Transcriptional and Epigenomic Markers of the Arterial-Venous and Micro/Macro-Vascular Endothelial Heterogeneity within the Umbilical-Placental Bed
Abstract
1. Introduction
2. Results
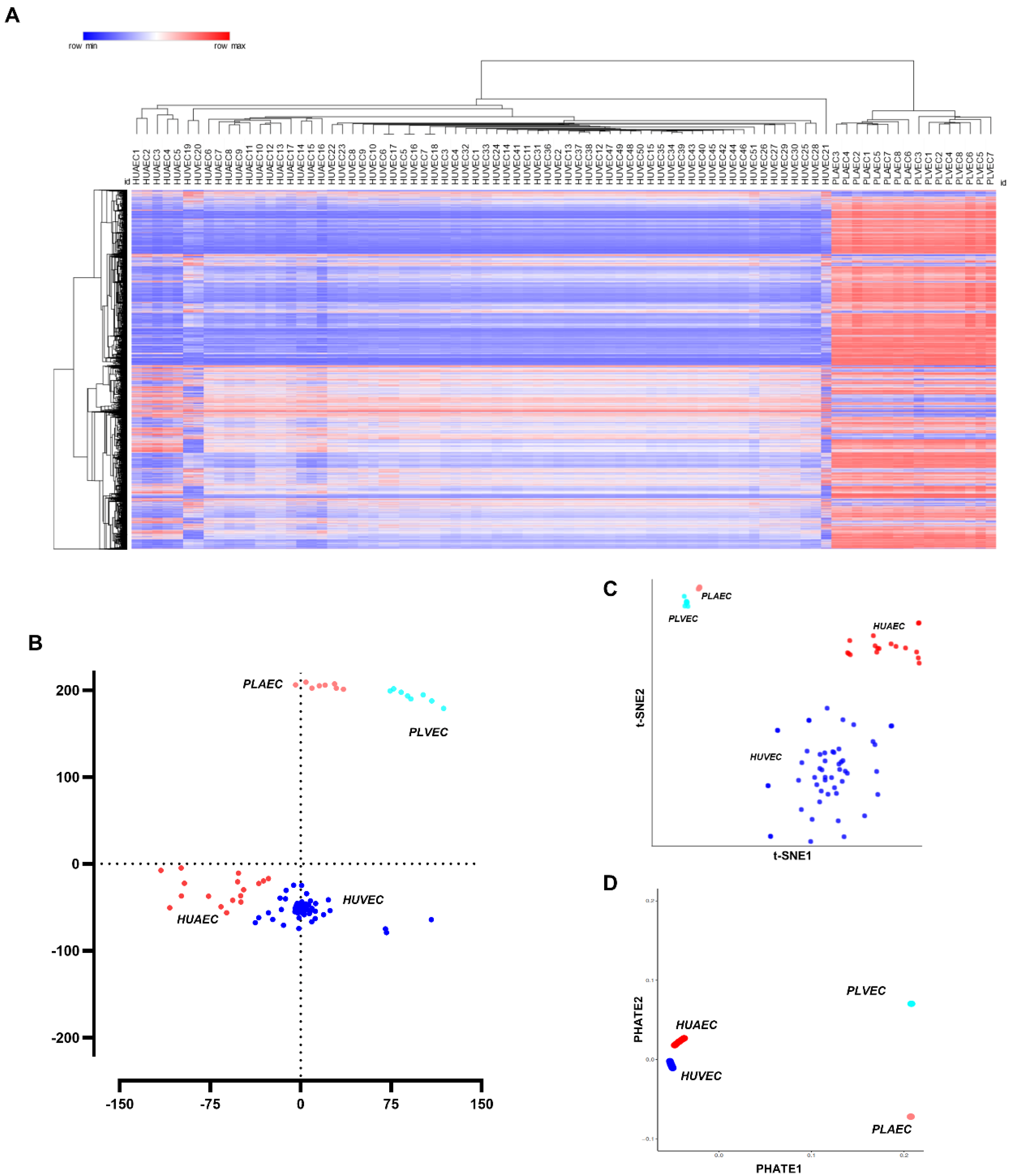
2.1. Transcriptional Profiling of Umbilical and Placental Endothelial Cells
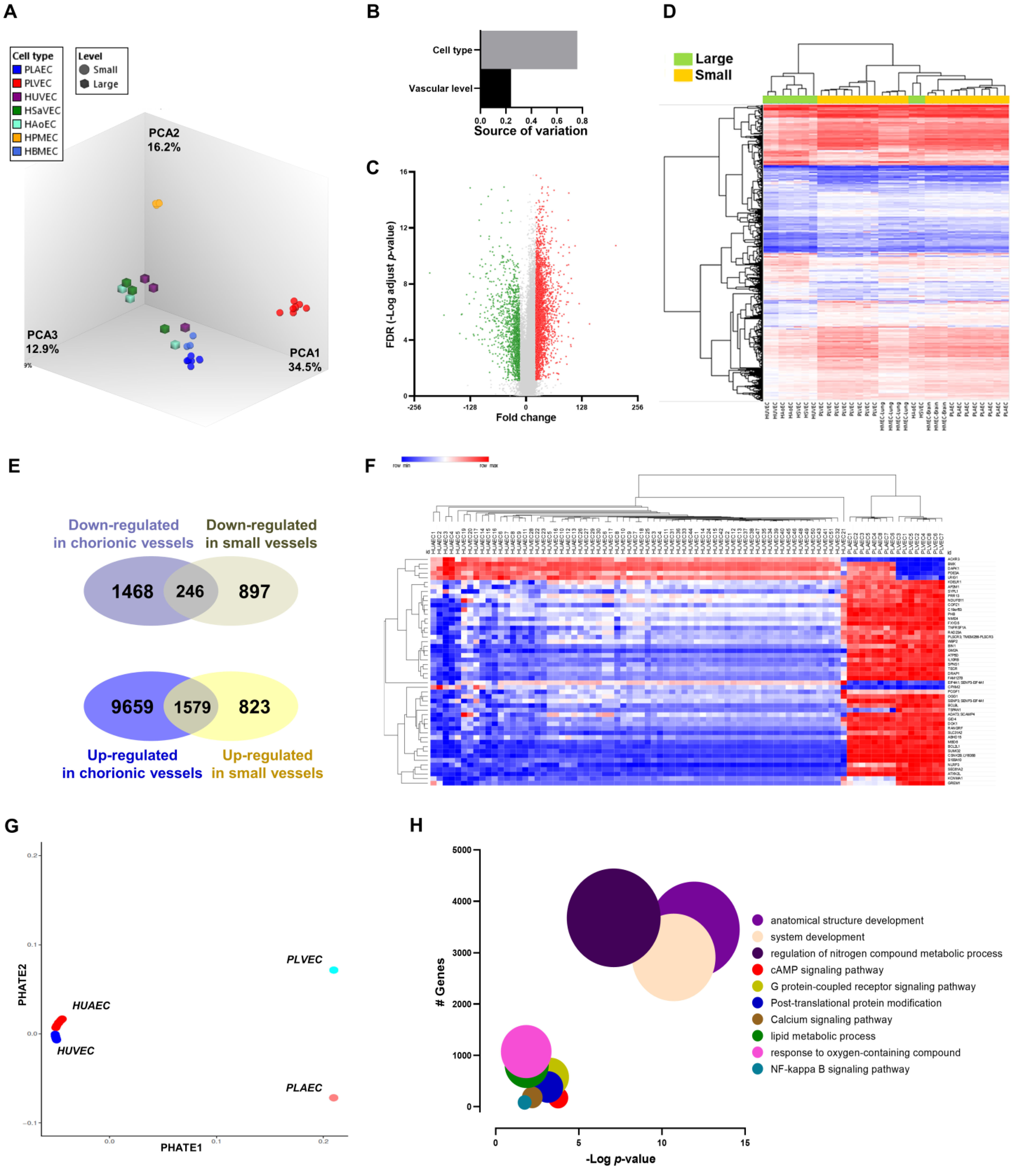
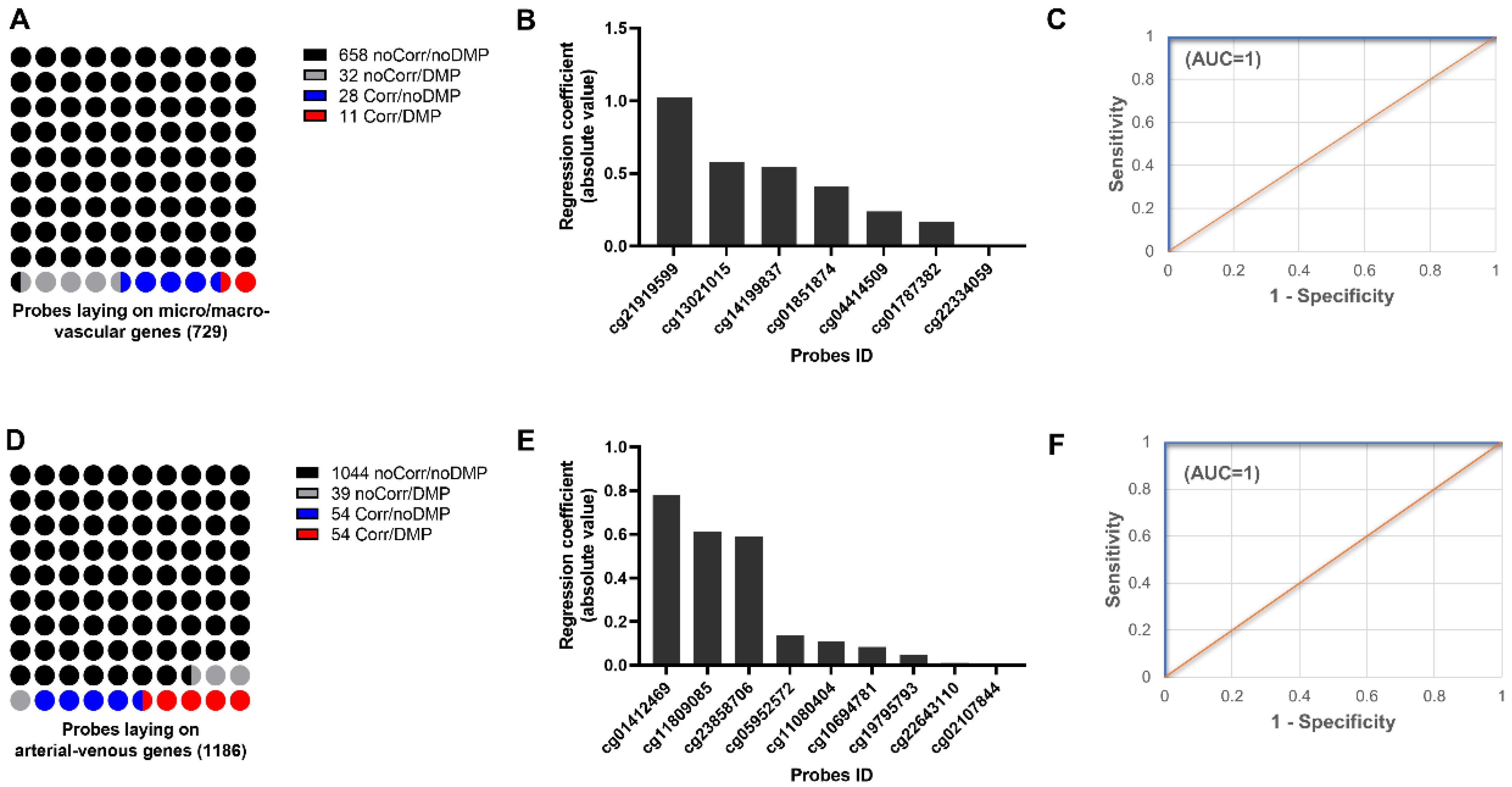
2.2. Discovering Microvascular-Specific Transcripts
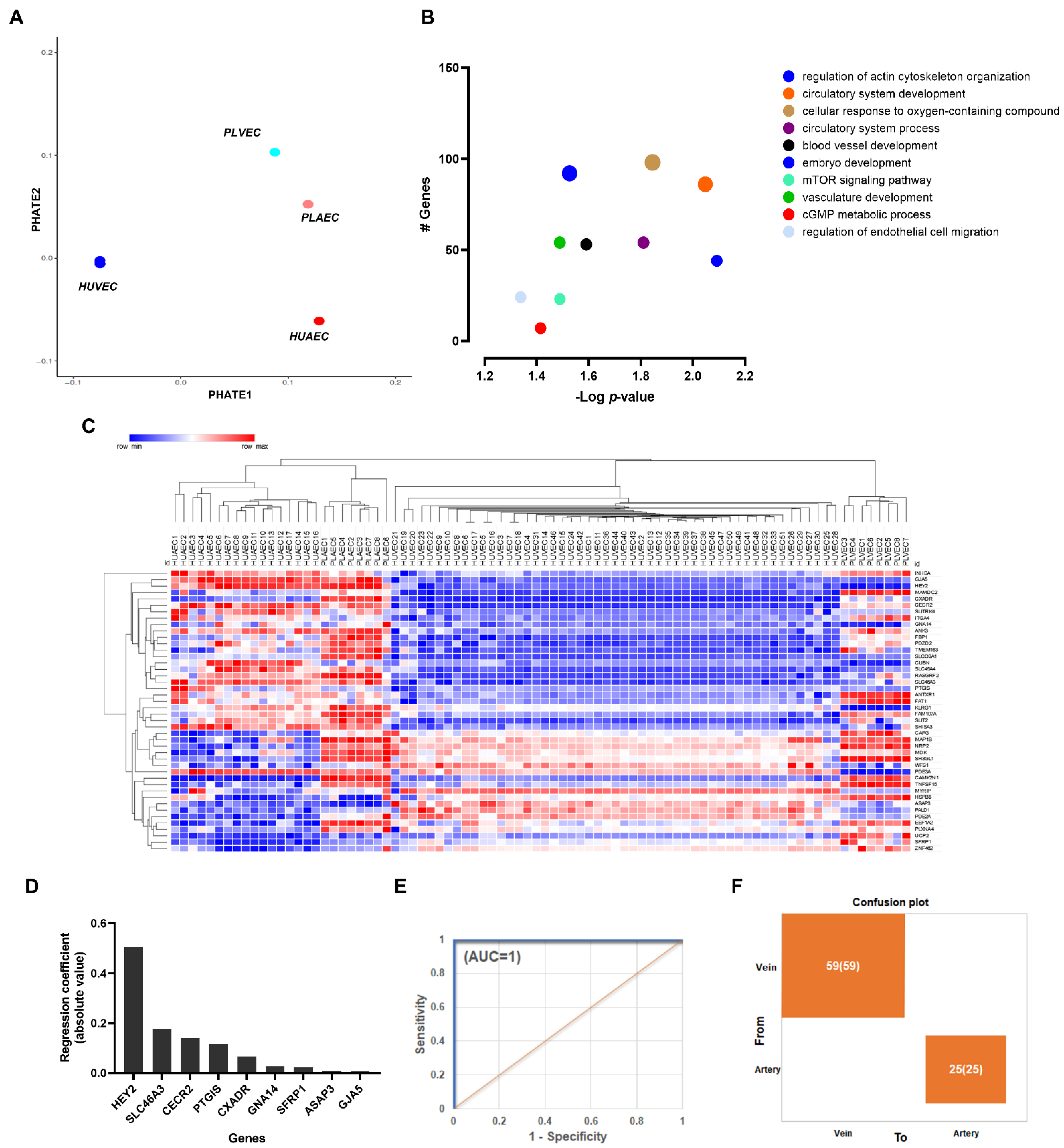
2.3. Transcriptional Markers of Arterial-Venous Identity for Umbilical and Placental Vessels
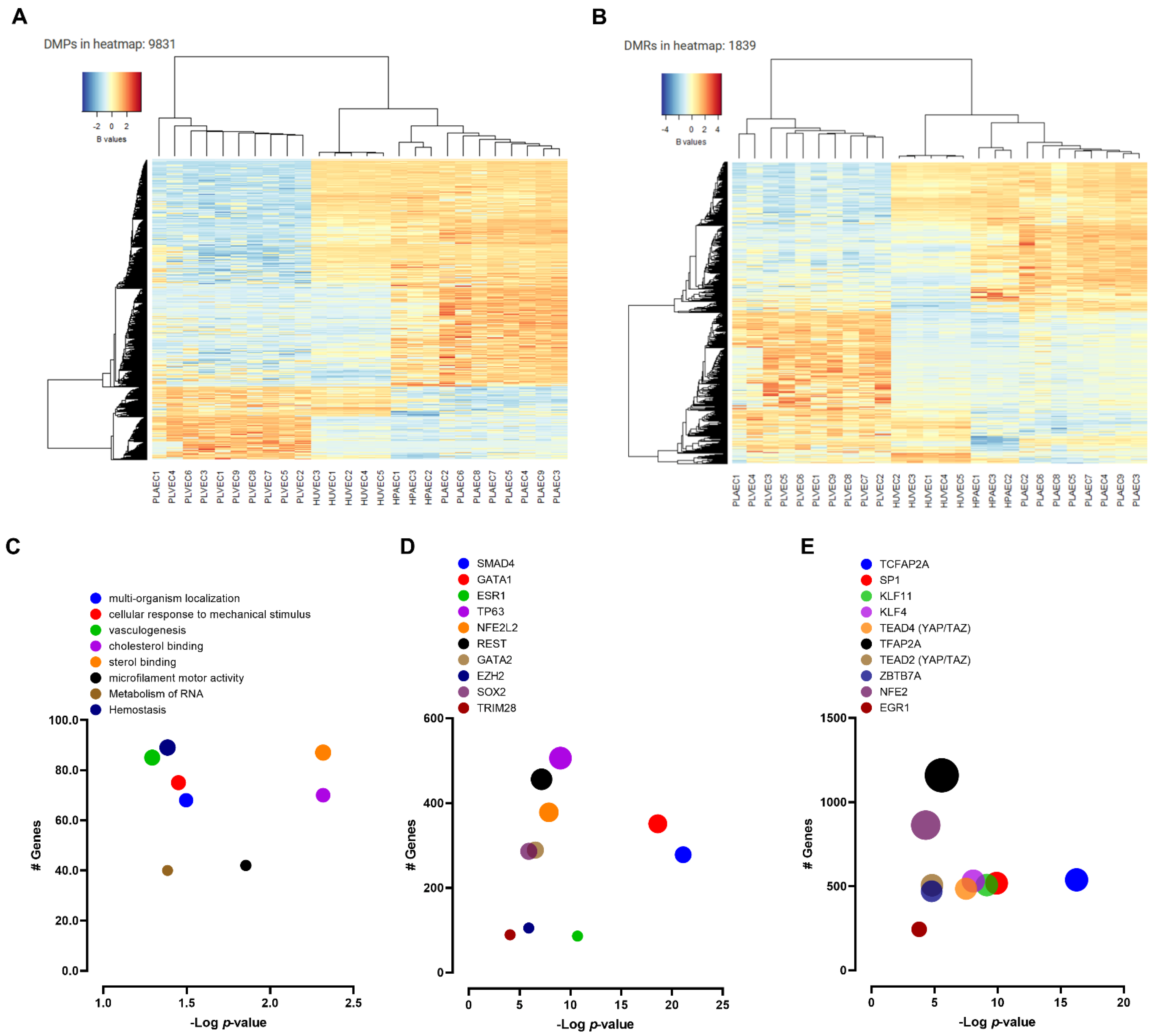
2.4. DNA Methylation Profiling of Fetal and Placental EC
2.5. Validation of Epigenetic Markers for Fetal, Umbilical, and Placental EC Heterogeneity
3. Discussion
4. Methods
4.1. Transcriptional Profiling
4.2. DNA Methylation Profiling
4.3. Functional Enrichment
4.4. Clustering and Dimensionality Reduction
4.5. Discovery and Validation of Molecular Markers
4.6. Statistical Analysis
4.7. Data Availability
4.8. Code Availability
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krause, B.J. Novel insights for the role of nitric oxide in placental vascular function during and beyond pregnancy. J. Cell. Physiol. 2021, 236(12), 7984–7999. [Google Scholar] [CrossRef] [PubMed]
- Casanello, P.; Schneider, D.; Herrera, E.A.; Uauy, R.; Krause, B.J. Endothelial heterogeneity in the umbilico-placental unit: DNA methylation as an innuendo of epigenetic diversity. Front. Pharmacol. 2014, 5, 49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aird, W.C. Endothelial cell heterogeneity. Cold Spring Harb. Perspect. Med. 2012, 2, a006429. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.W.; Baluk, P.; Pan, L.; Kwan, M.; Holash, J.; DeChiara, T.M.; McDonald, D.M.; Yancopoulos, G.D. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev. Biol. 2001, 230, 151–160. [Google Scholar] [CrossRef]
- Foo, S.S.; Turner, C.J.; Adams, S.; Compagni, A.; Aubyn, D.; Kogata, N.; Lindblom, P.; Shani, M.; Zicha, D.; Adams, R.H. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 2006, 124, 161–173. [Google Scholar] [CrossRef]
- Matouk, C.C.; Marsden, P.A. Epigenetic regulation of vascular endothelial gene expression. Circ. Res. 2008, 102, 873–887. [Google Scholar] [CrossRef]
- Sissaoui, S.; Yu, J.; Yan, A.; Li, R.; Yukselen, O.; Kucukural, A.; Zhu, L.J.; Lawson, N.D. Genomic Characterization of Endothelial Enhancers Reveals a Multifunctional Role for NR2F2 in Regulation of Arteriovenous Gene Expression. Circ. Res. 2020, 126, 875–888. [Google Scholar] [CrossRef]
- Vega-Tapia, F.; Peñaloza, E.; Krause, B.J. Specific arterio-venous transcriptomic and ncRNA-RNA-interactions in human umbilical endothelial cells: A meta-analysis. iScience, 2021, in press. [CrossRef]
- Kalucka, J.; de Rooij, L.; Goveia, J.; Rohlenova, K.; Dumas, S.J.; Meta, E.; Conchinha, N.V.; Taverna, F.; Teuwen, L.A.; Veys, K.; et al. Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell 2020, 180, 764–779.e20. [Google Scholar] [CrossRef] [PubMed]
- Cleuren, A.C.A.; van der Ent, M.A.; Jiang, H.; Hunker, K.L.; Yee, A.; Siemieniak, D.R.; Molema, G.; Aird, W.C.; Ganesh, S.K.; Ginsburg, D. The in vivo endothelial cell translatome is highly heterogeneous across vascular beds. Proc. Natl. Acad. Sci. USA 2019, 116, 23618–23624. [Google Scholar] [CrossRef]
- Jambusaria, A.; Hong, Z.; Zhang, L.; Srivastava, S.; Jana, A.; Toth, P.T.; Dai, Y.; Malik, A.B.; Rehman, J. Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. Elife 2020, 9, e51413. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Ruggieri, S.; Annese, T.; Crivellato, E. Surface markers: An identity card of endothelial cells. Microcirculation 2020, 27, e12587. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.; Hartmann, M.; Blaschitz, A.; Dohr, G.; Skofitsch, G.; Desoye, G. Immunohistochemical evidence for the heterogeneity of maternal and fetal vascular endothelial cells in human full-term placenta. Cell Tissue Res. 1993, 274, 211–218. [Google Scholar] [CrossRef]
- Lang, I.; Schweizer, A.; Hiden, U.; Ghaffari-Tabrizi, N.; Hagendorfer, G.; Bilban, M.; Pabst, M.A.; Korgun, E.T.; Dohr, G.; Desoye, G. Human fetal placental endothelial cells have a mature arterial and a juvenile venous phenotype with adipogenic and osteogenic differentiation potential. Differentiation 2008, 76, 1031–1043. [Google Scholar] [CrossRef]
- Joo, J.E.; Hiden, U.; Lassance, L.; Gordon, L.; Martino, D.J.; Desoye, G.; Saffery, R. Variable promoter methylation contributes to differential expression of key genes in human placenta-derived venous and arterial endothelial cells. BMC Genom. 2013, 14, 475. [Google Scholar] [CrossRef] [PubMed]
- Erlich, A.; Pearce, P.; Mayo, R.P.; Jensen, O.E.; Chernyavsky, I.L. Physical and geometric determinants of transport in fetoplacental microvascular networks. Sci. Adv. 2019, 5, eaav6326. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Charnock-Jones, D.S.; Jauniaux, E. Regulation of vascular growth and function in the human placenta. Reproduction 2009, 138, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.U.; Chen, Z.F.; Anderson, D.J. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 1998, 93, 741–753. [Google Scholar] [CrossRef]
- Shin, D.; Garcia-Cardena, G.; Hayashi, S.; Gerety, S.; Asahara, T.; Stavrakis, G.; Isner, J.; Folkman, J.; Gimbrone, M.A., Jr.; Anderson, D.J. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev. Biol. 2001, 230, 139–150. [Google Scholar] [CrossRef]
- Lawson, N.D.; Scheer, N.; Pham, V.N.; Kim, C.H.; Chitnis, A.B.; Campos-Ortega, J.A.; Weinstein, B.M. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 2001, 128, 3675–3683. [Google Scholar] [CrossRef]
- Chen, X.; Qin, J.; Cheng, C.M.; Tsai, M.J.; Tsai, S.Y. COUP-TFII is a major regulator of cell cycle and Notch signaling pathways. Mol. Endocrinol. 2012, 26, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- You, L.R.; Lin, F.J.; Lee, C.T.; DeMayo, F.J.; Tsai, M.J.; Tsai, S.Y. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 2005, 435, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Lang, I. Heterogeneity of microvascular endothelial cells isolated from human term placenta and macrovascular umbilical vein endothelial cells. Eur. J. Cell Biol. 2003, 82, 163–173. [Google Scholar] [CrossRef]
- Bueter, W.; Dammann, O.; Zscheppang, K.; Korenbaum, E.; Dammann, C.E. ErbB receptors in fetal endothelium--a potential linkage point for inflammation-associated neonatal disorders. Cytokine 2006, 36, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Murthi, P.; Hiden, U.; Rajaraman, G.; Liu, H.; Borg, A.J.; Coombes, F.; Desoye, G.; Brennecke, S.P.; Kalionis, B. Novel homeobox genes are differentially expressed in placental microvascular endothelial cells compared with macrovascular cells. Placenta 2008, 29, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jia, L.; Qian, Z.; Jia, Y.; Chen, X.; Xu, X.; Chang, X.; Liu, M.; Wang, K. Diversity in human placental microvascular endothelial cells and macrovascular endothelial cells. Cytokine 2018, 111, 287–294. [Google Scholar] [CrossRef]
- Pavlicev, M.; Wagner, G.P.; Chavan, A.R.; Owens, K.; Maziarz, J.; Dunn-Fletcher, C.; Kallapur, S.G.; Muglia, L.; Jones, H. Single-cell transcriptomics of the human placenta: Inferring the cell communication network of the maternal-fetal interface. Genome Res. 2017, 27, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Sendler, E.D.; Xu, Y.; Garcia-Flores, V.; Leng, Y.; Luca, F.; Hassan, S.S.; Gomez-Lopez, N. Single cell transcriptional signatures of the human placenta in term and preterm parturition. Elife 2019, 8, e52004. [Google Scholar] [CrossRef] [PubMed]
- Ugele, B.; Lange, F. Isolation of endothelial cells from human placental microvessels: Effect of different proteolytic enzymes on releasing endothelial cells from villous tissue. Vitr. Cell. Dev. Biology. Anim. 2001, 37, 408–413. [Google Scholar] [CrossRef]
- Nakato, R.; Wada, Y.; Nakaki, R.; Nagae, G.; Katou, Y.; Tsutsumi, S.; Nakajima, N.; Fukuhara, H.; Iguchi, A.; Kohro, T.; et al. Comprehensive epigenome characterization reveals diverse transcriptional regulation across human vascular endothelial cells. Epigenetics Chromatin 2019, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Krause, B.J.; Costello, P.M.; Munoz-Urrutia, E.; Lillycrop, K.A.; Hanson, M.A.; Casanello, P. Role of DNA methyltransferase 1 on the altered eNOS expression in human umbilical endothelium from intrauterine growth restricted fetuses. Epigenetics Off. J. DNA Methylation Soc. 2013, 8, 944–952. [Google Scholar] [CrossRef]
- Herrera, E.A.; Cifuentes-Zuniga, F.; Figueroa, E.; Villanueva, C.; Hernandez, C.; Alegria, R.; Arroyo-Jousse, V.; Penaloza, E.; Farias, M.; Uauy, R.; et al. N-Acetylcysteine, a glutathione precursor, reverts vascular dysfunction and endothelial epigenetic programming in intrauterine growth restricted guinea pigs. J. Physiol. 2017, 595, 1077–1092. [Google Scholar] [CrossRef]
- Krause, B.J.; Penaloza, E.; Candia, A.; Canas, D.; Hernandez, C.; Arenas, G.A.; Peralta-Scholz, M.J.; Valenzuela, R.; Garcia-Herrera, C.; Herrera, E.A. Adult vascular dysfunction in foetal growth-restricted guinea-pigs is associated with a neonate-adult switching in Nos3 DNA methylation. Acta Physiol. 2019, 227, e13328. [Google Scholar] [CrossRef]
- Postberg, J.; Kanders, M.; Forcob, S.; Willems, R.; Orth, V.; Hensel, K.O.; Weil, P.P.; Wirth, S.; Jenke, A.C. CpG signalling, H2A.Z/H3 acetylation and microRNA-mediated deferred self-attenuation orchestrate foetal NOS3 expression. Clin. Epigenetics 2015, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Long, T.I.; Arakawa, K.; Wang, R.; Yu, M.C.; Laird, P.W. DNA methylation as a biomarker for cardiovascular disease risk. PLoS ONE 2010, 5, e9692. [Google Scholar] [CrossRef]
- Tumova, S.; Kerimi, A.; Porter, K.E.; Williamson, G. Transendothelial glucose transport is not restricted by extracellular hyperglycaemia. Vascul. Pharmacol. 2016, 87, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Fruhwurth, S.; Pavelka, M.; Bittman, R.; Kovacs, W.J.; Walter, K.M.; Rohrl, C.; Stangl, H. High-density lipoprotein endocytosis in endothelial cells. World J. Biol. Chem. 2013, 4, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yang, X.; Jia, X.; Rong, Y.; Chen, L.; Zeng, T.; Deng, X.; Li, W.; Wu, G.; Wang, L.; et al. CAV1-CAVIN1-LC3B-mediated autophagy regulates high glucose-stimulated LDL transcytosis. Autophagy 2020, 16, 1111–1129. [Google Scholar] [CrossRef]
- De Val, S.; Black, B.L. Transcriptional control of endothelial cell development. Dev. Cell 2009, 16, 180–195. [Google Scholar] [CrossRef]
- Dobrzycki, T.; Lalwani, M.; Telfer, C.; Monteiro, R.; Patient, R. The roles and controls of GATA factors in blood and cardiac development. IUBMB Life 2020, 72, 39–44. [Google Scholar] [CrossRef]
- Meadows, S.M.; Myers, C.T.; Krieg, P.A. Regulation of endothelial cell development by ETS transcription factors. Semin. Cell Dev. Biol. 2011, 22, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Morante-Palacios, O.; Ballestar, E. shinyEPICo: A graphical pipeline to analyze Illumina DNA methylation arrays. Bioinformatics 2021, 37, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Kuan, P.F. methylGSA: A Bioconductor package and Shiny app for DNA methylation data length bias adjustment in gene set testing. Bioinformatics 2019, 35, 1958–1959. [Google Scholar] [CrossRef] [PubMed]
- Maksimovic, J.; Oshlack, A.; Phipson, B. Gene set enrichment analysis for genome-wide DNA methylation data. Genome Biol. 2021, 22, 173. [Google Scholar] [CrossRef]
- Anowar, F.; Sadaoui, S.; Selim, B. Conceptual and empirical comparison of dimensionality reduction algorithms (PCA, KPCA, LDA, MDS, SVD, LLE, ISOMAP, LE, ICA, t-SNE). Comput. Sci. Rev. 2021, 40, 100378. [Google Scholar] [CrossRef]
- Moon, K.R.; van Dijk, D.; Wang, Z.; Gigante, S.; Burkhardt, D.B.; Chen, W.S.; Yim, K.; Elzen, A.V.D.; Hirn, M.J.; Coifman, R.R.; et al. Visualizing structure and transitions in high-dimensional biological data. Nat. Biotechnol. 2019, 37, 1482–1492. [Google Scholar] [CrossRef]

| Gene | Probe ID | r2 | p-Value |
|---|---|---|---|
| ACKR3 | cg27529004 | 0.5049 | 0.002 |
| cg26960322 | 0.4845 | 0.0027 | |
| cg27529004 | 0.4179 | 0.0068 | |
| ATP5D | cg25732045 | 0.5784 | 0.0006 |
| C14orf2 | cg24129873 | 0.4053 | 0.008 |
| CFH | cg23557926 | 0.4591 | 0.0039 |
| DCXR | cg07847925 | 0.4022 | 0.0083 |
| MBD6 | cg09687907 | 0.402 | 0.0084 |
| HAS3 | cg00451194 | 0.6541 | 0.0001 |
| cg02562299 | 0.6135 | 0.0003 | |
| cg02730714 | 0.5877 | 0.0005 | |
| cg12108730 | 0.5639 | 0.0008 | |
| cg26818159 | 0.5668 | 0.0008 | |
| MBD6 | cg09687907 | 0.402 | 0.0084 |
| MYH10 | cg08493106 | 0.501 | 0.0022 |
| cg23163754 | 0.5323 | 0.0013 | |
| MECOM | cg01787382 | 0.6061 | 0.0004 |
| cg04414509 | 0.597 | 0.0005 | |
| cg12847986 | 0.4631 | 0.0037 | |
| cg13021015 | 0.4572 | 0.004 | |
| cg20201475 | 0.6523 | 0.0002 | |
| cg20203114 | 0.4009 | 0.0085 | |
| NLRP3 | cg11422335 | 0.5182 | 0.0017 |
| cg18183941 | 0.4599 | 0.0039 | |
| cg18793688 | 0.488 | 0.0026 | |
| cg21919599 | 0.4156 | 0.007 | |
| PCGF4 | cg18826743 | 0.3991 | 0.0087 |
| PLSCR3 | cg13775913 | 0.4792 | 0.003 |
| RUNX1T1 | cg00045118 | 0.4515 | 0.0044 |
| cg03760919 | 0.556 | 0.0009 | |
| cg07120544 | 0.5728 | 0.0007 | |
| cg18457433 | 0.5135 | 0.0018 | |
| cg22334059 | 0.6667 | 0.0001 | |
| SENP3 | cg01733795 | 0.5143 | 0.0018 |
| VPS72 | cg14199837 | 0.5746 | 0.0007 |
| cg22513511 | 0.5242 | 0.0015 | |
| cg24694326 | 0.6687 | 0.0001 | |
| WBP2 | cg19824334 | 0.5601 | 0.0009 |
| ZNF561 | cg23227837 | 0.5248 | 0.0015 |
| Gene | Probe ID | r2 | p-Value |
|---|---|---|---|
| ANTXR1 | cg00240205 | 0.6279 | 0.0003 |
| cg10803714 | 0.7891 | 0.0001 | |
| ASAP3 | cg20847090 | 0.5708 | 0.0007 |
| CUBN | cg11298524 | 0.4825 | 0.0028 |
| cg17436460 | 0.4577 | 0.004 | |
| CXADR | cg20541233 | 0.5772 | 0.0006 |
| EEF1A2 | cg10786876 | 0.4521 | 0.0043 |
| cg11080404 | 0.4124 | 0.0073 | |
| cg23858706 | 0.5094 | 0.0019 | |
| FAT1 | cg01337940 | 0.4239 | 0.0063 |
| cg01454936 | 0.571 | 0.0007 | |
| cg01585180 | 0.5632 | 0.0008 | |
| cg02325160 | 0.487 | 0.0026 | |
| cg02596645 | 0.4254 | 0.0062 | |
| cg02998591 | 0.5382 | 0.0012 | |
| cg03844826 | 0.5928 | 0.0005 | |
| cg08100122 | 0.4206 | 0.0066 | |
| cg08288016 | 0.4092 | 0.0076 | |
| cg11970085 | 0.3911 | 0.0096 | |
| cg12876620 | 0.476 | 0.0031 | |
| cg15374133 | 0.6197 | 0.0003 | |
| cg15815948 | 0.4592 | 0.0039 | |
| cg17755082 | 0.6884 | 0.0001 | |
| cg24792658 | 0.3975 | 0.0088 | |
| cg24820270 | 0.4635 | 0.0037 | |
| cg24890045 | 0.6032 | 0.0004 | |
| FBP1 | cg01210663 | 0.5108 | 0.0019 |
| cg13982688 | 0.4929 | 0.0024 | |
| GJA5 | cg02103822 | 0.4502 | 0.0044 |
| cg27546670 | 0.404 | 0.0081 | |
| cg00421368 | 0.4832 | 0.0028 | |
| cg06617692 | 0.4726 | 0.0033 | |
| cg14146751 | 0.4954 | 0.0023 | |
| cg14232851 | 0.4072 | 0.0078 | |
| GNA14 | cg14232851 | 0.4519 | 0.0043 |
| HEY2 | cg00066750 | 0.5169 | 0.0017 |
| cg22060817 | 0.6908 | 0.0001 | |
| HSPB8 | cg11187110 | 0.5173 | 0.0017 |
| cg16425829 | 0.6418 | 0.0002 | |
| cg24694702 | 0.5929 | 0.0005 | |
| cg05361406 | 0.5221 | 0.0016 | |
| INHBA | cg01412469 | 0.6064 | 0.0004 |
| ITGA4 | cg16057262 | 0.476 | 0.0031 |
| cg20415809 | 0.407 | 0.0078 | |
| KLRG1 | cg00443307 | 0.7357 | 0.0001 |
| cg14913610 | 0.7502 | 0.0001 | |
| cg26806779 | 0.8603 | 0.0001 | |
| MAMDC2 | cg13870494 | 0.583 | 0.0006 |
| cg14649449 | 0.5675 | 0.0008 | |
| MYRIP | cg23024967 | 0.3905 | 0.0096 |
| NRP2 | cg05348875 | 0.4383 | 0.0052 |
| cg14157435 | 0.4339 | 0.0055 | |
| cg01154445 | 0.4072 | 0.0078 | |
| cg19795793 | 0.3987 | 0.0087 | |
| cg26422981 | 0.3942 | 0.0092 | |
| PALD1 | cg01464515 | 0.5916 | 0.0005 |
| cg14683490 | 0.4362 | 0.0054 | |
| cg20418394 | 0.391 | 0.0096 | |
| cg22643110 | 0.6028 | 0.0004 | |
| PDE2A | cg05952572 | 0.4508 | 0.0044 |
| PDE3A | cg04724646 | 0.4541 | 0.0042 |
| cg12136731 | 0.816 | 0.0001 | |
| cg13063900 | 0.6577 | 0.0001 | |
| cg13446110 | 0.6396 | 0.0002 | |
| cg18639524 | 0.3884 | 0.0099 | |
| cg21913301 | 0.7737 | 0.0001 | |
| cg23015991 | 0.6685 | 0.0001 | |
| cg24336686 | 0.5668 | 0.0008 | |
| PLXNA4 | cg04126760 | 0.4102 | 0.0075 |
| cg10694781 | 0.5089 | 0.0019 | |
| RASGRF2 | cg09571630 | 0.394 | 0.0092 |
| cg15341601 | 0.4573 | 0.004 | |
| SFRP1 | cg00000321 | 0.4361 | 0.0054 |
| cg06166767 | 0.4027 | 0.0083 | |
| SH3GL1 | cg11592634 | 0.4155 | 0.007 |
| cg22305516 | 0.5871 | 0.0005 | |
| SHISA3 | cg11065575 | 0.4044 | 0.0081 |
| SLC45A4 | cg01081737 | 0.6167 | 0.0003 |
| cg07769015 | 0.4194 | 0.0067 | |
| cg15586392 | 0.5482 | 0.001 | |
| SLCO3A1 | cg00314343 | 0.6501 | 0.0002 |
| cg01240823 | 0.5338 | 0.0013 | |
| cg02107844 | 0.5093 | 0.0019 | |
| cg02633036 | 0.4318 | 0.0057 | |
| cg03756778 | 0.5045 | 0.002 | |
| cg04200243 | 0.5267 | 0.0015 | |
| cg07725123 | 0.5644 | 0.0008 | |
| cg11330307 | 0.6864 | 0.0001 | |
| cg11680590 | 0.5256 | 0.0015 | |
| cg11859489 | 0.4483 | 0.0046 | |
| cg12034869 | 0.5715 | 0.0007 | |
| cg12667732 | 0.7015 | 0.0001 | |
| cg13039199 | 0.5038 | 0.0021 | |
| cg17266475 | 0.3995 | 0.0086 | |
| cg19621065 | 0.545 | 0.0011 | |
| cg19687529 | 0.4041 | 0.0081 | |
| cg20464948 | 0.3904 | 0.0097 | |
| cg21656246 | 0.4978 | 0.0023 | |
| cg21715599 | 0.4385 | 0.0052 | |
| cg26467725 | 0.3902 | 0.0097 | |
| cg26677406 | 0.5366 | 0.0013 | |
| cg27070951 | 0.6864 | 0.0001 | |
| SLIT2 | cg07290920 | 0.4456 | 0.0047 |
| TMEM163 | cg17319374 | 0.4106 | 0.0075 |
| TNFSF15 | cg10791260 | 0.4555 | 0.0041 |
| cg11809085 | 0.7195 | 0.0001 | |
| ZNF462 | cg13635022 | 0.3988 | 0.0087 |
| cg13576904 | 0.4373 | 0.0053 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arenas, G.A.; Santander, N.; Krause, B.J. Transcriptional and Epigenomic Markers of the Arterial-Venous and Micro/Macro-Vascular Endothelial Heterogeneity within the Umbilical-Placental Bed. Int. J. Mol. Sci. 2022, 23, 11873. https://doi.org/10.3390/ijms231911873
Arenas GA, Santander N, Krause BJ. Transcriptional and Epigenomic Markers of the Arterial-Venous and Micro/Macro-Vascular Endothelial Heterogeneity within the Umbilical-Placental Bed. International Journal of Molecular Sciences. 2022; 23(19):11873. https://doi.org/10.3390/ijms231911873
Chicago/Turabian StyleArenas, German A., Nicolas Santander, and Bernardo J. Krause. 2022. "Transcriptional and Epigenomic Markers of the Arterial-Venous and Micro/Macro-Vascular Endothelial Heterogeneity within the Umbilical-Placental Bed" International Journal of Molecular Sciences 23, no. 19: 11873. https://doi.org/10.3390/ijms231911873
APA StyleArenas, G. A., Santander, N., & Krause, B. J. (2022). Transcriptional and Epigenomic Markers of the Arterial-Venous and Micro/Macro-Vascular Endothelial Heterogeneity within the Umbilical-Placental Bed. International Journal of Molecular Sciences, 23(19), 11873. https://doi.org/10.3390/ijms231911873






