Controlled Release of Epigenetically-Enhanced Extracellular Vesicles from a GelMA/Nanoclay Composite Hydrogel to Promote Bone Repair
Abstract
:1. Introduction
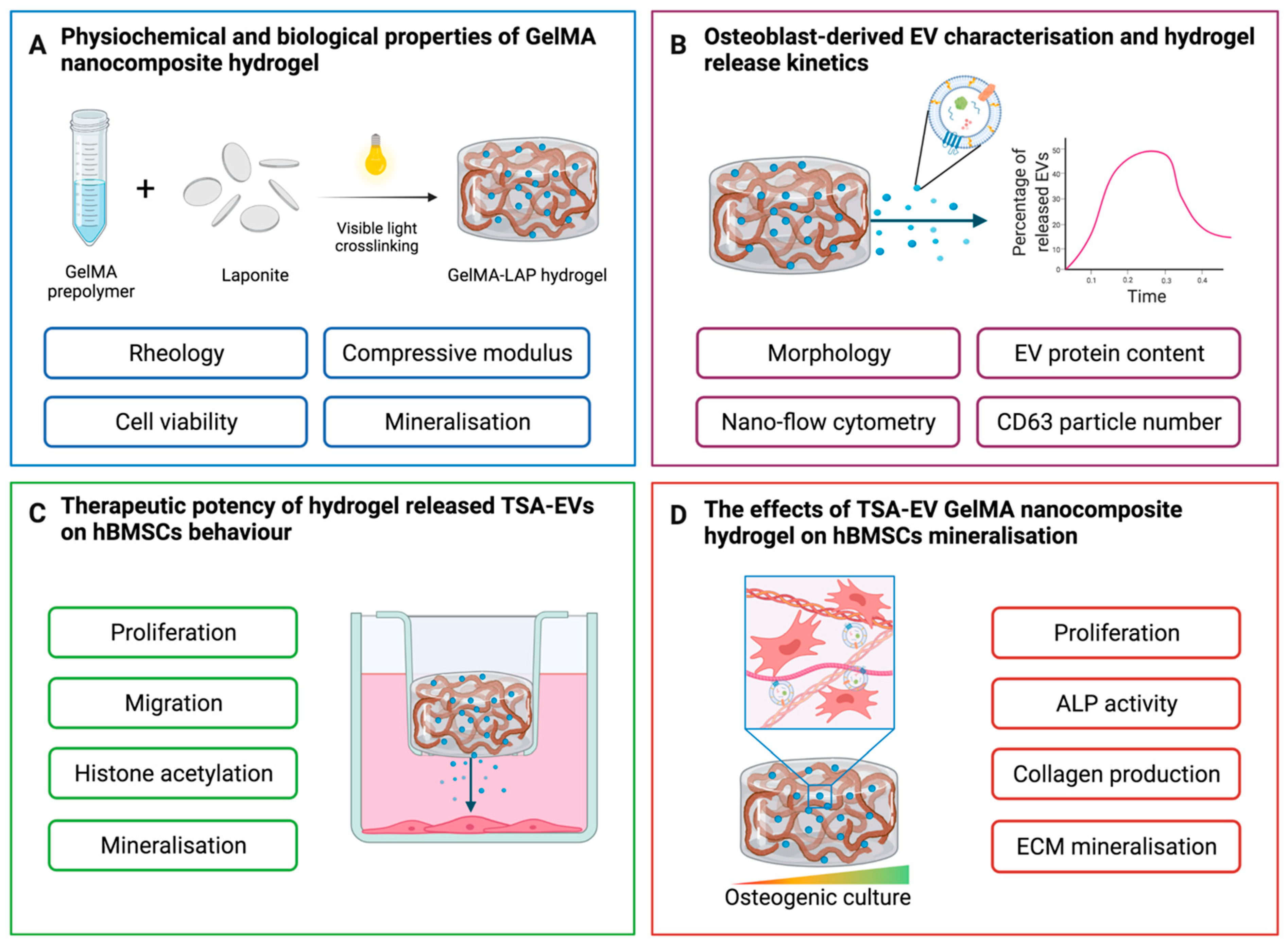
2. Results
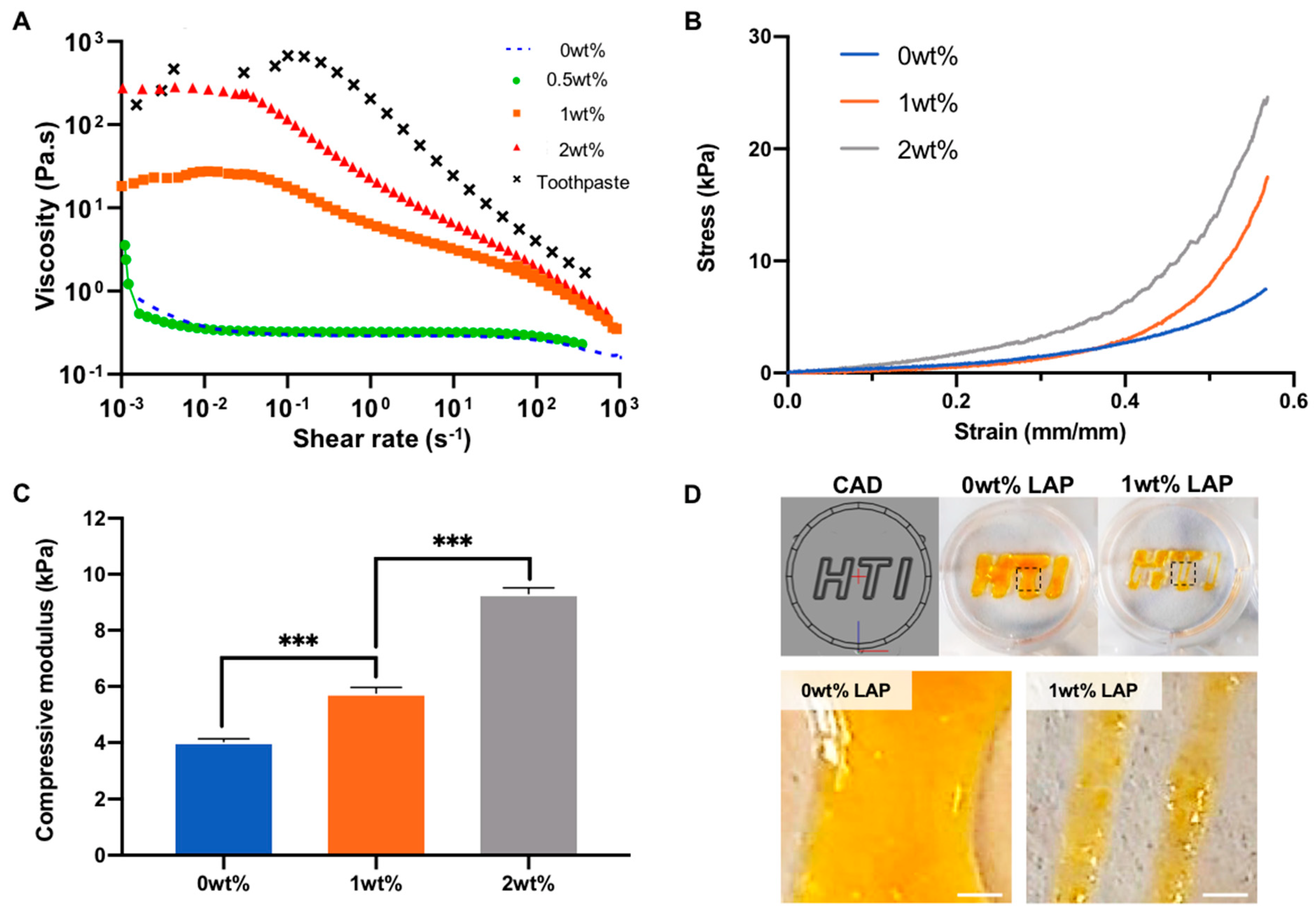
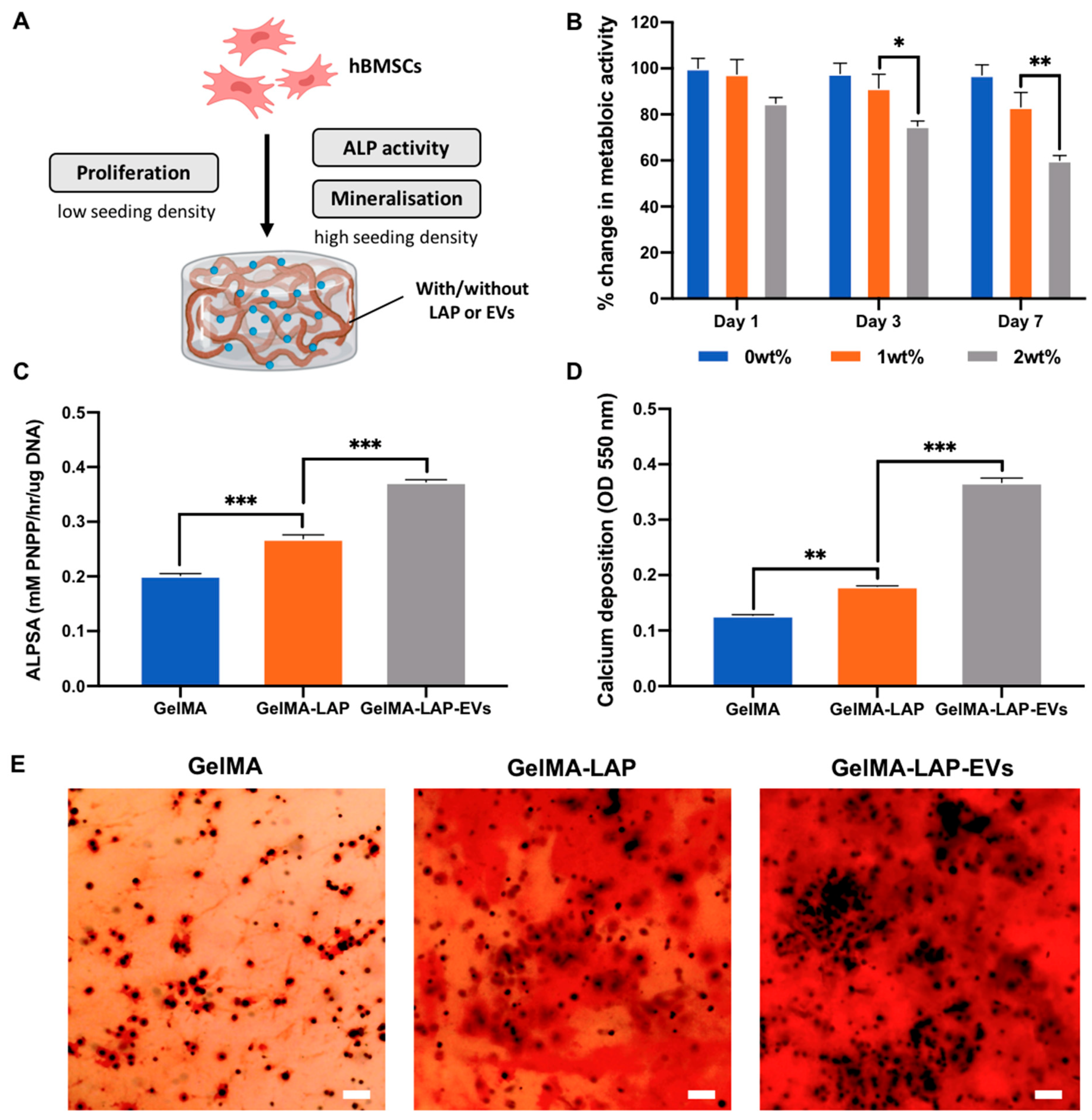
2.1. Nanosilicate Inclusion Augments GelMA Physicochemical and Osteogenic Properties
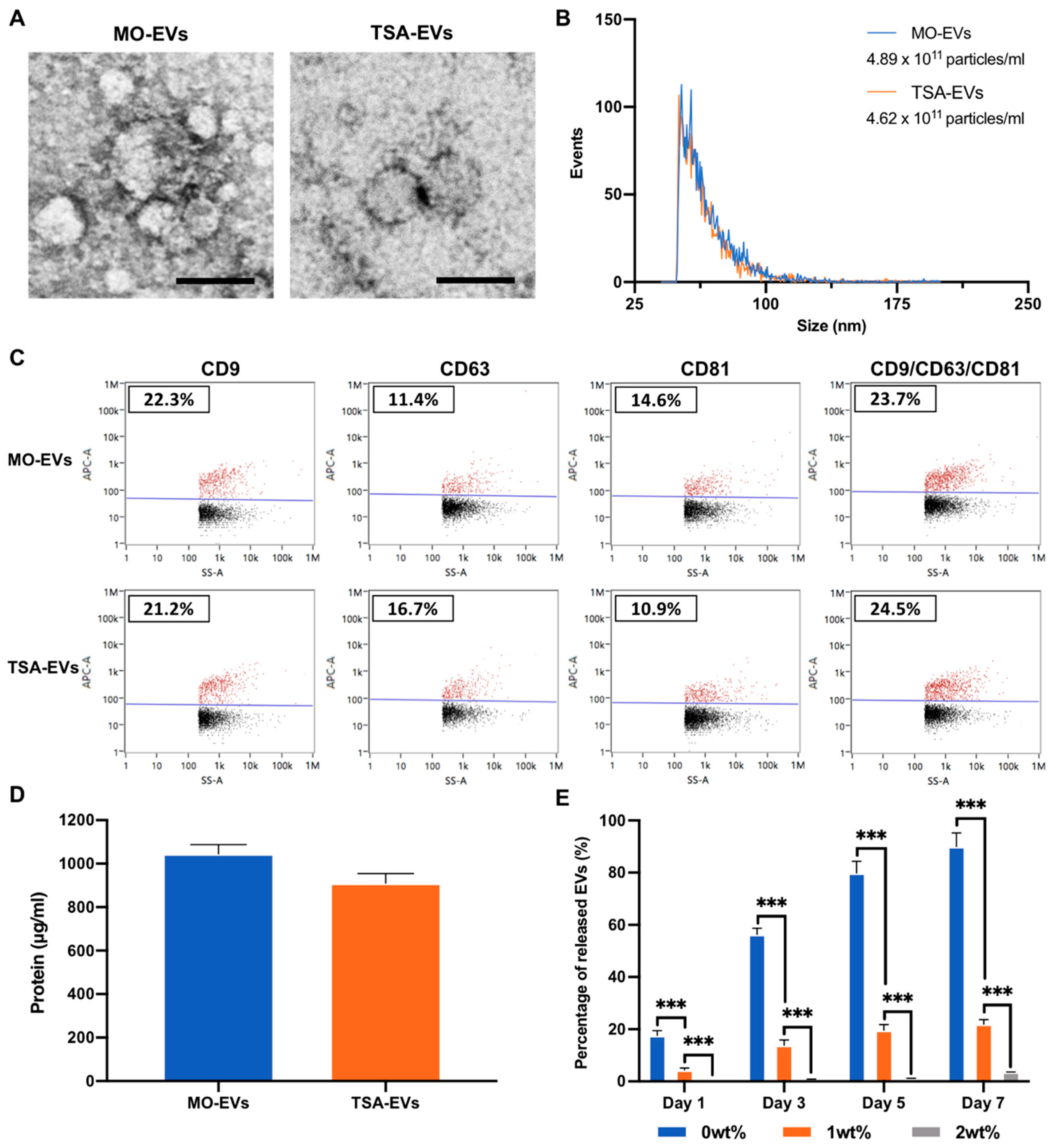
2.2. Characterisation of EVs Derived from TSA Treated Osteoblasts
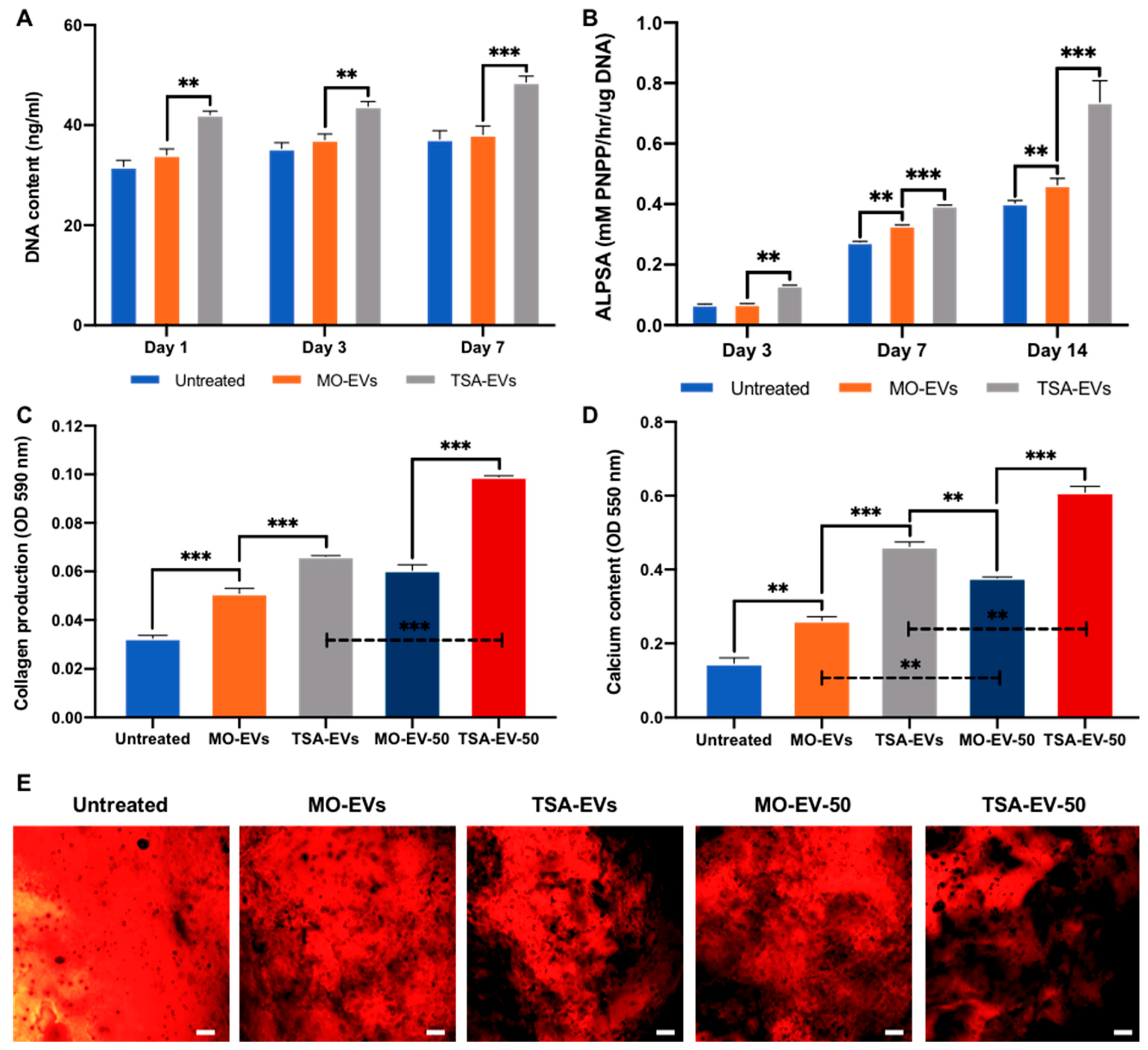
2.3. Hydrogel-Released TSA-EVs Enhance hBMSCs Osteogenic Differentiation
2.4. TSA-EVs Promote hBMSCs Extracellular Matrix Mineralisation within the GelMA-LAP Hydrogel
3. Discussion
4. Materials and Methods
4.1. GelMA and Nanocomposite Synthesis
4.2. Fabrication of GelMA-LAP Hydrogel
4.3. Rheology
4.4. Mechanical Testing
4.5. Fabrication of 3D Printed GelMA-LAP Construct
4.6. Cell Culture and Reagents
4.7. The Biological Efficacy of GelMA Nanocomposite
4.8. EV Isolation and Characterisation
4.8.1. EV Isolation
4.8.2. Transmission Electron Microscopy (TEM)
4.8.3. EV Particle Size, Concentration and Tetraspanin Analysis
4.9. EV Release Kinetics from Hydrogels
4.10. The Impact of Hydrogel-Released EVs on hBMSCs Proliferation, Migration and Mineralisation
4.10.1. EV Cell Uptake
4.10.2. Proliferation Assay
4.10.3. Migration Assay
4.10.4. H3K9 Acetylation Assay
4.10.5. Osteoinduction
4.11. TSA-EV Functionalised Hydrogels on hBMSCs Proliferation and Mineralisation
4.12. Alkaline Phosphatase (ALP) Activity
4.13. Collagen Production
4.14. Mineral Deposition
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chance-Larsen, K.; Backhouse, M.R.; Collier, R.; Wright, C.; Gosling, S.; Harden, B.; Marsh, S.; Kay, P.; Wyles, H.; Erwin, J.; et al. Developing a national musculoskeletal core capabilities framework for first point of contact practitioners. Rheumatol. Adv. Pract. 2019, 3, rkz036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous bone graft: Properties and techniques. J. Orthop. Trauma 2010, 24 (Suppl. S1), S36–S40. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics the bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gresham, R.C.H.; Bahney, C.S.; Leach, J.K. Growth factor delivery using extracellular matrix-mimicking substrates for musculoskeletal tissue engineering and repair. Bioact. Mater. 2021, 6, 1945–1956. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N.E. Complications due to the use of bmp/infuse in spine surgery: The evidence continues to mount. Surg. Neurol. Int. 2013, 4 (Suppl. S5), S343–S352. [Google Scholar] [CrossRef]
- Hustedt, J.W.; Blizzard, D.J. The controversy surrounding bone morphogenetic proteins in the spine: A review of current research. Yale J. Biol. Med. 2014, 87, 549–561. [Google Scholar]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Calori, G.M.; Begue, T.; Schmidmaier, G. Bone regeneration strategies: Current trends but what the future holds? Inj.-Int. J. Care Inj. 2013, 44, S1–S2. [Google Scholar] [CrossRef]
- Tatara, A.M.; Mikos, A.G. Tissue engineering in orthopaedics. J. Bone Joint Surg. Am. 2016, 98, 1132–1139. [Google Scholar] [CrossRef] [Green Version]
- Amariglio, N.; Hirshberg, A.; Scheithauer, B.W.; Cohen, Y.; Loewenthal, R.; Trakhtenbrot, L.; Paz, N.; Koren-Michowitz, M.; Waldman, D.; Leider-Trejo, L.; et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009, 6, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Herberts, C.A.; Kwa, M.S.G.; Hermsen, H.P.H. Risk factors in the development of stem cell therapy. J. Transl. Med. 2011, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Heathman, T.R.; Nienow, A.W.; McCall, M.J.; Coopman, K.; Kara, B.; Hewitt, C.J. The translation of cell-based therapies: Clinical landscape and manufacturing challenges. Regen. Med. 2015, 10, 49–64. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Song, S.H.; Kim, K.L.; Ko, J.J.; Im, J.E.; Yie, S.W.; Ahn, Y.K.; Kim, D.K.; Suh, W. Human cord blood-derived endothelial progenitor cells and their conditioned media exhibit therapeutic equivalence for diabetic wound healing. Cell Transplant. 2010, 19, 1635–1644. [Google Scholar] [CrossRef] [Green Version]
- Li, X.C.; Chen, C.Y.; Wei, L.M.; Li, Q.; Niu, X.; Xu, Y.J.; Wang, Y.; Zhao, J.G. Exosomes derived from endothelial progenitor cells attenuate vascular repair and accelerate reendothelialization by enhancing endothelial function. Cytotherapy 2016, 18, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Brunet, M.Y.; Jones, M.C.; Cox, S.C. Engineered extracellular vesicles: Tailored-made nanomaterials for medical applications. Nanomaterials 2020, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borger, V.; Bremer, M.; Ferrer-Tur, R.; Gockeln, L.; Stambouli, O.; Becic, A.; Giebel, B. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int. J. Mol. Sci. 2017, 18, 1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnecchi, M.; He, H.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine action accounts for marked protection of ischemic heart by akt-modified mesenchymal stem cells. Nat. Med. 2005, 11, 367–368. [Google Scholar] [CrossRef]
- Xin, H.Q.; Li, Y.; Chopp, M. Exosomes/mirnas as mediating cell-based therapy of stroke. Front. Cell. Neurosci. 2014, 8, 377. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.C.; Guo, S.C. Extracellular vesicles in bone: “Dogrobbers” in the “eternal battle field”. Cell Commun. Signal. 2019, 17, 6. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Gao, W.; Papadimitriou, J.M.; Zhang, C.; Gao, J.; Zheng, M. Exosomes-the enigmatic regulators of bone homeostasis. Bone Res. 2018, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.; Porter, R.M. Harnessing extracellular vesicles to direct endochondral repair of large bone defects. Bone Joint Res. 2018, 7, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; de Wildt, B.W.M.; Vis, M.A.M.; de Korte, C.E.; Ito, K.; Hofmann, S.; Yuana, Y. Matrix vesicles: Role in bone mineralization and potential use as therapeutics. Pharmaceuticals 2021, 14, 289. [Google Scholar] [CrossRef]
- Eichholz, K.F.; Woods, I.; Johnson, G.P.; Shen, N.; Corrigan, M.; Labour, M.N.; Wynne, K.; Lowery, M.C.; O’Driscoll, L.; Hoey, D.A.; et al. Human bone marrow stem/stromal cell osteogenesis is regulated via mechanically activated osteocyte-derived extracellular vesicles. Stem Cells Transl. Med. 2020, 9, 1431–1447. [Google Scholar] [CrossRef] [PubMed]
- Gholami, L.; Nooshabadi, V.T.; Shahabi, S.; Jazayeri, M.; Tarzemany, R.; Afsartala, Z.; Khorsandi, K. Extracellular vesicles in bone and periodontal regeneration: Current and potential therapeutic applications. Cell Biosci. 2021, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Brunet, M.Y.; Louth, S.; Robinson, T.E.; Fernandez-Rhodes, M.; Williams, S.; Federici, A.S.; Davies, O.G.; Hoey, D.A.; Cox, S.C. Development of a bone-mimetic 3d printed ti6al4v scaffold to enhance osteoblast-derived extracellular vesicles’ therapeutic efficacy for bone regeneration. Front. Bioeng. Biotechnol. 2021, 9, 757220. [Google Scholar] [CrossRef] [PubMed]
- Lunyak, V.V.; Rosenfeld, M.G. Epigenetic regulation of stem cell fate. Hum. Mol. Genet. 2008, 17, R28–R36. [Google Scholar] [CrossRef]
- Nashun, B.; Hill, P.W.S.; Hajkova, P. Reprogramming of cell fate: Epigenetic memory and the erasure of memories past. Embo J. 2015, 34, 1296–1308. [Google Scholar] [CrossRef]
- Man, K.; Mekhileri, N.; Lim, K.; Jiang, L.H.; Woodfield, T.; Yang, X.B. Mi192 induced epigenetic reprogramming enhances the therapeutic efficacy of human bone marrows stromal cells for bone regeneration. Bone 2021, 153, 116138. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Lawlor, L.; Jiang, L.-H.; Yang, X.B. The selective histone deacetylase inhibitor mi192 enhances the osteogenic differentiation efficacy of human dental pulp stromal cells. Int. J. Mol. Sci. 2021, 22, 5224. [Google Scholar] [CrossRef] [PubMed]
- Paino, F.; la Noce, M.; Tirino, V.; Naddeo, P.; Desiderio, V.; Pirozzi, G.; de Rosa, A.; Laino, L.; Altucci, L.; Papaccio, G. Histone deacetylase inhibition with valproic acid downregulates osteocalcin gene expression in human dental pulp stem cells and osteoblasts: Evidence for hdac2 involvement. Stem Cells 2014, 32, 279–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, K.; Brunet, M.Y.; Fernandez-Rhodes, M.; Williams, S.; Heaney, L.M.; Gethings, L.A.; Federici, A.; Davies, O.G.; Hoey, D.; Cox, S.C. Epigenetic reprogramming enhances the therapeutic efficacy of osteoblast-derived extracellular vesicles to promote human bone marrow stem cell osteogenic differentiation. J. Extracell. Vesicles 2021, 10, e12118. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Takahashi, Y.; Nishikawa, M.; Kato, K.; Morishita, M.; Yamashita, T.; Matsumoto, A.; Charoenviriyakul, C.; Takakura, Y. Macrophage-dependent clearance of systemically administered b16bl6-derived exosomes from the blood circulation in mice. J. Extracell. Vesicles 2015, 4, 26238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef] [Green Version]
- Brennan, M.A.; Layrolle, P.; Mooney, D.J. Biomaterials functionalized with msc secreted extracellular vesicles and soluble factors for tissue regeneration. Adv. Funct. Mater. 2020, 30, 1909125. [Google Scholar] [CrossRef] [PubMed]
- Nikravesh, N.; Davies, O.G.; Azoidis, I.; Moakes, R.J.A.; Marani, L.; Turner, M.; Kearney, C.J.; Eisenstein, N.M.; Grover, L.M.; Cox, S.C. Physical structuring of injectable polymeric systems to controllably deliver nanosized extracellular vesicles. Adv. Healthc. Mater. 2019, 8, e1801604. [Google Scholar] [CrossRef] [Green Version]
- Mol, E.A.; Lei, Z.; Roefs, M.T.; Bakker, M.H.; Goumans, M.J.; Doevendans, P.A.; Dankers, P.Y.W.; Vader, P.; Sluijter, J.P.G. Injectable supramolecular ureidopyrimidinone hydrogels provide sustained release of extracellular vesicle therapeutics. Adv. Healthc. Mater. 2019, 8, e1900847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, K.; Santiago, G.T.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (gelma) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.N.; Zhao, T.F.; Wang, J.K.; Wang, C.G.; Du, J.N.; Ying, L.W.; Lin, J.T.; Zhang, C.H.; Hu, W.L.; Wang, L.N.; et al. Gelatin methacrylate (gelma)-based hydrogels for cell transplantation: An effective strategy for tissue engineering. Stem Cell Rev. Rep. 2019, 15, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Cidonio, G.; Alcala-Orozco, C.R.; Lim, K.S.; Glinka, M.; Mutreja, I.; Kim, Y.H.; Dawson, J.I.; Woodfield, T.B.F.; Oreffo, R.O.C. Osteogenic and angiogenic tissue formation in high fidelity nanocomposite laponite-gelatin bioinks. Biofabrication 2019, 11, 035027. [Google Scholar] [CrossRef] [PubMed]
- Zoratto, N.; di Lisa, D.; de Rutte, J.; Sakib, M.N.; Silva, A.R.A.E.; Tamayol, A.; di Carlo, D.; Khademhosseini, A.; Sheikhi, A. In situ forming microporous gelatin methacryloyl hydrogel scaffolds from thermostable microgels for tissue engineering. Bioeng. Transl. Med. 2020, 5, e10180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, Z.; Li, Y.; Wang, Y.; Li, Q.; Han, D. Gelma combined with sustained release of huvecs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J. Mol. Histol. 2020, 51, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Celikkin, N.; Mastrogiacomo, S.; Jaroszewicz, J.; Walboomers, X.F.; Swieszkowski, W. Gelatin methacrylate scaffold for bone tissue engineering: The influence of polymer concentration. J. Biomed. Mater. Res. Part A 2018, 106, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, N.; Kharaziha, M.; Emadi, R.; Zarrabi, A.; Mokhtari, H.; Salehi, S. An adhesive and injectable nanocomposite hydrogel of thiolated gelatin/gelatin methacrylate/laponite (r) as a potential surgical sealant. J. Colloid Interface Sci. 2020, 564, 155–169. [Google Scholar] [CrossRef]
- Nadernezhad, A.; Caliskan, O.S.; Topuz, F.; Afghah, F.; Erman, B.; Koc, B. Nanocomposite bioinks based on agarose and 2d nanosilicates with tunable flow properties and bioactivity for 3d bioprinting. ACS Appl. Bio Mater. 2019, 2, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.I.; Kanczler, J.M.; Yang, X.B.B.; Attard, G.S.; Oreffo, R.O.C. Clay gels for the delivery of regenerative microenvironments. Adv. Mater. 2011, 23, 3304–3308. [Google Scholar] [CrossRef] [PubMed]
- Koshy, S.T.; Zhang, D.K.Y.; Grolman, J.M.; Stafford, A.G.; Mooney, D.J. Injectable nanocomposite cryogels for versatile protein drug delivery. Acta Biomater. 2018, 65, 36–43. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Mihaila, S.M.; Swami, A.; Patel, A.; Sant, S.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells. Adv. Mater. 2013, 25, 3329–3336. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, J.Q.; Lei, X.; Miao, S.; Zhang, S.S.; Cheng, P.Z.; Song, Y.; Wu, H.; Gao, Y.; Bi, L.; et al. Cell-loaded injectable gelatin/alginate/laponite (r) nanocomposite hydrogel promotes bone healing in a critical-size rat calvarial defect model. RSC Adv. 2020, 10, 25652–25661. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P.; Darder, M.; Rytwo, G. Hybrid materials based on clays for environmental and biomedical applications. J. Mater. Chem. 2010, 20, 9306–9321. [Google Scholar] [CrossRef]
- Davila, J.L.; d’Avila, M.A. Laponite as a rheology modifier of alginate solutions: Physical gelation and aging evolution. Carbohydr. Polym. 2017, 157, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Zhang, K.Y.; Zhao, X.N.; Kong, D.L.; Zhao, Q.; Liu, N.; Ma, F.X. Enhanced therapeutic effects of MSC-derived exosomes with an injectable hydrogel for hindlimb ischemia treatment. Circ. Res. 2018, 10, 30081–30091. [Google Scholar] [CrossRef]
- Liu, X.L.; Yang, Y.L.; Li, Y.; Niu, X.; Zhao, B.Z.; Wang, Y.; Bao, C.Y.; Xie, Z.P.; Lin, Q.N.; Zhu, L.Y. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 2017, 9, 4430–4438. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, S.; Yildirimer, L.; Zhao, H.; Ding, R.H.; Wang, H.N.; Cui, W.G.; Weitz, D. Injectable stem cell-laden photocrosslinkable microspheres fabricated using microfluidics for rapid generation of osteogenic tissue constructs. Adv. Funct. Mater. 2016, 26, 2809–2819. [Google Scholar] [CrossRef]
- Xavier, J.R.; Thakur, T.; Desai, P.; Jaiswal, M.K.; Sears, N.; Cosgriff-Hernandez, E.; Kaunas, R.; Gaharwar, A.K. Bioactive nanoengineered hydrogels for bone tissue engineering: A growth-factor-free approach. ACS Nano 2015, 9, 3109–3118. [Google Scholar] [CrossRef]
- Paul, A.; Manoharan, V.; Krafft, D.; Assmann, A.; Uquillas, J.A.; Shin, S.R.; Hasan, A.; Hussain, M.A.; Memic, A.; Gaharwar, A.K.; et al. Nanoengineered biomimetic hydrogels for guiding human stem cell osteogenesis in three dimensional microenvironments. J. Mater. Chem. B 2016, 4, 3544–3554. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Yang, X.; Shi, L.Y.; Lanham, S.A.; Hilborn, J.; Oreffo, R.O.C.; Ossipov, D.; Dawson, J.I. Bisphosphonate nanoclay edge-site interactions facilitate hydrogel self-assembly and sustained growth factor localization. Nat. Commun. 2020, 11, 1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; Erdem, T.; Eiser, E. A simple approach to prepare self-assembled, nacre-inspired clay/polymer nanocomposites. Soft Matter 2020, 16, 5497–5505. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Byun, M.R.; Kim, A.R.; Kim, K.M.; Cho, H.J.; Lee, Y.H.; Kim, J.; Jeong, M.G.; Hwang, E.S.; Hong, J.H. Extracellular matrix stiffness regulates osteogenic differentiation through mapk activation. PLoS ONE 2015, 10, e0135519. [Google Scholar]
- Zandi, N.; Sani, E.S.; Mostafavi, E.; Ibrahim, D.M.; Saleh, B.; Shokrgozar, M.A.; Tamjid, E.; Weiss, P.S.; Simchi, A.; Annabi, N. Nanoengineered shear-thinning and bioprintable hydrogel as a versatile platform for biomedical applications. Biomaterials 2021, 267, 120476. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.F.; Heid, S.; Boccaccini, A.R. Potential of laponite(r) incorporated oxidized alginate-gelatin (ada-gel) composite hydrogels for extrusion-based 3d printing. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1090–1104. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hahn, L.; Yang, M.S.; Altmann, A.; Stahlhut, P.; Groll, J.; Luxenhofer, R. Improving printability of a thermoresponsive hydrogel biomaterial ink by nanoclay addition. J. Mater. Sci. 2021, 56, 691–705. [Google Scholar] [CrossRef]
- Goto, R.; Nishida, E.; Kobayashi, S.; Aino, M.; Ohno, T.; Iwamura, Y.; Kikuchi, T.; Hayashi, J.I.; Yamamoto, G.; Asakura, M.; et al. Gelatin methacryloyl-riboflavin (gelma-rf) hydrogels for bone regeneration. Int. J. Mol. Sci. 2021, 22, 1635. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Wang, W.; Liu, X.; Xu, C.; Wang, Y.; Wang, Z.; Xu, J.; Guan, J.; Zhou, P.; Mao, Y. Gelatin methacrylate hydrogel scaffold carrying resveratrol-loaded solid lipid nanoparticles for enhancement of osteogenic differentiation of bmscs and effective bone regeneration. Regen. Biomater. 2021, 8, rbab044. [Google Scholar] [CrossRef]
- Davies, O.G.; Cox, S.C.; Williams, R.L.; Tsaroucha, D.; Dorrepaal, R.M.; Lewis, M.P.; Grover, L.M. Annexin-enriched osteoblast-derived vesicles act as an extracellular site of mineral nucleation within developing stem cell cultures. Sci. Rep. 2017, 7, 12639. [Google Scholar] [CrossRef]
- Eichholz, K.F.; Federici, A.; Riffault, M.; Woods, I.; Mahon, O.R.; O’Driscoll, L.; Hoey, D.A. Extracellular vesicle functionalized melt electrowritten scaffolds for bone tissue engineering. Adv. NanoBiomed Res. 2021, 1, 2100037. [Google Scholar] [CrossRef]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.; Evans, B.A.; Thompson, R.P.; Powell, J.J.; Hampson, G.N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Golafshan, N.; Rezahasani, R.; Esfahani, M.T.; Kharaziha, M.; Khorasani, S.N. Nanohybrid hydrogels of laponite: Pva-alginate as a potential wound healing material. Carbohydr. Polym. 2017, 176, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Golafshan, N.; Gharibi, H.; Kharaziha, M.; Fathi, M. A facile one-step strategy for development of a double network fibrous scaffold for nerve tissue engineering. Biofabrication 2017, 9, 025008. [Google Scholar] [CrossRef]
- Muller, A.S.; Artner, M.; Janjic, K.; Edelmayer, M.; Kurzmann, C.; Moritz, A.; Agis, H. Synthetic clay-based hypoxia mimetic hydrogel for pulp regeneration: The impact on cell activity and release kinetics based on dental pulp-derived cells in vitro. J. Endod. 2018, 44, 1263–1269. [Google Scholar] [CrossRef]
- Sun, M.; Chi, G.; Xu, J.; Tan, Y.; Xu, J.; Lv, S.; Xu, Z.; Xia, Y.; Li, L.; Li, Y. Extracellular matrix stiffness controls osteogenic differentiation of mesenchymal stem cells mediated by integrin alpha5. Stem Cell Res. Ther. 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Rice, G.E.; Tapia, J.; Mitchell, M.D.; Salomon, C. Exosomes are fingerprints of originating cells: Potential biomarkers for ovarian cancer. Res. Rep. Biochem. 2015, 5, 101–109. [Google Scholar]
- Perez, R.A.; Seo, S.J.; Won, J.E.; Lee, E.J.; Jang, J.H.; Knowles, J.C.; Kim, H.W. Therapeutically relevant aspects in bone repair and regeneration. Mater. Today 2015, 18, 573–589. [Google Scholar] [CrossRef]
- De Jong, O.G.; van Balkom, B.W.; Schiffelers, R.M.; Bouten, C.V.; Verhaar, M.C. Extracellular vesicles: Potential roles in regenerative medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriebel, P.W.; Majumdar, R.; Jenkins, L.M.; Senoo, H.; Wang, W.; Ammu, S.; Chen, S.; Narayan, K.; Iijima, M.; Parent, C.A. Extracellular vesicles direct migration by synthesizing and releasing chemotactic signals. J. Cell Biol. 2018, 217, 2891–2910. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Cui, X.; Zhang, Z.; Xu, Y.; Guo, J.; Soliman, B.G.; Lu, Y.; Qin, Z.; Wang, Q.; Zhang, H.; et al. Injection-free delivery of msc-derived extracellular vesicles for myocardial infarction therapeutics. Adv. Healthc. Mater. 2021, e2100312. [Google Scholar] [CrossRef]
- Freeman, F.E.; Pitacco, P.; van Dommelen, L.H.A.; Nulty, J.; Browe, D.C.; Shin, J.Y.; Alsberg, E.; Kelly, D.J. 3d bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration. Sci. Adv. 2020, 6, eabb5093. [Google Scholar] [CrossRef] [PubMed]
- Cross, L.M.; Carrow, J.K.; Ding, X.C.; Singh, K.A.; Gaharwar, A.K. Sustained and prolonged delivery of protein therapeutics from two-dimensional nanosilicates. ACS Appl. Mater. Interfaces 2019, 11, 6741–6750. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Dong, L.; Bu, Z.; Shen, Y.; Luo, J.; Zhang, H.; Zhao, S.; Lv, F.; Liu, Z. Mir-23a-3p-abundant small extracellular vesicles released from gelma/nanoclay hydrogel for cartilage regeneration. J. Extracell. Vesicles 2020, 9, 1778883. [Google Scholar] [CrossRef]
- Sterner, D.E.; Berger, S.L. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 2000, 64, 435–459. [Google Scholar] [CrossRef] [Green Version]
- Soutoglou, E.; Katrakili, N.; Talianidis, I. Acetylation regulates transcription factor activity at multiple levels. Mol. Cell 2000, 5, 745–751. [Google Scholar] [CrossRef]
- Nieuwoudt, M.; Woods, I.; Eichholz, K.F.; Martins, C.; McSweeney, K.; Shen, N.; Hoey, D.A. Functionalization of electrospun polycaprolactone scaffolds with matrix-binding osteocyte-derived extracellular vesicles promotes osteoblastic differentiation and mineralization. Ann. Biomed. Eng. 2021, 49, 3621–3635. [Google Scholar] [CrossRef]
- Holkar, K.; Kale, V.; Ingavle, G. Hydrogel-assisted 3d model to investigate the osteoinductive potential of mc3t3-derived extracellular vesicles. ACS Biomater. Sci. Eng. 2021, 7, 2687–2700. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension—How 3d culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef] [Green Version]
- Jensen, C.; Teng, Y. Is it time to start transitioning from 2d to 3d cell culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (misev2018): A position statement of the international society for extracellular vesicles and update of the misev2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, K.; Barroso, I.A.; Brunet, M.Y.; Peacock, B.; Federici, A.S.; Hoey, D.A.; Cox, S.C. Controlled Release of Epigenetically-Enhanced Extracellular Vesicles from a GelMA/Nanoclay Composite Hydrogel to Promote Bone Repair. Int. J. Mol. Sci. 2022, 23, 832. https://doi.org/10.3390/ijms23020832
Man K, Barroso IA, Brunet MY, Peacock B, Federici AS, Hoey DA, Cox SC. Controlled Release of Epigenetically-Enhanced Extracellular Vesicles from a GelMA/Nanoclay Composite Hydrogel to Promote Bone Repair. International Journal of Molecular Sciences. 2022; 23(2):832. https://doi.org/10.3390/ijms23020832
Chicago/Turabian StyleMan, Kenny, Inês A. Barroso, Mathieu Y. Brunet, Ben Peacock, Angelica S. Federici, David A. Hoey, and Sophie C. Cox. 2022. "Controlled Release of Epigenetically-Enhanced Extracellular Vesicles from a GelMA/Nanoclay Composite Hydrogel to Promote Bone Repair" International Journal of Molecular Sciences 23, no. 2: 832. https://doi.org/10.3390/ijms23020832
APA StyleMan, K., Barroso, I. A., Brunet, M. Y., Peacock, B., Federici, A. S., Hoey, D. A., & Cox, S. C. (2022). Controlled Release of Epigenetically-Enhanced Extracellular Vesicles from a GelMA/Nanoclay Composite Hydrogel to Promote Bone Repair. International Journal of Molecular Sciences, 23(2), 832. https://doi.org/10.3390/ijms23020832







