c | 1.17 ± 0.01 a | 1.54 ± 0.03 a | 1.08 ± 0.04 b | 1.03 ± 0.04 c |
| Ct.Po (%) | 0.06 ± 0.05 b | 2.3 ± 0.83 a | 0.02 ± 0.02 b | 0.13 ± 0.10 c | 4.61 ± 1.29 a | 3.30 ± 1.15 b |
| Po.N (n) | 3.83 ± 2.14 b | 21 ± 7.42 a | 1.83 ± 0.98 b | 1.6 ± 1.07 b | 34.45 ± 17.10 a | 28.00 ± 14.35 a |
| Po.V (mm3) | 0.01 ± 0.01 b | 0.2 ± 0.1 a | 0.003 ± 0.003 b | 0.02 ± 0.01 b | 0.33 ± 0.10 a | 0.26 ± 0.09 a |
| Three point bending test | Control | UPF + CSD | UPF + CSD + Ca | Control | UPF | UPF + Cu |
| Stiffness (N/mm) | 239.56 ± 45.30 a | 36.18 ± 14.38 b | 282.44 ± 39.52 a | 273.77 ± 23.53 a | 51.59 ± 13.70 b | 40.64 ± 7.88 b |
| yield (N) | 49.49 ± 3.07 a | 17.47 ± 2.81 b | 50.94 ± 6.88 a | 42.02 ± 4.38 a | 14.02 ± 2.50 b | 14.77 ± 1.78 b |
| fracture load (N) | 72.00 ± 10.14 a | 12.86 ± 7.28 b | 71.56 ± 12.52 a | 74.95 ± 10.08 a | 18.11 ± 6.65 b | 16.78 ± 6.66 b |
| Max load (N) | 79.31 ± 8.40 a | 22.03 ± 3.45 b | 82.18 ± 8.04 a | 87.04 ± 8.16 a | 25.28 ± 3.76 b | 23.35 ± 3.33 b |
| Energy to fracture (Nxmm) | 54.29 ± 7.39 a | 36.14 ± 11.58 b | 50.23 ± 7.92 a | 66.57 ± 14.35 a | 40.86 ± 7.82 b | 41.35 ± 6.50 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griess-Fishheimer, S.; Zaretsky, J.; Travinsky-Shmul, T.; Zaretsky, I.; Penn, S.; Shahar, R.; Monsonego-Ornan, E. Nutritional Approaches as a Treatment for Impaired Bone Growth and Quality Following the Consumption of Ultra-Processed Food. Int. J. Mol. Sci. 2022, 23, 841. https://doi.org/10.3390/ijms23020841
Griess-Fishheimer S, Zaretsky J, Travinsky-Shmul T, Zaretsky I, Penn S, Shahar R, Monsonego-Ornan E. Nutritional Approaches as a Treatment for Impaired Bone Growth and Quality Following the Consumption of Ultra-Processed Food. International Journal of Molecular Sciences. 2022; 23(2):841. https://doi.org/10.3390/ijms23020841
Chicago/Turabian StyleGriess-Fishheimer, Shelley, Janna Zaretsky, Tamara Travinsky-Shmul, Irina Zaretsky, Svetlana Penn, Ron Shahar, and Efrat Monsonego-Ornan. 2022. "Nutritional Approaches as a Treatment for Impaired Bone Growth and Quality Following the Consumption of Ultra-Processed Food" International Journal of Molecular Sciences 23, no. 2: 841. https://doi.org/10.3390/ijms23020841
APA StyleGriess-Fishheimer, S., Zaretsky, J., Travinsky-Shmul, T., Zaretsky, I., Penn, S., Shahar, R., & Monsonego-Ornan, E. (2022). Nutritional Approaches as a Treatment for Impaired Bone Growth and Quality Following the Consumption of Ultra-Processed Food. International Journal of Molecular Sciences, 23(2), 841. https://doi.org/10.3390/ijms23020841






