Abstract
Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME) and Cancer-Related Fatigue (CRF) are syndromes with considerable overlap with respect to symptoms. There have been many studies that have compared the two conditions, and some of this research suggests that the etiologies of the conditions are linked in some cases. In this narrative review, CFS/ME and cancer are introduced, along with their known and putative mechanistic connections to multiple stressors including ionizing radiation. Next, we summarize findings from the literature that suggest the involvement of HPA-axis dysfunction, the serotonergic system, cytokines and inflammation, metabolic insufficiency and mitochondrial dysfunction, and genetic changes in CRF and CFS/ME. We further suspect that the manifestation of fatigue in both diseases and its causes could indicate that CRF and CFS/ME lie on a continuum of potential biological effects which occur in response to stress. The response to this stress likely varies depending on predisposing factors such as genetic background. Finally, future research ideas are suggested with a focus on determining if common biomarkers exist in CFS/ME patients and those afflicted with CRF. Both CFS/ME and CRF are relatively heterogenous syndromes, however, it is our hope that this review assists in future research attempting to elucidate the commonalities between CRF and CFS/ME.
1. Introduction to Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME)
Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME) is a variably severe disease that presents clinically as a multi-symptom, recurring illness [1]. Patients commonly report a panel of symptoms, with persistent fatigue being the most indicative diagnostic sign [2,3,4]. While the disease is heterogenous in nature and diverse biological effects appear differently between patients, the underlying syndrome is generally hypothesized to be the result of some combination of cognitive, immune, and endocrine dysfunction [5,6,7]. Common symptoms include sleep disturbances, orthostatic intolerance, chronic pain, challenges to memory and cognition (or “brain fog”), weakness, general malaise, tender lymph nodes, digestive problems including irritable bowel syndrome, night sweats and chills, allergies, irregular heartbeat, shortness of breath, and sore throat [8,9,10,11,12,13]. The onset of CFS/ME has been observed to manifest following exposure to an acute stressor in many patients, such as a viral or bacterial infection [12,14]. The onset of the syndrome can be gradual or sudden. CFS/ME patients often report exacerbation of their symptoms following relatively minor physical or mental activity; this is known as post-exertional malaise (PEM) [15]. This oftentimes results in the complete inability—or significantly reduced capacity—of those suffering from the disease to perform routine tasks, consequently diminishing performance at school and work. CFS/ME appears more frequently in those of European heritage but is not believed to be necessarily more common in this population. CFS/ME is believed to affect millions internationally with varying degrees of medical recognition [8,16,17,18,19]. The disease is significantly more common in women compared to men, and usually affects those between 40 and 60 years of age. However, studies suggest that the disease can develop in children as well, with greater frequency in adolescents than younger children [20]. The cause of this distribution is unknown; however, it may suggest that aging or repeated exposure to inciting factors over time can cause or contribute to the appearance or progression of the disease.
Currently, a diagnosis of CFS/ME is always made based on symptomatology, as no confirmed biomarker or diagnostic test exists [3,21]. The Fukuda and the US Center for Disease Control and Prevention (CDC) criteria are most frequently used to make diagnoses of CFS/ME based on symptoms related to fatigue, PEM, cognitive dysfunction, and sleep disturbances [22,23]. Other diagnostic criteria exist, including the Institute of Medicine (IOM) and U.S. CFS/ME Expert Physician Coalition criteria. While it is estimated that the disease affects millions internationally, physician skepticism of the disease and consequent dismissal of patients was and continues to be a significant obstacle for diagnosis [24,25,26,27]. A survey of approximately 800 general physicians in the United Kingdom revealed that 48% did not feel confident diagnosing the disease and 41% did not feel confident in the available treatments [28]. Another survey of physicians in the United States from 2010 found that the majority (80%) could correctly identify CFS/ME symptoms, while 40% had given a diagnosis of CFS/ME at some point in their medical practice; this indicates that attitudes towards the illness in healthcare may be changing, with increased awareness and greatly reduced negative attitudes compared to previous years [29]. However, it is known that CFS/ME patients are medically underserved in the United States with regard to specialist care access; the majority cited geographic and financial barriers that precluded access to medical specialists, even though nearly all participants expressed interest in such care [30].
Often, it is very difficult to separately assess the metabolic, immunologic, and neurologic manifestations of the disease [31,32]. Usually, effects falling into one or more of these categories influence processes and outcomes in other categories, which is discussed in further detail in later sections of this review. Among epidemiological studies, it is difficult to compare reports because there is no universal set of criteria used to diagnose CFS/ME [5] and the biomedical assays performed that may be indicative of CFS/ME can vary greatly from study to study. These factors significantly complicate the investigation, diagnosis, and treatment of CFS/ME. In this narrative review, we hypothesize that CFS/ME could be connected to cancer-related fatigue.
CFS/ME, Ionizing Radiation, and Multiple Stressors
It is also of note that similar syndromes have been reported in other groups exposed to acute stressors, like Atomic and Gulf War Veterans, radiotherapy patients and cancer survivors, and survivors of nuclear catastrophes like the one that occurred in Chernobyl [33,34,35,36]. This may suggest that the underlying pathophysiology of CFS/ME and syndromes associated with exposure to ionizing radiation may share some commonalities. Furthermore, it may suggest that ionizing radiation exposure can cause CFS/ME [33]; however, this has not been conclusively demonstrated.
The first paper that proposed that CFS/ME may result following exposure to ionizing radiation studied Chernobyl liquidators following the disaster [33]. In the aftermath of the 1986 nuclear disaster in the Soviet Union that precipitated the uncontrolled release of over one hundred types of radioisotopes into the environment, teams made up of civilians, police, military, and firefighters, were tasked with organizing and executing clean-up efforts. This action was undertaken to mitigate further uncontrolled release and subsequent contamination. A subset of this group, containing liquidators which were estimated to have been exposed to less than 300 mSv, was studied for health effects. In a sample of 100 workers, 26 fit the diagnostic criteria for CFS/ME, with the authors noting persistent fatigue, malaise, and immune dysfunction. This was initially suspected to be the result of neurological damage (vegetative-vascular dystonia) linked to low-dose radiation exposure [37], which would otherwise be considered a subclinical effect of exposure in this context due to its presentation. Another study by the same group sought to identify biological markers associated with radiation exposure in Chernobyl liquidators [38]. In this later study, the authors found several markers associated with functional neurological dysfunction, which was proposed to be a cause of the symptoms. It was also observed that the prevalence of CFS/ME in Chernobyl liquidators decreased significantly after 10 years. Together, these reports described that the extent of neurodegeneration associated with participating in the liquidation was correlated with estimated dose as well as time spent in high radiation areas, indicating a direct connection between CFS/ME symptoms and the extent of radiation exposure.
It should be noted that other survivors of radiation accidents, Atomic and Gulf War veterans, and radiotherapy patients have experienced similar symptoms following ionizing radiation exposure [13,34,39,40,41,42,43,44,45,46]. A clear dose-dependence was not always observed in the literature—consistent with low dose radiation effects such as radiation-induced bystander effects—but suggestions that ionizing radiation exposure may be linked to fatigue have been historically dismissed as unfounded. This may be due, in part, to the lack of clear dose-dependence, the greater prevalence of deterministic effects with higher doses, and assumptions of radiophobia in those reporting symptoms [26,47,48,49,50]. There are other characteristics of non-targeted effects that make them a candidate mechanism for explaining CFS/ME in some cases [35]. Non-targeted effects are most prevalent following exposure to lower doses of ionizing radiation (below 0.5 Gy) and they saturate at higher doses [51,52,53,54]. These effects are also known to persist over time and across generations due to their induction of genomic instability and additional signaling following receipt of a primary signal [55,56,57]. It is therefore proposed that non-targeted effects like radiation-induced bystander effects could promote the inflammatory responses [58,59,60] seen in CFS/ME patients and contribute in yet undiscovered ways to promoting fatigue and related symptoms. This could occur by modulation of oxidative metabolism for example, which has been observed in several studies of a subset of non-targeted effects known as radiation-induced bystander effects (RIBE) [61,62]. This is discussed in further detail later in this review.
Furthermore, we suspect that CFS/ME may be connected mechanistically to cancer and believe that fatigue as a common symptom may indicate this connection. To understand why this is a possibility, a review of the fundamentals of cancer biology is required. Here, we will review the distinctive features of cancer, what biological changes are associated with cancer, and how cancer is linked to multiple stressors and ionizing radiation exposure. A pictorial summary of our hypotheses is presented in Figure 1.
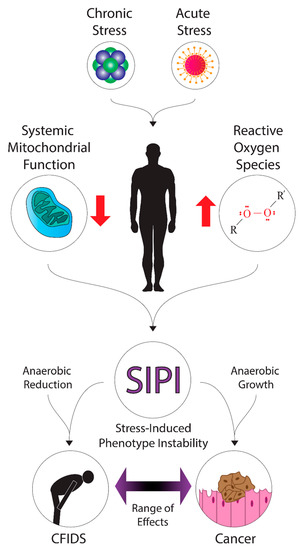
Figure 1.
A diagram of the hypothetical mechanisms and effects of stress-induced phenotype instability in cancer and CFS/ME. Various forms of acute stress (such as radiation exposure or infection) or chronic stress may contribute to the systemic dysregulation of energy metabolism and upregulation of oxidative stress. These factors may promote stress-induced phenotype instability in patients, where down- or up-regulation of metabolic pathways could contribute to energy deficits or growth, leading to fatigue symptoms in cancer and CFIDS patients.
2. Proposed Link between CFS/ME and Cancer
We suspect that CFS/ME and cancer are linked. Some authors have proposed a link between CFS/ME and cancer, although this link has yet to be conclusively demonstrated. One study noted that fatigue symptoms in CFS/ME patients and those suffering from cancer-related fatigue (CRF) show some similarities [63].
Most cancers arise spontaneously and may be associated with genetic predisposition and environmental factors [64,65,66]. Environmental causes include viral and bacterial infection [67,68,69,70,71,72], smoking [73,74], diet, and lifestyle choices such as sedentation [75,76]. Certain syndromes are known to predispose individuals to specific types of cancer, and these are typically inherited. Comparatively, a few authors proposed that CFS/ME may have a basis, or is at least “buffered”, by genetics.
Tumors modulate their metabolism to allow for further growth and invasion of surrounding tissues. It is now known that cancer cells perform fermentation after glycolysis over oxidative phosphorylation because transformed cells have different nutritional demands than normal cells. Tumor cells shunt pyruvate to a fermentative pathway because this is conducive to the accumulation of biomass required for growth and division, even though catabolism and extensive oxidation to carbon dioxide produces more energy in the form of ATP [77,78,79]. The metabolites generated in glycolysis can be utilized to synthesize a diverse number of biomolecules and can be used in the pentose phosphate pathway (glucose-6-phosphate) [80,81]. Glycogen synthesis also starts with metabolites from glycolysis (glucose-6-phosphate) [82,83], as does the synthesis of glycerol (glyceraldehyde-3-phosphate), fatty acid synthesis (pyruvate) [84,85], and cholesterol synthesis (pyruvate). Further, the metabolites of the citric acid cycle—which is supplied by the products of glycolysis and the dehydrogenation of pyruvate—can be used for nucleic and amino acid synthesis [86]. It is suspected that the modulation of cellular metabolism is also at play in CFS/ME; this is further described in Section 3.5.
Connections to Multiple Stressors
As previously discussed, carcinogenesis can usually be linked to some combination of genetic or environmental factors. This may also be the case for CFS/ME, and this is discussed in further detail in later sections. Certain inherited conditions can predispose individuals to cancer formation [87,88]. Specific mutations in certain genes can greatly predispose an individual to oncogenesis, like mutations in the tumor suppressors BRCA1 and BRCA2, which greatly increase the risk of developing breast and ovarian cancers [66,89,90].
Furthermore, a variety of environmental factors are important in the induction of tumorigenesis. It is now widely recognized that smoking significantly increases one’s risk of developing many different types of cancer, from cancers of the upper and lower respiratory tract to cancers of the bladder, kidney, pancreas, and stomach [91,92,93]. It is known that chemical carcinogens present in cigarette smoke promote DNA damage and allow the accumulation of mutations in tumor suppressors and oncogenes. Moreover, radioactive carcinogens are also present in cigarette smoke that contribute to oncogenesis. Finally, the liberation of free radicals and pro-oxidant chemicals in cigarette smoke damages blood vessels and promotes oncogenesis through changes in the tissue microenvironment, causing inflammation and damage to structures like bronchi and alveoli [91,92,93]. Other environmental causes are known to promote tumorigenesis. For example, microparticle and nanoparticle exposure is an occupational hazard for some, including those in the mining industry. Furthermore, naturally-occurring radon is another hazard that is prevalent in many parts of the world [94]. Viral and bacterial infection is another cause of some types of cancer. For example, Helicobacter pylori can cause gastric cancers via chronic inflammation of stomach mucosa and the secretion of carcinogenic virulence factors [68,69]. Chronic inflammation can lead to DNA damage over time, which can cause random alterations to DNA if not repaired. The same link to environmental toxins and stressors has not been conclusively demonstrated in CFS/ME yet, although some evidence is present in the literature [95,96].
Cancer and Radiation Damage
Radiation exposure has been widely observed as a risk factor for oncogenesis for many years. Ionizing radiation damages DNA and produces double-strand breaks; these breaks, if not repaired, lead to mutations in the cell and its progeny [97]. If the error is in an essential gene, or if there is an accumulation of many mutations, death of the cell may occur. This could also damage genetic information that, in turn, leads to the manifestation of CFS/ME through several mechanisms, which are described in further detail in Section 3. Alternatively, a cell may become transformed if the mutations occur in tumor suppressors or proto-oncogenes [98,99]. Although this direct damage is believed to be the primary cause of mutations resulting in oncogenesis, other radiation interactions promote DNA damage or a microenvironment conducive to transformation. These and other forms of indirect damage are collectively referred to as the non-targeted effects of ionizing radiation (non-targeted effects) [100,101], which were discussed at some length earlier in this review. These are known as “non-targeted” because the damage occurs in cells that have not received a direct deposition of energy and are therefore outside of the field of radiation classically associated with damage to biomolecules [97]. It is also possible that non-targeted effects could account for the etiology of CFS/ME in some cases, and could also be the reason why a dose-dependent relationship between radiation exposure and CFS/ME has not yet been identified [34].
The term non-targeted effects encompasses a broad range of effects in cells and tissues in vitro and in vivo. RIBE were demonstrated in vitro and named as such by a few groups in the early and late nineties; however, the evidence for radiation-induced bystander effects appears to be almost as old as the discovery of radioactivity [102,103,104]. Next, a demonstration of bystander signals as soluble factors secreted by exposed cells into a culture medium was performed by Mothersill and Seymour [53]. Shortly thereafter, a great number of reports were published indicating roles for mitochondria, reactive oxygen species, and metabolism in the manifestation of bystander effects in recent decades [61,105,106,107].
As previously discussed, tumor cells typically alter cellular metabolism such that metabolites are directed to anabolic pathways, while catabolic pathways, such as the energy-generating reactions in the citric acid cycle and mitochondrial oxidative phosphorylation, are down-regulated [108,109,110]. RIBE are known to modulate aerobic metabolism in vitro, specifically acting on mitochondria and repressing mitochondrial respiratory chain activity [61,62]. Additionally, a number of mitochondrial changes are implicated in bystander signaling, including changes in morphology, mutations in mitochondrial DNA, and loss of the inner mitochondrial membrane potential required to drive ATP synthesis [61,107,111,112,113]. The result of this signaling is the loss of ATP production [77], generation of oxidative stress [114], and ultimately apoptosis [107,115]. Apoptosis can be initiated through a signaling pathway specific to mitochondria; proapoptotic members of the BCL-2 family facilitate the permeabilization of the mitochondrial membrane [115,116] which is usually coincident with the leakage of electrons from enzymes in the electron transport chain [117]. Cytochrome c, the terminal electron carrier in the transport chain, is liberated from the mitochondria and activates apoptotic enzymes [118]. As discussed below, all of these changes to mitochondrial and aerobic metabolism could potentially be implicated in CFS/ME as well, although further research is still required to conclusively demonstrate mitochondrial dysfunction or biomarkers.
4. Possible Implications to Clinical Practice in the Management of Fatigue
If a connection between CRF and CFS/ME is established, the context under which the fatigue symptoms emerge is important for diagnosis and determining treatment. For example, in the case of a cancer patient reporting fatigue symptoms, it could be initially assumed that cancer itself is the primary cause, however, this is not necessarily the case —as described previously—if other possible causes are present. If the fatigue is debilitating and interferes with daily activities, then counseling and basic education are usually prescribed as a first step [217]. Following this, the fatigue can be assessed in depth by an examination of associated systems, and then several treatable causes must be discounted; these include pain related to cancer, emotional distress possibly related to cancer, anemia, cancer-related sleep disturbances, nutritional deficiencies, comorbidities, and a possible link to a treatment regime. Assuming none of these causes can be identified, then the best course of action may be symptom management.
It is proposed that, whether or not CRF and CFS/ME have an underlying cause or set of causes, that CFS/ME management should be approached in a similar fashion in clinical practice. First, a diagnosis of CFS/ME should be made based on excluding other potential criteria that may cause fatigue [218]. It is therefore important to have a complete medical history of the patient and perform a complete physical examination. As previously described, fatigue may be caused by another medical condition, emotional distress, sleep disturbances, and nutritional deficiencies; therefore, the exclusion of these factors is important in determining an appropriate approach. To further complicate matters, the CFS/ME condition is oftentimes associated with sleep disturbances [170], so teasing out the likely cause of these disturbances would be very useful for the management of the symptoms. In adolescents, cognitive behavioral therapy appears to be helpful in a majority of cases, so this could be indicated depending on the underlying cause [218]. In cases where this approach is not helpful, then the possibility of a metabolic or other physiological cause may be evidenced. Because the research on possible biomedical markers is still highly controversial in the literature, it is difficult to recommend specific assays to be used in diagnosis, as more research and evidence are required. Future research efforts should focus on standardizing methods for metabolomic screening of CFS/ME such that findings can be better compared between studies in the future [219].
5. Conclusions
In CFS/ME and CRF, there is significant overlap in terms of symptoms and presentation. Both syndromes could conceivably result from exposure to some stressor, such as ionizing radiation, and there appears to be evidence in the literature that not only supports this but also the notion that CFS/ME and CRF may be connected etiologically. Neurological dysfunction, changes in serotonin, the circadian clock, elevated inflammation, metabolic deficiencies, and genetic changes are candidates for mechanisms in both diseases, although further research is still needed. At present, the effect of radiation dose on the induction of fatigue and these conditions is still unclear, and further research is needed to link radiation to the two diseases—particularly CFS. Considering that both CFS/ME and cancer are very heterogenous and diverse conditions, we suspect that a combination of these factors may be present in CFS/ME patients and those suffering from CRF. Identification of common biomarkers in patients suffering from one disease or another is required, as this may determine where effective therapy can be applied.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/ijms23020691/s1.
Author Contributions
A.R.: Conception and design of the manuscript. Investigation and acquisition of manuscripts and literature review. Interpretation of key findings and summary. Verification of report accuracy. Writing—original draft preparation. Writing—review and editing. Visualization of concepts and graphic design. A.C.: Conception and design of the manuscript. Investigation and acquisition of manuscripts and literature review. Interpretation of key findings and summary. Verification of report accuracy. Writing—review and editing. C.S.: Conception and design of the manuscript. Interpretation of key findings and summary. Supervision of project. Project administration. C.M.: Conception and design of the manuscript. Investigation and acquisition of manuscripts and literature review. Interpretation of key findings and summary. Verification of report accuracy. Writing—review and editing. Supervision of project. Project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mandarano, A.H.; Maya, J.; Giloteaux, L.; Peterson, D.L.; Maynard, M.; Gottschalk, C.G.; Hanson, M.R. Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations. J. Clin. Investig. 2020, 130, 1491–1505. [Google Scholar] [CrossRef]
- Lorusso, L.; Mikhaylova, S.V.; Capelli, E.; Ferrari, D.; Ngonga, G.K.; Ricevuti, G. Immunological aspects of chronic fatigue syndrome. Autoimmun. Rev. 2009, 8, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.; Kaplan, J.; Gantz, N.; Komaroff, A.; Schonberger, B. Chronic fatigue syndrome: A working case definition. Annu. Intern. Med. 1988, 108, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Afari, N.; Buchwald, D. Chronic fatigue syndrome: A review. Am. J. Psychiatry 2003, 160, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-J.; Ahn, Y.-C.; Jang, E.-S.; Lee, S.-W.; Lee, S.-H.; Son, C.-G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 100. [Google Scholar] [CrossRef]
- Wood, E.; Hall, K.H.; Tate, W. Role of mitochondria, oxidative stress and the response to antioxidants in myalgic encephalomyelitis/chronic fatigue syndrome: A possible approach to SARS-CoV-2 ‘long-haulers’? Chronic Dis. Transl. Med. 2020, 7, 14–26. [Google Scholar] [CrossRef]
- Lim, E.-J.; Son, C.-G. Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2020, 18, 289. [Google Scholar] [CrossRef]
- Słomko, J.; Newton, J.L.; Kujawski, S.; Tafil-Klawe, M.; Klawe, J.; Staines, D.; Marshall-Gradisnik, S.; Zalewski, P. Prevalence and characteristics of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) in Poland: A cross-sectional study. BMJ Open 2019, 9, e023955. [Google Scholar] [CrossRef]
- Simani, L.; Ramezani, M.; Darazam, I.A.; Sagharichi, M.; Aalipour, M.A.; Ghorbani, F.; Pakdaman, H. Prevalence and correlates of chronic fatigue syndrome and post-traumatic stress disorder after the outbreak of the COVID-19. J. Neurovirol. 2021, 27, 154–159. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Pen, J.J.; Chirumbolo, S.; Aaseth, J. Chronic fatigue syndrome (CFS): Suggestions for a nutritional treatment in the therapeutic approach. Biomed. Pharmacother. 2019, 109, 1000–1007. [Google Scholar] [CrossRef]
- Krupp, L.B.; Sliwinski, M.; Masur, D.M.; Friedberg, F.; Coyle, P.K. Cognitive functioning and depression in patients with chronic fatigue syndrome and multiple sclerosis. Arch. Neurol. 1994, 51, 705–710. [Google Scholar] [CrossRef]
- Whelton, C.L.; Salit, I.; Moldofsky, H. Sleep, Epstein-Barr virus infection, musculoskeletal pain, and depressive symptoms in chronic fatigue syndrome. J. Rheumatol. 1992, 19, 939–943. [Google Scholar] [PubMed]
- Kang, H.K.; Natelson, B.H.; Mahan, C.M.; Lee, K.Y.; Murphy, F.M. Post-traumatic stress disorder and chronic fatigue syndrome-like illness among Gulf War veterans: A population-based survey of 30,000 veterans. Am. J. Epidemiol. 2003, 157, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.S.; Bradley, A.S.; Bishop, K.N.; Kiani-Alikhan, S.; Ford, B. Chronic fatigue syndrome, the immune system and viral infection. Brain Behav. Immun. 2012, 26, 24–31. [Google Scholar] [CrossRef]
- McGregor, N.R.; Armstrong, C.W.; Lewis, D.P.; Gooley, P.R. Post-exertional malaise is associated with hypermetabolism, hypoacetylation and purine metabolism deregulation in ME/CFS cases. Diagnostics 2019, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Mothersill, C.; Seymour, C. Uncomfortable issues in radiation protection posed by low-dose radiobiology. Radiat. Environ. Biophys. 2013, 52, 293–298. [Google Scholar] [CrossRef]
- Bhui, K.S.; Dinos, S.; Ashby, D.; Nazroo, J.; Wessely, S.; White, P.D. Chronic fatigue syndrome in an ethnically diverse population: The influence of psychosocial adversity and physical inactivity. BMC Med. 2011, 9, 26. [Google Scholar] [CrossRef]
- Buchwald, D.; Manson, S.M.; Pearlman, T.; Umali, J.; Kith, P. Race and ethnicity in patients with chronic fatigue. J. Chronic Fatigue Syndr. 1996, 2, 53–66. [Google Scholar] [CrossRef]
- Dinos, S.; Khoshaba, B.; Ashby, D.; White, P.D.; Nazroo, J.; Wessely, S.; Bhui, K.S. A systematic review of chronic fatigue, its syndromes and ethnicity: Prevalence, severity, co-morbidity and coping. Int. J. Epidemiol. 2009, 38, 1554–1570. [Google Scholar] [CrossRef]
- Daniel, S.; Nylander, V.; Ingerslev, L.R.; Zhong, L.; Fabre, O.; Clifford, B.; Johnston, K.; Cohn, R.J.; Barres, R.; Simar, D. T cell epigenetic remodeling and accelerated epigenetic aging are linked to long-term immune alterations in childhood cancer survivors. Clin. Epigenetics 2018, 10, 138. [Google Scholar] [CrossRef]
- Itoh, Y.; Fukunaga, Y.; Igarashi, T.; Imai, T.; Yoshida, J.; Tsuchiya, M.; Fujino, O.; Murakami, M.; Yamamoto, M. Autoimmunity in chronic fatigue syndrome in children. Jpn. J. Rheumatol. 1998, 8, 429–437. [Google Scholar]
- Naviaux, R.K.; Naviaux, J.C.; Li, K.; Bright, A.T.; Alaynick, W.A.; Wang, L.; Baxter, A.; Nathan, N.; Anderson, W.; Gordon, E. Metabolic features of chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, 1607571113. [Google Scholar] [CrossRef]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Griffith, J.P.; Zarrouf, F.A. A systematic review of chronic fatigue syndrome: Don’t assume it’s depression. Prim. Care Companion J. Clin. Psychiatry 2008, 10, 120–128. [Google Scholar] [CrossRef]
- Chew-Graham, C.; Dowrick, C.; Wearden, A.; Richardson, V.; Peters, S. Making the diagnosis of Chronic Fatigue Syndrome/Myalgic Encephalitis in primary care: A qualitative study. BMC Fam. Pract. 2010, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Terman, J.; Cotler, J.; Jason, L.A. How psychiatric referrals influence stigmatization in patients with myalgic encephalomyelitis and chronic fatigue syndrome: An examination of American and British models. Community Psychol. Glob. Perspect. 2019, 5, 19–29. [Google Scholar]
- Green, J.; Romei, J.; Natelson, B.H. Stigma and chronic fatigue syndrome. J. Chronic Fatigue Syndr. 1999, 5, 63–75. [Google Scholar] [CrossRef]
- Bowen, J.; Pheby, D.; Charlett, A.; McNulty, C. Chronic Fatigue Syndrome: A survey of GPs’ attitudes and knowledge. Fam. Pract. 2005, 22, 389–393. [Google Scholar] [CrossRef]
- Brimmer, D.J.; Fridinger, F.; Lin, J.-M.S.; Reeves, W.C. US healthcare providers’ knowledge, attitudes, beliefs, and perceptions concerning Chronic Fatigue Syndrome. BMC Fam. Pract. 2010, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Sunnquist, M.; Nicholson, L.; Jason, L.A.; Friedman, K.J. Access to medical care for individuals with myalgic encephalomyelitis and chronic fatigue syndrome: A call for centers of excellence. Mod. Clin. Med. Res. 2017, 1, 28. [Google Scholar]
- Holden, S.; Maksoud, R.; Eaton-Fitch, N.; Cabanas, H.; Staines, D.; Marshall-Gradisnik, S. A systematic review of mitochondrial abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome/systemic exertion intolerance disease. J. Transl. Med. 2020, 18, 290. [Google Scholar] [CrossRef]
- Smith, A.K.; White, P.D.; Aslakson, E.; Vollmer-Conna, U.; Rajeevan, M.S. Polymorphisms in genes regulating the HPA axis associated with empirically delineated classes of unexplained chronic fatigue. Pharmacogenomics 2006, 7, 110647. [Google Scholar] [CrossRef] [PubMed]
- Loganovsky, K.N. Chronic fatigue syndrome in the Chernobyl accident consequences liquidators. Int. J. Radiat. Med. 2001, 3, 76. [Google Scholar]
- Loganovsky, K.N. Vegetative-vascular dystonia and osteoalgetic syndrome or Chronic Fatigue Syndrome as a characteristic after-effect of radioecological disaster: The Chernobyl accident experience. J. Chronic Fatigue Syndr. 2000, 7, 3–16. [Google Scholar] [CrossRef]
- Rusin, A.; Seymour, C.; Mothersill, C. Chronic fatigue and immune deficiency syndrome (CFIDS), cellular metabolism, and ionizing radiation: A review of contemporary scientific literature and suggested directions for future research. Int. J. Radiat. Biol. 2018, 94, 212–228. [Google Scholar] [CrossRef] [PubMed]
- Rusin, A.; Li, M.; Cocchetto, A.; Seymour, C.; Mothersill, C. Radiation exposure and mitochondrial insufficiency in Chronic Fatigue and Immune Dysfunction Syndrome. Med. Hypotheses 2021, 154, 110647. [Google Scholar] [CrossRef] [PubMed]
- Loganovsky, K. Do low doses of ionizing radiation affect the human brain? Data Sci. J. 2009, 8, BR13–BR35. [Google Scholar] [CrossRef]
- Bazyka, D.; Loganovsky, K.; Ilyenko, I.; Volovyk, S.; Perchuk, I.; Pleskach, O.; Nechayev, S. Psychophysiological, neuroimmune and gene expression changes in chronic fatigue syndrome after low-dose radiation exposure. Int. J. Psychophysiol. 2010, 77, 340. [Google Scholar] [CrossRef]
- McCurry, J. Hiroshima survivors remember. Lancet 2015, 386, 417–418. [Google Scholar] [CrossRef][Green Version]
- Yamada, M.; Izumi, S. Psychiatric sequelae in atomic bomb survivors in Hiroshima and Nagasaki two decades after the explosions. Soc. Psychiatry Psychiatr. Epidemiol. 2002, 37, 409–415. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, X.-D.; Denny, T.; Ottenweller, J.E.; Lange, G.; LaManca, J.J.; Lavietes, M.H.; Pollet, C.; Gause, W.C.; Natelson, B.H. Changes in immune parameters seen in Gulf War veterans but not in civilians with chronic fatigue syndrome. Clin. Diagn. Lab. Immunol. 1999, 6, 6–13. [Google Scholar] [CrossRef]
- Irvine, D.; Vincent, L.; Graydon, J.E.; Bubela, N.; Thompson, L. The prevalence and correlates of fatigue in patients receiving treatment with chemotherapy and radiotherapy. A comparison with the fatigue experienced by healthy individuals. Cancer Nurs. 1994, 17, 367–378. [Google Scholar] [CrossRef]
- Broeckel, J.A.; Jacobsen, P.B.; Horton, J.; Balducci, L.; Lyman, G.H. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J. Clin. Oncol. 1998, 16, 1689–1696. [Google Scholar] [CrossRef]
- Irvine, D.M.; Vincent, L.; Graydon, J.E.; Bubela, N. Fatigue in women with breast cancer receiving radiation therapy. Cancer Nurs. 1998, 21, 127–135. [Google Scholar] [CrossRef]
- McCauley, L.A.; Joos, S.K.; Barkhuizen, A.; Shuell, T.; Tyree, W.A.; Bourdette, D.N. Chronic fatigue in a population-based study of Gulf War veterans. Arch. Environ. Health 2002, 57, 340–348. [Google Scholar] [CrossRef]
- Jereczek-Fossa, B.A.; Marsiglia, H.R.; Orecchia, R. Radiotherapy-related fatigue. Crit. Rev. Oncol. Hematol. 2002, 41, 317–325. [Google Scholar] [CrossRef]
- Dickson, A.; Knussen, C.; Flowers, P. Stigma and the delegitimation experience: An interpretative phenomenological analysis of people living with chronic fatigue syndrome. Psychol. Health 2007, 22, 851–867. [Google Scholar] [CrossRef]
- Åsbring, P.; Närvänen, A.-L. Women’s experiences of stigma in relation to chronic fatigue syndrome and fibromyalgia. Qual. Health Res. 2002, 12, 148–160. [Google Scholar]
- Pastel, R.H. Radiophobia: Long-term psychological consequences of Chernobyl. Mil. Med. 2002, 167, 134–136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jaworowski, Z. Observations on Chernobyl after 25 Years of Radiophobia. 21st Century Sci. Technol. 2010, 2010, 30–46. [Google Scholar]
- Seymour, C.B.; Mothersill, C. Relative contribution of bystander and targeted cell killing to the low-dose region of the radiation dose-response curve. Radiat. Res. 2000, 153, 508–511. [Google Scholar] [CrossRef]
- Liu, Z.F.; Mothersill, C.E.; McNeill, F.E.; Lyng, F.M.; Byun, S.H.; Seymour, C.B.; Prestwich, W. V A dose threshold for a medium transfer bystander effect for a human skin cell line. Radiat. Res. 2006, 166, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Mothersill, C.; Seymour, C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int. J. Radiat. Biol. 1997, 71, 421–427. [Google Scholar]
- Prise, K.M.; Belyakov, O.V.; Newman, H.C.; Patel, S.; Schettino, G.; Folkard, M.; Michael, B.D. Non-targeted effects of radiation: Bystander responses in cell and tissue models. Radiat. Prot. Dosim. 2002, 99, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Mothersill, C.; Seymour, C. Lethal mutations and genomic instability. Int. J. Radiat. Biol. 1997, 71, 751–758. [Google Scholar] [PubMed]
- Seymour, C.B.; Mothersill, C. Delayed expression of lethal mutations and genomic instability in the progeny of human epithelial cells that survived in a bystander-killing environment. Radiat. Oncol. Investig. 1997, 5, 106–110. [Google Scholar] [CrossRef]
- Seymour, C.B.; Mothersill, C.E.; Alper, T. High Yields of Lethal Mutations in Somatic Mammalian-Cells that Survive Ionizing-Radiation. Int. J. Radiat. Biol. 1986, 50, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Lorimore, S.A.; Coates, P.J.; Scobie, G.E.; Milne, G.; Wright, E.G. Inflammatory-type responses after exposure to ionizing radiation in vivo: A mechanism for radiation-induced bystander effects? Oncogene 2001, 20, 7085. [Google Scholar] [CrossRef]
- Lorimore, S.A.; Coates, P.J.; Wright, E.G. Radiation-induced genomic instability and bystander effects: Inter-related nontargeted effects of exposure to ionizing radiation. Oncogene 2003, 22, 7058–7069. [Google Scholar] [CrossRef]
- Rodel, F.; Frey, B.; Multhoff, G.; Gaipl, U. Contribution of the immune system to bystander and non-targeted effects of ionizing radiation. Cancer Lett. 2015, 356, 105–113. [Google Scholar] [CrossRef]
- Nugent, S.; Mothersill, C.E.; Seymour, C.; McClean, B.; Lyng, F.M.; Murphy, J.E.J. Altered mitochondrial function and genome frequency post exposure to gamma-radiation and bystander factors. Int. J. Radiat. Biol. 2010, 86, 829–841. [Google Scholar] [CrossRef]
- Le, M.; McNeill, F.E.; Seymour, C.B.; Rusin, A.; Diamond, K.; Rainbow, A.J.; Murphy, J.; Mothersill, C.E. Modulation of oxidative phosphorylation (OXPHOS) by radiation-induced biophotons. Environ. Res. 2018, 163, 80–87. [Google Scholar] [CrossRef]
- Servaes, P.; van der Werf, S.; Prins, J.; Verhagen, S.; Bleijenberg, G. Fatigue in disease-free cancer patients compared with fatigue in patients with chronic fatigue syndrome. Support. Care Cancer 2001, 9, 11–17. [Google Scholar] [CrossRef]
- Preston-Martin, S.; Pike, M.C.; Ross, R.K.; Jones, P.A.; Henderson, B.E. Increased cell division as a cause of human cancer. Cancer Res. 1990, 50, 7415–7421. [Google Scholar]
- Kaufmann, S.H.; Gores, G.J. Apoptosis in cancer: Cause and cure. Bioessays 2000, 22, 1007–1017. [Google Scholar] [CrossRef]
- Hulka, B.S.; Stark, A.T. Breast cancer: Cause and prevention. Lancet 1995, 346, 883–887. [Google Scholar] [CrossRef]
- Parsonnet, J. Helicobacter pylori and gastric cancer. Gastroenterol. Clin. N. Am. 1993, 22, 89–104. [Google Scholar] [CrossRef]
- Polk, D.B.; Peek, R.M. Helicobacter pylori: Gastric cancer and beyond. Nat. Rev. Cancer 2010, 10, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human papillomavirus and cervical cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef]
- Manns, A.; Hisada, M.; La Grenade, L. Human T-lymphotropic virus type I infection. Lancet 1999, 353, 1951–1958. [Google Scholar] [CrossRef]
- Verdonck, K.; González, E.; Van Dooren, S.; Vandamme, A.-M.; Vanham, G.; Gotuzzo, E. Human T-lymphotropic virus 1: Recent knowledge about an ancient infection. Lancet Infect. Dis. 2007, 7, 266–281. [Google Scholar] [CrossRef]
- Hanu, C.; Timotin, E.; Wong, R.; Sur, R.K.; Hayward, J.E.; Seymour, C.B.; Mothersill, C.E. The influence of smoking on radiation-induced bystander signal production in esophageal cancer patients. Environ. Res. 2016, 147, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Lyng, F.M.; de Feijter-Rupp, H.L.; Hayashi, T.; OMalley, K.; Murphy, D.M.; Cottell, D.C.; Trosko, J.E.; Seymour, C.B.; Mothersill, C. Effect of a tobacco-related nitrosamine on intercellular communication in human urothelial cells: A possible factor in smoking-related bladder carcinogenesis. Oncol. Res. 1996, 8, 371–378. [Google Scholar] [PubMed]
- Calle, E.E.; Thun, M.J. Obesity and cancer. Oncogene 2004, 23, 6365–6378. [Google Scholar] [CrossRef] [PubMed]
- Demeyer, D.; Mertens, B.; De Smet, S.; Ulens, M. Mechanisms linking colorectal cancer to the consumption of (processed) red meat: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2747–2766. [Google Scholar] [CrossRef]
- Vander Heiden, M.; Cantley, L.; Thompson, C. Understanding the Warburg effect: The metabolic Requiremetns of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.M.; Krüger, A.; Tauqeer Alam, M.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2015, 90, 927–963. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.M.R. Cancer metabolism: The Warburg effect today. Exp. Mol. Pathol. 2010, 89, 372–380. [Google Scholar] [CrossRef]
- Bolaños, J.P.; Delgado-Esteban, M.; Herrero-Mendez, A.; Fernandez-Fernandez, S.; Almeida, A. Regulation of glycolysis and pentose–phosphate pathway by nitric oxide: Impact on neuronal survival. Biochim. Biophys. Acta (BBA)-Bioenergetics 2008, 1777, 789–793. [Google Scholar] [CrossRef]
- Patra, K.C.; Hay, N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 2014, 39, 347–354. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Sattler, U.G.A.; Mueller-Klieser, W. Lactate: A metabolic key player in cancer. Cancer Res. 2011, 71, 6921–6925. [Google Scholar] [CrossRef] [PubMed]
- Birkeland, E.S.; Koch, L.M.; Dechant, R. Another consequence of the Warburg effect? Metabolic regulation of Na+/H+ exchangers may link aerobic glycolysis to cell growth. Front. Oncol. 2020, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2014, 4, 81. [Google Scholar] [CrossRef] [PubMed]
- Numa, S.; Bortz, W.M.; Lynen, F. Regulation of fatty acid synthesis at the acetyl-CoA carboxylation step. Adv. Enzym. Regul. 1965, 3, 407–423. [Google Scholar] [CrossRef]
- Vakifahmetoglu-Norberg, H.; Ouchida, A.T.; Norberg, E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017, 482, 426–431. [Google Scholar] [CrossRef]
- Powell, S.M.; Petersen, G.M.; Krush, A.J.; Booker, S.; Jen, J.; Giardiello, F.M.; Hamilton, S.R.; Vogelstein, B.; Kinzler, K.W. Molecular diagnosis of familial adenomatous polyposis. N. Engl. J. Med. 1993, 329, 1982–1987. [Google Scholar] [CrossRef]
- Knudson, A.G. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef]
- MacMahon, B.; Cole, P.; Brown, J. Etiology of human breast cancer: A review. J. Natl. Cancer Inst. 1973, 50, 21–42. [Google Scholar] [CrossRef]
- Eustermann, S.; Wu, W.F.; Langelier, M.F.; Yang, J.C.; Easton, L.E.; Riccio, A.A.; Pascal, J.M.; Neuhaus, D. Structural Basis of Detection and Signaling of DNA Single-Strand Breaks by Human PARP-1. Mol. Cell 2015, 60, 742–754. [Google Scholar] [CrossRef]
- Loeb, L.A.; Emster, V.L.; Warner, K.E.; Abbotts, J.; Laszlo, J. Smoking and lung cancer: An overview. Cancer Res. 1984, 44, 5940–5958. [Google Scholar] [PubMed]
- Newcomb, P.A.; Carbone, P.P. The health consequences of smoking: Cancer. Med. Clin. N. Am. 1992, 76, 305–331. [Google Scholar] [CrossRef]
- Fisher, R.A. Cancer and smoking. Nature 1958, 182, 596. [Google Scholar] [CrossRef]
- Huntington-Moskos, L.; Rayens, M.K.; Wiggins, A.; Hahn, E.J. Radon, Secondhand Smoke, and Children in the Home: Creating a Teachable Moment for Lung Cancer Prevention. Public Health Nurs. 2016, 33, 529–538. [Google Scholar] [CrossRef]
- Racciatti, D.; Vecchiet, J.; Ceccomancini, A.; Ricci, F.; Pizzigallo, E. Chronic fatigue syndrome following a toxic exposure. Sci. Total Environ. 2001, 270, 27–31. [Google Scholar] [CrossRef]
- Underhill, R.A. Myalgic encephalomyelitis, chronic fatigue syndrome: An infectious disease. Med. Hypotheses 2015, 85, 765–773. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; ISBN 0781741513. [Google Scholar]
- Preston, D.L.; Pierce, D.A.; Shimizu, Y.; Cullings, H.M.; Fujita, S.; Funamoto, S.; Kodama, K. Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates. Radiat. Res. 2004, 162, 377–389. [Google Scholar] [CrossRef]
- Clutton, S.M.; Townsend, K.M.S.; Walker, C.; Ansell, J.D.; Wright, E.G. Radiation-induced genomic instability and persisting oxidative stress in primary bone marrow cultures. Carcinogenesis 1996, 17, 1633–1639. [Google Scholar] [CrossRef]
- Mothersill, C.; Seymour, C. Implications for human and environmental health of low doses of ionising radiation. J. Environ. Radioact. 2014, 133, 5–9. [Google Scholar] [CrossRef]
- Mothersill, C.; Rusin, A.; Seymour, C. Relevance of Non-Targeted Effects for Radiotherapy and Diagnostic Radiology; A Historical and Conceptual Analysis of Key Players. Cancers 2019, 11, 1236. [Google Scholar] [CrossRef] [PubMed]
- Mothersill, C.E.; Rusin, A.; Fernandez-Palomo, C.; Seymour, C.B. History of bystander effects research 1905-present; what is in a name? Int. J. Radiat. Biol. 2018, 94, 696–707. [Google Scholar] [CrossRef]
- Nagasawa, H.; Little, J.B. Induction of sister chromatid exchanges by extremely low doses of α-particles. Cancer Res. 1992, 52, 6394–6396. [Google Scholar]
- Little, J.B.; Azzam, E.I.; de Toledo, S.M.; Nagasawa, H. Bystander effects: Intercellular transmission of radiation damage signals. Radiat. Prot. Dosim. 2002, 99, 159–162. [Google Scholar] [CrossRef]
- Lyng, F.M.; Semour, C.B.; Mothersill, C. Early events in the apoptotic cascade initiated in cells treated with medium from the progeny of irradiated cells. Radiat. Prot. Dosim. 2002, 99, 169–172. [Google Scholar] [CrossRef]
- Maguire, P.; Mothersill, C.; McClean, B.; Seymour, C.; Lyng, F.M. Modulation of radiation responses by pre-exposure to irradiated cell conditioned medium. Radiat. Res. 2007, 167, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Lyng, F.M.; Maguire, P.; Kilmurray, N.; Mothersill, C.; Shao, C.; Folkard, M.; Prise, K.M. Apoptosis is initiated in human keratinocytes exposed to signalling factors from microbeam irradiated cells. Int. J. Radiat. Biol. 2006, 82, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B. Tumor microenvironment. Medicina 2020, 56, 15. [Google Scholar] [CrossRef]
- Tatum, J.L.; Kelloff, G.J.; Gillies, R.J.; Arbeit, J.M.; Brown, J.M.; Chao, K.S.C.; Chapman, J.D.; Eckelman, W.C.; Fyles, A.W.; Giaccia, A.J.; et al. Hypoxia: Importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int. J. Radiat. Biol. 2006, 82, 699–757. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.A. Therapeutic targeting of the tumor microenvironment. Cancer Cell 2005, 7, 513–520. [Google Scholar] [CrossRef]
- Lyng, F.M.; Seymour, C.B.; Mothersill, C. Initiation of apoptosis in cells exposed to medium from the progeny of irradiated cells: A possible mechanism for bystander-induced genomic instability? Radiat. Res. 2002, 157, 365–370. [Google Scholar] [CrossRef]
- Lyng, F.M.; Seymour, C.B.; Mothersill, C. Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. Br. J. Cancer 2000, 83, 1223–1230. [Google Scholar] [CrossRef]
- Murphy, J.E.J.; Nugent, S.; Seymour, C.; Mothersill, C. Mitochondrial DNA point mutations and a novel deletion induced by direct low-LET radiation and by medium from irradiated cells. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 585, 127–136. [Google Scholar] [CrossRef]
- Shimura, T.; Kunugita, N. Mitochondrial reactive oxygen species-mediated genomic instability in low-dose irradiated human cells through nuclear retention of cyclin D1. Cell Cycle 2016, 15, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Furlong, H.; Mothersill, C.; Lyng, F.M.; Howe, O. Apoptosis is signalled early by low doses of ionising radiation in a radiation-induced bystander effect. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2013, 741–742, 35–43. [Google Scholar] [CrossRef]
- Vines, A.M.; Lyng, F.M.; McClean, B.; Seymour, C.; Mothersill, C.E. Bystander effect induced changes in apoptosis related proteins and terminal differentiation in invitro murine bladder cultures. Int. J. Radiat. Biol. 2009, 85, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ragheb, K.; Lawler, G.; Sturgis, J.; Rajwa, B.; Melendez, J.A.; Robinson, J.P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003, 278, 8516–8525. [Google Scholar] [CrossRef] [PubMed]
- Yang, J. Prevention of Apoptosis by Bcl-2: Release of Cytochrome c from Mitochondria Blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Maes, M. Increased nuclear factor-κB and loss of p53 are key mechanisms in Myalgic Encephalomyelitis/chronic fatigue syndrome (ME/CFS). Med. Hypotheses 2012, 79, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Jeon, H.J.; Bang, Y.R.; Yoon, I.-Y. Multidimensional Comparison of Cancer-Related Fatigue and Chronic Fatigue Syndrome: The Role of Psychophysiological Markers. Psychiatry Investig. 2019, 16, 71. [Google Scholar] [CrossRef]
- Franc, M.; Michalski, B.; Kuczerawy, I.; Szuta, J.; Skrzypulec-Plinta, V. Cancer related fatigue syndrome in neoplastic diseases. Prz. Menopauzalny Menopause Rev. 2014, 13, 352. [Google Scholar] [CrossRef]
- Ryan, J.L.; Carroll, J.K.; Ryan, E.P.; Mustian, K.M.; Fiscella, K.; Morrow, G.R. Mechanisms of cancer-related fatigue. Oncologist 2007, 12 (Suppl. 1), 22–34. [Google Scholar] [CrossRef] [PubMed]
- Saligan, L.N.; Olson, K.; Filler, K.; Larkin, D.; Cramp, F.; Sriram, Y.; Escalante, C.P.; Del Giglio, A.; Kober, K.M.; Kamath, J. The biology of cancer-related fatigue: A review of the literature. Support. Care Cancer 2015, 23, 2461–2478. [Google Scholar] [CrossRef]
- Berger, A.M. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncol. Nurs. Forum 1998, 25, 51–62. [Google Scholar] [PubMed]
- Kamal, M.; Rosenthal, D.I.; Batra, A.; Volpe, S.; Elgohari, B.; Goepfert, R.P.; Garden, A.S.; Phan, J.; Eraj, S.; Dursteler, A. Fatigue following radiation therapy in nasopharyngeal cancer survivors: A dosimetric analysis incorporating patient report and observer rating. Radiother. Oncol. 2019, 133, 35–42. [Google Scholar] [CrossRef]
- Marcucci, G.; Haferlach, T.; Döhner, H. Molecular genetics of adult acute myeloid leukemia: Prognostic and therapeutic implications. J. Clin. Oncol. 2011, 29, 475–486. [Google Scholar] [CrossRef] [PubMed]
- McManimen, S.L.; Devendorf, A.R.; Brown, A.A.; Moore, B.C.; Moore, J.H.; Jason, L.A. Mortality in patients with myalgic encephalomyelitis and chronic fatigue syndrome. Fatigue Biomed. Health Behav. 2016, 4, 195–207. [Google Scholar] [CrossRef]
- Levine, P.H.; Pilkington, D.; Strickland, P.; Peterson, D. Chronic fatigue syndrome and cancer. J. Chronic Fatigue Syndr. 2000, 7, 29–38. [Google Scholar] [CrossRef]
- Levine, P.H.; Atherton, M.; Fears, T.; Hoover, R. An approach to studies of cancer subsequent to clusters of chronic fatigue syndrome: Use of data from the Nevada State Cancer Registry. Clin. Infect. Dis. 1994, 18, S49–S53. [Google Scholar] [CrossRef] [PubMed]
- Servaes, P.; Prins, J.; Verhagen, S.; Bleijenberg, G. Fatigue after breast cancer and in chronic fatigue syndrome: Similarities and differences. J. Psychosom. Res. 2002, 52, 453–459. [Google Scholar] [CrossRef]
- Chang, C.M.; Warren, J.L.; Engels, E.A. Chronic fatigue syndrome and subsequent risk of cancer among elderly US adults. Cancer 2012, 118, 5929–5936. [Google Scholar] [CrossRef]
- Light, K.C.; Agarwal, N.; Iacob, E.; White, A.T.; Kinney, A.Y.; VanHaitsma, T.A.; Aizad, H.; Hughen, R.W.; Bateman, L.; Light, A.R. Differing leukocyte gene expression profiles associated with fatigue in patients with prostate cancer versus chronic fatigue syndrome. Psychoneuroendocrinology 2013, 38, 2983–2995. [Google Scholar] [CrossRef]
- Cleare, A.J. The HPA axis and the genesis of chronic fatigue syndrome. Trends Endocrinol. Metab. 2004, 15, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Holtorf, K. Diagnosis and treatment of hypothalamic-pituitary-adrenal (HPA) axis dysfunction in patients with chronic fatigue syndrome (CFS) and fibromyalgia (FM). J. Chronic Fatigue Syndr. 2007, 14, 59–88. [Google Scholar] [CrossRef]
- Papadopoulos, A.S.; Cleare, A.J. Hypothalamic–pituitary–adrenal axis dysfunction in chronic fatigue syndrome. Nat. Rev. Endocrinol. 2012, 8, 22–32. [Google Scholar] [CrossRef]
- Vangeel, E.; Van Den Eede, F.; Hompes, T.; Izzi, B.; Del Favero, J.; Moorkens, G.; Lambrechts, D.; Freson, K.; Claes, S. Chronic fatigue syndrome and DNA hypomethylation of the glucocorticoid receptor gene promoter 1F region: Associations with HPA axis hypofunction and childhood trauma. Psychosom. Med. 2015, 77, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Van Houdenhove, B.; Van Den Eede, F.; Luyten, P. Does hypothalamic–pituitary–adrenal axis hypofunction in chronic fatigue syndrome reflect a ‘crash’in the stress system? Med. Hypotheses 2009, 72, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Van Den Eede, F.; Moorkens, G. HPA-axis dysfunction in chronic fatigue syndrome: Clinical implications. Psychosomatics 2008, 49, 450. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Anderson, G.; Maes, M. Hypothalamic-pituitary-adrenal hypofunction in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS) as a consequence of activated immune-inflammatory and oxidative and nitrosative pathways. Mol. Neurobiol. 2017, 54, 6806–6819. [Google Scholar] [CrossRef]
- Andrykowski, M.A.; Schmidt, J.E.; Salsman, J.M.; Beacham, A.O.; Jacobsen, P.B. Use of a case definition approach to identify cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. J. Clin. Oncol. 2005, 23, 6613. [Google Scholar] [CrossRef] [PubMed]
- Ganz, P.A.; Bower, J.E. Cancer related fatigue: A focus on breast cancer and Hodgkin’s disease survivors. Acta Oncol. 2007, 46, 474–479. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Kupelnick, B.; Miller, K.; Devine, D.; Lau, J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J. Natl. Cancer Inst. Monogr. 2004, 2004, 40–50. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Desmond, K.A.; Rowland, J.H.; Meyerowitz, B.E.; Belin, T.R. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J. Clin. Oncol. 2000, 18, 743. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Cancer-related fatigue: Links with inflammation in cancer patients and survivors. Brain Behav. Immun. 2007, 21, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S. Pathophysiology of cancer-related fatigue. Clin. J. Oncol. Nurs. 2008, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.-B.; Morgan, C.J.; Llewelyn, M.B.; Albuquerque, S.R.J.; Farmer, A. Heterogeneity of serum tryptophan concentration and availability to the brain in patients with the chronic fatigue syndrome. J. Psychopharmacol. 2005, 19, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Morrow, G.R.; Hickok, J.T.; Roscoe, J.A.; Raubertas, R.F.; Andrews, P.L.R.; Flynn, P.J.; Hynes, H.E.; Banerjee, T.K.; Kirshner, J.J.; King, D.K. Differential effects of paroxetine on fatigue and depression: A randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J. Clin. Oncol. 2003, 21, 4635–4641. [Google Scholar] [CrossRef]
- Barsevick, A.; Frost, M.; Zwinderman, A.; Hall, P.; Halyard, M. I’m so tired: Biological and genetic mechanisms of cancer-related fatigue. Qual. Life Res. 2010, 19, 1419–1427. [Google Scholar] [CrossRef]
- Roscoe, J.A.; Morrow, G.R.; Hickok, J.T.; Mustian, K.M.; Griggs, J.J.; Matteson, S.E.; Bushunow, P.; Qazi, R.; Smith, B. Effect of paroxetine hydrochloride (Paxil®) on fatigue and depression in breast cancer patients receiving chemotherapy. Breast Cancer Res. Treat. 2005, 89, 243–249. [Google Scholar] [CrossRef]
- Kurz, K.; Fiegl, M.; Holzner, B.; Giesinger, J.; Pircher, M.; Weiss, G.; Denz, H.A.; Fuchs, D. Fatigue in patients with lung cancer is related with accelerated tryptophan breakdown. PLoS ONE 2012, 7, e36956. [Google Scholar] [CrossRef]
- O’Higgins, C.M.; Brady, B.; O’Connor, B.; Walsh, D.; Reilly, R.B. The pathophysiology of cancer-related fatigue: Current controversies. Support. Care Cancer 2018, 26, 3353–3364. [Google Scholar] [CrossRef]
- Dantzer, R.; Heijnen, C.J.; Kavelaars, A.; Laye, S.; Capuron, L. The neuroimmune basis of fatigue. Trends Neurosci. 2014, 37, 39–46. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Y.; Liu, T.; Li, R.; Xie, M. Serotonin regulation in a rat model of exercise-induced chronic fatigue. Neuroscience 2017, 349, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Pinho, C.; Wong, R.; Sur, R.K.; Hayward, J.E.; Farrell, T.J.; Seymour, C.; Mothersill, C. The involvement of serum serotonin levels producing radiation-induced bystander effects for an in vivo assay with fractionated high dose-rate (HDR) brachytherapy. Int. J. Radiat. Biol. 2012, 88, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Lyng, F.M.; Desplanques, M.; Jella, K.K.; Garcia, A.; McClean, B. The importance of serum serotonin levels in the measurement of radiation-induced bystander cell death in HaCaT cells. Int. J. Radiat. Biol. 2012, 88, 770–772. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mothersill, C.; Saroya, R.; Smith, R.W.; Singh, H.; Seymour, C.B. Serum serotonin levels determine the magnitude and type of bystander effects in medium transfer experiments. Radiat. Res. 2010, 174, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.J.; Seymour, C.B.; Mothersill, C.E. Cell Line-Specific Direct Irradiation and Bystander Responses are Influenced by Fetal Bovine Serum Serotonin Concentrations. Radiat. Res. 2018, 190, 262–270. [Google Scholar] [CrossRef]
- Kalanxhi, E.; Dahle, J. The role of serotonin and p53 status in the radiation-induced bystander effect. Int. J. Radiat. Biol. 2012, 88, 773–776. [Google Scholar] [CrossRef]
- Ciarleglio, C.M.; Resuehr, H.E.S.; McMahon, D.G. Interactions of the serotonin and circadian systems: Nature and nurture in rhythms and blues. Neuroscience 2011, 197, 8–16. [Google Scholar] [CrossRef]
- Lee, J.; Choo, H. Serotonin Receptors for Treatment of Insomnia. Chronobiol. Med. 2019, 1, 14–20. [Google Scholar] [CrossRef]
- Shan, Z.Y.; Barnden, L.R.; Kwiatek, R.A.; Bhuta, S.; Hermens, D.F.; Lagopoulos, J. Neuroimaging characteristics of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A systematic review. J. Transl. Med. 2020, 18, 335. [Google Scholar] [CrossRef]
- Tryon, W.W.; Jason, L.; Frankenberry, E.; Torres-Harding, S. Chronic fatigue syndrome impairs circadian rhythm of activity level. Physiol. Behav. 2004, 82, 849–853. [Google Scholar] [CrossRef]
- Focan, C.; Focan-Henrard, D.; Collette, J.; Mechkouri, M.; Levi, F.; Hrushesky, W.; Touitou, Y.; Franchimont, P. Cancer-associated alteration of circadian rhythms in carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP) in humans. Anticancer Res. 1986, 6, 1137–1144. [Google Scholar]
- Reinberg, A.; Halberg, F. Circadian chronopharmacology. Annu. Rev. Pharmacol. 1971, 11, 455–492. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.D.; Daehler, M.A.; Grutsch, J.F.; Quiton, J.; Lis, C.G.; Peterson, C.; Gupta, D.; Watson, K.; Layer, D.; Huff-Adams, S. Circadian function in patients with advanced non-small-cell lung cancer. Br. J. Cancer 2005, 93, 1202–1208. [Google Scholar] [CrossRef]
- Sephton, S.; Spiegel, D. Circadian disruption in cancer: A neuroendocrine-immune pathway from stress to disease? Brain Behav. Immun. 2003, 17, 321–328. [Google Scholar] [CrossRef]
- Mormont, M.C.; De Prins, J.; Levi, F. Study of circadian rhythms of activity by actometry: Preliminary results in 30 patients with metastatic colorectal cancer. Pathol. Biol. 1996, 44, 165–171. [Google Scholar] [PubMed]
- Singh, R.; Singh, R.K.; Mahdi, A.A.; Misra, S.; Rai, S.P.; Singh, D.; Cornélissen, G.; Halberg, F. Studies on circadian periodicity of urinary corticoids in carcinoma of the breast. In Vivo 1998, 12, 69–73. [Google Scholar]
- Bower, J.E.; Ganz, P.A.; Dickerson, S.S.; Petersen, L.; Aziz, N.; Fahey, J.L. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology 2005, 30, 92–100. [Google Scholar] [CrossRef]
- Evengård, B.; Schacterle, R.S.; Komaroff, A.L. Chronic fatigue syndrome: New insights and old ignorance. J. Intern. Med. 1999, 246, 455–469. [Google Scholar] [CrossRef]
- Roscoe, J.A.; Kaufman, M.E.; Matteson-Rusby, S.E.; Palesh, O.G.; Ryan, J.L.; Kohli, S.; Perlis, M.L.; Morrow, G.R. Cancer-related fatigue and sleep disorders. Oncologist 2007, 12, 35–42. [Google Scholar] [CrossRef]
- Mormont, M.; Hecquet, B.; Bogdan, A.; Benavides, M.; Touitou, Y.; Lévi, F. Non-invasive estimation of the circadian rhythm in serum cortisol in patients with ovarian or colorectal cancer. Int. J. Cancer 1998, 78, 421–424. [Google Scholar] [CrossRef]
- Petrovsky, N.; McNair, P.; Harrison, L.C. Diurnal rhythms of pro-inflammatory cytokines: Regulation by plasma cortisol and therapeutic implications. Cytokine 1998, 10, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Kronfol, Z.; Nair, M.; Zhang, Q.; Hill, E.E.; Brown, M.B. Circadian immune measures in healthy volunteers: Relationship to hypothalamic-pituitary-adrenal axis hormones and sympathetic neurotransmitters. Psychosom. Med. 1997, 59, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Sephton, S.E.; Sapolsky, R.M.; Kraemer, H.C.; Spiegel, D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J. Natl. Cancer Inst. 2000, 92, 994–1000. [Google Scholar] [CrossRef]
- Touitou, Y.; Bogdan, A.; Levi, F.; Benavides, M.; Auzeby, A. Disruption of the circadian patterns of serum cortisol in breast and ovarian cancer patients: Relationships with tumour marker antigens. Br. J. Cancer 1996, 74, 1248–1252. [Google Scholar] [CrossRef]
- DeFreitas, E.; Hilliard, B.; Cheney, P.R.; Bell, D.S.; Kiggundu, E.; Sankey, D.; Wroblewska, Z.; Palladino, M.; Woodward, J.P.; Koprowski, H. Retroviral sequences related to human T-lymphotropic virus type II in patients with chronic fatigue immune dysfunction syndrome. Proc. Natl. Acad. Sci. USA 1991, 88, 2922–2926. [Google Scholar] [CrossRef] [PubMed]
- Kannian, P.; Green, P.L. Human T lymphotropic virus type 1 (HTLV-1): Molecular biology and oncogenesis. Viruses 2010, 2, 2037–2077. [Google Scholar] [CrossRef]
- Roucoux, D.F.; Murphy, E.L. The epidemiology and disease outcomes of human T-lymphotropic virus type II. AIDS Rev. 2004, 6, 144–154. [Google Scholar]
- Beilke, M.A.; Theall, K.P.; Clayton, J.L.; Benjamin, S.M.; Winsor, E.L.; Kissinger, P.J. Clinical outcomes and disease progression among patients coinfected with HIV and human T lymphotropic virus types 1 and 2. Clin. Infect. Dis. 2004, 39, 256–263. [Google Scholar] [CrossRef]
- Rasa, S.; Nora-Krukle, Z.; Henning, N.; Eliassen, E.; Shikova, E.; Harrer, T.; Scheibenbogen, C.; Murovska, M.; Prusty, B.K. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2018, 16, 268. [Google Scholar] [CrossRef]
- Khan, A.S.; Heneine, W.M.; Chapman, L.E.; Gary, H.E.; Woods, T.C.; Folks, T.M.; Schonberger, L.B. Assessment of a retrovirus sequence and other possible risk factors for the chronic fatigue syndrome in adults. Ann. Intern. Med. 1993, 118, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Gow, J.W.; Simpson, K.; Schliephake, A.; Behan, W.M.; Morrison, L.J.; Cavanagh, H.; Rethwilm, A.; Behan, P.O. Search for retrovirus in the chronic fatigue syndrome. J. Clin. Pathol. 1992, 45, 1058–1061. [Google Scholar] [CrossRef]
- Meeus, M.; Mistiaen, W.; Lambrecht, L.; Nijs, J. Immunological similarities between cancer and chronic fatigue syndrome: The common link to fatigue? Anticancer Res. 2009, 29, 4717–4726. [Google Scholar] [PubMed]
- Noda, M.; Ifuku, M.; Hossain, M.; Katafuchi, T. Glial activation and expression of the serotonin transporter in chronic fatigue syndrome. Front. Psychiatry 2018, 9, 589. [Google Scholar] [CrossRef] [PubMed]
- Ojo-Amaize, E.A.; Conley, E.J.; Peter, J.B. Decreased natural killer cell activity is associated with severity of chronic fatigue immune dysfunction syndrome. Clin. Infect. Dis. 1994, 18, S157–S159. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.; Fuite, J.; Kreitz, A.; Vernon, S.D.; Klimas, N.; Fletcher, M.A. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav. Immun. 2010, 24, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.; Katz, B.Z.; Fernandes, H.; Fletcher, M.A.; Klimas, N.; Smith, F.A.; O’Gorman, M.R.G.; Vernon, S.D.; Taylor, R. Cytokine expression profiles of immune imbalance in post-mononucleosis chronic fatigue. J. Transl. Med. 2012, 10, 191. [Google Scholar] [CrossRef]
- Pusztai, L.; Mendoza, T.R.; Reuben, J.M.; Martinez, M.M.; Willey, J.S.; Lara, J.; Syed, A.; Fritsche, H.A.; Bruera, E.; Booser, D. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine 2004, 25, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-H.; Chiang, C.-S.; Campbell, I.L.; Sun, J.-R.; Withers, H.R.; McBride, W.H. Induction of acute phase gene expression by brain irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 619–626. [Google Scholar] [CrossRef]
- Hallahan, D.E.; Haimovitz-Friedman, A.; Kufe, D.W.; Fuks, Z.; Weichselbaum, R.R. The role of cytokines in radiation oncology. Important Adv. Oncol. 1993, 71–80. [Google Scholar]
- Greenberg, D.B.; Gray, J.L.; Mannix, C.M.; Eisenthal, S.; Carey, M. Treatment-related fatigue and serum interleukin-1 levels in patients during external beam irradiation for prostate cancer. J. Pain Symptom Manag. 1993, 8, 196–200. [Google Scholar] [CrossRef]
- Bianco, J.A.; Appelbaum, F.R.; Nemunaitis, J.; Almgren, J.; Andrews, F.; Kettner, P.; Shields, A.; Singer, J.W. Phase I-II trial of pentoxifylline for the prevention of transplant-related toxicities following bone marrow transplantation [published erratum appears in Blood 1992 Jun 15; 79 (12): 3397][see comments]. Blood 1991, 78, 1205–1211. [Google Scholar] [CrossRef]
- Benzing, T.; Brandes, R.; Sellin, L.; Schermer, B.; Lecker, S.; Walz, G.; Kim, E. Upregulation of RGS7 may contribute to tumor necrosis factor-induced changes in central nervous function. Nat. Med. 1999, 5, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Demetrashvili, M.; Capuron, L.; Miller, A.H. Neuropsychiatric adverse effects of interferon-α. CNS Drugs 2005, 19, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Kumar, A.; Laskar, S.; Pandey, B.N. Cytokine profile of conditioned medium from human tumor cell lines after acute and fractionated doses of gamma radiation and its effect on survival of bystander tumor cells. Cytokine 2013, 61, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Pasi, F.; Facoetti, A.; Nano, R. IL-8 and IL-6 bystander signalling in human glioblastoma cells exposed to gamma radiation. Anticancer Res. 2010, 30, 2769–2772. [Google Scholar] [PubMed]
- Facoetti, A.; Ballarini, F.; Cherubini, R.; Gerardi, S.; Nano, R.; Ottolenghi, A.; Prise, K.M.; Trott, K.R.; Zilio, C. Gamma ray-induced bystander effect in tumour glioblastoma cells: A specific study on cell survival, cytokine release and cytokine receptors. Radiat. Prot. Dosim. 2006, 122, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, L.G.; Bertolotti, A.; Ranza, E.; Babini, G.; Ottolenghi, A. Investigation of the mechanisms underpinning IL-6 cytokine release in bystander responses: The roles of radiation dose, radiation quality and specific ROS/RNS scavengers. Int. J. Radiat. Biol. 2012, 88, 751–762. [Google Scholar] [CrossRef]
- Armstrong, C.W.; McGregor, N.R.; Lewis, D.P.; Butt, H.L.; Gooley, P.R. Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients. Metabolomics 2015, 11, 1626–1639. [Google Scholar] [CrossRef]
- Sargent, C.; Scroop, G.C.; Nemeth, P.M.; Burnet, R.B.; Buckley, J.D. Maximal oxygen uptake and lactate metabolism are normal in chronic fatigue syndrome. Med. Sci. Sports Exerc. 2002, 34, 51–56. [Google Scholar] [CrossRef]
- Tomas, C.; Newton, J. Metabolic abnormalities in chronic fatigue syndrome/myalgic encephalomyelitis: A mini-review. Biochem. Soc. Trans. 2018, 46, 547–553. [Google Scholar] [CrossRef]
- Averbeck, D. Non-targeted effects as a paradigm breaking evidence. Mutat. Res. 2010, 687, 7–12. [Google Scholar] [CrossRef]
- Sawal, H.A.; Asghar, K.; Bureik, M.; Jalal, N. Bystander signaling via oxidative metabolism. OncoTargets Ther. 2017, 10, 3925–3940. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Balliet, R.M.; Rivadeneira, D.B.; Chiavarina, B.; Pavlides, S.; Wang, C.; Whitaker-Menezes, D.; Daumer, K.M.; Lin, Z.; Witkiewicz, A.K.; et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle 2010, 9, 3256–3276. [Google Scholar] [CrossRef] [PubMed]
- Gorman, S.; Tosetto, M.; Lyng, F.; Howe, O.; Sheahan, K.; O’Donoghue, D.; Hyland, J.; Mulcahy, H.; O’Sullivan, J. Radiation and chemotherapy bystander effects induce early genomic instability events: Telomere shortening and bridge formation coupled with mitochondrial dysfunction. Mutat. Res. 2009, 669, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Prue, G.; Rankin, J.; Allen, J.; Gracey, J.; Cramp, F. Cancer-related fatigue: A critical appraisal. Eur. J. Cancer 2006, 42, 846–863. [Google Scholar] [CrossRef] [PubMed]
- Hokama, Y.; Empey-Campora, C.; Hara, C.; Higa, N.; Siu, N.; Lau, R.; Kuribayashi, T.; Yabusaki, K. Acute phase phospholipids related to the cardiolipin of mitochondria in the sera of patients with chronic fatigue syndrome (CFS), chronic Ciguatera fish poisoning (CCFP), and other diseases attributed to chemicals, Gulf War, and marine toxins. J. Clin. Lab. Anal. 2008, 22, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Tharmalingam, S.; Sreetharan, S.; Brooks, A.L.; Boreham, D.R. Re-evaluation of the linear no-threshold (LNT) model using new paradigms and modern molecular studies. Chem. Biol. Interact. 2019, 301, 54–67. [Google Scholar] [CrossRef]
- Glaser, R.; Kiecolt-Glaser, J.K. Stress-associated immune modulation: Relevance to viral infections and chronic fatigue syndrome. Am. J. Med. 1998, 105, 35S–42S. [Google Scholar] [CrossRef]
- Munoz, N. Human papillomavirus and cancer: The epidemiological evidence. J. Clin. Virol. 2000, 19, 1–5. [Google Scholar] [CrossRef]
- Cleare, A.J. The neuroendocrinology of chronic fatigue syndrome. Endocr. Rev. 2003, 24, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L.; Friberg, D. Natural killer cells and natural killer cell activity in chronic fatigue syndrome. Am. J. Med. 1998, 105, 27S–34S. [Google Scholar] [CrossRef]
- LaVoy, E.C.P.; Fagundes, C.P.; Dantzer, R. Exercise, inflammation, and fatigue in cancer survivors. Exerc. Immunol. Rev. 2016, 22, 82. [Google Scholar] [PubMed]
- Wessely, S. The neuropsychiatry of chronic fatigue syndrome. Chronic Fatigue Syndr. 1993, 173, 212–237. [Google Scholar]
- Larkin, D.; Martin, C.R. The interface between chronic fatigue syndrome and depression: A psychobiological and neurophysiological conundrum. Neurophysiol. Clin. Clin. Neurophysiol. 2017, 47, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Mock, V.; Abernethy, A.P.; Atkinson, A.; Barsevick, A.M.; Berger, A.M.; Cella, D.; Cimprich, B.; Cleeland, C.; Eisenberger, M.A.; Escalante, C.P. Cancer-related fatigue clinical practice guidelines in oncology. JNCCN J. Natl. Compr. Cancer Netw. 2007, 5, 1054–1078. [Google Scholar]
- Werker, C.L.; Nijhof, S.L.; van de Putte, E.M. Clinical practice: Chronic fatigue syndrome. Eur. J. Pediatr. 2013, 172, 1293–1298. [Google Scholar] [CrossRef]
- Nilsson, I.; Palmer, J.; Apostolou, E.; Gottfries, C.-G.; Rizwan, M.; Dahle, C.; Rosén, A. Metabolic dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome not due to anti-mitochondrial antibodies. Front. Med. 2020, 7, 108. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
