Abstract
Prunus mume, a famous perennial ornamental plant and fruit tree in Asia, blooms in winter or early spring in the Yangtze River area. The flowering time directly determines its ornamental and economic value, so it is of great significance to study the molecular mechanism of flowering time. SQUAMOSA PROMOTER BINDING PROTEIN (SBP), often regulated by miR156, is an important flowering regulator, although its function is unknown in P. mume. Here, 11 miR156 precursors were analyzed and located in five chromosomes of the P. mume genome. The expression pattern showed that PmSBP1/6 was negatively correlated with miR156. The promoters of PmSBP1/6 were specifically expressed in the apical meristem. Overexpression of PmSBP1/6 in tobacco promoted flowering and changed the length ratio of pistil and stamen. Moreover, PmSBP1 also affected the number and vitality of pollen and reduced the fertility of transgenic tobacco. Furthermore, ectopic expression of PmSBP1/6 caused up-regulated expression of endogenous SUPPRESSOR OF OVEREXPRESSION OF CO1 (NtSOC1). The yeast-one hybrid assay showed that PmSBP1 was bonded to the promoters of PmSOC1s. In conclusion, a miR156-PmSBP1-PmSOC1s pathway was formed to participate in the regulation of flowering time in P. mume, which provided references for the molecular mechanism of flowering time regulation and molecular breeding of P. mume.
1. Introduction
Flowering time is a very important agronomic trait, which directly determines the ornamental value and economic benefits of ornamental plants. Plant flowering marks the end of vegetative growth and the beginning of reproductive growth, which is a very complex and important link in the process of plant growth and development. It is not only affected by external environmental factors (light, temperature, etc.) but also regulated by internal physiological factors. So far, six pathways regulating the flowering time of plants have been identified. These pathways include environmental factor pathways: photoperiod pathway, vernalization pathway, and temperature pathway; and the physiological factor pathways: autonomous pathway, gibberellin pathway, and aging pathway. Flowering time is jointly regulated by these pathways, which can affect each other and integrated by a series of genes, such as SUPPRESSOR OF OVEREXPRESSION OF CO1 (SOC1), LEAFY (LFY), FLOWERING LOCUS T (FT), CONSTANS (CO), FLOWERING LOCUS C (FLC), etc. [1].
The aging pathway is based on the phenomenon that plants must grow to a certain age before the commencement of reproductive growth. The aging pathway is mainly regulated by microRNA156 (miR156) and its target gene SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL), which was also named SQUAMOSA PROMOTER-BINDING PROTEIN (SBP). The expression of miR156 and SPL genes is negatively correlated. For example, from the juvenile to adult phase of Arabidopsis thaliana, the expression level of miR156 was gradually decreased, while the expression level of its target gene AtSPL, increased with the increase of age, and reached a high level in adulthood [2,3,4]. In addition, their expression patterns were conservative in many species [5,6,7,8]. The miR156-SPL mode plays an important role in the flowering process of plants. In Arabidopsis, constitutive expression of miR156 could prolong the vegetative growth, and overexpression of its target gene SPL resulted in early flowering [3,4,9,10,11]. Furthermore, the aging pathway can mediate photoperiod, gibberellic acid, and endogenous flowering pathways, and co-regulate plant flowering by affecting flowering integrators, such as SOC1 [12,13], CO [14], FT [2,15], AP1, FUL, and LFY [11,16]. However, AtSPL8, a member of SBP-box genes in Arabidopsis, does not affect the flowering time, but affects the development of the flower organs and reduces the fertility of plants [17].
Mei (Prunus mume), which belongs to Prunus in Rosaceae, is an important perennial woody ornamental plant and fruit tree. P. mume is widely spread in temperate regions of Asia and has been cultivated for more than 3000 years in China. The early flowering determines the ornamental, economic, and cultural value of Mei. So far, some flowering-related genes in P. mume have been studied, such as PmSOC1 [18], PmSVP [19], and PmLFY [20]. The SBP-box genes have been identified in P. mume [21]. However, the molecular mechanism of PmSBPs in flowering regulation is still not clear. Here, two members of the SBP-box gene family (PmSBP1 and PmSBP6) were cloned, and their expression patterns, gene function, and regulation mechanism were studied. The outcomes, thus, lay a foundation for clarifying the molecular mechanism of flowering time regulation of P. mume and other Prunus species.
2. Results
2.1. Chromosomal Location, Phylogenetic Analysis and Sequence Analysis of miR156 from P. mume
In P. mume, the precursors of pmu-miR156 were encoded by 11 genomic sequences (pmu-MIR156a-k), which were located in five chromosomes (Supplementary Figure S1A) and cut into four types of mature sequences (pmu-miR156a–d, e–f, g–j, k). An ML phylogenetic tree of 36 precursor sequences (11 from P. mume, 10 from Arabidopsis, and 15 from tobacco) was built and the miR156 family was divided into three subgroups (Supplementary Figure S1B). Among them, the pmu-MIR156i/j/g/h were clustered with ath-MIR156a–f and nta-MIR156c–i, and the pmu-MIR156a/b/c/d/f/k were clustered with ath-MIR157a–d and nta-MIR156a/b/j–o, but the pmu-MIR156e was a subgroup alone. Sequence alignment showed that only two differentiated bases were found between the miR156 mature sequences from P. mume, Arabidopsis, and tobacco (Supplementary Figure S1C). The pmu-miR156f can fully bind to the miR156 site on the mRNA of PmSBP1, and they are located on the same chromosome.
2.2. Expression Patterns of PmSBP1/6 and miR156f during the Flower Development Period
The flower development period of P. mume consists of two phases with overlapping parts: flower bud morphological differentiation and dormancy, which were divided into nine stages (S1–S9): flower primordium forming stage (S1), sepal forming stage (S2), petal forming stage (S3), stamen forming stage (S4), pistil forming stage (S5), anther and ovule forming stage (S6), morphological differentiation finished stage (S7), alabastrum intumescence stage (S8), and flower upcoming to bloom stage (S9) (Figure 1A,B). Among them, S1–S7 belong to the flower bud morphological differentiation phase, and S6–S9 belong to the flower bud dormancy phase. To verify the function of PmSBP1/6 and the regulation relationship with miR156f, their expression patterns in flower development were analyzed. The expression level of PmSBP1 gradually increased (S1–S4) and remained stable (S4–S6), but decreased sharply when flower bud morphological differentiation was completed (S7), then recovered slightly at S8 and then maintained a very low level before blooming (S9) (Figure 1C). The expression level of PmSBP6 increased first (S1–S2) and decreased gradually from S2 to S4, then suddenly raised at pistil forming stage (S5) and then dropped when flower bud morphological differentiation was completed (S7), and maintained at a low level (S8–S9) (Figure 1D). In general, PmSBP1/6 shows relatively high expression in the flower bud morphological differentiation phase, but low expression after differentiation. On the other hand, the expression level of pmu-miR156f decreased from S1 to S4, but suddenly increased at S5, then fell back and remained at a comparatively stable level (S6–S9). In conclusion, the expression patterns of PmSBP1/6 and pmu-miR156f were negatively correlated.
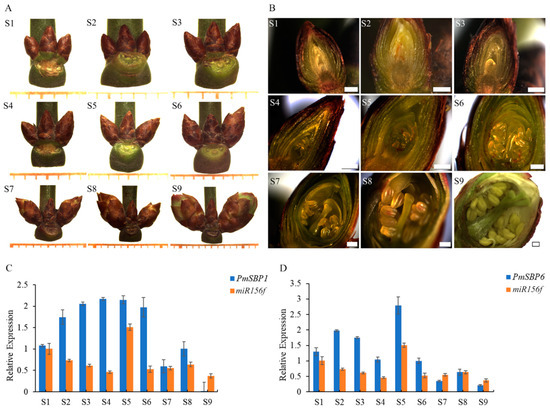
Figure 1.
The flower development stages and the expression patterns of PmSBP1/6 and miR156f. (A,B) Flower buds at different development stages. S1: flower primordium forming stage, S2: sepal forming stage, S3: petal forming stage, S4: stamen forming stage, S5: pistil forming stage, S6: anther and ovule forming stage, S7: morphological differentiation finished stage, S8: alabastrum intumescence stage, S9: flower upcoming to bloom. S1–S7 belong to the flower bud morphological differentiation phase, and S6–S9 belong to the flower bud dormancy phase, the two phases have overlapping parts. The minimum scale in (A) = 1 mm, and the scale bar in (B) = 400 μm. (C,D) Expression patterns of PmSBP1, PmSBP6 and miR156f during the flower development period.
2.3. The Activity Analysis of PmSBP1/6 Promoters
To verify the activity of the PmSBP1/6 promoters, the 2000 bp upstream sequences of PmSBP1/6 were cloned and fused with the β-glucuronidase (GUS) gene and transiently transformed into the N. benthamiana leaves by A. tumefaciens. Finally, clear blue spots were observed in the tobacco leaves with PmSBP1/6 promoters (Figure 2A,B) compared with the control (Figure 2C,D). For further study of the tissue spatial expression profile of PmSBP1/6 promoters, the promoters with GUS were stably overexpressed in tobacco. In the PmSBP1-promoter transgenic tobacco, obvious blue tissues were detected in the apical meristem (Figure 2E). While in the PmSBP6-promoter transgenic tobacco, the blue tissues were detected in both the apical meristem and lateral bud (Figure 2F).
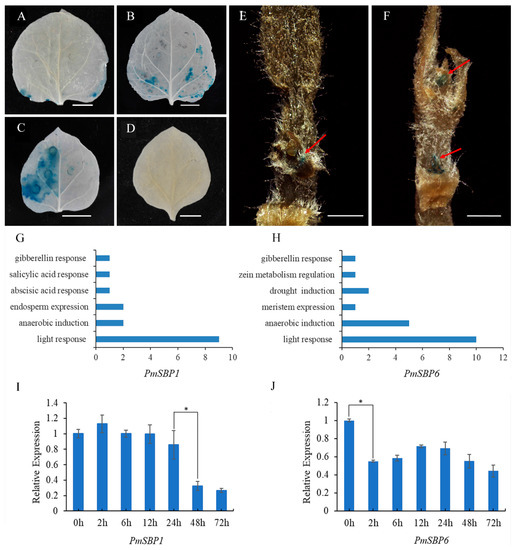
Figure 2.
Analysis of the PmSBP1/6 promoter function and their response to GA3. (A,B) The activity of the PmSBP1/6 promoters. (C) The 35S::GUS positive control. (D)The negative control without any vector. The scale bar in (A–D) is 1 cm. (E,F) The spatial expression profile of PmSBP1/6. The red arrows indicate the location of GUS staining. The scale bar is 1 mm. (G,H). The promoter cis-elements composition of PmSBP1/6. (I,J) The expression of PmSBP1/6 under GA3 treatment. The asterisk indicates significant differences (p < 0.01).
2.4. The Promoter Cis-Elements Composition of PmSBP1/6 and Their Response to GA3
The promoter cis-elements can affect the gene expression. The cloned promoter sequences of PmSBP1/6 (about 2000 bp) were predicted on the PlantCARE website (Supplementary Table S2 and Supplementary Data S1). Statistical results of cis-elements showed that gibberellin response elements, anaerobic induction elements, and light response elements were contained in the promoters of both PmSBP1 and PmSBP6 (Figure 2G,H). In addition, the promoter of PmSBP6 also contained meristem expression elements (Figure 2H). To analyze whether the expression of PmSBP1/6 is regulated by gibberellin, the qRT-PCR was carried out with the flower buds treated with 100 mg/L GA3 solutions. The expression patterns of PmSBP1 and PmSBP6 are shown in Figure 2I,J. The expression level of PmSBP1 and PmSBP6 significantly decreased after gibberellin treatment for 48 h and 2 h, respectively (Figure 2I,J).
2.5. Subcellular Localization of PmSBP1/6 Proteins
The subcellular localization of PmSBP1/6 was detected by the transient transformation of the PmSBP1/6-GFP fusion proteins into tobacco leaves. In the control 35S::GFP, the GFP fluorescence signals were examined in the cytoplasm and nucleus. In the 35S::PmSBP1/6-GFP, although the GFP fluorescence signals were detected mainly in the nucleus and cytoplasm, the fluorescence intensity is weaker than the control (Figure 3).
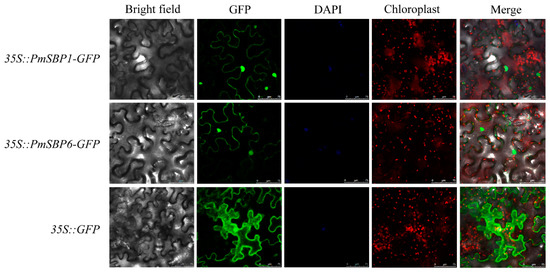
Figure 3.
Subcellular localization of PmSBP1/6. The green, blue, and red fluorescence represent GFP fusion protein, nucleus, and chloroplast positions. The merge pictures were made of bright field, GFP, DAPI, and chloroplast pictures. The scale bar = 75 μm.
2.6. Overexpression of PmSBP1/1tb and PmSBP6 in Tobacco Promotes Flowering and Affects Fertility
The PmSBP1, PmSBP1tb, and PmSBP6 were overexpressed in tobacco under CaMV35S. To confirm the function of PmSBP1 and miR156, the miR156 site of PmSBP1 was synonymously mutated and named PmSBP1tb. The schematic map of PmSBP1/1tb recombinant vectors is shown in Figure 4A. More than 10 transgenic lines of each gene were obtained and confirmed by a reverse transcription polymerase chain reaction (RT-PCR) assay, and three lines were selected for subsequent analyses. In the juvenile period of transgenic T3 generation plants, the vegetative growth of the 35S::PmSBP1tb transgenic seedlings was significantly less than that of the wild type (WT) (Figure 4C), but the 35S::PmSBP1 transgenic lines were slightly larger than that of the WT (Figure 4B). Besides, the vegetative growth of the three 35S::PmSBP6 transgenic lines was also a little larger than that of the WT (Figure 5A). As plants grew older, the difference in vegetative growth between all the transgenic seedlings and wild type gradually disappeared (Figure 4D,E and Figure 5B).
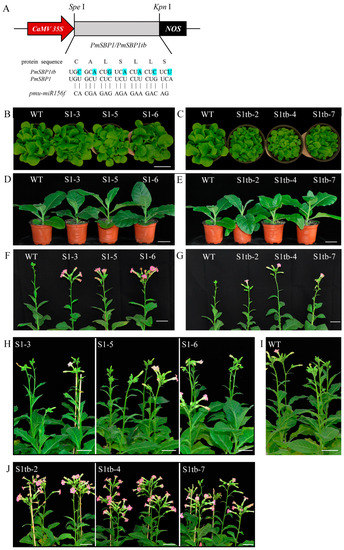
Figure 4.
Overexpression of PmSBP1/1tb in tobacco. (A) The structure of PmSBP1/1tb recombinant vectors. (B–E) The phenotype of PmSBP1 and PmSBP1tb transgenic plants in the vegetative growth period (50 d and 80 d). (F,G) The flowering time of PmSBP1 and PmSBP1tb transgenic plants under short-day conditions (100 d). (H–J) The flowering time of PmSBP1, PmSBP1tb transgenic plants and WT under long-day conditions (110 d). The scale bar = 5 cm.
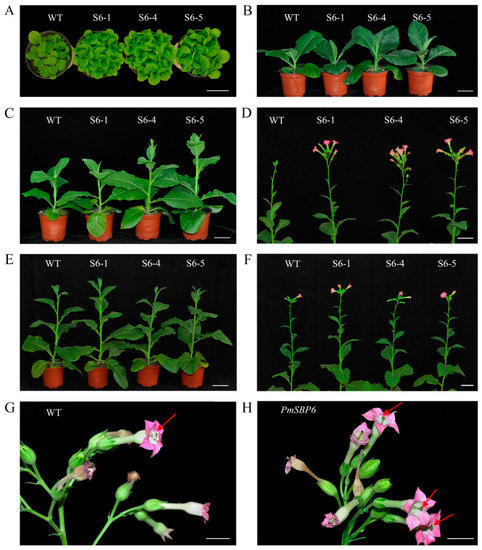
Figure 5.
The phenotype of PmSBP6 transgenic plants. (A,B) The vegetative growth period of PmSBP6 transgenic plants (50 d and 80 d). (C,D) The flowering time of PmSBP6 transgenic plants grown in short-day conditions (90 d and 100 d). (E,F) The flowering time of PmSBP6 transgenic plants grown in long-day conditions (100 d and 110 d). (G,H) The flower phenotype of WT and the PmSBP6 transgenic tobacco. The red arrows indicate the relative height of stigma and stamen. The scale bar = 1 cm.
The flowering time of transgenic plants was different when they under different photoperiod conditions. In short-day conditions, both PmSBP1 (Figure 4F), PmSBP1tb (Figure 4G), and PmSBP6 (Figure 5C,D) transgenic plants bloomed earlier than WT. In addition, the flowering time of PmSBP1 transgenic plants was earlier than that of PmSBP1tb. While under the long-day conditions, the flowering time of PmSBP1 (Figure 4H) and PmSBP6 (Figure 5E, F) transgenic lines was similar to that of WT (Figure 4I). Interestingly, the flowering time of the PmSBP1tb transgenic lines was significantly earlier than that of WT (Figure 4J).
In the reproductive growth stage, the 35S::PmSBP1tb transgenic tobacco was normal, but the 35S::PmSBP1 and 35S::PmSBP6 transgenic tobacco were different from the wild-type tobacco. In the wild-type tobacco, the length of style and filaments were the same, allowing them to be self-bred and bear fruit (Figure 5G). While in the 35S::PmSBP1 (Figure 6A) and 35S::PmSBP6 (Figure 5H) transgenic tobacco, the length of style and filaments were inconsistent. In the 35S::PmSBP1 transgenic tobacco, the length ratio of style to filament showed two phenotypes: one was the high-style (the style was higher than the filament) and the other was the short-style (the style was shorter than the filament). Among them, the high-style flower was the main phenotype (96%), with smaller size, withered anthers, less pollen, and fast-drying filaments (Figure 6A,B), which could not be self-pollinated. The pods became smaller and the seed number in one pod was less after artificial self-pollination (Figure 6C). The pollen viability of the high-style flower was tested by TTC staining and pollen germination in vitro. As shown in Figure 6D and Supplementary Figure S2, in the wild-type tobacco and three 35S::PmSBP1 transgenic lines S1-3, S1-5, and S1-6, the pollen staining rates were 85.8%, 41%, 32.5%, and 13.8%, respectively, and the pollen germination rates were 78.8%, 18.2%, 13.7%, and 12.5%, respectively. In short, the results of the two methods showed that the pollen viability of 35S::PmSBP1 transgenic plants was lower than that of WT. The flower phenotype in the 35S::PmSBP6 transgenic tobacco was partly different from the 35S::PmSBP1 transgenic tobacco. In the 35S::PmSBP6 transgenic tobacco, all the flowers showed a high-style phenotype with normal size, but the flowers could bear fruit normally after artificial self-pollination (Figure 5H).
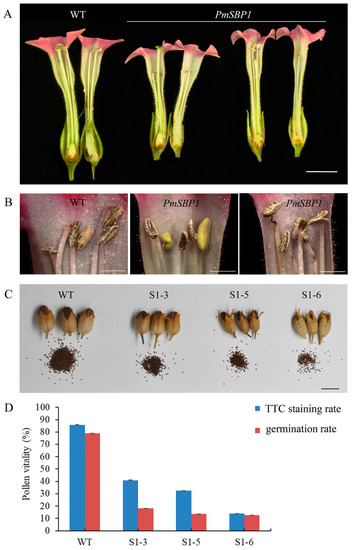
Figure 6.
The phenotype of reproductive organs and pollen vitality of PmSBP1 transgenic tobacco. (A) The floral organs of PmSBP1 transgenic plants. (B) The anther morphology of PmSBP1 transgenic plants. (C) The pod size and seed number from one pod in the PmSBP1 transgenic plants. (D) The pollen vitality of PmSBP1 transgenic plants. The scale bar in (A,C) = 1 cm. The scale bar in (B) = 2 mm.
2.7. Expression Pattern of Endogenous Flowering-Related Genes in PmSBP1/1tb and PmSBP6 Transgenic Tobacco
To investigate the early flowering transgenic tobacco, the expression level of eight endogenous flowering-related genes (NtSOC1, NtCO, NtAP1, NtMADS3, NtMADS4, NtMADS11, NtNFL1, and NtNFL2) in the shoot tip of tobacco (90 d in short-day conditions, 100 d in long-day conditions) was detected by qRT-PCR. The expression level of these eight endogenous flowering-related genes in the three PmSBP1 transgenic tobacco lines is shown in Figure 7A. In short-day conditions, their expression was higher than that of the wild-type tobacco; while in long-day conditions, the expression level was lower than that of wild-type tobacco, except for NtSOC1 showing similar expression to wild-type tobacco. As shown in Figure 7B, the expression level of the endogenous NtSOC1 in the PmSBP1tb transgenic tobacco was increased under both short-day and long-day conditions. In short-day conditions, the expression of the other seven flowering-related genes was similar to WT. In long-day conditions, the expression of NtCO only increased sharply in the PmSBP1tb transgenic tobacco line S1tb-7; however, it changed slightly in the other two lines. The expression level of NtAP1, NtMADS3, NtMADS4, and NtMADS11 in all three lines decreased; however, the expression of NtNFL1 and NtNFL2 fluctuated marginally. As shown in Figure 8, the expression level of these eight flowering-related genes in the three PmSBP6 transgenic lines under short-day conditions was higher than that of WT. Under long-day conditions, their expression levels in the three lines fluctuated up and down at the relative expression value of WT. The expression level of NtSOC1 was directly proportional to the phenotype of early flowering in these transgenic plants.
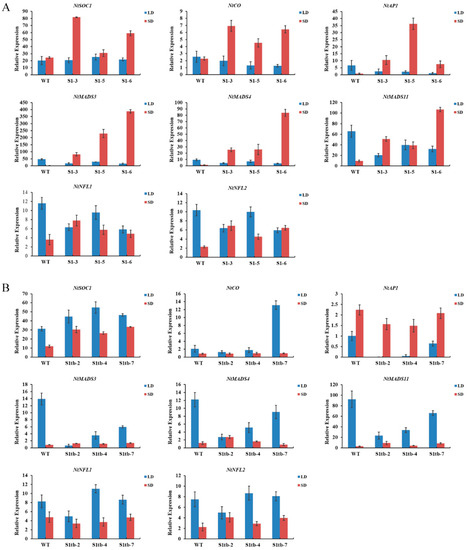
Figure 7.
The relative expression of endogenous flowering-related genes in PmSBP1 and PmSBP1tb transgenic tobacco under long and short conditions. (A) The expression of flowering-related genes in PmSBP1 transgenic tobacco. S1-3, S1-6, and S1-7 were three transgenic lines. (B) The expression of flowering-related genes in PmSBP1tb transgenic tobacco. S1tb-2, S1tb-4, and S1tb-7 were three transgenic lines.
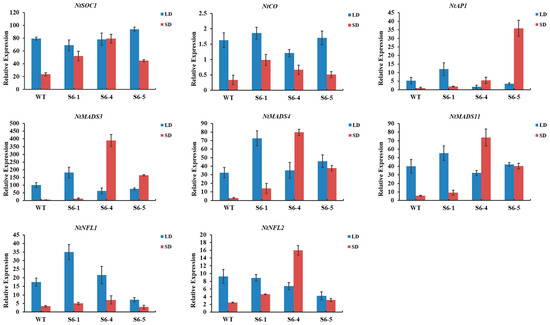
Figure 8.
The relative expression of endogenous flowering-related genes in PmSBP6 transgenic tobacco under long and short conditions. S6-1, S6-4, and S6-5 were three transgenic lines.
2.8. PmSBP1 Binding to the PmSOC1s Promoters
To verify the regulatory relationship between PmSBP1 and PmSOC1s, the promoter sequences of PmSOC1s (about 850 bp) were cloned and analyzed (Supplementary Data S1). Among them, except for PmSOC1-1, the promoters of PmSOC1-2 and PmSOC1-3 contained four SBP binding sites ‘GTAC’ respectively (Figure 9A). The 100 bp fragments of PmSOC1s promoters containing SBP binding sites were used as baits. The B1 fragment of PmSOC1-2 (C2B1) and the B2 fragment of PmSOC1-3 (C3B2) contained two and three SBP binding sites, respectively. The interactions between PmSBP1 and the fragments of PmSOC1s promoters were detected by yeast one-hybrid (Figure 9B). All transformed yeast grew normally on the SD/-Leu solid medium. As shown in Figure 9B, PmSBP1 could bind to the B2 fragments of PmSOC1-2 (C2B2) and PmSOC1-3 promoters (C3B2) but could not associate with the other fragments (C2B1, C2B3, and C3B1). This result suggested that PmSBP1 could activate PmSOC1-2 and PmSOC1-3 by binding to the B2 sites of their promoters. Furthermore, we compared the eight SBP-binding fragments containing the core ‘GTAC’ (11 bp) with the SBP-binding sequence of Arabidopsis AtSPL9 (ID: MA1322.1, homologous with PmSBP1), and found that the three bases adjacent to the core ‘GTAC’ in C2B2 and C3B2 were consistent with the high-frequency bases in the SBP-binding sequence of AtSPL9 (Figure 9C), which indicated that the three bases adjacent to ‘GTAC’ were critical for the binding with PmSBP1.
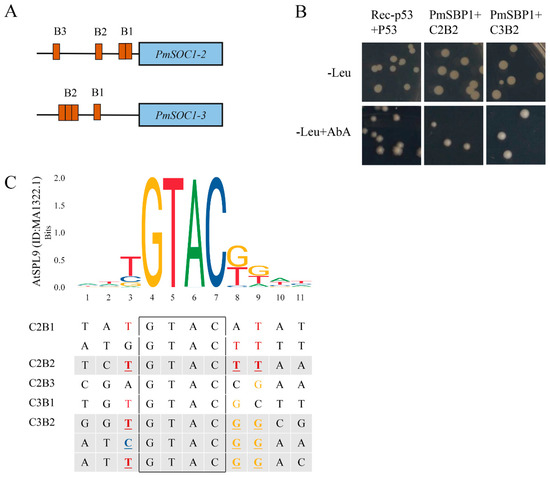
Figure 9.
PmSBP1 binding to the PmSOC1s promoter. (A) The SBP binding sites on the PmSOC1s promoters. (B) The Y1H results of PmSBP1 and the PmSOC1s promoter fragments. (C) The analysis of the SBP-binding fragments in PmSOC1s promoters. The black box is the core component ‘GTAC’ of the SBP-binding fragments. The sequence logo of the SBP-domain in AtSPL9 (ID: MA1322.1) was downloaded from JASPAR (http://jaspar.genereg.net, accessed on 16 September 2022).
3. Discussion
Plant microRNAs (miRNAs), 19–25 nt long, are highly conserved small non-coding RNAs, and play important role in juvenile-to-adult phase transition and flowering time in Arabidopsis [22,23]. The miR156 is quite conservative among plant species [24]. In this study, the sequence alignment results showed that the miR156 mature sequences in P. mume, Arabidopsis and tobacco were indeed highly conserved, with only two SNPs (Supplementary Figure S1A). The miR156-SPL is the famous age pathway; SPL is the target gene of miR156, and is regulated by miR156 mainly through complementation base-pairing at the post-transcriptional level [23]. In our previous study, the miR156-mediated PmSBPs mRNA cleavage was detected by 5′-RACE [25].
In Arabidopsis, there are 16 AtSPLs, which are clustered into two clades: clade I contains two subclades AtSPL7 and AtSPL1/12/14/16; clade II contains four subclades AtSPL3/4/5, AtSPL2/10/11, AtSPL9/15/6/13, and AtSPL8, and all members in clade II have miR156/157 sites except for AtSPL8 [26]. In this study, PmSBP1 were clustered with the AtSPL9/15, and PmSBP6 were clustered with AtSPL3/4/5 together with PmSBP7/8 [21]. Both PmSBP1 and PmSBP6 have miR156 binding sites, but the difference is that the miR156 binding site of PmSBP1 is in the coding region, while the miR156 binding site of PmSBP6 is in the 3′ UTR region [25]. Here, the expression patterns of pmu-miR156f and PmSBP1/6 during the flower development period were negatively correlated.
The spatiotemporal expression pattern of genes is closely related to gene function. So far, the expression pattern of SBP in the flower development period has been studied in some perennial plants. The expression profile of AtSPL9/15 homologous genes in some species is as follows. In loquat, the expression of EjSPL9 decreased with the development of flower bud [27]. In chestnut, the expression level of CmSPL9 was increased with the development of flower bud [28], and so does the JrSBP23 in walnut [29]. In Betula luminifera, BlSPL8 was highly expressed in the early and middle stages of male inflorescence [30]. Unlike them, the expression of PmSBP1 was relatively stable and high in the flower bud morphological differentiation phase of P. mume, which indicated that PmSBP1 may play a vital role in flower bud differentiation. The expression pattern of AtSPL3/4/5 homologous genes in other species is as follows. In loquat, the expression of EjSPL3 and EjSPL 4 reached the peak at flower bud initiation, the expression level of EjSPL4 and EjSPL5 was suddenly raised in the middle stage of flower bud development [27]. The BlSPL15 of Betula luminifera was significant highly expressed in the early stage of female inflorescence [30]. But partly like EjSPL4, EjSPL5 and BlSPL15, in this study, the expression level of PmSBP6 was significantly high at the stamen differentiation stage (S5), which implied that it may participate in the stamen differentiation or regulate fertility. Different expression patterns in different species indicated that they may have different functions during flower bud development.
The promoter is the switch of the gene, which can regulate the gene expression, or bind to the transcription factors to start or close the gene expression. In this study, the 2000 bp promoter sequences of PmSBP1/6 were cloned, both had typical promoter core structure regions, and the GUS staining results showed they had driven activity. Some fragment deletions were found in the cloned promoter sequences of PmSBP1/6 when compared with the genome sequence of wild P. mume. This may be the evolutionary result of the changes in environmental factors during the long-term cultivation process. In addition, the two promoter sequences contained multiple cis-acting elements, such as the light response elements, the anaerobic inducing elements, hormone response elements, and the endosperm expression element or meristem expression element. This suggested that PmSBP1 and PmSBP6 can be regulated by external environmental stimuli (light, water, and hormones), and may be expressed in some specific plant tissues. Besides, the exogenous GA3 downregulated the expression of PmSBP1/6, which was similar to the result of CmSPL9 in chestnut [28]. In addition, the tissue spatial expression profile showed that the PmSBP1 promoter was initiated in the apical meristem, and the PmSBP6 promoter was initiated in both the apical meristem and lateral bud. This displayed that they may play an important role in the initiation of flower buds.
Recently, the function of AtSPLs in clade II of Arabidopsis was extensively studied. AtSPL8 functions in both the male and female fertility of Arabidopsis by affecting the anther development [17,31,32,33] and gynoecium patterning [33]. Based on the function research results, the other ten AtSPLs with miR156/157 sites are divided into three groups. Group 1 contains AtSPL2/9/10/11/13/15, which play role in both the transition from childhood to adulthood and from nutrition to reproduction, and AtSPL9/13/15 [34] play more important roles than AtSPL2/10/11 [10]. Group 2 contains AtSPL3/4/5, which can promote the transformation of flower meristem, but play a minor role in the change of vegetative stage or flower induction [16,35]. Group 3 contains AtSPL6, which does not play a major role in shoot morphogenesis, but may play an important role in some physiological processes [11]. As is known, the miR156-SPL is an important regulatory model in plant growth. Overexpression of miR156 in Arabidopsis prolongs childhood, while silencing miR156 makes plants mature early [3]. In Arabidopsis [2], tobacco [36], and several other plants [6,30,37], the content of miR156 was high and the content of SPL was low in childhood. With the increase of plant age, the content of miR156 decreased and the content of SPL increased. In addition, their expression can be regulated by each other [3,9].
In our study, PmSBP1 and its synonymous mutation PmSBP1tb and PmSBP6 were constitutively overexpressed in tobacco and showed their role in the regulation of plant growth, flowering time, and reproductive organ development. As our results showed, in the childhood stage, the 35S::PmSBP1tb transgenic tobacco was significantly smaller than that of the wild-type tobacco (Figure 4C), while the 35S::PmSBP1 transgenic seedlings were only slightly larger than the wild-type tobacco (Figure 4B). This may be related to the regulatory balance between the content of excessive SBP transcript and miR156. Unexpectedly, the 35S::PmSBP6 transgenic seedlings were also slightly larger than WT in the childhood stage (Figure 5A). Like PmSBP1tb, The CDS region of PmSBP6 does not contain a miR156 binding site, which cannot be regulated by nta-miR156 in tobacco, but they do have opposite phenotypes (Figure 4B and Figure 5A). We speculated that there was still a regulatory relationship between PmSBP6 and miR156 after removing the 3′ UTR sequence (containing miR156 binding site); however, further research should be conducted to confirm the new findings.
The development of floral organs is very important for the sexual reproduction of plants, especially the stamen and pistil. In Arabidopsis, the AtSPL8 (without miR156 binding site) and other AtSPLs (with miR156 binding site) are necessary for the production of fully fertile flowers [23,26]. The knocking out of AtSPL8 resulted in abnormal anther development [17], but the overexpressing of AtSPL8 caused anther non-dehiscence [32]. When overexpressed miR156 in the spl8 mutant, the plant showed complete male sterility, but the overexpression of other AtSPL with miR156 binding sites in the spl8 mutant can alleviate the semi-sterile phenotype to a certain extent [26]. Another miR156 targeted gene AtSPL2 can affect plant fertility by affecting pollen production and fertilization rate. The fertility of both the gain-of-function mutant 35S::SPL2SRDX and the loss-of-function mutant spl2 decreased, and the fertility of the loss-of-function mutant was lower [38]. In this research, the overexpression of PmSBP1 (homologous to AtSPL9/15) and PmSBP6 (homologous to AtSPL3/4/5) in tobacco caused changes in the length of style and filament, and led to changes in the pollination mode, especially PmSBP1, which caused pollen abortion by reducing the number and vitality of pollen. However, overexpression of the PmSBP1tb gene neither caused changes in flower organs nor affected the pollen number and activity, and the fertility of PmSBP1tb transgenic plants was not affected. This indicated that both PmSBP1 and PmSBP6 can change the length ratio of style to the filament and affect the pollination mode, and the miR156 locus of PmSBP1 is indispensable in affecting plant fertility. Similar to Arabidopsis AtSPL8 [23,26] and AtSPL2 [38], all these genes affect plant fertility, but in different ways. Among these SBPs from different species, those not clustered together by phylogenetic analysis also have similar functions, this may be due to the species evolution.
Ectopic expression of PmSBP1 and PmSBP6 in tobacco caused early flowering, which was similar to their homologous genes in Arabidopsis [15,34,35]. However, in our study, the flowering time of each transgenic plant was not consistent under different photoperiod conditions. The flowering time of PmSBP1 and PmSBP6 transgenic tobacco was earlier than that of WT only in short-day conditions, but the flowering time of PmSBP1tb transgenic tobacco was earlier in both long-day and short-day conditions. This indicated that PmSBP1 and PmSBP6 were affected by miR156 in promoting flowering in tobacco through mediating the photoperiod pathway. Furthermore, we examined the expression pattern of eight endogenous flowering-related genes in transgenic plants under different photoperiod conditions. Combined with the phenotype and expression level, we found that the expression level of endogenous NtSOC1 in early flowering transgenic plants was significantly higher than that of wild-typeunder corresponding photoperiod conditions. This suggested that PmSBP1 and PmSBP6 can regulate flowering time by regulating NtSOC1. Furthermore, the results of the yeast-one hybrid showed that PmSBP1 can regulate PmSOC1-2 and PmSOC1-3 by directly binding to their promoters. However, the relationship between PmSBP6 and the promoter of PmSOC1s cannot be verified by yeast-one hybrid because the introduction of PmSBP6 makes yeast grow abnormally. In previous reports, AtSPL3/4/5 mediated flowering time regulation by cooperating with FT-FD complexes in the photoperiod pathway [15]. Moreover, the AtSPL3/4/5 can be regulated by AtSOC1 by directly binding to their promoters [13]. However, the study on how SBP protein regulates flowering time by directly binding to the promoter of SOC1 has not been reported. Our findings opened a new way for SBP to regulate flowering time and laid a foundation for molecular breeding of P. mume and its research in other species.
4. Materials and Methods
4.1. Plant Materials
Prunus mume ‘Sanlun Yudie’, grown in the campus of Beijing Forestry University was used in this study. The flower buds and young leaves were collected for gene and promoter cloning, respectively. The developmental periods of flower buds were identified by hand sectioning and flower buds were collected from July (initiation of flower bud differentiation) to March (flowering) in the next year for expression pattern analysis. To detect the response of PmSBP1 on gibberellin, the 100 mg/L GA3 was sprayed on the flower buds of cut-off one-year-old branches after pistil formation and during bud dormancy, the buds were sampled after 0 h, 2 h, 6 h, 12 h, 24 h, 48 h, and 72 h. The Nicotiana tabacum and Nicotiana benthamiana were grown at 25 °C, 16 h light/8 h dark (long-day conditions) or 8 h light/16 h dark (short-day conditions) in the greenhouse. The stem tips of transgenic and wild-type tobacco were sampled for endogenous gene detection at 100 d in short-day conditions and 110 d in long-day conditions. All samples were collected in liquid nitrogen and stored at −80 °C for RNA isolation.
4.2. Bioinformatics Analysis of pmu-miR156
The mature sequences and precursor sequences of pmu-miR156 in P. mume, ath-miR156 in Arabidopsis, and nta-miR156 in Nicotiana tabacum were obtained from our previous study [25], the miRbase (www.mirbase.org/ftp.shtmlmL, accessed on 2 June 2021), and published article [39], respectively. The precursor sequences of pmu-miR156 were aligned to the genome data of P. mume by blastn, and the chromosome position was drawn through the MG2Cv2 (http://mg2c.iask.in/mg2c_v2.0, accessed on 2 June 2021). The DNAMAN software was used to analyze the differences of miR156 mature sequence in P. mume, Arabidopsis and tobacco. The phylogenetic tree of the precursor sequences of three species was obtained through the maximum likelihood method in MEGA software after Clustalx alignment.
4.3. Cloning and Analysis of PmSBP Gene and Promoter
The total RNA of flower buds was isolated by EASYspin Plus Plant RNA Kit (Aidlab, Beijing, China), and the first strand of cDNA was synthesized by TransScript RT Kit (TIANGEN, Beijing, China). The genome DNA of young leaves was extracted by DNAsecure Plant Kit (TIANGEN). The cDNA was used as a template to amplify the coding sequences (CDS) of PmSBP1 and PmSBP6. The promoter sequences of PmSBP1/6 (about 2000 bp) and PmSOC1s (about 850 bp) were cloned from the genome DNA by specific primers. The primers were designed by Oligo7 and are listed in Supplementary Table S1. The total volume of the 50 μL polymerase chain reaction (PCR) system includes 25 μL PrimeSTAR HS (Premix) (TaKaRa, Beijing, China), 1 μL forward primer, and 1 μL reverse primer, 2 μL cDNA or 1 μL genome DNA. The PCR procedure was 94 °C pre-denaturation for 2 min; 35 cycles of 98 °C for 10 s, 55 °C for 5 s, 72 °C for 90 s; and 72 °C extensions for 10 min. The PCR products were separated from 0.8% agarose gel using TIANgel Midi Purification Kit (TIANGEN) and cloned into the pCloneEZ-NRS-Omni-Amp/HC vector (Clone Smarter, Houston, TX, USA) and transformed into Escherichia coli DH5α for sequencing (TIANGEN). The cis-acting element composition of the PmSBP1/6 (about 2000 bp) promoter sequences was predicted on the PlantCARE website [40].
4.4. Expression Pattern Analysis
The total RNA of flower buds in different flower development stages and treated with GA3, and the stem tip of tobacco was isolated by the EASYspin Plus Plant RNA Kit (Aidlab). The FastQuant RT Kit (with gDNase) (TIANGEN) was used to synthesize the first strand of cDNA, and the SYBR Premix ExTaq II (TaKaRa) was used for qRT-PCR following the instructions. The miRNA RT/qPCR Detection kit (Aidlab) was used to synthesize cDNA and complete Poly(A) tailed qRT-PCR of miR156f. The qRT-PCR was performed on the PikoReal real-time PCR system (Thermo Fisher Scientific, Waltham, MA, USA). The primers of the housekeeping gene (protein phosphatase 2A, PmPP2A) [41], miR156f [25], and PmSBP1/6 [21] of P. mume were referred to in the previous study. The reaction with 10 µL volume (1 µL of cDNA, 5 µL of SYBR Premix ExTaq II (Takara), and 0.2 µL of each primer) were conducted as follow: 30 s in 95 °C, 40 cycles of 5 s in 95 °C and 30 s in 60 °C, and finally end in 20 °C, and each reaction was repeated in triplicate. The qRT-PCR primers of the housekeeping gene (NtActin) and eight endogenous flowering-related genes in tobacco used were also from the previous study [42,43,44]. The relative expression level was calculated by the 2−∆∆Ct method [45]. The error line was drawn according to the standard deviation (calculated by EXCEL) of the three technical repetitions. The significant difference analysis of the gene expression level under GA3 treatment was performed by F-test in EXCEL.
4.5. Subcellular Localization
To analyze the subcellular localization of PmSBP1/6, the CDSs of PmSBP1/6 were cloned into the vector pSuper1300-GFP between Sal I and Spe I restriction sites to generate the PmSBP1/6-GFP fusion gene driven by CaMV35S. The recombinant plasmid was transformed into Agrobacterium tumefaciens GV3101, cultured in 15 mL liquid LB medium (with 50 mg/L kanamycin and 50 mg/L rifampicin) until the OD600 = 0.8, then diluted to OD600 = 0.5, and injected into the leaves of 4–6 weeks old Nicotiana benthamiana. After injection for 24–72 h, the leaves were sectioned and stained in 4′,6-diamino-2-phenylindole (DAPI) solution for 10 min, and observed under TCS SP8 (Leica, Wetzlar, Germany) confocal laser scanning microscope. The fluorescence of GFP and DAPI were detected at 488 nm and 405 nm excitation wavelengths, respectively.
4.6. Promoter Activity Analysis
To analyze the promoter activity, the 2000 bp promoters of PmSBP1/6 were inserted into the vector pCAMBIA1305.4-GUS through Sal I/Bam HI restriction sites. After instantaneous transformation in N. benthamiana leaves, the leaves were cut and stained in histochemical GUS staining reagent for 24 h, and then rinsed in 75% alcohol to remove chlorophyll.
4.7. Overexpression of PmSBP1/6 and Their Promoters in Tobacco
The miR156-sensitive gene PmSBP1, miR156-insensitive genes PmSBP1tb, and PmSBP6 were cloned into the plant expression vector pSuper1300 using Spe I/Kpn I restriction sites under the CaMV35S. PmSBP1tb is a synonymous mutation in the miR156 binding site of PmSBP1, and this mutation was completed by Sangon Biotech Company (Shanghai, China). The recombinants 35S::PmSBP1/6 and 35S::PmSBP1tb were transformed into A. tumefaciens GV3101, respectively. The A. tumefaciens contain 35S::PmSBP1/6, 35S::PmSBP1tb, and PmSBP1/6-promoter::GUS vectors were separately cultured in 30 mL liquid LB medium (with 50 mg/L kanamycin and 50 mg/L rifampicin) until the OD600 = 0.6–0.8, then diluted to OD600 = 0.2–0.5 and infected tobacco by leaf disc method [46]. The phenotypes of T3 generation of PmSBP1/6 and PmSBP1tb transgenic seedlings were observed and photographed. To determine the tissue expression specificity of the promoter of PmSBP1/6, different tissues of T1 generation of PmSBP1/6-promoter transgenic seedlings were stained in GUS solution for GUS activity detection.
4.8. Phenotypic Statistical Analysis
The phenotype changes of these transgenic and wild-type tobacco were observed, recorded, and photographed. The flower morphology was observed throughout the flowering period, and the anthers were collected when the flower was just opened in each transgenic line. The 2,3,5-triphenyl tetrazolium chloride (TTC) staining and pollen germination in vitro were used to test the pollen viability. Three plants were used in each transgenic line. The TTC staining and pollen germination were observed under the microscope. The number of pollen grains in each field was more than 100, and six fields of each plant were counted. The average value and standard deviation of the three technical repetitions were calculated by EXCEL, and the error line was drawn according to the standard deviation. For transgenic plants of PmSBP1 and PmSBP6, artificial self-pollination was performed at the beginning of flower blooming. The pods were harvested when their color turned brown and cracked. The seeds and pods were photographed, and the seeds were stored in bags with desiccant.
4.9. Yeast One-Hybrid Assay
In the cloned promoter sequences of PmSOC1s, there are several ‘GTAC’ SBP binding sites. Fragments of about 100 bp including the SBP binding sites during the 850 bp promoters were inserted into the Sac I/Sal I-cleaved pAbAi vector as baits. The prey vector pGADT7-PmSBP1/6 was constructed by Bam H I/Eco R I sites. The primers were designed by Oligo7 and shown in Supplementary Table S1. The bait recombinants plasmid was transformed into the yeast Y1HGold strains after being digested by Bst BI, and their tolerance to Aureobasidin A (AbA) was detected in the SD/-Ura/AbA medium. Subsequently, the prey vector was transferred into the yeast-containing bait vectors by the Quick & Easy Yeast Transformation Mix (TaKaRa) following its procedure, which was selected on the SD/-Leu/AbA medium.
5. Conclusions
Overall, PmSBP1/6 were important regulatory genes in flowering time and fertility, negatively regulated by miR156 and exogenous gibberellin. Meanwhile, PmSBP1 can directly bind to the promoters of PmSOC1-2 and PmSOC1-3 to regulate flowering. In conclusion, a miR156-PmSBP1-PmSOC1s pathway was formed to participate in the regulation of flowering time in P. mume. This study lays a foundation for revealing the molecular mechanism of flowering time regulation in P. mume.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms231911976/s1.
Author Contributions
Conceptualization, Q.Z. and X.Y.; methodology, X.Y.; software, T.Z., T.C. (Tianci Cong) and P.L.; validation, Q.Z., J.W. and Y.H.; formal analysis, T.Z. and W.L.; investigation, X.Y. and T.Z.; resources, Q.Z.; data curation, Y.H. and T.C. (Tangren Cheng); writing—original draft preparation, X.Y.; writing—review and editing, Q.Z., T.Z. and Y.H.; visualization, A.D.; supervision, T.C. (Tangren Cheng) and J.W.; project administration, T.Z. and J.W.; funding acquisition, Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Research Funds for the Central Universities (grant No. 2021ZY39), the Beijing Municipal Science and Technology Project (grant No. Z181100002418006), and the Special Fund for Beijing Common Construction Project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Moon, J.; Lee, H.; Kim, M.; Lee, I. Analysis of Flowering Pathway Integrators in Arabidopsis. Plant Cell Physiol. 2005, 46, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-W.; Czech, B.; Weigel, D. miR156-Regulated SPL Transcription Factors Define an Endogenous Flowering Pathway in Arabidopsis thaliana. Cell 2009, 138, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Park, M.Y.; Conway, S.R.; Wang, J.-W.; Weigel, D.; Poethig, R.S. The Sequential Action of miR156 and miR172 Regulates Developmental Timing in Arabidopsis. Cell 2009, 138, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Poethig, R.S. Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 2006, 133, 3539–3547. [Google Scholar] [CrossRef]
- Wang, J.-W.; Park, M.Y.; Wang, L.-J.; Koo, Y.; Chen, X.-Y.; Weigel, D.; Poethig, R.S. MiRNA Control of Vegetative Phase Change in Trees. PLoS Genet. 2011, 7, e1002012. [Google Scholar] [CrossRef]
- Xie, K.; Shen, J.; Hou, X.; Yao, J.; Li, X.; Xiao, J.; Xiong, L. Gradual Increase of miR156 Regulates Temporal Expression Changes of Numerous Genes during Leaf Development in Rice. Plant Physiol. 2012, 158, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Bergonzi, S.; Albani, M.C.; van Themaat, E.V.L.; Nordström, K.J.V.; Wang, R.; Schneeberger, K.; Moerland, P.D.; Coupland, G. Mechanisms of Age-Dependent Response to Winter Temperature in Perennial Flowering of Arabis alpina. Science 2013, 340, 1094–1097. [Google Scholar] [CrossRef]
- Zhou, C.-M.; Zhang, T.-Q.; Wang, X.; Yu, S.; Lian, H.; Tang, H.; Feng, Z.-Y.; Zozomova-Lihová, J.; Wang, J.-W. Molecular Basis of Age-Dependent Vernalization in Cardamine flexuosa. Science 2013, 340, 1097–1100. [Google Scholar] [CrossRef]
- Wei, S.; Gruber, M.Y.; Yu, B.; Gao, M.-J.; Khachatourians, G.G.; Hegedus, D.D.; AP Parkin, I.; Hannoufa, A. Arabidopsis mutant sk156 reveals complex regulation of SPL15 in a miR156-controlled gene network. BMC Plant Biol. 2012, 12, 169. [Google Scholar] [CrossRef]
- Shikata, M.; Koyama, T.; Mitsuda, N.; Ohme-Takagi, M. Arabidopsis SBP-Box Genes SPL10, SPL11 and SPL2 Control Morphological Change in Association with Shoot Maturation in the Reproductive Phase. Plant Cell Physiol. 2009, 50, 2133–2145. [Google Scholar] [CrossRef]
- Xu, M.; Hu, T.; Zhao, J.; Park, M.-Y.; Earley, K.W.; Wu, G.; Yang, L.; Poethig, R.S. Developmental Functions of miR156-Regulated SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) Genes in Arabidopsis thaliana. PLoS Genet. 2016, 12, e1006263. [Google Scholar] [CrossRef] [PubMed]
- Hyun, Y.; Richter, R.; Vincent, C.; Martinez-Gallegos, R.; Porri, A.; Coupland, G. Multi-layered Regulation of SPL15 and Cooperation with SOC1 Integrate Endogenous Flowering Pathways at the Arabidopsis Shoot Meristem. Dev. Cell 2016, 37, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-H.; Ju, Y.; Seo, P.J.; Lee, J.-H.; Park, C.-M. The SOC1-SPL module integrates photoperiod and gibberellic acid signals to control flowering time in Arabidopsis. Plant J. 2012, 69, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Seo, Y.H.; Seo, P.J.; Reyes, J.L.; Yun, J.; Chua, N.H.; Park, C.M. The GIGANTEA-regulated microRNA172 mediates photoperiodic flowering independent of CONSTANS in Arabidopsis. Plant Cell 2007, 19, 2736–2748. [Google Scholar] [CrossRef]
- Jung, J.-H.; Lee, H.-J.; Ryu, J.Y.; Park, C.-M. SPL3/4/5 Integrate Developmental Aging and Photoperiodic Signals into the FT-FD Module in Arabidopsis Flowering. Mol. Plant 2016, 9, 1647–1659. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Wu, M.-F.; Yang, L.; Wu, G.; Poethig, R.S.; Wagner, D. The MicroRNA-Regulated SBP-Box Transcription Factor SPL3 Is a Direct Upstream Activator of LEAFY, FRUITFULL, and APETALA1. Dev. Cell 2009, 17, 268–278. [Google Scholar] [CrossRef]
- Unte, U.S.; Sorensen, A.-M.; Pesaresi, P.; Gandikota, M.; Leister, D.; Saedler, H.; Huijser, P. SPL8, an SBP-Box Gene That Affects Pollen Sac Development in Arabidopsis. Plant Cell 2003, 15, 1009–1019. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Yang, W.; Cheng, T.; Wang, J.; Zhang, Q. Isolation and Functional Characterization of SOC1-like Genes in Prunus mume. J. Am. Soc. Hortic. Sci. 2016, 141, 315–326. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Yang, W.; Cheng, T.; Wang, J.; Zhang, Q. Isolation and functional characterization of SVP-like genes in Prunus mume. Sci. Hortic. 2017, 215, 91–101. [Google Scholar] [CrossRef]
- Ahmad, S.; Li, Y.; Yang, Y.; Zhou, Y.; Zhao, K.; Zhang, Q. Isolation, functional characterization and evolutionary study of LFY1 gene in Prunus mume. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 136, 523–536. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, L.; Zhou, Y.; Yang, W.; Cheng, T.; Wang, J.; Zhang, Q. Identification and expression analysis of the SQUAMOSA promoter-binding protein (SBP)-box gene family in Prunus mume. Mol. Genet. Genom. 2015, 290, 1701–1715. [Google Scholar] [CrossRef] [PubMed]
- Gramzow, L.; Theißen, G. Plant miRNA Conservation and Evolution. Methods Mol. Biol. 2019, 1932, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Ye, M.; Sang, M.; Wu, R. A Regulatory Network for miR156-SPL Module in Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 6166. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, Z.; Li, L. Evolutionary conservation of microRNA regulatory programs in plant flower development. Dev. Biol. 2013, 380, 133–144. [Google Scholar] [CrossRef]
- Wang, T.; Pan, H.; Wang, J.; Yang, W.; Cheng, T.; Zhang, Q. Identification and profiling of novel and conserved microRNAs during the flower opening process in Prunus mume via deep sequencing. Mol. Genet. Genom. 2014, 289, 169–183. [Google Scholar] [CrossRef]
- Xing, S.; Salinas, M.; Höhmann, S.; Berndtgen, R.; Huijser, P. miR156-Targeted and Nontargeted SBP-Box Transcription Factors Act in Concert to Secure Male Fertility in Arabidopsis. Plant Cell 2010, 22, 3935–3950. [Google Scholar] [CrossRef]
- Jiang, Y.; Peng, J.; Wang, M.; Su, W.; Gan, X.; Jing, Y.; Yang, X.; Lin, S.; Gao, Y. The Role of EjSPL3, EjSPL4, EjSPL5, and EjSPL9 in Regulating Flowering in Loquat (Eriobotrya japonica Lindl.). Int. J. Mol. Sci. 2020, 21, 248. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Liu, Y.; Zhang, Q.; Gao, Y.; Fang, K.; Cao, Q.; Qin, L.; Xing, Y. Roles of the GA-mediated SPL Gene Family and miR156 in the Floral Development of Chinese Chestnut (Castanea mollissima). Int. J. Mol. Sci. 2019, 20, 1577. [Google Scholar] [CrossRef]
- Zhou, L.; Quan, S.; Ma, L.; Xu, H.; Yang, J.; Niu, J. Molecular characterization of SBP-box gene family during floral induction in walnut (Juglans regia L.). Tree Genet. Genomes 2020, 16, 12. [Google Scholar] [CrossRef]
- Li, X.-Y.; Lin, E.-P.; Huang, H.-H.; Niu, M.-Y.; Tong, Z.-K.; Zhang, J.-H. Molecular Characterization of SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) Gene Family in Betula luminifera. Front. Plant Sci. 2018, 9, 608. [Google Scholar] [CrossRef]
- Xing, S.; Quodt, V.; Chandler, J.; Höhmann, S.; Berndtgen, R.; Huijser, P. SPL8 Acts Together with the Brassinosteroid-Signaling Component BIM1 in Controlling Arabidopsis thaliana Male Fertility. Plants 2013, 2, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Schwarz, S.; Saedler, H.; Huijser, P. SPL8, a local regulator in a subset of gibberellin-mediated developmental processes in Arabidopsis. Plant Mol. Biol. 2007, 63, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Salinas, M.; Garcia-Molina, A.; Höhmann, S.; Berndtgen, R.; Huijser, P. SPL8and miR156-targetedSPLgenes redundantly regulate Arabidopsis gynoecium differential patterning. Plant J. 2013, 75, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Grande, A.V.; Bujdoso, N.; Saedler, H.; Huijser, P. The microRNA regulated SBP-box genes SPL9 and SPL15 control shoot maturation in Arabidopsis. Plant Mol. Biol. 2008, 67, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-H.; Seo, P.J.; Kang, S.K.; Park, C.-M. miR172 signals are incorporated into the miR156 signaling pathway at the SPL3/4/5 genes in Arabidopsis developmental transitions. Plant Mol. Biol. 2011, 76, 35–45. [Google Scholar] [CrossRef]
- Feng, S.; Xu, Y.; Guo, C.; Zheng, J.; Zhou, B.; Zhang, Y.; Ding, Y.; Zhang, L.; Zhu, Z.; Wang, H.; et al. Modulation of miR156 to identify traits associated with vegetative phase change in tobacco (Nicotiana tabacum). J. Exp. Bot. 2016, 67, 1493–1504. [Google Scholar] [CrossRef]
- Wang, B.; Geng, S.; Wang, D.; Feng, N.; Zhang, D.; Wu, L.; Hao, C.; Zhang, X.; Li, A.; Mao, L. Characterization of Squamosa Promoter Binding Protein-Like genes in wheat. J. Plant Biol. 2015, 58, 220–229. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Kohalmi, S.E.; Amyot, L.; Hannoufa, A. SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 2 controls floral organ development and plant fertility by activating ASYMMETRIC LEAVES 2 in Arabidopsis thaliana. Plant Mol. Biol. 2016, 92, 661–674. [Google Scholar] [CrossRef]
- Frazier, T.P.; Xie, F.; Freistaedter, A.; Burklew, C.E.; Zhang, B. Identification and characterization of microRNAs and their target genes in tobacco (Nicotiana tabacum). Planta 2010, 232, 1289–1308. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Wang, T.; Hao, R.; Pan, H.; Cheng, T.; Zhang, Q. Selection of Suitable Reference Genes for Quantitative Real-time Polymerase Chain Reaction in Prunus mume during Flowering Stages and under Different Abiotic Stress Conditions. J. Am. Soc. Hortic. Sci. 2014, 139, 113–122. [Google Scholar] [CrossRef]
- Eli, C.; Ezhang, Y.; Ezhang, K.; Eguo, D.; Ecui, B.; Ewang, X.; Ehuang, X. Promoting flowering, lateral shoot outgrowth, leaf development, and flower abscission in tobacco plants overexpressing cotton FLOWERING LOCUS T (FT)-like gene GhFT1. Front. Plant Sci. 2015, 6, 454. [Google Scholar] [CrossRef]
- Liao, W.-Y.; Lin, L.-F.; Lin, M.-D.; Hsieh, S.-C.; Li, A.Y.-S.; Tsay, Y.-S.; Chou, M.-L. Overexpression of Lilium formosanum MADS-box (LFMADS) Causing Floral Defects While Promoting Flowering in Arabidopsis thaliana, Whereas Only Affecting Floral Transition Time in Nicotiana tabacum. Int. J. Mol. Sci. 2018, 19, 2217. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Li, S.; Zang, L.; Dai, L.; Yang, C.; Qu, G.-Z. Overexpression of Two PsnAP1 Genes from Populus simonii × P. nigra Causes Early Flowering in Transgenic Tobacco and Arabidopsis. PLoS ONE 2014, 9, e111725. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Maleki, S.S.; Mohammadi, K.; Ji, K. Influencing factors of genetic transformation efficiency in tobacco (Nicotiana tabacum ‘NC89′) using Agrobacterium tumefacience. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2018, 42, 27–34. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).