Abstract
Mesothelial cells (MCs) play a classic role in maintaining homeostasis in pleural, peritoneal, and pericardial cavities. MCs work as lubricants to reduce friction between organs, as regulators of fluid transport, and as regulators of defense mechanisms in inflammation. MCs can differentiate into various cells, exhibiting epithelial and mesenchymal characteristics. MCs have a high potential for differentiation during the embryonic period when tissue development is active, and this potential decreases through adulthood. The expression of the Wilms’ tumor suppressor gene (Wt1), one of the MC markers, decreased uniformly and significantly from the embryonic period to adulthood, suggesting that it plays a major role in the differentiation potential of MCs. Wt1 deletion from the embryonic period results in embryonic lethality in mice, and even Wt1 knockout in adulthood leads to death with rapid organ atrophy. These findings suggest that MCs expressing Wt1 have high differentiation potential and contribute to the formation and maintenance of various tissues from the embryonic period to adulthood. Because of these properties, MCs dynamically transform their characteristics in the tumor microenvironment as cancer-associated MCs. This review focuses on the relationship between the differentiation potential of MCs and Wt1, including recent reports using lineage tracing using the Cre-loxP system.
1. Introduction
Mesothelial cells (MCs) form the surface layer of serosa-like paving stones, including the peritoneum, pleura, and pericardium, with a cell diameter of ~25 μm []. In addition, the surface of visceral organs, such as the lung, heart, liver, intestine, colon, uterus, and tunica vaginalis testis, is lined with MCs. So far, there is much evidence of MC differentiation from the mesoderm during the embryo stage. However, in the adult stage, the capacity and capability that MCs have are confusing.
Many genes expressed in MCs have been reported in the previous literature. Among these genes, the Wilms’ tumor suppressor gene (Wt1) is expressed in nearly all MCs []. Thus, Wt1-expressing MCs (Wt1+ MCs) have multipotent progenitor cells that can transdifferentiate into epithelial and mesenchymal cells. In the inflammatory condition, hepatic stellate cells (HSCs) and pancreatic stellate cells (PSCs) are partially derived from MCs in adult mice [,,,]. Although the capacity is limited in the adult stage compared to the embryo stage, adult MCs are likely to transdifferentiate other cell types in specific conditions (i.e., inflammation or tumorigenesis in somatic body cavities).
This study reviewed the differences between embryonic and adult stages in the capacity of MC differentiation, particularly related to Wt1.
2. MCs and Wt1 Gene
2.1. General Information on MCs
The roles of MCs include reducing friction between organs as a lubricant, regulating fluid transport as a semipermeable membrane, and regulating immune function []. MCs produce hyaluronic acid and sialomucin, which reduce the coefficient of kinetic friction, contribute to lubrication between organs, and protect injured tissues [,]. Tight junctions involved in cell–cell junctions are important for developing cell-surface polarity and maintaining a semipermeable diffusion barrier, which passively transports fluid across the peritoneum []. Furthermore, MCs trigger inflammatory responses to bacteria and viruses, induce and activate immune cells, and participate in tissue repair after inflammation by regulating coagulation and preventing reinvasion from damaged areas [].
On the structure of MCs, they form specific glycocalyx (a complex composed of glycolipids, proteoglycans, and glycosaminoglycans that coat the cell surface) to maintain homeostasis in the body cavity, and most glycosaminoglycans in the glycocalyx are composed of the hyaluronan family [,,]. Hyaluronic acid (HA) is composed of repeating D-glucuronic acid and N-acetyl-D-glucosamine, which is synthesized by uridine diphosphate glucose dehydrogenase and HA synthase (HAS) []. HAS has three major isozymes, HAS I, HAS II, and HAS III, in which HAS II is necessary for survival []. The role of hyaluronan depends on the molecular weight, with high-molecular-weight synthesis mainly driven by HAS II and low-molecular-weight synthesis particularly by HAS III. Even so, the mechanism of its regulation remains unclear []. Further studies are needed to clarify the role of HA in the developmental and pathological processes of MCs. The glycocalyx formed by MCs lubricates and protects the serosal surface from frictional damage arising from the movement of organs and other surfaces. However, the non-cross-linked organization of the mesothelial glycocalyx is poor in the recognition of, or as a physical barrier against, bacteria and viruses. Therefore, MCs display multiple pattern-recognition receptors that recognize carbohydrates and lipopolysaccharides on the surface of microbial pathogens, such as bacteria and viruses, to release inflammatory mediators and initiate inflammation [,]. MCs lead to the activation of macrophages mobilized from mesothelial subcutaneous tissue by CSF1 secretion [], express class II major histocompatibility complex molecules, and, therefore, modulate lymphocytes [].
2.2. Wt1 in MC Differentiation
MCs derived from the embryonic mesoderm exhibit characteristics such as epithelial cells forming tight and gap junctions and desmosomes, while exhibiting mesenchymal features expressing vimentin and N-cadherin [,,]. Wt1 may be adjusting for these characteristics []. The Wt1 gene maps to chromosome 11p13, which was first identified in 1990 as a strong candidate predisposition gene for the Wilms’ tumor []. Wt1 is widely expressed in mesodermal progenitors during embryogenesis, and the loss of Wt1 in mice lacks kidney, adrenal, gland, gonad, spleen, and coronary vasculature [,,]. In the kidneys, nephron progenitor cells derived from the intermediate mesoderm led to underdeveloped kidneys which reduced the expression of Wnt4, a regulator of the mesenchymal-to-epithelial transition to nephrons due to the loss of Wt1 [,]. In contrast, in the heart, epicardial MCs contribute to the formation of coronary vessels through epithelial-to-mesenchymal transition (EMT), and the loss of Wt1 leads to underdeveloped coronary vasculature with no effect on epicardial formation [,,].
Although Wt1 is widely expressed during embryogenesis, Wt1 expression in adult mice is restricted to a small percentage of cells, such as the podocyte cells of the kidney and gonads, ~1% of cells in the bone marrow, and MCs [,]. Nevertheless, adult mice with induced knockout of Wt1 have undeveloped glomerulosclerosis, spleen and exocrine pancreatic atrophy, loss of bone and fat mass, and defects in red blood cell formation [,]. These findings suggest that Wt1-expressing cells have stem-cell-like properties and maintain homeostasis in adulthood. Today, it is possible to trace the lineage of specific cells by generating conditional knockout experimental animals with the Cre-loxP system. As a result, there is an increasing number of reports on the lineage tracing of Wt1+ MCs with respect to their differentiation and function during the embryonic period and adulthood.
3. Differentiation of MCs into Fibroblasts
3.1. Differentiation of MCs into Fibroblasts Related to Peritoneal Dialysis (PD)
PD is an effective and affordable renal replacement therapy, resulting in less frequent hospital visits and significantly improved quality of life compared to hemodialysis []. However, it is used by only ~11% of the total global dialysis population []. One cause is nonphysiological PD solutions that are bioincompatible for sustained peritoneal exposure and provoke MC injury and peritoneal inflammation [,]. In a study comparing the thickness of the submesothelial compact collagenous zone in patients undergoing PD, PD-induced fibrosis of the peritoneum leads to reduced dialysis efficiency []. Normal peritoneal fibroblasts are scattered in the submesothelial connective tissue and express neither myofibroblastic nor MC markers. However, many fibroblasts associated with PD showed a myofibroblast phenotype expressing α-smooth muscle actin (α-SMA) and accompanied by the expression of cytokeratins suggestive of MC [,].
MCs that transition to myofibroblasts upregulate the transcription factors of Snail, ZEB, and Twist. Wt1 promotes EMT by directly activating the Snai1 promoter and directly repressing the Cdh1 (E-cadherin) promoter during development in the mesenchymal progenitor cells []. Transforming growth factor-β (TGF-β) has been reported as a representative and important mediator in inducing EMT [,,]. Normal MCs homeostatically produce factors, such as bone morphogenetic protein, that counteract the induction of EMT and inhibit TGF-β expression [,]. In vitro and in vivo, PD fluids with a high glucose degradation product (GDP) concentration induce TGF-β production and EMT in MCs [,]. MC stimulation by TGF-β1 and interleukin (IL)-1β activates TGF-β-activated kinase 1, increases the expression of extracellular signal-regulated kinase 1/2, nuclear factor-κB, and Snail, and promotes EMT [,,]. Hepatocyte growth factor, BMP7, vitamin D analogs, and corticosteroids inhibit EMT and peritoneal fibrosis [,,,]. In a study that used Wt1-dependent CreER-expressing mice for the lineage tracing of Wt1+ MCs, the intraperitoneal injection of hypochlorite or dialysis solution, containing 4.25% glucose and 40 mM GDP, induced peritoneal fibrosis, and Wt1+ MCs underwent tissue repair through cell cycle upregulation, as analyzed by increased Ki-67 expression. In contrast, within the submesothelial scar, ~15.9% and 16.5% of the cells differentiated into myofibroblasts expressing α-SMA []. In addition, myofibroblasts derived from Wt1+ MC express platelet-derived growth factor receptor-β (PDGFR-β), and the PDGFR tyrosine kinase inhibitor imatinib significantly attenuated the accumulation of α-SMA myofibroblasts and reduced the fibrotic thickening of the peritoneum.
3.2. Differentiation of Wt1+ MCs into Fibroblasts
Wt1+ MCs in the pancreas are important for the formation and maintenance of PSCs, the fibroblasts of the pancreas. PSCs are usually located around the acinar cells, ducts, and vessels, and comprise ~4% of the total pancreas, and quiescent PSCs are necessary for pancreatic exocrine stability and regeneration [,,]. In a study that used Wt1-dependent CreER-expressing mice for the lineage tracing of Wt1+ MCs, E9.5 and E15.5 showed epithelial features of MCs, whereas E10.5 to E14.5 showed a puncture pattern characteristic of mesenchymal cells in the submesothelium and EMT features []. The induction of Wt1 conditional knockout in MCs between E9.5 and E12.5 showed a significant delay in the development of ventral pancreatic buds at E16.5, characterized by fewer and less dense gland tufts compared to controls. In adult mice, Wt1+ cells are confined to MCs only and do not contribute to PSCs. During Wt1 conditional knockout in adult mice, glandular structures show reduced intercellular adhesion, a rounded shape, and disrupted exocrine tissue structure []. Caerulein-induced pancreatitis in these mice partially rescues glandular tissue by causing de novo Wt1 expression in PSCs, even when Wt1 is deleted from MCs.
HSCs, the fibroblasts of the liver, are also thought to be derived from Wt1+ MCs. A study that used Wt1-dependent CreER-expressing mice for the lineage tracing of Wt1+ MCs supports the contribution of MCs to HSCs and perivascular mesenchymal cells at E11.5 to E12.5. Liver or biliary injury by carbon tetrachloride (CCl4) or bile duct ligation in mice also results in the differentiation of Wt1+ MCs into HSCs or myofibroblasts. Still, the antagonization of TGF-β1 and TGF-β3 by soluble TGF-β receptor 2 decreases the differentiation of Wt1+ MCs []. Previous studies using collagen-driven Cre or Wt1-Cre to track myofibroblasts all tracked collagen-producing myofibroblasts and showed differentiation as part of their composition, but their role remains unclear [,]. Recent studies have shown that activated HSCs derived from Wt1+ MCs act to suppress classical myofibroblasts involved in fibrogenesis []. In this study, to define the subpopulation of active HSCs, Wt1 expression was separated into high-, intermediate-, and negative-expressing cells and cultured. Only high-expressing cells showed a rounded morphology that does not exhibit classic myofibroblast characteristics, and transcriptome data showed a high incidence of mesothelium-related profiles. Wt1 deletion reduced the mean circularity of these cells by 17.5% and was accompanied by a 67.56% increase in fibrosis due to CCl4 injury, indicating that HSCs derived from Wt1+ MCs are inhibitory to fibrosis.
3.3. Differentiation of MCs into Myofibroblasts in Cancer
Peritoneal dissemination is the primary metastatic route for gastric, ovarian, and pancreatic cancers, and contact is necessarily made with MCs during seeding formation in other organs. MCs have been thought to act as a protective barrier against cancer progression [,]. Cancer with peritoneal dissemination often results in ascites effusions that contain numerous cytokines, including TGF-β, which differentiate MCs into the cancer-associated fibroblast (CAF) phenotype, suggesting their involvement in the tumor microenvironment [,,,]. Integrin α5 is involved in the enhanced adhesion of ovarian cancer in peritoneal dissemination and is highly expressed in single cells in ascites fluid or spheroids formed from multiple cells [,]. Recent reports have shown that CAFs in spheroids that receive TGF-β signaling from ovarian cancer secrete epidermal growth factor and induce integrin α5 expression in ovarian cancer, promoting adhesion to the peritoneum []. PDGF signaling is important for CAF survival [], and imatinib reduces CAF activity and leads to a rapid decrease in peritoneal adhesion []. MCs differentiated into CAF-like phenotype by TGF-β stimulation acquire platinum resistance by upregulating FN1 expression and activating Akt1 signaling in ovarian cancer cells []. MCs may also be involved in angiogenesis within tumors [].
Malignant ascites contain high vascular endothelial growth factor (VEGF) concentrations [,], a source not only of cancer but also of MCs []. Normal MCs also produce VEGF homeostatically, and MC stimulation with TGF-β, IL-1β, and fibroblast growth factor 2 increases VEGF secretion, suggesting tumor angiogenesis [,,,]. Bevacizumab, a monoclonal antibody targeting all VEGF-A isoforms, is also useful in platinum-resistant ovarian cancer, suggesting a strong involvement of MC in the tumor [,]. These reports support the need to target MC in the tumor microenvironment as a novel therapeutic strategy.
A novel strategy for preventing peritoneal dissemination has recently been reported, focusing on the ability of vitamin D to recover MC function by inhibiting TGF-β-stimulated MC mesenchymal transition secreted from ovarian cancer cells. In this study, vitamin D recovers MC function and significantly reduces the peritoneal dissemination of ovarian cancer by suppressing thrombospondin-1 expression []. A study using mice spontaneously developing pancreatic cancer found a population of podoplanin-positive CAFs in the stroma that is suggested to be derived from MCs []. Subpopulations involved in the poor response to anti-PD-L1 therapy have been identified by profiling the population with single-cell RNA sequencing (RNAseq). A recent study that used Wt1-dependent CreER-expressing mice for the lineage tracing of Wt1+ MCs identified MC-derived CAFs in the cancer stroma. This study found antigen-presenting CAFs from MCs that induce regulatory T cells and contribute to immune evasion [].
4. Contribution of MCs to Blood Vessels
4.1. Wt1+ MCs in Heart Development
Wt1 is highly expressed in epicardial MCs during fetal heart development and is downregulated as epicardial EMT progresses. Some MCs undergoing EMT after E12.5 in mice form epicardial-derived progenitor cells (EPDC) []. EPDCs migrate into the myocardium and differentiate into stromal fibroblasts and smooth muscle cells of the coronary vasculature [,]. Wt1 deletion during the embryonic period in mice causes death by E14.5 with pericardial effusion at E13.5 []. In the mice model where the Wt1 gene was conditionally knocked out by Gata5-Cre, a decrease in mesenchymal progenitor cells and their derivatives resulted in coronary artery dysplasia and death by E16.5 due to the accumulation of edema and blood in systemic veins []. A study that used Wt1-dependent CreER-expressing mice for the lineage tracing of Wt1+ MCs in the epicardium has shown that they form part of the coronary arteries in neonatal and adult stages []. In addition, lineage tracing data of Wt1+ MCs in the epicardium in adulthood have confirmed not only the expression of α-SMA, a vascular smooth muscle marker, but also CD31, a vascular endothelial cell marker. However, it is not possible to exclude that Wt1-expressing endothelial cells may have contributed to these labeled vascular cells.
Wt1 expression in the heart is not restricted to the epicardium but is also present in the developing myocardial layer after E12.5 in mice and at 5 weeks postfertilization in humans []. In human and mouse myocardial layers, Wt1 expression is observed in the endothelial cells of small capillaries and larger coronary vessels, and this decreases from the prenatal stage to adulthood [,]. In mice with myocardial infarction, Wt1 expression is upregulated in endothelial cells in the infarct zone and border zone of the heart []. Over time, vessels in the infarcted region mature and form a fibrotic scar, and Wt1 expression disappears. Endogenous Wt1 expression in cardiac endothelial cells prevents the use of Wt1-Cre for the cell lineage tracing of epicardial MCs to coronary endothelial cells [,]. A study using Apln-CreER mice engineered to lineage trace subepicardial endothelial cells has shown that subepicardial endothelial cells contribute to the formation of coronary endothelial cells during the process of cardiogenesis [].
4.2. Role of Wt1 in MC Differentiation Related to Tumor Angiogenesis
The process of angiogenesis in the tumor microenvironment is considered one of the key features of cancer [,]. Tumor and bone-marrow-derived cells, and other stromal cells, release paracrine VEGF, which increases vascular branching and promotes tumor vascular development []. The combination of conventional chemotherapy and the VEGF inhibitor bevacizumab has increased the survival rates of patients with advanced colorectal and lung cancers [,].
Pericytes are specialized mesenchymal cells with finger-like processes that wrap around the endothelial tubes of blood vessels []. PDGFR-β-positive pericytes are recruited to the periphery of endothelial cells by PDGF-B released from endothelial cells, tumors, and platelets, providing mechanical and physiological support for the vessel. Experiments using PDGFR inhibitors, in combination with VEGFR inhibitors, prevent islet cancer growth by inducing pericyte detachment and tumor vascularity disruption in mice with end-stage islet cancer []. In contrast, using anti-VEGF drugs in mice deficient in pericytes does not affect antitumor efficacy []. In addition, a study using mice with conditional knockout of PDGF-B in platelets has shown that the impaired pericyte coverage of tumor blood vessels promotes metastasis and, thus, the need for vascular integrity maintenance [].
High-level Wt1-expressing tumors correlate with promoted angiogenesis, demonstrating that Wt1 regulates VEGF expression [,]. In a study using the Ewing’s sarcoma cell line, Wt1-expressing tumors increased the expression of antiangiogenesis-promoting molecules, such as VEGF, MMP9, Ang-1, and Tie-2 []. In addition, in vivo experiments have shown that Wt1 deletion in tumors significantly suppresses blood vessel and tumor formation.
Previously, it was found that Wt1 is upregulated in endothelial cells of various tumors [,]. Mice with endothelial-cell-specific Wt1 knockout using Tie2-CreER or VE-cadherin-CreER have reduced vascular density and tumor volume in melanoma and lung cancer []. In this study, mice with the Wt1-CreER genotype showed that ~50% of tumor stromal cells undergo Wt1-Cre-mediated recombination. In addition, Wt1 coexpression with markers of endothelial cells, hematopoietic progenitor cells, myeloid cells, and pericytes has been observed in human tumor samples, supporting a high Wt1 contribution to the tumor stroma.
5. MCs and Adipocytes
Adipose tissue is mainly classified into white adipose tissue (WAT) and brown adipose tissue (BAT) []. WAT further exists as subcutaneous and visceral WAT. Subcutaneous WAT is located under the skin and accounts for the highest percentage of adipose tissue []. Visceral WAT surrounds the perirenal, gonad, epicardium, retroperitoneum, cartilage, and mesentery []. WAT consists mainly of white fat cells with monoblastic fat droplets, which store excess energy in fat droplets as triglycerides and decompose them into glycerol and free fatty acids as necessary to resupply them throughout the body as an energy source []. BAT exists in locally limited quantities composed mainly of brown fat cells with the morphological feature of having small fat droplets that are multifocal, and numerous mitochondria are present around the fat droplets []. Uncoupling protein 1 (UCP1) on the inner mitochondrial membrane of brown adipocytes has the function of deconjugating oxidative phosphorylation in mitochondria, eliminating the proton concentration gradient without involving ATP synthesis, dissipating it as heat energy, and contributing to high heat production [].
Wt1 expression in adipose tissue is restricted to visceral WAT and is not detectable in subcutaneous WAT and BAT []. Wt1 represses the BAT gene signature, and thermogenic genes, such as Ucp1 and Prdm16, are expressed in visceral WAT in mice with adipocyte-specific Wt1 deletion []. It is presumed that Wt1 leads to increased WAT and decreased BAT, and is associated with obesity, diabetes, and cardiovascular disease due to metabolic dysfunction. In general, the accumulation of subcutaneous WAT has no significant effect on mortality risk, but the accumulation of visceral WAT with Wt1 expression leads to increased mortality risk []. BAT can be readily induced in subcutaneous WAT, but visceral WAT expressing Wt1 is resistant to induction []. A study using heterozygous Wt1 knockout mice has shown that the expression of thermogenic genes, such as Ucp1, is enhanced in visceral WAT []. Retroviral Wt1 expression in brown preadipocytes also reduces the expression of thermogenic genes, such as Ucp1, during in vitro differentiation. These findings suggest that reducing Wt1 expression in visceral WAT, a more intra-abdominal fat reservoir, may improve the percentage of brown preadipocytes and be a novel therapeutic strategy in metabolic diseases.
In a study of lineage tracing in adipose tissue, using mice expressing CreER in a Wt1-dependent manner, induction with tamoxifen administration at E14.5 and tissue analysis at 1.2 years of age showed that the epididymal fat (77%), epicardial (66%), visceral (47%), and mesenteric (28%) contributions of Wt1+ cells are confirmed in WAT. In contrast, no positive adipocytes derived from Wt1-expressing cells are present in subcutaneous WAT and BAT []. In addition, adipose progenitor cell-surface markers are expressed in Wt1-positive cells in adipose tissue in adulthood. Thus, the role of Wt1+ MCs as a source of visceral progenitor adipocytes during embryonic and adulthood is widely accepted, and subsequent studies have used Wt1 for lineage tracing analysis []. In a recent study, MCs have been reported not to be a source of adipocytes in adults []. In this study, RNAseq of WAT in the epididymis identifies MC populations and progenitor adipocyte populations, and identifies that not only MC populations but also progenitor adipocyte populations express Wt1. In a previous study, single-cell RNAseq of WAT confirmed Wt1 expression in the non-MC stromal population [,]. They also identified keratin 19 (Krt19) as a more MC-specific marker in the process of single-cell RNAseq []. Interestingly, data from lineage tracing analysis with Wt1 and Krt19, respectively, found no involvement of Krt19+ cells in adipocytes. These data show that Wt1 expressed by progenitor adipocytes should be distinguished from Wt1+ MCs. In visceral WAT, Wt1 is detected in the stromal vascular fraction (e.g., endothelial cells, immune cells, and progenitor adipocytes). There, it differentiates not only into adipocytes but also into muscle cells and osteoblasts in vitro, although their role during adulthood remains unclear []. In tumor microenvironments, such as breast and pancreatic cancers, adipocytes differentiate into fibroblast-like cells by Wnt signaling and promote tumor development [,,]. These are currently unknown in terms of their association with Wt1, which is interesting.
6. Conclusions
Finally, we summarized the capacity of MC differentiation during the embryonic and adult stages (Figure 1).
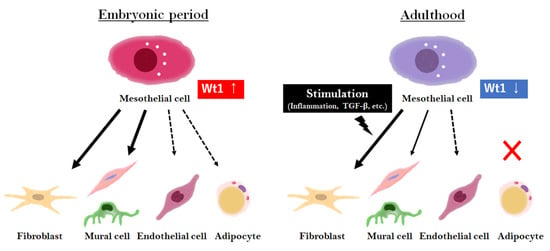
Figure 1.
Overview of MC differentiation in the embryonic and adult stages. In the embryonic period, MCs highly express Wt1, contributing to various tissue formations. In adulthood, Wt1 expression in MCs is low and limited to a few populations, but is involved in tissue repair with differentiation upon stimulation, such as inflammation or injury.
The control of MC, especially when expressing Wt1 differentiation, is an important issue in the treatment of human diseases, such as inflammation, cancer, and injury, as well as PD. The recovery or modification of MC function may be one of the potential novel therapeutic strategies for these diseases and situations. Although the availability of MC control is still unknown, MC definitely has the potential for therapeutic development, and research on MCs needs to be further accelerated.
Author Contributions
T.T., H.T. and A.H. wrote and revised the manuscript. T.K., K.M., Y.K., Y.Y., M.Y. and H.K. provided specific advice to us on this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank all the members of our laboratory.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Van Baal, J.O.; Van de Vijver, K.K.; Nieuwland, R.; van Noorden, C.J.; van Driel, W.J.; Sturk, A.; Kenter, G.G.; Rikkert, L.G.; Lok, C.A. The histophysiology and pathophysiology of the peritoneum. Tissue Cell 2017, 49, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Carmona, R.; Gonzalez-Iriarte, M.; Perez-Pomares, J.M.; Munoz-Chapuli, R. Localization of the Wilm’s tumour protein WT1 in avian embryos. Cell Tissue Res. 2001, 303, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Affo, S.; Nair, A.; Brundu, F.; Ravichandra, A.; Bhattacharjee, S.; Matsuda, M.; Chin, L.; Filliol, A.; Wen, W.; Song, X.; et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell 2021, 39, 866–882.e11. [Google Scholar] [CrossRef]
- Dominguez, C.X.; Muller, S.; Keerthivasan, S.; Koeppen, H.; Hung, J.; Gierke, S.; Breart, B.; Foreman, O.; Bainbridge, T.W.; Castiglioni, A.; et al. Single-Cell RNA Sequencing Reveals Stromal Evolution into LRRC15(+) Myofibroblasts as a Determinant of Patient Response to Cancer Immunotherapy. Cancer Discov. 2020, 10, 232–253. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lua, I.; French, S.W.; Asahina, K. Role of TGF-beta signaling in differentiation of mesothelial cells to vitamin A-poor hepatic stellate cells in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G262–G272. [Google Scholar] [CrossRef] [PubMed]
- Lua, I.; Li, Y.; Zagory, J.A.; Wang, K.S.; French, S.W.; Sevigny, J.; Asahina, K. Characterization of hepatic stellate cells, portal fibroblasts, and mesothelial cells in normal and fibrotic livers. J. Hepatol. 2016, 64, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, K. Diverse properties of the mesothelial cells in health and disease. Pleura Peritoneum 2016, 1, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Bodega, F.; Pecchiari, M.; Sironi, C.; Porta, C.; Arnaboldi, F.; Barajon, I.; Agostoni, E. Lubricating effect of sialomucin and hyaluronan on pleural mesothelium. Respir. Physiol. Neurobiol. 2012, 180, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Pecchiari, M.; Sartori, P.; Conte, V.; D’Angelo, E.; Moscheni, C. Friction and morphology of pleural mesothelia. Respir. Physiol. Neurobiol. 2016, 220, 17–24. [Google Scholar] [CrossRef]
- Mutsaers, S.E. Mesothelial cells: Their structure, function and role in serosal repair. Respirology 2002, 7, 171–191. [Google Scholar] [CrossRef]
- Kothari, H.; Kaur, G.; Sahoo, S.; Idell, S.; Rao, L.V.; Pendurthi, U. Plasmin enhances cell surface tissue factor activity in mesothelial and endothelial cells. J. Thromb. Haemost. 2009, 7, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, S.E.; Prele, C.M.; Pengelly, S.; Herrick, S.E. Mesothelial cells and peritoneal homeostasis. Fertil. Steril. 2016, 106, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, A.; Yamana, S.; Murakami, T. Localization of membrane-associated sialomucin on the free surface of mesothelial cells of the pleura, pericardium, and peritoneum. Histochem. Cell. Biol. 1997, 107, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Soliman, F.; Ye, L.; Jiang, W.; Hargest, R. Targeting Hyaluronic Acid and Peritoneal Dissemination in Colorectal Cancer. Clin. Colorectal Cancer 2021, 21, e126–e134. [Google Scholar] [CrossRef] [PubMed]
- Itano, N.; Sawai, T.; Yoshida, M.; Lenas, P.; Yamada, Y.; Imagawa, M.; Shinomura, T.; Hamaguchi, M.; Yoshida, Y.; Ohnuki, Y.; et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 1999, 274, 25085–25092. [Google Scholar] [CrossRef]
- Camenisch, T.D.; Spicer, A.P.; Brehm-Gibson, T.; Biesterfeldt, J.; Augustine, M.L.; Calabro, A., Jr.; Kubalak, S.; Klewer, S.E.; McDonald, J.A. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J. Clin. Invest. 2000, 106, 349–360. [Google Scholar] [CrossRef]
- Yung, S.; Chan, T.M. Pathophysiology of the peritoneal membrane during peritoneal dialysis: The role of hyaluronan. J. Biomed. Biotechnol. 2011, 2011, 180594. [Google Scholar] [CrossRef]
- Yung, S.; Chan, T.M. Pathophysiological changes to the peritoneal membrane during PD-related peritonitis: The role of mesothelial cells. Mediat. Inflamm. 2012, 2012, 484167. [Google Scholar] [CrossRef]
- Ivanov, S.; Gallerand, A.; Gros, M.; Stunault, M.I.; Merlin, J.; Vaillant, N.; Yvan-Charvet, L.; Guinamard, R.R. Mesothelial cell CSF1 sustains peritoneal macrophage proliferation. Eur. J. Immunol. 2019, 49, 2012–2018. [Google Scholar] [CrossRef]
- Hausmann, M.J.; Rogachev, B.; Weiler, M.; Chaimovitz, C.; Douvdevani, A. Accessory role of human peritoneal mesothelial cells in antigen presentation and T-cell growth. Kidney Int. 2000, 57, 476–486. [Google Scholar] [CrossRef]
- Batra, H.; Antony, V.B. The pleural mesothelium in development and disease. Front. Physiol. 2014, 5, 284. [Google Scholar] [CrossRef] [PubMed]
- Zsiros, V.; Kiss, A.L. Cellular and molecular events of inflammation induced transdifferentiation (EMT) and regeneration (MET) in mesenteric mesothelial cells. Inflamm. Res. 2020, 69, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.Y.; Hastie, N.D. The role of Wt1 in regulating mesenchyme in cancer, development, and tissue homeostasis. Trends Genet. 2012, 28, 515–524. [Google Scholar] [CrossRef]
- Gessler, M.; Poustka, A.; Cavenee, W.; Neve, R.L.; Orkin, S.H.; Bruns, G.A. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature 1990, 343, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Kreidberg, J.A.; Sariola, H.; Loring, J.M.; Maeda, M.; Pelletier, J.; Housman, D.; Jaenisch, R. WT-1 Is Required for Early Kidney Development. Cell 1993, 74, 679–691. [Google Scholar] [CrossRef]
- Herzer, U. The Wilms tumor suppressor gene Wt1 is required for development of the spleen. Curr. Biol. 1999, 9, 837–840. [Google Scholar] [CrossRef]
- Moore, A.W.; McInnes, L.; Kreidberg, J.; Hastie, N.D.; Schedl, A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development 1999, 126, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Akpa, M.M.; Iglesias, D.M.; Chu, L.L.; Cybulsky, M.; Bravi, C.; Goodyer, P.R. Wilms tumor suppressor, WT1, suppresses epigenetic silencing of the beta-catenin gene. J. Biol. Chem. 2015, 290, 2279–2288. [Google Scholar] [CrossRef]
- Essafi, A.; Webb, A.; Berry, R.L.; Slight, J.; Burn, S.F.; Spraggon, L.; Velecela, V.; Martinez-Estrada, O.M.; Wiltshire, J.H.; Roberts, S.G.; et al. A Wt1-controlled chromatin switching mechanism underpins tissue-specific wnt4 activation and repression. Dev. Cell. 2011, 21, 559–574. [Google Scholar] [CrossRef]
- Martinez-Estrada, O.M.; Lettice, L.A.; Essafi, A.; Guadix, J.A.; Slight, J.; Velecela, V.; Hall, E.; Reichmann, J.; Devenney, P.S.; Hohenstein, P.; et al. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat. Genet. 2010, 42, 89–93. [Google Scholar] [CrossRef]
- Wu, M.; Smith, C.L.; Hall, J.A.; Lee, I.; Luby-Phelps, K.; Tallquist, M.D. Epicardial spindle orientation controls cell entry into the myocardium. Dev. Cell. 2010, 19, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ma, Q.; Rajagopal, S.; Wu, S.M.; Domian, I.; Rivera-Feliciano, J.; Jiang, D.; von Gise, A.; Ikeda, S.; Chien, K.R.; et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 2008, 454, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Hastie, N.D. Wilms’ tumour 1 (WT1) in development, homeostasis and disease. Development 2017, 144, 2862–2872. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.Y.; Brownstein, D.; Mjoseng, H.; Lee, W.C.; Buza-Vidas, N.; Nerlov, C.; Jacobsen, S.E.; Perry, P.; Berry, R.; Thornburn, A.; et al. Acute multiple organ failure in adult mice deleted for the developmental regulator Wt1. PLoS Genet. 2011, 7, e1002404. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, A.; Gioberge, S.; Moeller, S.; Brown, G. ESRD patients in 2004: Global overview of patient numbers, treatment modalities and associated trends. Nephrol. Dial. Transplant. 2005, 20, 2587–2593. [Google Scholar] [CrossRef]
- Morgan, L.W.; Wieslander, A.; Davies, M.; Horiuchi, T.; Ohta, Y.; Beavis, M.J.; Craig, K.J.; Williams, J.D.; Topley, N. Glucose degradation products (GDP) retard remesothelialization independently of D-glucose concentration. Kidney Int. 2003, 64, 1854–1866. [Google Scholar] [CrossRef][Green Version]
- Witowski, J.; Wisniewska, J.; Korybalska, K.; Bender, T.O.; Breborowicz, A.; Gahl, G.M.; Frei, U.; Passlick-Deetjen, J.; Jorres, A. Prolonged exposure to glucose degradation products impairs viability and function of human peritoneal mesothelial cells. J. Am. Soc. Nephrol. 2001, 12, 2434–2441. [Google Scholar] [CrossRef]
- Williams, J.D.; Craig, K.J.; Topley, N.; Von Ruhland, C.; Fallon, M.; Newman, G.R.; Mackenzie, R.K.; Williams, G.T. Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 2002, 13, 470–479. [Google Scholar] [CrossRef]
- Jimenez-Heffernan, J.A.; Aguilera, A.; Aroeira, L.S.; Lara-Pezzi, E.; Bajo, M.A.; del Peso, G.; Ramirez, M.; Gamallo, C.; Sanchez-Tomero, J.A.; Alvarez, V.; et al. Immunohistochemical characterization of fibroblast subpopulations in normal peritoneal tissue and in peritoneal dialysis-induced fibrosis. Virchows Arch. 2004, 444, 247–256. [Google Scholar] [CrossRef]
- Yáñez-Mó, M. Peritoneal Dialysis and Epithelial-to-Mesenchymal Transition of Mesothelial Cells. N. Engl. J. Med. 2003, 348, 403–413. [Google Scholar] [CrossRef]
- Nasreen, N.; Mohammed, K.A.; Mubarak, K.K.; Baz, M.A.; Akindipe, O.A.; Fernandez-Bussy, S.; Antony, V.B. Pleural mesothelial cell transformation into myofibroblasts and haptotactic migration in response to TGF-beta1 in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L115–L124. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.H.; Chen, J.Y.; Lin, J.K. Myofibroblastic conversion of mesothelial cells. Kidney Int. 2003, 63, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, M.; Lan, H.; Yu, X. miR-30a negatively regulates TGF-beta1-induced epithelial-mesenchymal transition and peritoneal fibrosis by targeting Snai1. Am. J. Pathol. 2013, 183, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.; Schilte, M.; Aguilera, A.; Albar-Vizcaino, P.; Ramirez-Huesca, M.; Perez-Lozano, M.L.; Gonzalez-Mateo, G.; Aroeira, L.S.; Selgas, R.; Mendoza, L.; et al. BMP-7 blocks mesenchymal conversion of mesothelial cells and prevents peritoneal damage induced by dialysis fluid exposure. Nephrol. Dial. Transplant. 2010, 25, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.A.; Shin, K.S.; Kim, J.H.; Kim, Y.I.; Chung, S.S.; Park, S.H.; Kim, Y.L.; Kang, D.H. HGF and BMP-7 ameliorate high glucose-induced epithelial-to-mesenchymal transition of peritoneal mesothelium. J. Am. Soc. Nephrol. 2009, 20, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Bajo, M.A.; Perez-Lozano, M.L.; Albar-Vizcaino, P.; del Peso, G.; Castro, M.J.; Gonzalez-Mateo, G.; Fernandez-Perpen, A.; Aguilera, A.; Sanchez-Villanueva, R.; Sanchez-Tomero, J.A.; et al. Low-GDP peritoneal dialysis fluid (‘balance’) has less impact in vitro and ex vivo on epithelial-to-mesenchymal transition (EMT) of mesothelial cells than a standard fluid. Nephrol. Dial. Transplant. 2011, 26, 282–291. [Google Scholar] [CrossRef]
- Hirahara, I.; Ishibashi, Y.; Kaname, S.; Kusano, E.; Fujita, T. Methylglyoxal induces peritoneal thickening by mesenchymal-like mesothelial cells in rats. Nephrol. Dial. Transplant. 2009, 24, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, R.; Benedicto, I.; Foronda, M.; Perez-Lozano, M.L.; Sanchez-Perales, S.; Lopez-Cabrera, M.; Del Pozo, M.A. p38 maintains E-cadherin expression by modulating TAK1-NF-kappa B during epithelial-to-mesenchymal transition. J. Cell Sci. 2010, 123 Pt 24, 4321–4331. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, R.; Benedicto, I.; Perez Lozano, M.L.; Cerezo, A.; Lopez-Cabrera, M.; del Pozo, M.A. Epithelial-to-mesenchymal transition of peritoneal mesothelial cells is regulated by an ERK/NF-kappaB/Snail1 pathway. Dis Models Mech 2008, 1, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, R.; Benedicto, I.; Perez Lozano, M.L.; Pellinen, T.; Sandoval, P.; Lopez-Cabrera, M.; del Pozo, M.A. Inhibition of transforming growth factor-activated kinase 1 (TAK1) blocks and reverses epithelial to mesenchymal transition of mesothelial cells. PLoS ONE 2012, 7, e31492. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, Y.; Liu, Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J. Am. Soc. Nephrol. 2006, 17, 3382–3393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lei, W.; Wang, X.; Tang, Y.; Song, J. Glucocorticoid induces mesenchymal-to-epithelial transition and inhibits TGF-beta1-induced epithelial-to-mesenchymal transition and cell migration. FEBS Lett. 2010, 584, 4646–4654. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Chang, Y.T.; Pan, S.Y.; Chou, Y.H.; Chang, F.C.; Yeh, P.Y.; Liu, Y.H.; Chiang, W.C.; Chen, Y.M.; Wu, K.D.; et al. Lineage tracing reveals distinctive fates for mesothelial cells and submesothelial fibroblasts during peritoneal injury. J. Am. Soc. Nephrol. 2014, 25, 2847–2858. [Google Scholar] [CrossRef]
- Jaster, R. Molecular regulation of pancreatic stellate cell function. Mol. Cancer 2004, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Riopel, M.M.; Li, J.; Liu, S.; Leask, A.; Wang, R. beta1 integrin-extracellular matrix interactions are essential for maintaining exocrine pancreas architecture and function. Lab. Invest. 2013, 93, 31–40. [Google Scholar] [CrossRef]
- Zimmermann, A.; Gloor, B.; Kappeler, A.; Uhl, W.; Friess, H.; Buchler, M.W. Pancreatic stellate cells contribute to regeneration early after acute necrotising pancreatitis in humans. Gut 2002, 51, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Ariza, L.; Canete, A.; Rojas, A.; Munoz-Chapuli, R.; Carmona, R. Role of the Wilms’ tumor suppressor gene Wt1 in pancreatic development. Dev. Dyn. 2018, 247, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Ariza, L.; Rojas, A.; Munoz-Chapuli, R.; Carmona, R. The Wilms’ tumor suppressor gene regulates pancreas homeostasis and repair. PLoS Genet. 2019, 15, e1007971. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Asahina, K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proc. Natl. Acad. Sci. USA 2013, 110, 2324–2329. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Cong, M.; Paik, Y.; Scholten, D.; Jiang, C.; Benner, C.; Iwaisako, K.; Moore-Morris, T.; Scott, B.; Tsukamoto, H.; et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl. Acad. Sci. USA 2012, 109, 9448–9453. [Google Scholar] [CrossRef]
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.P.; Schwabe, R.F. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 2013, 4, 2823. [Google Scholar] [CrossRef] [PubMed]
- Kendall, T.J.; Duff, C.M.; Boulter, L.; Wilson, D.H.; Freyer, E.; Aitken, S.; Forbes, S.J.; Iredale, J.P.; Hastie, N.D. Embryonic mesothelial-derived hepatic lineage of quiescent and heterogenous scar-orchestrating cells defined but suppressed by WT1. Nat. Commun. 2019, 10, 4688. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.A.; Dogan, S.; Zillhardt, M.; Mitra, A.; Yamada, S.D.; Krausz, T.; Lengyel, E. Organotypic models of metastasis: A three-dimensional culture mimicking the human peritoneum and omentum for the study of the early steps of ovarian cancer metastasis. Cancer Treat. Res. 2009, 149, 335–351. [Google Scholar] [PubMed]
- Kenny, H.A.; Krausz, T.; Yamada, S.D.; Lengyel, E. Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells to the omentum. Int. J. Cancer 2007, 121, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Matte, I. Role of malignant ascites on human mesothelial cells and their gene expression profiles. BMC Cancer 2014, 14, 288. [Google Scholar] [CrossRef] [PubMed]
- Matte, I.; Lane, D.; Laplante, C.; Rancourt, C.; Piche, A. Profiling of cytokines in human epithelial ovarian cancer ascites. Am. J. Cancer Res. 2012, 2, 566–580. [Google Scholar] [PubMed]
- Rynne-Vidal, A.; Au-Yeung, C.L.; Jimenez-Heffernan, J.A.; Perez-Lozano, M.L.; Cremades-Jimeno, L.; Barcena, C.; Cristobal-Garcia, I.; Fernandez-Chacon, C.; Yeung, T.L.; Mok, S.C.; et al. Mesothelial-to-mesenchymal transition as a possible therapeutic target in peritoneal metastasis of ovarian cancer. J. Pathol. 2017, 242, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.S.P.; Agarwal, R.; Kaye, S.B. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 2006, 7, 925–934. [Google Scholar] [CrossRef]
- Casey, R.C.; Burleson, K.M.; Skubitz, K.M.; Pambuccian, S.E.; Oegema, T.R.; Ruff, L.E.; Skubitz, A.P.N. β1-Integrins Regulate the Formation and Adhesion of Ovarian Carcinoma Multicellular Spheroids. Am. J. Pathol. 2001, 159, 2071–2080. [Google Scholar] [CrossRef]
- Ohyagi-Hara, C.; Sawada, K.; Kamiura, S.; Tomita, Y.; Isobe, A.; Hashimoto, K.; Kinose, Y.; Mabuchi, S.; Hisamatsu, T.; Takahashi, T.; et al. miR-92a inhibits peritoneal dissemination of ovarian cancer cells by inhibiting integrin alpha5 expression. Am. J. Pathol. 2013, 182, 1876–1889. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, Z.; Xu, S.; Li, X.; Yang, X.; Jin, P.; Liu, Y.; Zhou, X.; Zhang, T.; Gong, C.; et al. Heterotypic CAF-tumor spheroids promote early peritoneal metastatis of ovarian cancer. J. Exp. Med. 2019, 216, 688–703. [Google Scholar] [CrossRef]
- Murata, T.; Mizushima, H.; Chinen, I.; Moribe, H.; Yagi, S.; Hoffman, R.M.; Kimura, T.; Yoshino, K.; Ueda, Y.; Enomoto, T.; et al. HB-EGF and PDGF mediate reciprocal interactions of carcinoma cells with cancer-associated fibroblasts to support progression of uterine cervical cancers. Cancer Res. 2011, 71, 6633–6642. [Google Scholar] [CrossRef]
- Yoshihara, M.; Kajiyama, H.; Yokoi, A.; Sugiyama, M.; Koya, Y.; Yamakita, Y.; Liu, W.; Nakamura, K.; Moriyama, Y.; Yasui, H.; et al. Ovarian cancer-associated mesothelial cells induce acquired platinum-resistance in peritoneal metastasis via the FN1/Akt signaling pathway. Int. J. Cancer 2020, 146, 2268–2280. [Google Scholar] [CrossRef]
- Fujikake, K.; Kajiyama, H.; Yoshihara, M.; Nishino, K.; Yoshikawa, N.; Utsumi, F.; Suzuki, S.; Niimi, K.; Sakata, J.; Mitsui, H.; et al. A novel mechanism of neovascularization in peritoneal dissemination via cancer-associated mesothelial cells affected by TGF-beta derived from ovarian cancer. Oncol. Rep. 2018, 39, 193–200. [Google Scholar] [PubMed]
- Zebrowski, B.K.; Liu, W.; Ramirez, K.; Akagi, Y.; Mills, G.B.; Ellis, L.M. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann. Surg. Oncol. 1999, 6, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Mandl-Weber, S.; Cohen, C.D.; Haslinger, B.; Kretzler, M.; Sitter, T. Vascular endothelial growth factor production and regulation in human peritoneal mesothelial cells. Kidney Int. 2002, 61, 570–578. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liao, S.; Liu, J.; Lin, P.; Shi, T.; Jain, R.K.; Xu, L. TGF-beta blockade controls ascites by preventing abnormalization of lymphatic vessels in orthotopic human ovarian carcinoma models. Clin. Cancer Res. 2011, 17, 1415–1424. [Google Scholar] [CrossRef]
- Sako, A.; Kitayama, J.; Yamaguchi, H.; Kaisaki, S.; Suzuki, H.; Fukatsu, K.; Fujii, S.; Nagawa, H. Vascular endothelial growth factor synthesis by human omental mesothelial cells is augmented by fibroblast growth factor-2: Possible role of mesothelial cell on the development of peritoneal metastasis. J. Surg. Res. 2003, 115, 113–120. [Google Scholar] [CrossRef]
- Stadlmann, S.; Amberger, A.; Pollheimer, J.; Gastl, G.; Offner, F.A.; Margreiter, R.; Zeimet, A.G. Ovarian carcinoma cells and IL-1beta-activated human peritoneal mesothelial cells are possible sources of vascular endothelial growth factor in inflammatory and malignant peritoneal effusions. Gynecol. Oncol. 2005, 97, 784–789. [Google Scholar] [CrossRef]
- Davis, A.; Tinker, A.V.; Friedlander, M. “Platinum resistant” ovarian cancer: What is it, who to treat and how to measure benefit? Gynecol. Oncol. 2014, 133, 624–631. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A.; et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Kitami, K.; Yoshihara, M.; Tamauchi, S.; Sugiyama, M.; Koya, Y.; Yamakita, Y.; Fujimoto, H.; Iyoshi, S.; Uno, K.; Mogi, K.; et al. Peritoneal restoration by repurposing vitamin D inhibits ovarian cancer dissemination via blockade of the TGF-beta1/thrombospondin-1 axis. Matrix Biol. 2022, 109, 70–90. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, Z.; Zhang, Y.; Pradhan, R.N.; Ganguly, D.; Chandra, R.; Murimwa, G.; Wright, S.; Gu, X.; Maddipati, R.; et al. Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory T cells in pancreatic cancer. Cancer Cell 2022, 40, 656–673.e7. [Google Scholar] [CrossRef]
- Quijada, P.; Trembley, M.A.; Small, E.M. The Role of the Epicardium During Heart Development and Repair. Circ. Res. 2020, 126, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Lie-Venema, H.; van den Akker, N.M.; Bax, N.A.; Winter, E.M.; Maas, S.; Kekarainen, T.; Hoeben, R.C.; deRuiter, M.C.; Poelmann, R.E.; Gittenberger-de Groot, A.C. Origin, fate, and function of epicardium-derived cells (EPDCs) in normal and abnormal cardiac development. Sci. World J. 2007, 7, 1777–1798. [Google Scholar] [CrossRef] [PubMed]
- Vrancken Peeters, M.P.; Gittenberger-de Groot, A.C.; Mentink, M.M.; Poelmann, R.E. Smooth muscle cells and fibroblasts of the coronary arteries derive from epithelial-mesenchymal transformation of the epicardium. Anat. Embryol. 1999, 199, 367–378. [Google Scholar] [CrossRef]
- von Gise, A.; Zhou, B.; Honor, L.B.; Ma, Q.; Petryk, A.; Pu, W.T. WT1 regulates epicardial epithelial to mesenchymal transition through beta-catenin and retinoic acid signaling pathways. Dev. Biol. 2011, 356, 421–431. [Google Scholar] [CrossRef]
- Wilm, T.P.; Tanton, H.; Mutter, F.; Foisor, V.; Middlehurst, B.; Ward, K.; Benameur, T.; Hastie, N.; Wilm, B. Restricted differentiative capacity of Wt1-expressing peritoneal mesothelium in postnatal and adult mice. Sci. Rep. 2021, 11, 15940. [Google Scholar] [CrossRef]
- Sjoerd, N. Chapter 13—WT1 in cardiac development and disease. In Wilms Tumor; van den Heuvel-Eibrink, M.M., Ed.; Exon Publications: Brisbane, Australia, 2016; pp. 211–234. [Google Scholar]
- Duim, S.N.; Kurakula, K.; Goumans, M.J.; Kruithof, B.P. Cardiac endothelial cells express Wilms’ tumor-1: Wt1 expression in the developing, adult and infarcted heart. J. Mol. Cell. Cardiol. 2015, 81, 127–135. [Google Scholar] [CrossRef]
- Duim, S.N.; Smits, A.M.; Kruithof, B.P.; Goumans, M.J. The roadmap of WT1 protein expression in the human fetal heart. J. Mol. Cell. Cardiol. 2016, 90, 139–145. [Google Scholar] [CrossRef]
- Rudat, C.; Kispert, A. Wt1 and epicardial fate mapping. Circ. Res. 2012, 111, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Pu, W.T. Genetic Cre-loxP assessment of epicardial cell fate using Wt1-driven Cre alleles. Circ. Res. 2012, 111, e276–e280. [Google Scholar] [CrossRef]
- Tian, X.; Hu, T.; Zhang, H.; He, L.; Huang, X.; Liu, Q.; Yu, W.; He, L.; Yang, Z.; Zhang, Z.; et al. Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell. Res. 2013, 23, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Stockmann, C.; Doedens, A.; Weidemann, A.; Zhang, N.; Takeda, N.; Greenberg, J.I.; Cheresh, D.A.; Johnson, R.S. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 2008, 456, 814–818. [Google Scholar] [CrossRef]
- Hurwitz, H. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef]
- Bergers, G.; Song, S.; Meyer-Morse, N.; Bergsland, E.; Hanahan, D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest. 2003, 111, 1287–1295. [Google Scholar] [CrossRef]
- Nisancioglu, M.H.; Betsholtz, C.; Genove, G. The absence of pericytes does not increase the sensitivity of tumor vasculature to vascular endothelial growth factor-A blockade. Cancer Res. 2010, 70, 5109–5115. [Google Scholar] [CrossRef]
- Zhang, Y.; Cedervall, J.; Hamidi, A.; Herre, M.; Viitaniemi, K.; D’Amico, G.; Miao, Z.; Unnithan, R.V.M.; Vaccaro, A.; van Hooren, L.; et al. Platelet-Specific PDGFB Ablation Impairs Tumor Vessel Integrity and Promotes Metastasis. Cancer Res. 2020, 80, 3345–3358. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.; Gorman, J.; Reese, J.; Fraizer, G. Regulation of vascular endothelial growth factor, VEGF, gene promoter by the tumor suppressor, WT1. Front. Biosci. 2007, 12, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- McCarty, G.; Awad, O.; Loeb, D.M. WT1 protein directly regulates expression of vascular endothelial growth factor and is a mediator of tumor response to hypoxia. J. Biol. Chem. 2011, 286, 43634–43643. [Google Scholar] [CrossRef]
- Katuri, V.; Gerber, S.; Qiu, X.; McCarty, G.; Goldstein, S.D.; Hammers, H.; Montgomery, E.; Chen, A.R.; Loeb, D.M. WT1 regulates angiogenesis in Ewing Sarcoma. Oncotarget 2014, 5, 2436–2449. [Google Scholar] [CrossRef]
- Timar, J.; Meszaros, L.; Orosz, Z.; Albini, A.; Raso, E. WT1 expression in angiogenic tumours of the skin. Histopathology 2005, 47, 67–73. [Google Scholar] [CrossRef]
- Wagner, N. The Wilms’ tumour suppressor WT1 is involved in endothelial cell proliferation and migration: Expression in tumour vessels in vivo. Oncogene 2008, 27, 3662–3672. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.D.; Cherfils-Vicini, J.; Hosen, N.; Hohenstein, P.; Gilson, E.; Hastie, N.D.; Michiels, J.F.; Wagner, N. The Wilms’ tumour suppressor Wt1 is a major regulator of tumour angiogenesis and progression. Nat. Commun. 2014, 5, 5852. [Google Scholar] [CrossRef] [PubMed]
- Frigolet, M.E.; Gutierrez-Aguilar, R. The colors of adipose tissue. Gac. Med. Mex. 2020, 156, 142–149. [Google Scholar] [CrossRef]
- Thomas, E.L. Magnetic resonance imaging of total body fat. J. Appl. Physiol. 1998, 85, 1778–1785. [Google Scholar] [CrossRef]
- Despres, J.P. Is visceral obesity the cause of the metabolic syndrome? Ann. Med. 2006, 38, 52–63. [Google Scholar] [CrossRef]
- Kwok, K.H.; Lam, K.S.; Xu, A. Heterogeneity of white adipose tissue: Molecular basis and clinical implications. Exp. Mol. Med. 2016, 48, e215. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. Transdifferentiation properties of adipocytes in the adipose organ. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E977–E986. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell. Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, K.M.; Scholz, H. WT1 in Adipose Tissue: From Development to Adult Physiology. Front. Cell. Dev. Biol. 2022, 10, 854120. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Levy, J.D.; Zhang, Y.; Frontini, A.; Kolodin, D.P.; Svensson, K.J.; Lo, J.C.; Zeng, X.; Ye, L.; Khandekar, M.J.; et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014, 156, 304–316. [Google Scholar] [CrossRef]
- Pischon, T.; Boeing, H.; Hoffmann, K.; Bergmann, M.; Schulze, M.B.; Overvad, K.; van der Schouw, Y.T.; Spencer, E.; Moons, K.G.; Tjonneland, A.; et al. General and abdominal adiposity and risk of death in Europe. N. Engl. J. Med. 2008, 359, 2105–2120. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Shinoda, K.; Spiegelman, B.M.; Kajimura, S. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 2012, 15, 395–404. [Google Scholar] [CrossRef]
- Kirschner, K.M.; Foryst-Ludwig, A.; Gohlke, S.; Li, C.; Flores, R.E.; Kintscher, U.; Schupp, M.; Schulz, T.J.; Scholz, H. Wt1 haploinsufficiency induces browning of epididymal fat and alleviates metabolic dysfunction in mice on high-fat diet. Diabetologia 2022, 65, 528–540. [Google Scholar] [CrossRef]
- Chau, Y.Y.; Bandiera, R.; Serrels, A.; Martinez-Estrada, O.M.; Qing, W.; Lee, M.; Slight, J.; Thornburn, A.; Berry, R.; McHaffie, S.; et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat. Cell Biol. 2014, 16, 367–375. [Google Scholar] [CrossRef]
- Lee, K.Y.; Luong, Q.; Sharma, R.; Dreyfuss, J.M.; Ussar, S.; Kahn, C.R. Developmental and functional heterogeneity of white adipocytes within a single fat depot. EMBO J. 2019, 38, e99291. [Google Scholar] [CrossRef]
- Westcott, G.P.; Emont, M.P.; Li, J.; Jacobs, C.; Tsai, L.; Rosen, E.D. Mesothelial cells are not a source of adipocytes in mice. Cell Rep. 2021, 36, 109388. [Google Scholar] [CrossRef] [PubMed]
- Burl, R.B.; Ramseyer, V.D.; Rondini, E.A.; Pique-Regi, R.; Lee, Y.H.; Granneman, J.G. Deconstructing Adipogenesis Induced by beta3-Adrenergic Receptor Activation with Single-Cell Expression Profiling. Cell Metab. 2018, 28, 300–309.e4. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, R.; Rainer, P.; Deplancke, B. Toward a Consensus View of Mammalian Adipocyte Stem and Progenitor Cell Heterogeneity. Trends Cell Biol. 2020, 30, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Bochet, L.; Lehuede, C.; Dauvillier, S.; Wang, Y.Y.; Dirat, B.; Laurent, V.; Dray, C.; Guiet, R.; Maridonneau-Parini, I.; Le Gonidec, S.; et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013, 73, 5657–5668. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S.; Bjorklund, G. Can Wnt5a and Wnt non-canonical pathways really mediate adipocyte de-differentiation in a tumour microenvironment? Eur. J. Cancer 2016, 64, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Iyoshi, S.; Yoshihara, M.; Nakamura, K.; Sugiyama, M.; Koya, Y.; Kitami, K.; Uno, K.; Mogi, K.; Tano, S.; Tomita, H.; et al. Pro-tumoral behavior of omental adipocyte-derived fibroblasts in tumor microenvironment at the metastatic site of ovarian cancer. Int. J. Cancer 2021, 149, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).