Spontaneous Occurrence of Various Types of Hepatocellular Adenoma in the Livers of Metabolic Syndrome-Associated Steatohepatitis Model TSOD Mice
Abstract
1. Introduction
1.1. HNF1α-Inactivated HCA (H-HCA)
1.2. Inflammatory HCA (IHCA)
1.3. β-Catenin-Activated HCA (b-HCA)
1.4. β-Catenin-Activated IHCA (b-IHCA)
2. Results
2.1. Frequency of Liver Tumor
2.2. Characteristic Histopathological and Immunohistochemical Findings of GS-Positive Liver Tumors
2.3. Characteristics of GS-Negative Liver Tumors
2.3.1. HNF1a-Inactivated HCA Tumor Mimics
2.3.2. Inflammatory HCA Tumor Mimics
2.3.3. Both HNF1 α-Inactivated HCA and Inflammatory HCA Tumor Mimic
2.3.4. B-Catenin-Activated IHCA (B-IHCA) Tumor Mimic
3. Materials and Methods
4. Conclusions
4.1. Investigation of the Molecular Biological Features and Microenvironmental Differences Underlying the Development of HCA Subtypes
4.2. Investigation of Carcinogenic Mechanism for HCA
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takahashi, T.; Deuschle, U.; Taira, S.; Nishida, T.; Fujimoto, M.; Hijikata, T.; Tsuneyama, K. Tsumura-Suzuki obese diabetic mice-derived hepatic tumors closely resemble human hepatocellular carcinomas in metabolism-related genes expression and bile acid accumulation. Hepatol. Int. 2018, 12, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Nishida, T.; Baba, H.; Hatta, H.; Imura, J.; Sutoh, M.; Toyohara, S.; Hokao, R.; Watanabe, S.; Ogawa, H.; et al. Histopathological characteristics of glutamine synthetase-positive hepatic tumor lesions in a mouse model of spontaneous metabolic syndrome (TSOD mouse). Mol. Clin. Oncol. 2016, 5, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Tsuneyama, K.; Fujimoto, M.; Nomoto, K.; Hayashi, S.; Miwa, S.; Nakajima, T.; Nakanishi, Y.; Sasaki, Y.; Suzuki, W.; et al. Spontaneous onset of nonalcoholic steatohepatitis and hepatocellular carcinoma in a mouse model of metabolic syndrome. Lab. Investig. 2013, 93, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Beaufrère, A.; Paradis, V. Hepatocellular adenomas: Review of pathological and molecular features. Hum. Pathol. 2021, 112, 128–137. [Google Scholar] [CrossRef]
- Shafizadeh, N.; Genrich, G.; Ferrell, L.; Kakar, S. Hepatocellular adenomas in a large community population, 2000 to 2010: Reclassification per current World Health Organization classification and results of long-term follow-up. Hum. Pathol. 2014, 45, 976–983. [Google Scholar] [CrossRef]
- Moors, G.; Poels, H.; Vandecaveye, V.; Roskams, T.; Verslype, C. Regression of multiple hepatocellular adenomas after cessation contraceptive pills: A case report and review of the current literature. Acta Gastro-Enterol. Belg. 2021, 84, 505–508. [Google Scholar] [CrossRef]
- Kakar, S.; Grenert, J.P.; Paradis, V.; Pote, N.; Jakate, S.; Ferrell, L.D. Hepatocellular carcinoma arising in adenoma: Similar immunohistochemical and cytogenetic features in adenoma and hepatocellular carcinoma portions of the tumor. Mod. Pathol. 2014, 27, 1499–1509. [Google Scholar] [CrossRef]
- Ridder, D.A.; Duret, D.; Wörns, M.A.; Schotten, S.; Heinrich, S.; Kloth, M.; Springer, E.; Roth, W.; Straub, B.K. Hepatocellular carcinoma in an inflammatory adenoma with β-catenin mutation in a patient with hepatocellular adenomatosis due to germline mutation in HNF1α. Z. Fur Gastroenterol. 2019, 57, 46–51. [Google Scholar]
- Julien, C.; le Bail, B.; Chiche, L.; Balabaud, C.; Bioulac-Sage, P. Malignant transformation of hepatocellular adenoma. JHEP Rep. 2022, 4, 100430. [Google Scholar] [CrossRef]
- Kang, H.J.; Jeong, H.J.; Kim, S.W.; Yu, E.; Lee, Y.J.; Kim, S.Y.; Kim, J. Hepatocellular carcinoma arising in a huge hepatocellular adenoma with bone marrow metaplasia. J. Pathol. Transl. Med. 2018, 52, 226–231. [Google Scholar] [CrossRef]
- Zucman-Rossi, J. Human and mouse hepatocellular adenoma and carcinoma display similar tumorigenesis pathway alterations. J. Hepatol. 2008, 48, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, C.; Ip, B.C.; Hu, K.-Q.; Smith, D.E.; Greenberg, A.S.; Wang, X.-D. Tumor progression locus 2 ablation suppressed hepatocellular carcinoma development by inhibiting hepatic inflammation and steatosis in mice. J. Exp. Clin. Cancer Res. 2015, 34, 138. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Fang, X.; Tajima, A.; Geng, X.; Ranganathan, S.; Dong, H.; Trucco, M.; Sperling, M.A. Evolution of hepatic steatosis to fibrosis and adenoma formation in liver-specific growth hormone receptor knockout mice. Front. Endocrinol. 2014, 5, 218. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.Y.; Kwon, J.H.; Cho, J.H.; Zhang, L.; Mansfield, B.C.; Chou, J.Y. Downregulation of pathways implicated in liver inflammation and tumorigenesis of glycogen storage disease type Ia mice receiving gene therapy. Hum. Mol. Genet. 2017, 26, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Davis, C.; Shah, S.; Hughes, D.; Ryan, J.C.; Altomare, D.; Peña, M.M.O. IL-33 promotes growth and liver metastasis of colorectal cancer in mice by remodeling the tumor microenvironment and inducing angiogenesis. Mol. Carcinog. 2017, 56, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Paradis, V.; Cherqui, D.; Vilgrain, V.; Zucman-Rossi, J. Molecular classification of hepatocellular adenoma in clinical practice. J. Hepatol. 2017, 67, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, S.; Fiel, M.I. Update on the new classification of hepatic adenomas: Clinical, molecular, and pathologic characteristics. Arch. Pathol. Lab. Med. 2014, 138, 1090–1097. [Google Scholar] [CrossRef]
- Bioulac-Sage, P.; Sempoux, C.; Balabaud, C. Hepatocellular adenoma: Classification, variants and clinical relevance. Semin. Diagn. Pathol. 2017, 34, 112–125. [Google Scholar] [CrossRef]
- Yasir, S.; Chen, Z.E.; Jain, D.; Kakar, S.; Wu, T.T.; Yeh, M.M.; Torbenson, M.S. Hepatic denomas in Patients 60 and Older Are Enriched for HNF1A Inactivation and alignant Transformation. Am. J. Surg. Pathol. 2022, 46, 786–792. [Google Scholar] [CrossRef]
- Izu, A.; Sugitani, M.; Kinukawa, N.; Matsumura, H.; Ogawa, M.; Moriyama, M.; Yamazaki, S.; Takayama, T.; Hano, H.; Yao, T.; et al. Hepatocellular adenoma, approximately half and predominantly inflammatory subtype, in 38 Japanese patients with several differences in age, gender, and clinical background factors from Western populations. Hepatol. Res. 2021, 51, 336–342. [Google Scholar] [CrossRef]
- Brunt, E.M.; Sempoux, C.; Bioulac-Sage, P. Hepatocellular adenomas: The expanding pidemiology. Histopathology 2021, 79, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Bioulac-Sage, P.; Gouw, A.S.H.; Balabaud, C.; Sempoux, C. Hepatocellular adenoma: What we know, what we do not know, and why it matters. Histopathology 2022, 80, 878–897. [Google Scholar] [CrossRef] [PubMed]
- Tsuneyama, K.; Nishitsuji, K.; Matsumoto, M.; Kobayashi, T.; Morimoto, Y.; Tsunematsu, T.; Ogawa, H. Animal models for analyzing metabolic syndrome-associated liver diseases. Pathol. Int. 2017, 67, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Tsuneyama, K.; Fujimoto, M.; Nomoto, K.; Hayashi, S.; Miwa, S.; Nakajima, T.; Nakanishi, Y.; Hatta, H.; Imura, J. Aberrant iron metabolism might have an impact on progression of diseases in Tsumura Suzuki obese diabetes mice, a model of spontaneous metabolic syndrome. Pathol. Int. 2016, 66, 622–628. [Google Scholar] [CrossRef]
- Resaz, R.; Rosa, F.; Grillo, F.; Basso, L.; Segalerba, D.; Puglisi, A.; Bosco, M.C.; Mastracci, L.; Neumaier, C.E.; Varesio, L. Characterization of high- and low-risk hepatocellular adenomas by magnetic resonance imaging in an animal model of glycogen storage disease type 1A. Dis. Model Mech. 2019, 12, dmm038026. [Google Scholar] [CrossRef]
- Köhler, U.A.; Böhm, F.; Rolfs, F.; Egger, M.; Hornemann, T.; Pasparakis, M.; Weber, A.; Werner, S. NF-κB/RelA and Nrf2 cooperate to maintain hepatocyte integrity and to prevent development of hepatocellular adenoma. J. Hepatol. 2016, 64, 94–102. [Google Scholar] [CrossRef]
- Amano, Y.; Shimizu, F.; Yasuno, H.; Harada, A.; Tsuchiya, S.; Isono, O.; Nagabukuro, H.; Tozawa, R. Non-alcoholic steatohepatitis-associated hepatic fibrosis and hepatocellular carcinoma in a combined mouse model of genetic modification and dietary challenge. Hepatol. Res. 2017, 47, 103–115. [Google Scholar] [CrossRef]

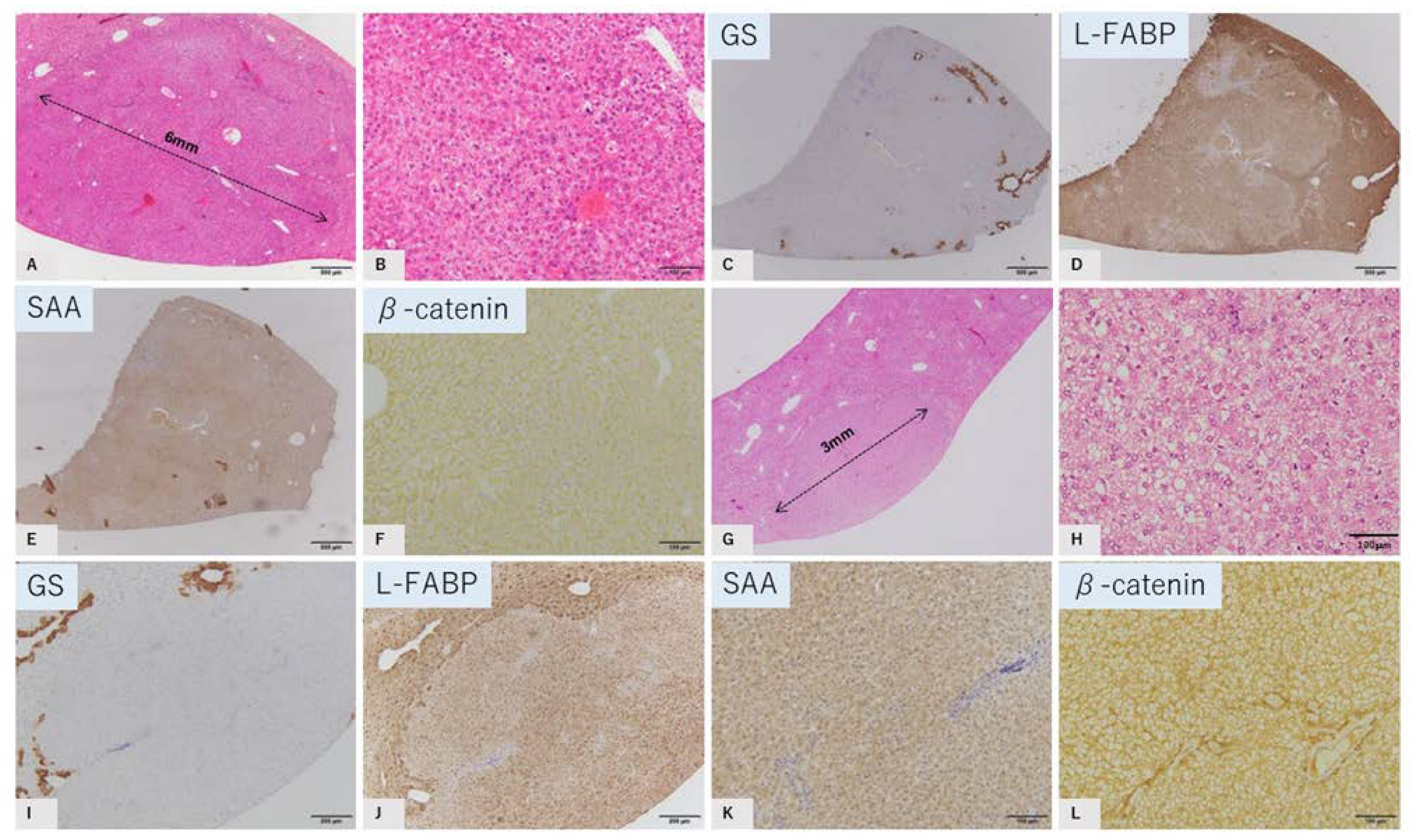
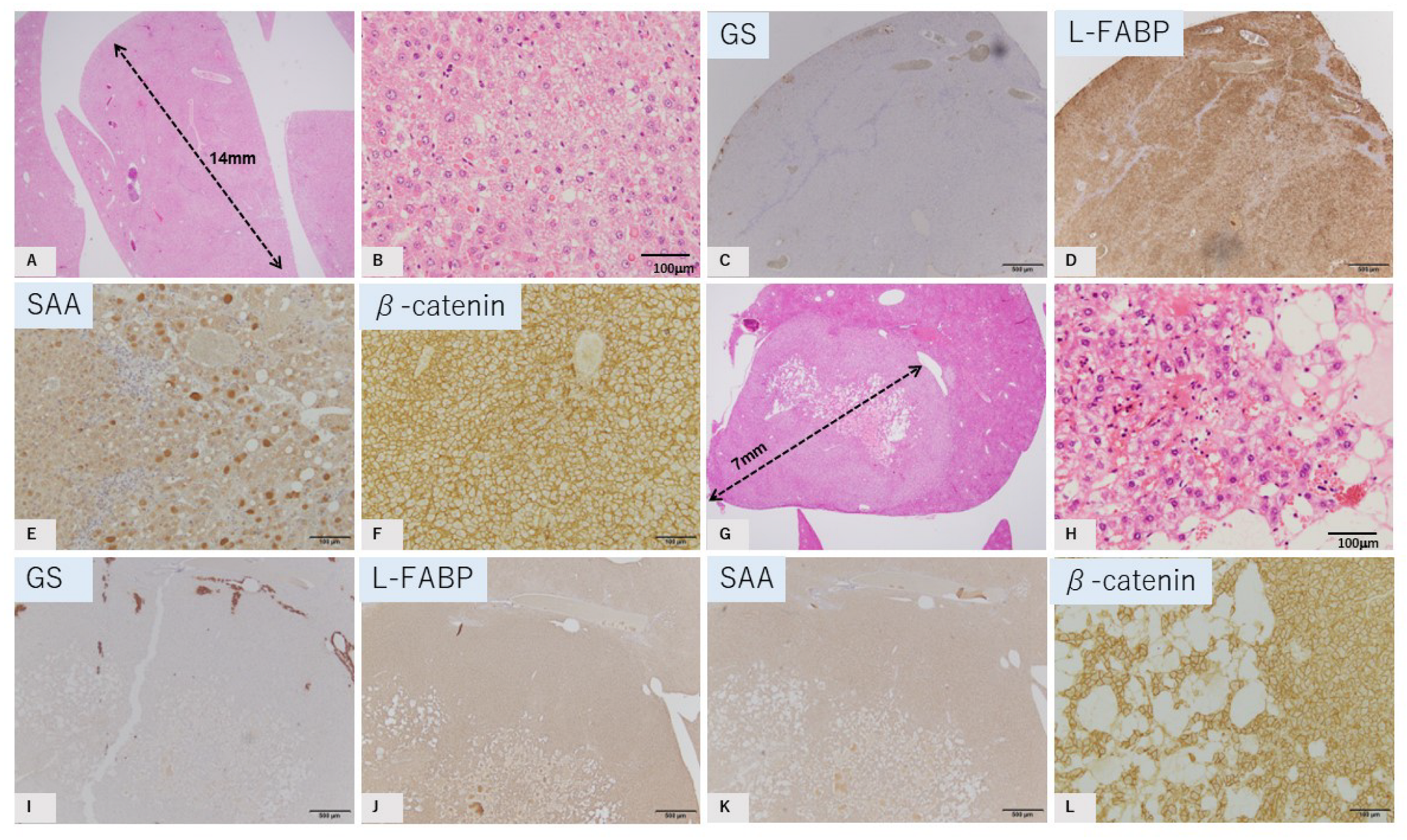

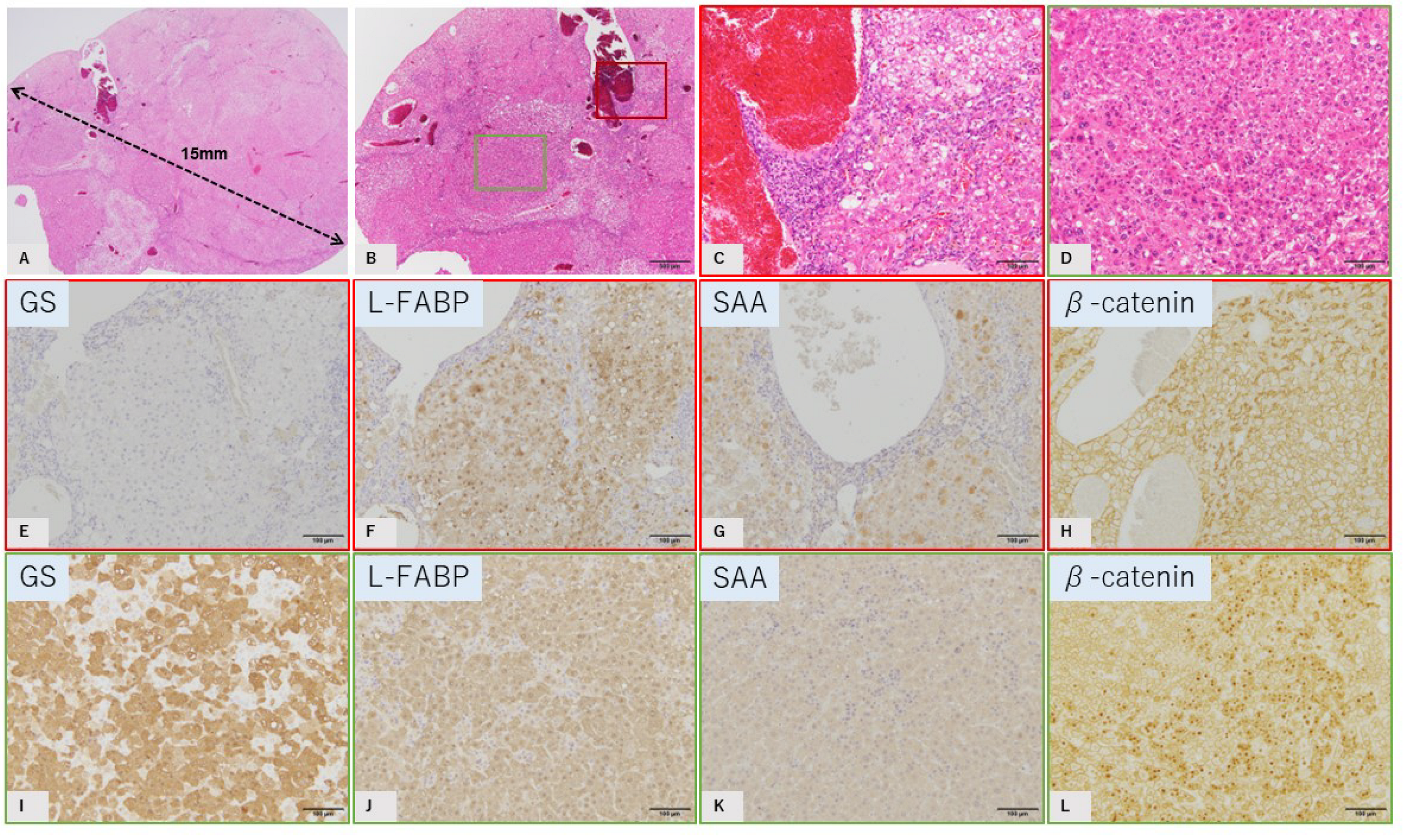
| No | Diameter | GS | L-FABP | SAA | β-Catenin |
|---|---|---|---|---|---|
| 1 | 7 mm | Negative | Reduced | Positive | Membrane |
| 2 | 14 mm | Negative | Positive | Positive | Membrane |
| 3 | 6 mm | Negative | Reduced | Negative | Membrane |
| 4 | 7 mm | Negative | Positive | Negative | Membrane |
| 5 | 3 mm | Negative | Reduced | Negative | Membrane |
| 6: Major | 15 mm | Negative | Positive | Positive | Membrane |
| 6: focal | 2 mm × 2 | Positive | Positive | Negative | Nucleus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, W.; Jargalsaikhan, O.; Ichimura-Shimizu, M.; Cai, Q.; Ogawa, H.; Miyakami, Y.; Atsumi, K.; Tomita, M.; Sutoh, M.; Toyohara, S.; et al. Spontaneous Occurrence of Various Types of Hepatocellular Adenoma in the Livers of Metabolic Syndrome-Associated Steatohepatitis Model TSOD Mice. Int. J. Mol. Sci. 2022, 23, 11923. https://doi.org/10.3390/ijms231911923
Shao W, Jargalsaikhan O, Ichimura-Shimizu M, Cai Q, Ogawa H, Miyakami Y, Atsumi K, Tomita M, Sutoh M, Toyohara S, et al. Spontaneous Occurrence of Various Types of Hepatocellular Adenoma in the Livers of Metabolic Syndrome-Associated Steatohepatitis Model TSOD Mice. International Journal of Molecular Sciences. 2022; 23(19):11923. https://doi.org/10.3390/ijms231911923
Chicago/Turabian StyleShao, Wenhua, Orgil Jargalsaikhan, Mayuko Ichimura-Shimizu, Qinyi Cai, Hirohisa Ogawa, Yuko Miyakami, Kengo Atsumi, Mitsuru Tomita, Mitsuko Sutoh, Shunji Toyohara, and et al. 2022. "Spontaneous Occurrence of Various Types of Hepatocellular Adenoma in the Livers of Metabolic Syndrome-Associated Steatohepatitis Model TSOD Mice" International Journal of Molecular Sciences 23, no. 19: 11923. https://doi.org/10.3390/ijms231911923
APA StyleShao, W., Jargalsaikhan, O., Ichimura-Shimizu, M., Cai, Q., Ogawa, H., Miyakami, Y., Atsumi, K., Tomita, M., Sutoh, M., Toyohara, S., Hokao, R., Kudo, Y., Oya, T., & Tsuneyama, K. (2022). Spontaneous Occurrence of Various Types of Hepatocellular Adenoma in the Livers of Metabolic Syndrome-Associated Steatohepatitis Model TSOD Mice. International Journal of Molecular Sciences, 23(19), 11923. https://doi.org/10.3390/ijms231911923







