The Anticancer Potential of Doxycycline and Minocycline—A Comparative Study on Amelanotic Melanoma Cell Lines
Abstract
1. Introduction
2. Results
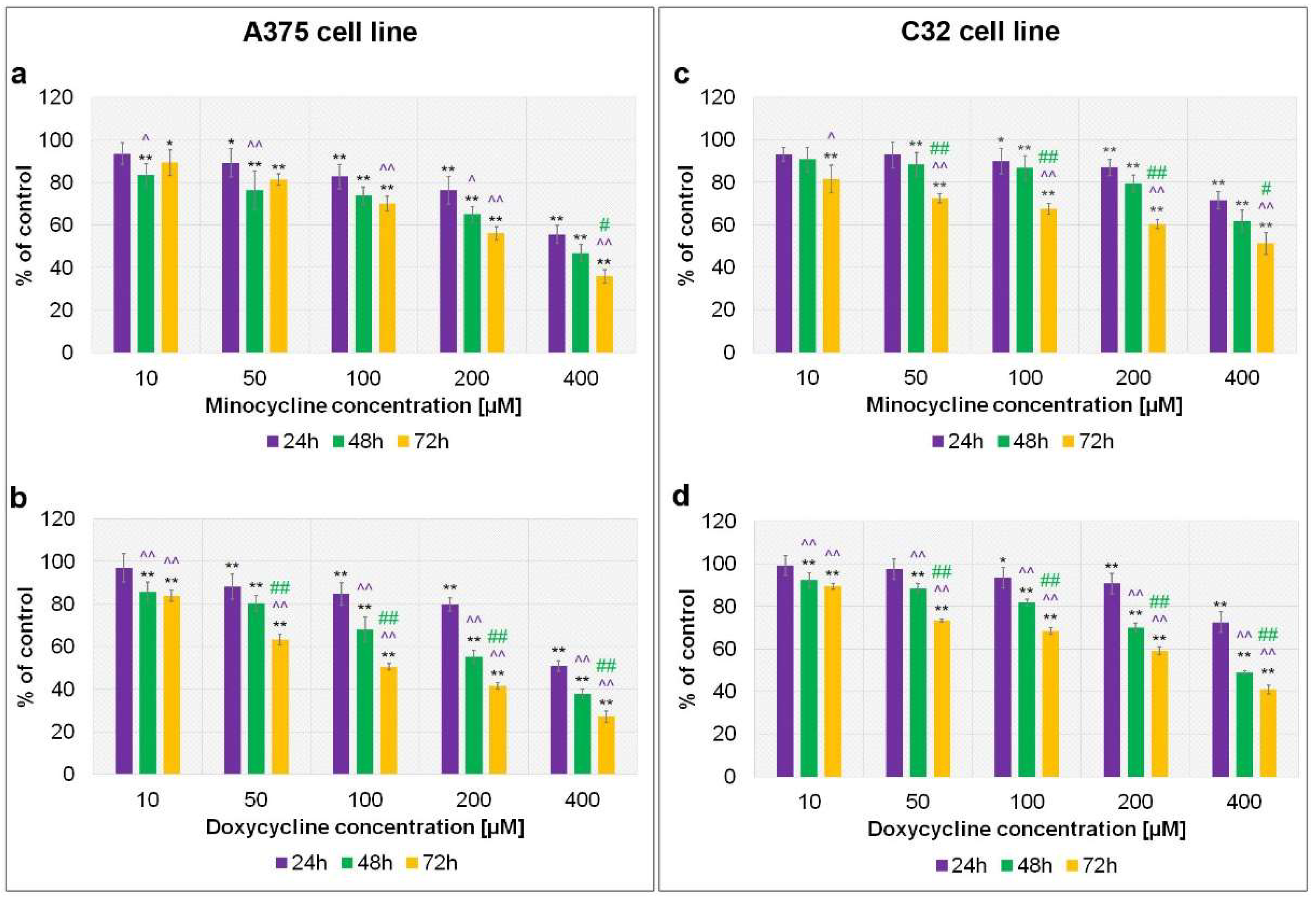
2.1. The Assessment of Melanoma Cell Proliferation after Exposure to Minocycline and Doxycycline
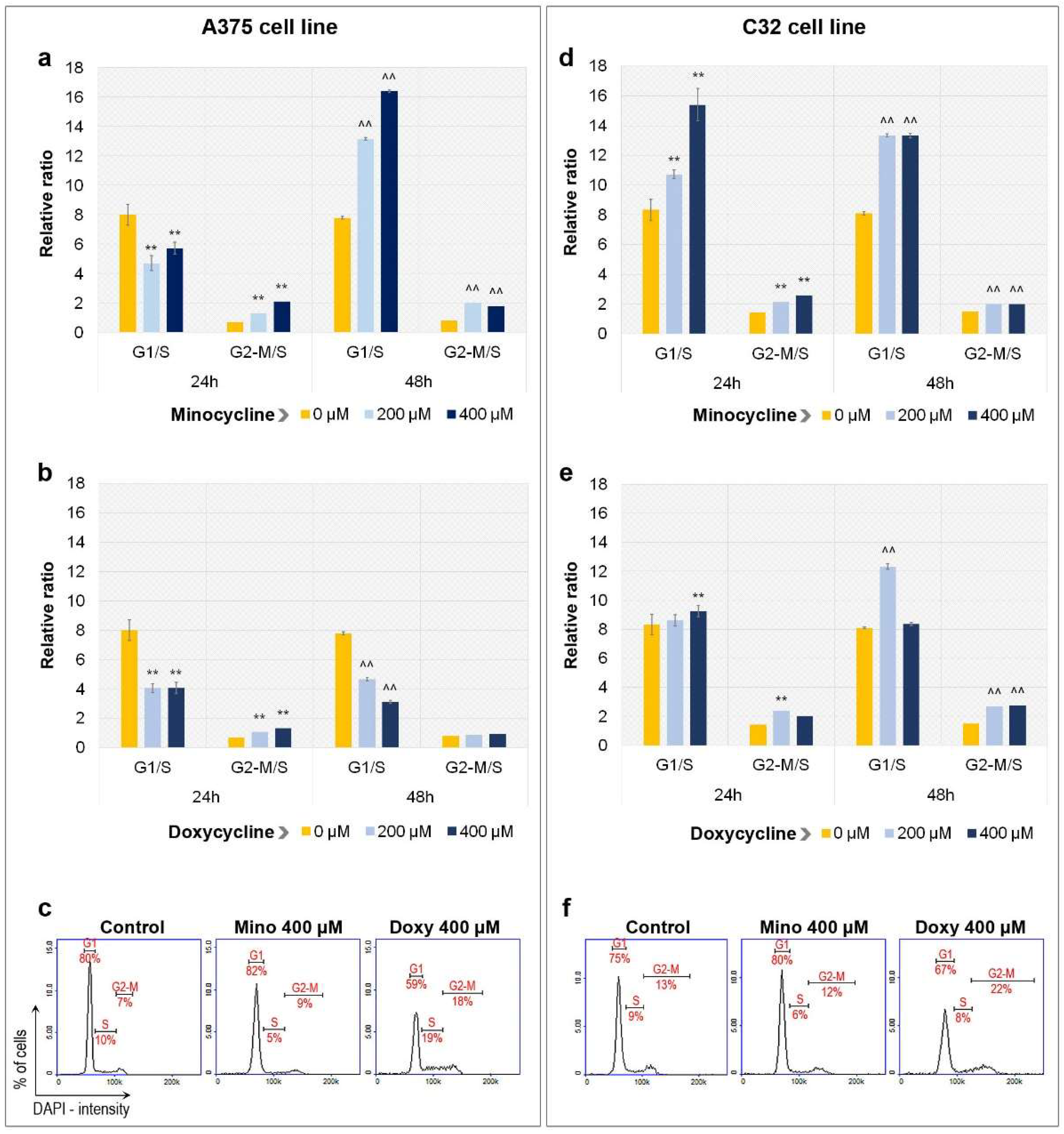
2.2. Analysis of Cell Cycle in A375 and C32 Melanoma Cells Incubated with Minocycline and Doxycycline
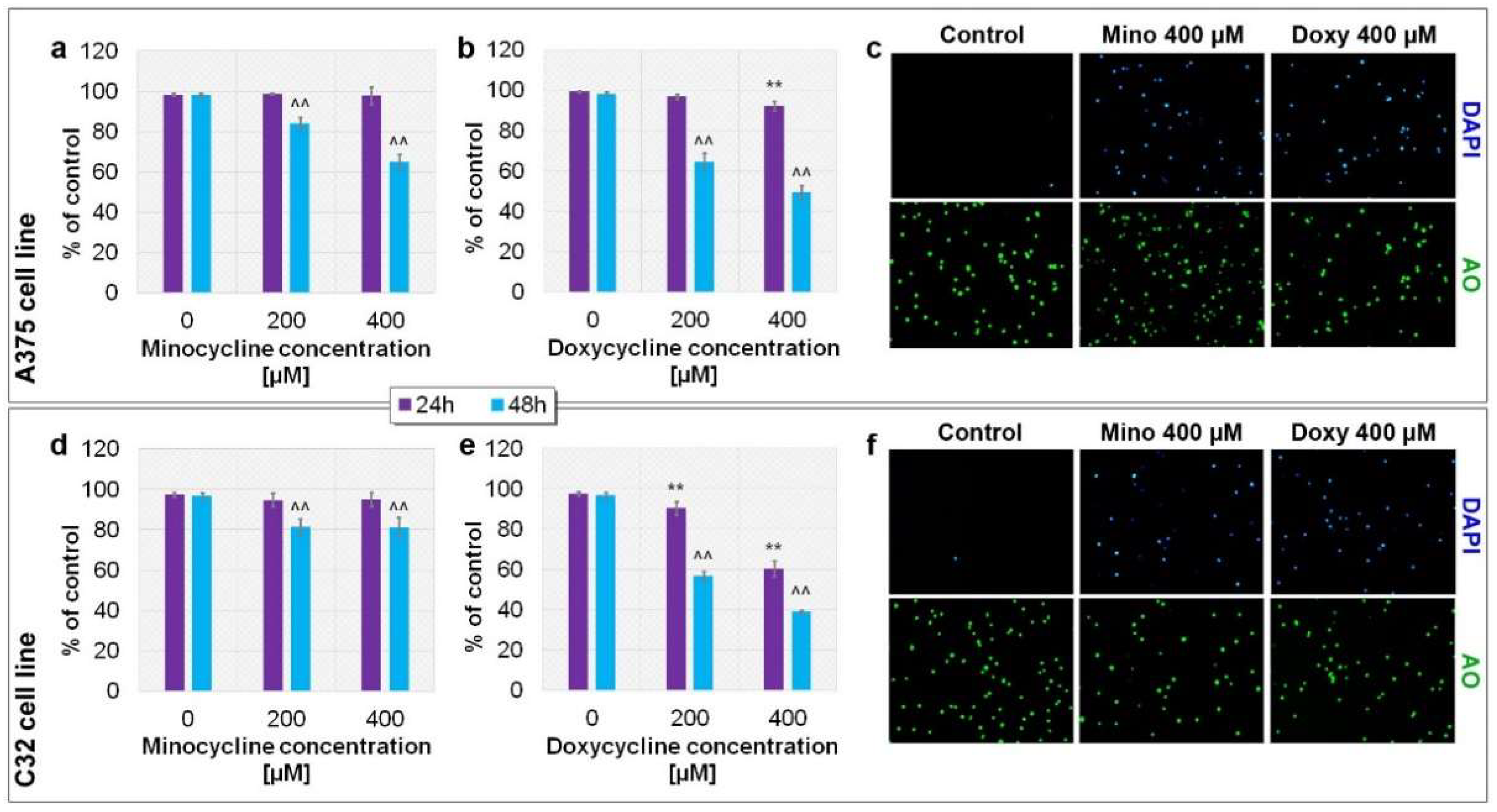
2.3. Evaluation of the Viability of A375 and C32 Melanoma Cells after Incubation with Minocycline and Doxycycline
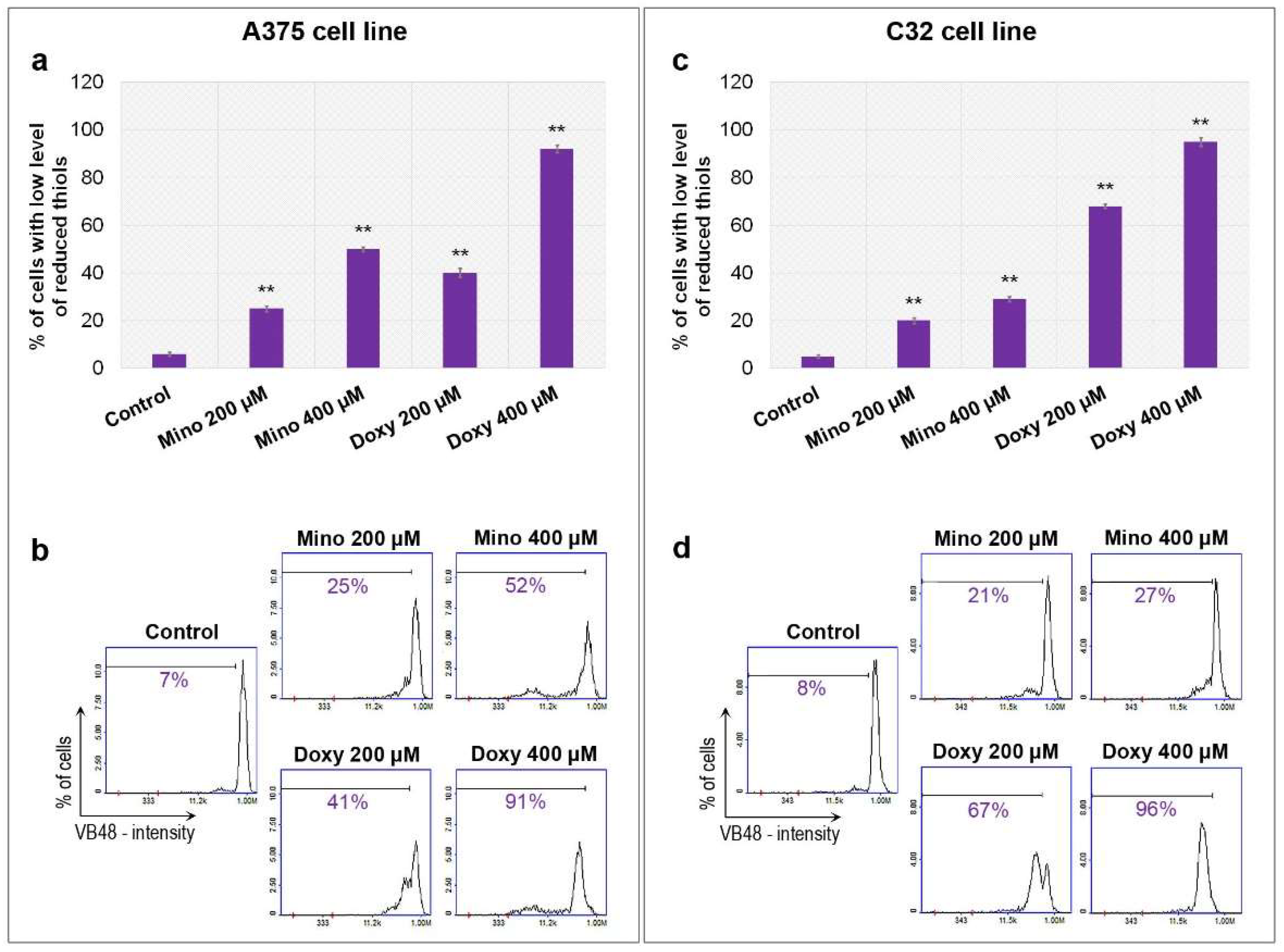
2.4. Analysis of Reduced Thiols in A375 and C32 Melanoma Cells Treated with Minocycline and Doxycycline
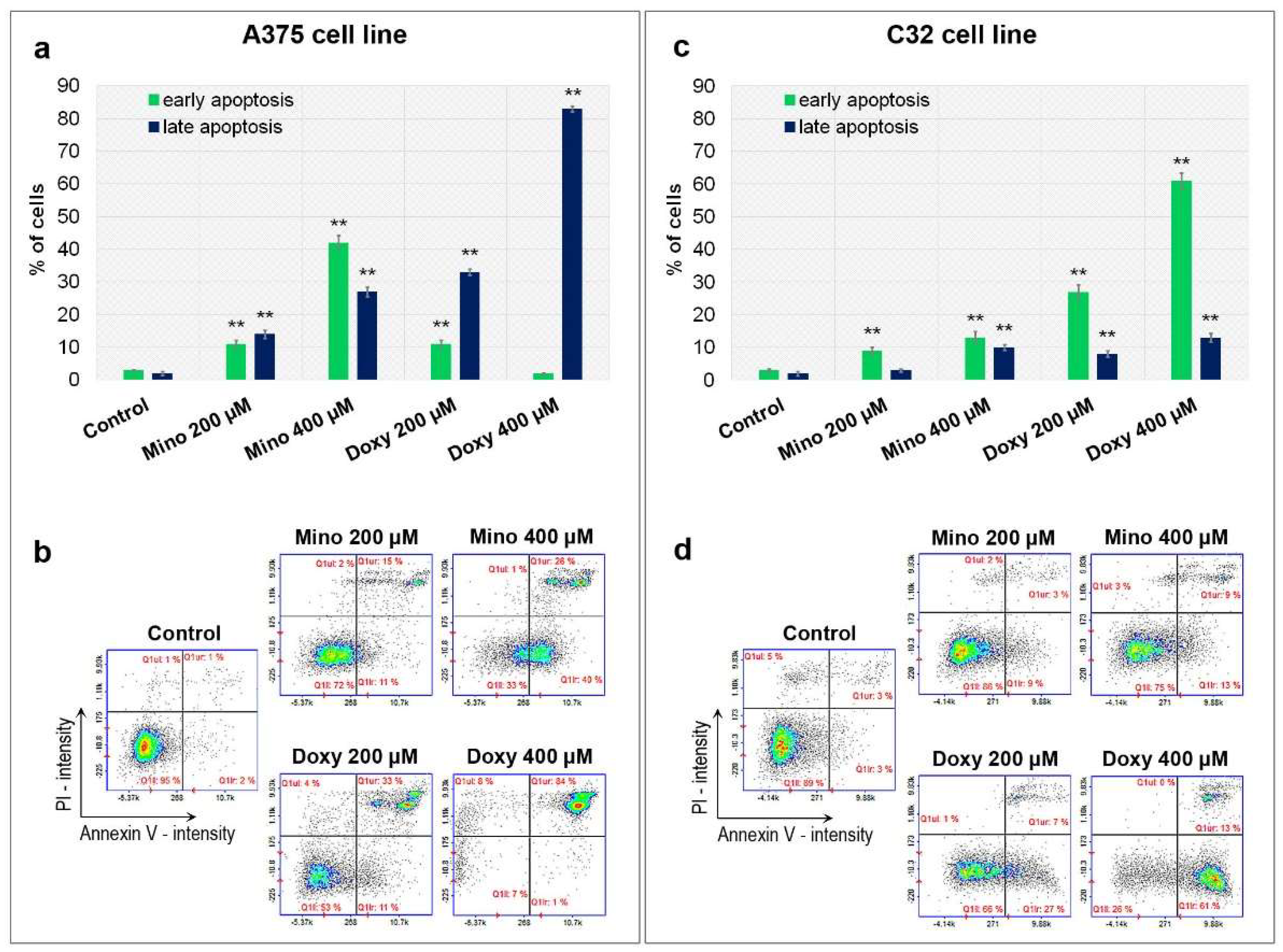
2.5. Analysis of Apoptosis of A375 and C32 Amelanotic Melanoma Cells Treated with Minocycline and Doxycycline
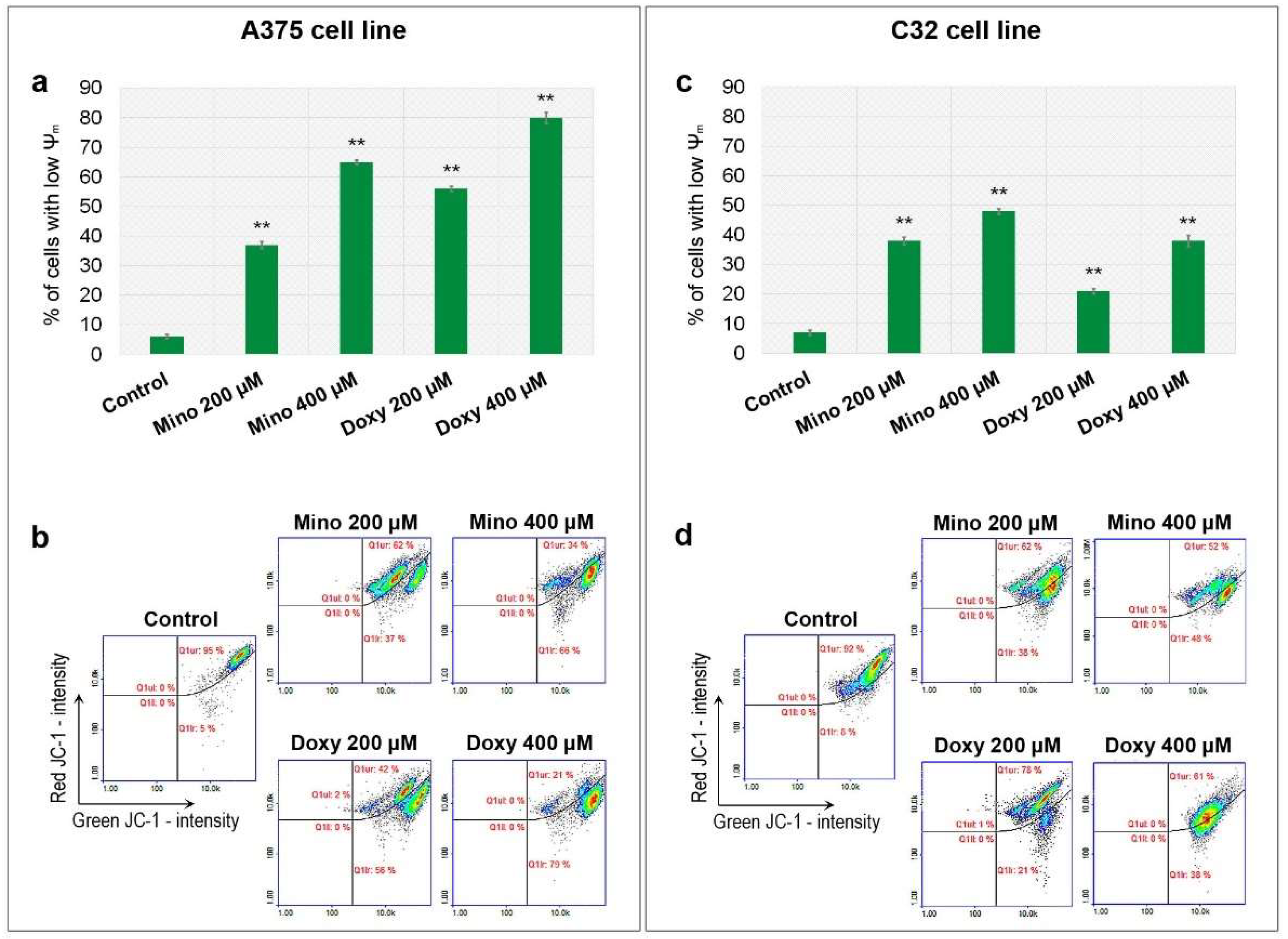
2.6. Analysis of Mitochondrial Membrane Potential (ψm) in A375 and C32 Melanoma Cells Treated with Minocycline and Doxycycline
2.7. Analysis of Caspases’ Activity in A375 and C32 Melanoma Cells Treated with Minocycline and Doxycycline
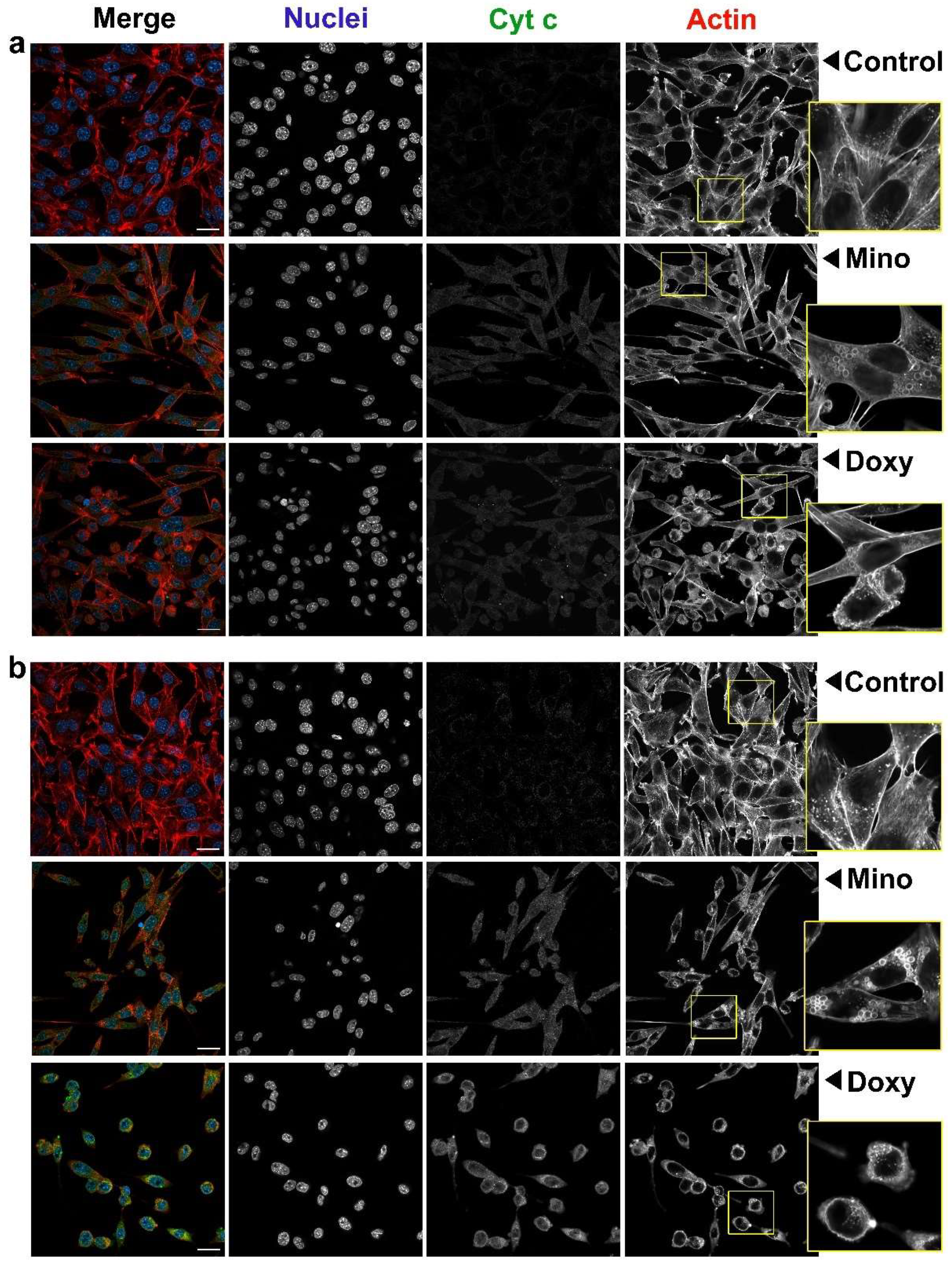
2.8. Analysis of Cell Morphology and Intracellular Level of Cytochrome c in A375 and C32 Melanoma Cells Treated with Minocycline and Doxycycline
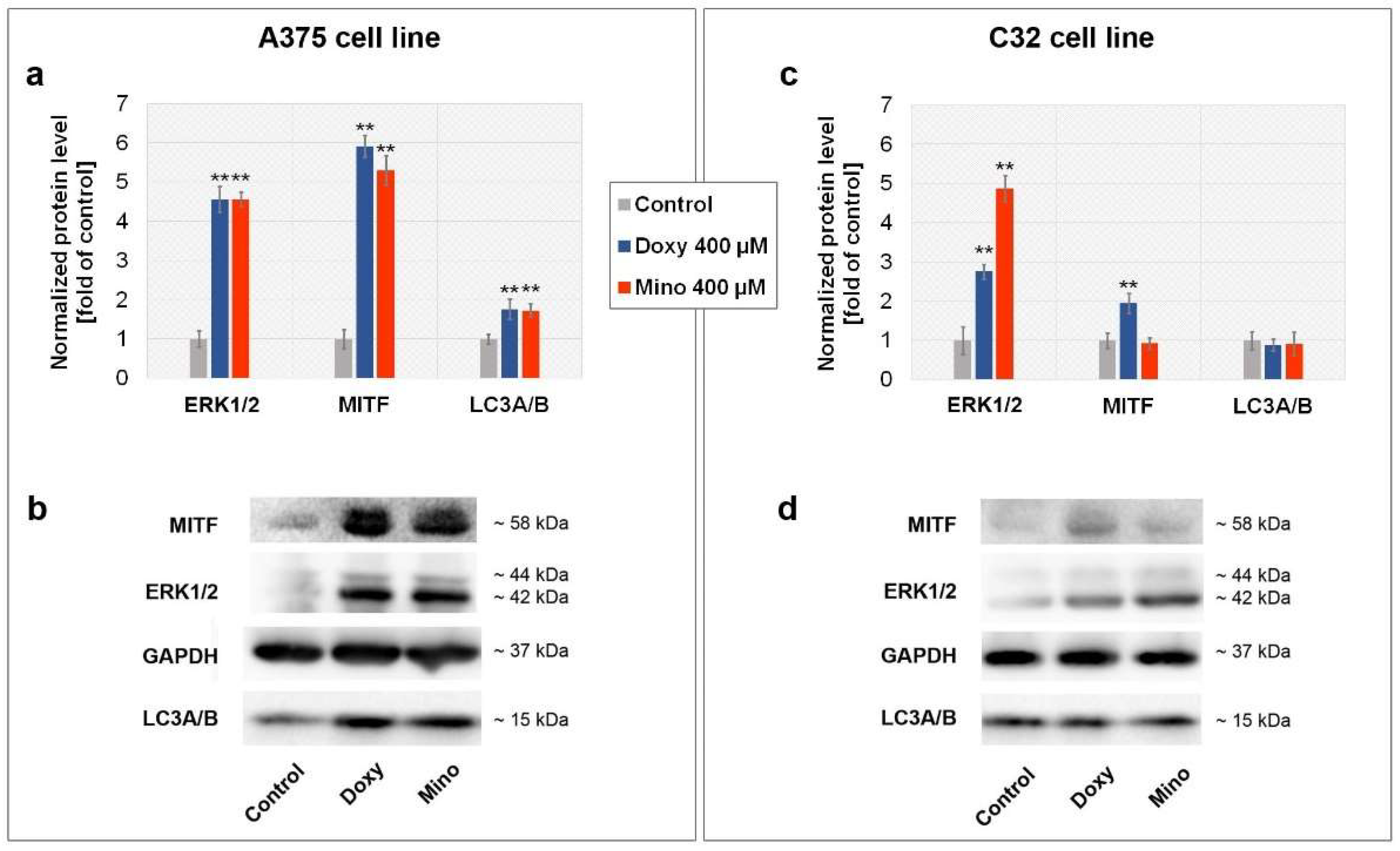
2.9. Analysis of MITF, ERK1/2, and LC3A/B Level in A375 and C32 Melanoma Cells Treated with Minocycline and Doxycycline
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and the Treatment
4.3. Screening Analysis of Cells Proliferation
4.4. Cell Cycle Analysis
4.5. The Evaluation of Cell Viability
4.6. Confocal Microscopy Imaging
4.7. Annexin V Assay
4.8. The Estimation of Intracellular Thiol Level
4.9. Mitochondrial Potential Analysis
4.10. Caspase Activity Assay
4.11. The Analysis of Protein Concentration
4.12. Western Blotting Analysis
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shain, A.H.; Bastian, B.C. From melanocytes to melanomas. Nat. Rev. Cancer 2016, 16, 345–358. [Google Scholar] [CrossRef]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection and skin pigmentation: Melanin-related molecules and some other new agents obtained from natural sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef]
- ElObeid, A.S.; Kamal-Eldin, A.; Abdelhalim, M.A.K.; Haseeb, A.M. Pharmacological properties of melanin and its function in health. Basic Clin. Pharmacol. Toxicol. 2017, 120, 515–522. [Google Scholar] [CrossRef]
- Strashilov, S.; Yordanov, A. Aetiology and pathogenesis of cutaneous melanoma: Current concepts and advances. Int. J. Mol. Sci. 2021, 22, 6395. [Google Scholar] [CrossRef]
- Teixido, C.; Castillo, P.; Martinez-Vila, C.; Arance, A.; Alos, L. Molecular markers and targets in melanoma. Cells 2021, 10, 2320. [Google Scholar] [CrossRef]
- Yeh, I.; Bastian, B.C. Melanoma pathology: New approaches and classification. Br. J. Dermatol. 2021, 185, 282–293. [Google Scholar] [CrossRef]
- Oba, J.; Woodman, S.E. The genetic and epigenetic basis of distinct melanoma types. J. Dermatol. 2021, 48, 925–939. [Google Scholar] [CrossRef]
- Davey, M.G.; Miller, N.; McInerney, N.M. A review of epidemiology and cancer biology of malignant melanoma. Cureus 2021, 13, e15087. [Google Scholar] [CrossRef]
- Tripp, M.K.; Watson, M.; Balk, S.J.; Swetter, S.M.; Gershenwald, J.E. State of the science on prevention and screening to reduce melanoma incidence and mortality: The time is now. CA Cancer J. Clin. 2016, 66, 460–480. [Google Scholar] [CrossRef]
- Bolick, N.L.; Geller, A.C. Epidemiology of melanoma. Hematol. Oncol. Clin. N. Am. 2021, 35, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Djavid, A.R.; Stonesifer, C.; Fullerton, B.T.; Wang, S.W.; Tartaro, M.A.; Kwinta, B.D.; Grimes, J.M.; Geskin, L.J.; Saenger, Y.M. Etiologies of melanoma development and prevention measures: A review of the current evidence. Cancers 2021, 13, 4914. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Tsao, H. Effect of the COVID-19 pandemic on delayed skin cancer services. Dermatol. Clin. 2021, 39, 627–637. [Google Scholar] [CrossRef]
- Stege, H.; Haist, M.; Nikfarjam, U.; Schultheis, M.; Heinz, J.; Pemler, S.; Loquai, C.; Grabbe, S. The status of adjuvant and neoadjuvant melanoma therapy, new developments and upcoming challenges. Target. Oncol. 2021, 16, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.; Lo, S.N.; Saw, R.P. Surgical excision margins in primary cutaneous melanoma: A systematic review and meta-analysis. Eur. J. Surg Oncol. 2021, 47, 1558–1574. [Google Scholar] [CrossRef]
- Dabestani, P.J.; Dawson, A.J.; Neumeister, M.W.; Bradbury, C.M. Radiation therapy for local cutaneous melanoma. Clin. Plast. Surg. 2021, 48, 643–649. [Google Scholar] [CrossRef]
- Curti, B.D.; Faries, M.B. Recent advances in the treatment of melanoma. N. Engl. J. Med. 2021, 384, 2229–2240. [Google Scholar] [CrossRef]
- Moreira, A.; Heinzerling, L.; Bhardwaj, N.; Friedlander, P. Current melanoma treatments: Where do we stand? Cancers 2021, 13, 221. [Google Scholar] [CrossRef]
- Burzi, L.; Alessandrini, A.M.; Quaglino, P.; Piraccini, B.M.; Dika, E.; Ribero, S. Cutaneous events associated with immunotherapy of melanoma: A review. J. Clin. Med. 2021, 10, 3047. [Google Scholar] [CrossRef]
- Peng, C.; Lei, J.-X. The incidence and risk of cutaneous toxicities associated with dabrafenib in melanoma patients: A systematic review and meta-analysis. Eur. J. Hosp. Pharm. 2021, 28, 182–189. [Google Scholar] [CrossRef]
- Vukadin, S.; Khaznadar, F.; Kizivat, T.; Vcev, A.; Smolic, M. Molecular mechanisms of resistance to immune checkpoint inhibitors in melanoma treatment: An update. Biomedicines 2021, 9, 835. [Google Scholar] [CrossRef]
- Patel, M.; Eckburg, A.; Gantiwala, S.; Hart, Z.; Dein, J.; Lam, K.; Puri, N. Resistance to molecularly targeted therapies in melanoma. Cancers 2021, 13, 1115. [Google Scholar] [CrossRef] [PubMed]
- Borgers, J.S.W.; Haanen, J.B.A.G. Cellular therapy and cytokine treatments for melanoma. Hematol. Oncol. Clin. N. Am. 2021, 35, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Jayaram, B.; Goliaei, B.; Masoudi-Nejad, A. Active repurposing of drug candidates for melanoma based on GWAS, PheWAS and a wide range of omics data. Mol. Med. 2019, 25, 30. [Google Scholar] [CrossRef]
- Nelson, M.L.; Levy, S.B. The history of the tetracyclines. Ann. N. Y. Acad. Sci. 2011, 1241, 17–32. [Google Scholar] [CrossRef]
- Barrenechea, V.; Vargas-Reyes, M.; Quiliano, M.; Milón, P. A complementary mechanism of bacterial mRNA translation inhibition by tetracyclines. Front. Microbiol. 2021, 12, 682682. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Leyden, J.J. Safety of doxycycline and minocycline: A systematic review. Clin. Ther. 2005, 27, 1329–1342. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Singh, S.; Khanna, D.; Kalra, S. Minocycline and doxycycline: More than antibiotics. Curr. Mol. Pharmacol. 2021, 14. [Google Scholar] [CrossRef]
- Platt, B.N.; Jacobs, C.A.; Conley, C.E.W.; Stone, A.V. Tetracycline use in treating osteoarthritis: A systematic review. Inflamm. Res. 2021, 70, 249–259. [Google Scholar] [CrossRef]
- Chaves Filho, A.J.M.; Mottin, M.; Soares, M.V.; Jucá, P.M.; Andrade, C.H.; Macedo, D.S. Tetracyclines, a promise for neuropsychiatric disorders: From adjunctive therapy to the discovery of new targets for rational drug design in psychiatry. Behav. Pharmacol. 2021, 32, 123–141. [Google Scholar] [CrossRef]
- Víctor-Carvalho, P.; Thome, R.; Rapôso, C. Can tetracyclines ensure help in multiple sclerosis immunotherapy? J. Clin. Transl. Res. 2021, 7, 22–33. [Google Scholar] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alqarni, M.; Cruz-Martins, N.; El-Saber Batiha, G. Pleiotropic effects of tetracyclines in the management of COVID-19: Emerging perspectives. Front. Pharmacol. 2021, 12, 642822. [Google Scholar] [CrossRef]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Barbie, D.A.; Kennedy, B.K. Doxycycline: New tricks for an old drug. Oncotarget 2015, 6, 19336–19337. [Google Scholar] [CrossRef]
- Cortés, H.; Reyes-Hernández, O.D.; Alcalá-Alcalá, S.; Bernal-Chávez, S.A.; Caballero-Florán, I.H.; González-Torres, M.; Sharifi-Rad, J.; González-Del Carmen, M.; Figueroa-González, G.; Leyva-Gómez, G. Repurposing of drug candidates for treatment of skin cancer. Front. Oncol. 2021, 10, 605714. [Google Scholar] [CrossRef] [PubMed]
- Rok, J.; Rzepka, Z.; Beberok, A.; Pawlik, J.; Wrześniok, D. Cellular and molecular aspects of anti-melanoma effect of minocycline—A study of cytotoxicity and apoptosis on human melanotic melanoma cells. Int. J. Mol. Sci. 2020, 21, 6917. [Google Scholar] [CrossRef] [PubMed]
- Rok, J.; Karkoszka, M.; Rzepka, Z.; Respondek, M.; Banach, K.; Beberok, A.; Wrześniok, D. Cytotoxic and proapoptotic effect of doxycycline—An in vitro study on the human skin melanoma cells. Toxicol. In Vitro 2020, 65, 104790. [Google Scholar] [CrossRef] [PubMed]
- Kaizer-Salk, K.A.; Herten, R.J.; Ragsdale, B.D.; Sengelmann, R.D. Amelanotic melanoma: A unique case study and review of the literature. BMJ Case Rep. 2018, 2018, bcr2017222751. [Google Scholar] [CrossRef]
- Stojkovic-Filipovic, J.; Kittler, H. Dermatoscopy of amelanotic and hypomelanotic melanoma. J. Dtsch. Dermatol. Ges. 2014, 12, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.Z.; Zheng, H.Y.; Li, J. Amelanotic melanoma. Melanoma Res. 2019, 29, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Chacón, M.; Pfluger, Y.; Angel, M.; Waisberg, F.; Enrico, D. Uncommon subtypes of malignant melanomas: A review based on clinical and molecular perspectives. Cancers 2020, 12, 2362. [Google Scholar] [CrossRef]
- Lan, J.; Wen, J.; Cao, S.; Yin, T.; Jiang, B.; Lou, Y.; Zhu, J.; An, X.; Suo, H.; Li, D.; et al. The diagnostic accuracy of dermoscopy and reflectance confocal microscopy for amelanotic/hypomelanotic melanoma: A systematic review and meta-analysis. Br. J. Dermatol. 2020, 183, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Banning, T.P.; Heard, C.M. Binding of doxycycline to keratin, melanin and human epidermal tissue. Int. J. Pharm. 2002, 235, 219–227. [Google Scholar] [CrossRef]
- Rok, J.; Rzepka, Z.; Respondek, M.; Beberok, A.; Wrześniok, D. Chlortetracycline and melanin biopolymer—The risk of accumulation and implications for phototoxicity: An in vitro study on normal human melanocytes. Chem. Biol. Interact. 2019, 303, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Rok, J.; Rzepka, Z.; Kowalska, J.; Banach, K.; Beberok, A.; Wrześniok, D. Molecular and biochemical basis of minocycline-induced hyperpigmentation-The study on normal human melanocytes exposed to UVA and UVB radiation. Int. J. Mol. Sci. 2021, 22, 3755. [Google Scholar] [CrossRef]
- Rok, J.; Buszman, E.; Beberok, A.; Delijewski, M.; Otręba, M.; Wrześniok, D. Modulation of melanogenesis and antioxidant status of melanocytes in response to phototoxic action of doxycycline. Photochem. Photobiol. 2015, 91, 1429–1434. [Google Scholar] [CrossRef]
- Rok, J.; Rzepka, Z.; Maszczyk, M.; Beberok, A.; Wrześniok, D. Minocycline impact on redox homeostasis of normal human melanocytes HEMn-LP exposed to UVA radiation and hydrogen peroxide. Int. J. Mol. Sci. 2021, 22, 1642. [Google Scholar] [CrossRef]
- Chong Teoh, T.; Al-Harbi, S.J.; Abdulrahman, A.Y.; Rothan, H.A. Doxycycline interferes with zika virus serine protease and inhibits virus replication in human skin fibroblasts. Molecules 2021, 26, 4321. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Park, J.H.; Park, I.H.; Lee, H.M. Doxycycline inhibits TGF-β1-induced extracellular matrix production in nasal polyp-derived fibroblasts. Int. Forum Allergy Rhinol. 2016, 6, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yagisawa, J.; Kumakura, S.; Tsutsui, T. Effects of minocycline and doxycycline on cell survival and gene expression in human gingival and periodontal ligament cells. J. Periodontal Res. 2006, 41, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ezra, D.G.; Burton, M.J.; Bailly, M. Doxycycline prevents matrix remodeling and contraction by trichiasis-derived conjunctival fibroblasts. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4675–4682. [Google Scholar] [CrossRef]
- Fouco, D. Cytotoxicity induced by tetracyclines via protein photooxidation. Adv. Toxicol. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Agwuh, K.N.; Gowan, M.A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 2006, 58, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Miliński, M.; Delijewski, M.; Rok, J.; Wrześniok, D.; Beberok, A.; Chełmecka, E.; Buszman, E. The pivotal effect of sulindac on melanin-containing cancers. Acta Pol. Pharm. 2017, 74, 1681–1689. [Google Scholar]
- Respondek, M.; Beberok, A.; Rzepka, Z.; Rok, J.; Wrześniok, D. MIM1 induces COLO829 melanoma cell death through mitochondrial membrane breakdown, GSH depletion, and DNA damage. Fundam. Clin. Pharmacol. 2020, 34, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Respondek, M.; Beberok, A.; Rzepka, Z.; Rok, J.; Wrześniok, D. Mcl-1 inhibitor induces cells death in BRAF-mutant amelanotic melanoma trough GSH depletion, DNA damage and cell cycle changes. Pathol. Oncol. Res. 2020, 26, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Al-Qatati, A.; Aliwaini, S. Combined pitavastatin and dacarbazine treatment activates apoptosis and autophagy resulting in synergistic cytotoxicity in melanoma cells. Oncol. Lett. 2017, 14, 7993–7999. [Google Scholar] [CrossRef] [PubMed]
- Charignon, E.; Bouché, M.; Clave-Darcissac, C.; Dahm, G.; Ichim, G.; Clotagatide, A.; Mertani, H.C.; Telouk, P.; Caramel, J.; Diaz, J.J.; et al. In cellulo evaluation of the therapeutic potential of NHC platinum compounds in metastatic cutaneous melanoma. Int. J. Mol. Sci 2020, 21, 7826. [Google Scholar] [CrossRef] [PubMed]
- Beberok, A.; Buszman, E.; Wrześniok, D.; Otręba, M.; Trzcionka, J. Interaction between ciprofloxacin and melanin: The effect on proliferation and melanization in melanocytes. Eur. J. Pharmacol. 2011, 669, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Beberok, A.; Wrześniok, D.; Minecka, A.; Rok, J.; Delijewski, M.; Rzepka, Z.; Respondek, M.; Buszman, E. Ciprofloxacin-mediated induction of S-phase cell cycle arrest and apoptosis in COLO829 melanoma cells. Pharmacol. Rep. 2018, 70, 6–13. [Google Scholar] [CrossRef]
- Beberok, A.; Rok, J.; Rzepka, Z.; Marciniec, K.; Boryczka, S.; Wrześniok, D. The role of MITF and Mcl-1 proteins in the antiproliferative and proapoptotic effect of ciprofloxacin in amelanotic melanoma cells: In silico and in vitro study. Toxicol. In Vitro 2020, 66, 104884. [Google Scholar] [CrossRef] [PubMed]
- Beberok, A.; Rzepka, Z.; Respondek, M.; Rok, J.; Stradowski, M.; Wrześniok, D. Moxifloxacin as an inducer of apoptosis in melanoma cells: A study at the cellular and molecular level. Toxicol. In Vitro 2019, 55, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, X.; Geng, M.; Huang, M. Targeting ERK, an Achilles’ Heel of the MAPK pathway, in cancer therapy. Acta Pharm. Sin. B 2018, 8, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W.; Somasundaram, S.; Moore, D.T.; Shi, Y.Y.; Orlowski, R.Z. Repression of mitogen-activated protein kinase (MAPK) phosphatase-1 by anthracyclines contributes to their antiapoptotic activation of p44/42-MAPK. J. Pharmacol. Exp. Ther. 2003, 307, 861–869. [Google Scholar] [CrossRef]
- Mirmohammadsadegh, A.; Mota, R.; Gustrau, A.; Hassan, M.; Nambiar, S.; Marini, A.; Bojar, H.; Tannapfel, A.; Hengge, U.R. ERK1/2 is highly phosphorylated in melanoma metastases and protects melanoma cells from cisplatin-mediated apoptosis. J. Investig. Dermatol. 2007, 127, 2207–2215. [Google Scholar] [CrossRef][Green Version]
- Kang, M.; Park, S.H.; Park, S.J.; Oh, S.W.; Yoo, J.A.; Kwon, K.; Kim, J.; Yu, E.; Cho, J.Y.; Lee, J. p44/42 MAPK signaling is a prime target activated by phenylethyl resorcinol in its anti-melanogenic action. Phytomedicine 2019, 58, 152877. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK signaling in cancer: Mechanisms of drug resistance and sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef]
- Dent, P.; Jarvis, W.D.; Birrer, M.J.; Fisher, P.B.; Schmidt-Ullrich, R.K.; Grant, S. The roles of signaling by the p42/p44 mitogen-activated protein (MAP) kinase pathway; a potential route to radio- and chemo-sensitization of tumor cells resulting in the induction of apoptosis and loss of clonogenicity. Leukemia 1998, 12, 1843–1850. [Google Scholar] [CrossRef]
- Bristot, I.J.; Kehl Dias, C.; Chapola, H.; Parsons, R.B.; Klamt, F. Metabolic rewiring in melanoma drug-resistant cells. Crit. Rev. Oncol. Hematol. 2020, 153, 102995. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Kim, Y.; Park, T.J.; Kang, H.Y. Dasatinib, a second-generation tyrosine kinase inhibitor, induces melanogenesis via ERK-CREB-MITF-tyrosinase signaling in normal human melanocytes. Biochem. Biophys. Res. Commun. 2020, 523, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.L.; Czyz, M. MITF in melanoma: Mechanisms behind its expression and activity. Cell. Mol. Life Sci. 2015, 72, 1249–1260. [Google Scholar] [CrossRef]
- Hartman, M.L.; Czyz, M. Pro-survival role of MITF in melanoma. J. Investig. Dermatol. 2015, 135, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, A.M.; Bartnik, E.; Fiedorowicz, M.; Rutkowski, P. Targeted therapy in melanoma and mechanisms of resistance. Int. J. Mol. Sci. 2020, 21, 4576. [Google Scholar] [CrossRef]
- Möller, K.; Sigurbjornsdottir, S.; Arnthorsson, A.O.; Pogenberg, V.; Dilshat, R.; Fock, V.; Brynjolfsdottir, S.H.; Bindesboll, C.; Bessadottir, M.; Ogmundsdottir, H.M.; et al. MITF has a central role in regulating starvation-induced autophagy in melanoma. Sci. Rep. 2019, 9, 1055. [Google Scholar] [CrossRef]
- Ballotti, R.; Cheli, Y.; Bertolotto, C. The complex relationship between MITF and the immune system: A melanoma immunotherapy (response) factor? Mol. Cancer 2020, 19, 170. [Google Scholar] [CrossRef]
- Kawakami, A.; Fisher, D.E. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab. Investig. 2017, 97, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Yan, X.; Song, L.; Yi, H.; Li, P.; Sun, G.; Yu, D.; Li, L.; Zeng, Z.; Guo, Z. Induction of mitochondrial dysfunction and oxidative damage by antibiotic drug doxycycline enhances the responsiveness of glioblastoma to chemotherapy. Med. Sci. Monit. 2017, 23, 4117–4125. [Google Scholar] [CrossRef]
- Ozben, T. Oxidative stress and apoptosis: Impact on cancer therapy. J. Pharm. Sci. 2007, 96, 2181–2196. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Sandhu, J.K.; Harper, M.E.; Cuperlovic-Culf, M. Role of glutathione in cancer: From mechanisms to therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.L.; Mena, S.; Estrela, J.M. Glutathione in cancer cell death. Cancers 2011, 3, 1285–1310. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Cidlowski, J.A. Apoptosis and glutathione: Beyond an antioxidant. Cell Death Differ. 2009, 16, 1303–1314. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.E. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef]
- Strasser, A.; Vaux, D.L. Cell death in the origin and treatment of cancer. Mol. Cell. 2020, 78, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Lin, L.; Baehrecke, E.H. Autophagy, cell death, and cancer. Mol. Cell. Oncol. 2015, 2, e985913. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.; Kögel, D. Autophagy in cancer cell death. Biology 2019, 8, 82. [Google Scholar] [CrossRef]
- Su, M.; Mei, Y.; Sinha, S. Role of the crosstalk between autophagy and apoptosis in cancer. J. Oncol. 2013, 2013, 102735. [Google Scholar] [CrossRef] [PubMed]
- An, H.K.; Chung, K.M.; Park, H.; Hong, J.; Gim, J.E.; Choi, H.; Lee, Y.W.; Choi, J.; Mun, J.Y.; Yu, S.W. CASP9 (caspase 9) is essential for autophagosome maturation through regulation of mitochondrial homeostasis. Autophagy 2020, 16, 1598–1617. [Google Scholar] [CrossRef]
- Han, J.; Hou, W.; Goldstein, L.A.; Stolz, D.B.; Watkins, S.C.; Rabinowich, H. A Complex between Atg7 and Caspase-9: A novel mechanism of cross-regulation between autophagy and apoptosis. J. Biol. Chem. 2014, 289, 6485–6497. [Google Scholar] [CrossRef]
- Choi, C.Y.; Vo, M.T.; Nicholas, J.; Choi, Y.B. Autophagy-competent mitochondrial translation elongation factor TUFM inhibits caspase-8-mediated apoptosis. Cell Death Differ. 2021. [Google Scholar] [CrossRef]
- Sun, J.; Shigemi, H.; Cao, M.; Qin, E.; Tang, J.; Shen, J.; Iwasaki, H. Minocycline induces autophagy and inhibits cell proliferation in LPS-stimulated THP-1 cells. Biomed. Res. Int. 2020, 2020, 5459209. [Google Scholar] [CrossRef]
- Liu, W.T.; Lin, C.H.; Hsiao, M.; Gean, P.W. Minocycline inhibits the growth of glioma by inducing autophagy. Autophagy 2011, 7, 166–175. [Google Scholar] [CrossRef]
- Dong, W.; Xiao, S.; Cheng, M.; Ye, X.; Zheng, G. Minocycline induces protective autophagy in vascular endothelial cells exposed to an in vitro model of ischemia/reperfusion-induced injury. Biomed. Rep. 2016, 4, 173–177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, E.; Zhao, X.; Zhang, L.; Li, N.; Yan, J.; Tu, K.; Yan, R.; Hu, J.; Zhang, M.; Sun, D.; et al. Minocycline promotes cardiomyocyte mitochondrial autophagy and cardiomyocyte autophagy to prevent sepsis-induced cardiac dysfunction by Akt/mTOR signaling. Apoptosis 2019, 24, 369–381. [Google Scholar] [CrossRef]
- Valero-Muñoz, M.; Wilson, R.M.; Bretón-Romero, R.; Croteau, D.; Seldin, D.C.; Sam, F. Doxycycline decreases amyloidogenic light chain-induced autophagy in isolated primary cardiac myocytes. Int. J. Cardiol. 2020, 321, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Desjarlais, M.; Pratt, J.; Lounis, A.; Mounier, C.; Haidara, K.; Annabi, B. Tetracycline derivative minocycline inhibits autophagy and inflammation in concanavalin-a-activated human hepatoma cells. Gene Regul. Syst. Bio. 2014, 8, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, L.; Zhang, F.; Vlashi, E. Doxycycline inhibits the cancer stem cell phenotype and epithelial-to-mesenchymal transition in breast cancer. Cell Cycle 2017, 16, 737–745. [Google Scholar] [CrossRef] [PubMed]


| Cell Line | A375 | C32 | ||||
|---|---|---|---|---|---|---|
| Incubation time | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h |
| Minocycline (µM) | 528.4 | 307.8 | 234.0 | 1040.0 | 651.9 | 273.1 |
| Doxycycline (µM) | 518.5 | 226.1 | 110.4 | 1270.0 | 416.0 | 238.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rok, J.; Rzepka, Z.; Kowalska, J.; Banach, K.; Beberok, A.; Wrześniok, D. The Anticancer Potential of Doxycycline and Minocycline—A Comparative Study on Amelanotic Melanoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 831. https://doi.org/10.3390/ijms23020831
Rok J, Rzepka Z, Kowalska J, Banach K, Beberok A, Wrześniok D. The Anticancer Potential of Doxycycline and Minocycline—A Comparative Study on Amelanotic Melanoma Cell Lines. International Journal of Molecular Sciences. 2022; 23(2):831. https://doi.org/10.3390/ijms23020831
Chicago/Turabian StyleRok, Jakub, Zuzanna Rzepka, Justyna Kowalska, Klaudia Banach, Artur Beberok, and Dorota Wrześniok. 2022. "The Anticancer Potential of Doxycycline and Minocycline—A Comparative Study on Amelanotic Melanoma Cell Lines" International Journal of Molecular Sciences 23, no. 2: 831. https://doi.org/10.3390/ijms23020831
APA StyleRok, J., Rzepka, Z., Kowalska, J., Banach, K., Beberok, A., & Wrześniok, D. (2022). The Anticancer Potential of Doxycycline and Minocycline—A Comparative Study on Amelanotic Melanoma Cell Lines. International Journal of Molecular Sciences, 23(2), 831. https://doi.org/10.3390/ijms23020831






