Neuroprotective Effect of Azithromycin Following Induction of Optic Nerve Crush in Wild Type and Immunodeficient Mice
Abstract
1. Introduction
2. Results
2.1. Histological Analysis (Day 21): RGC Survival
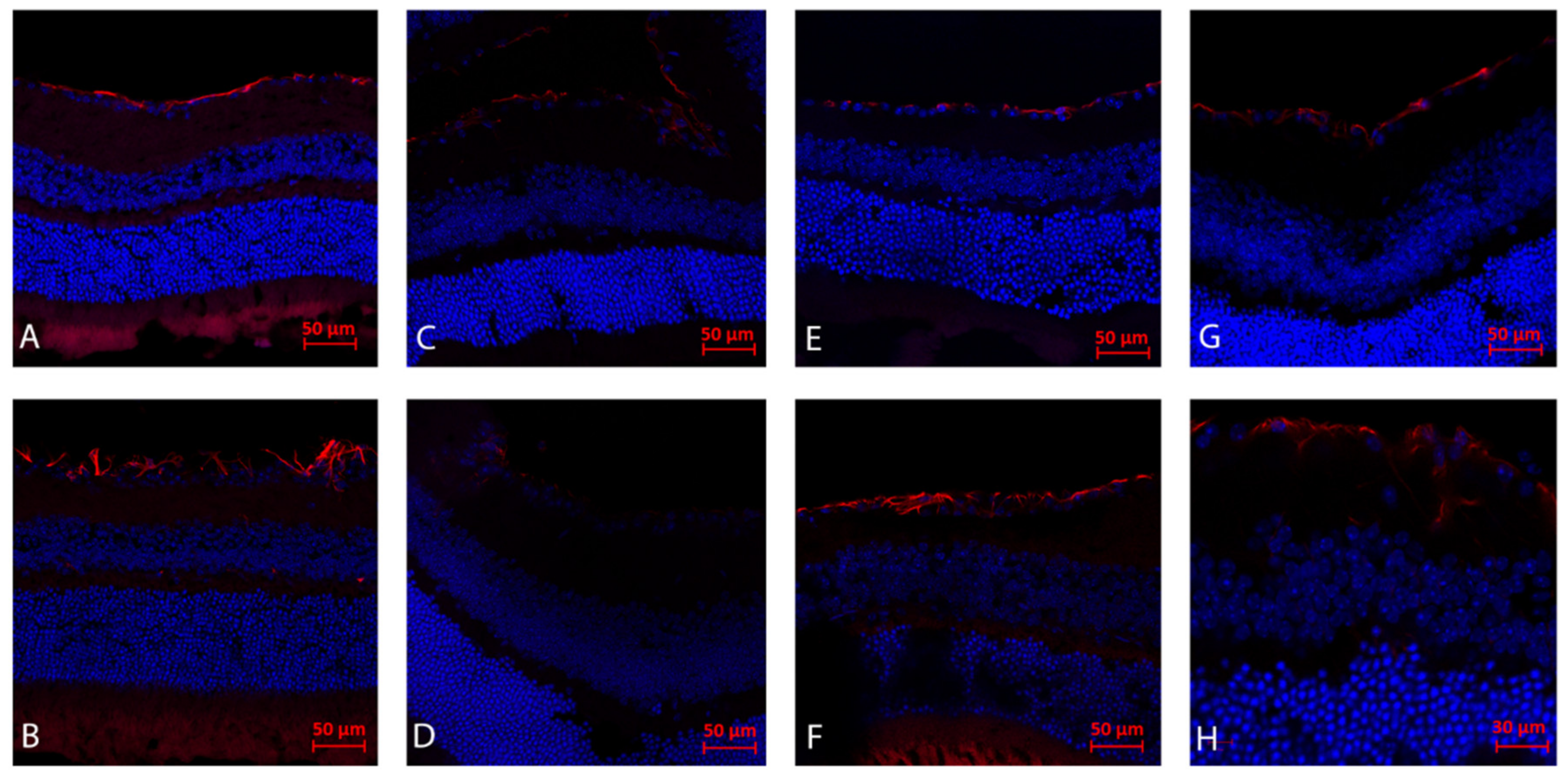
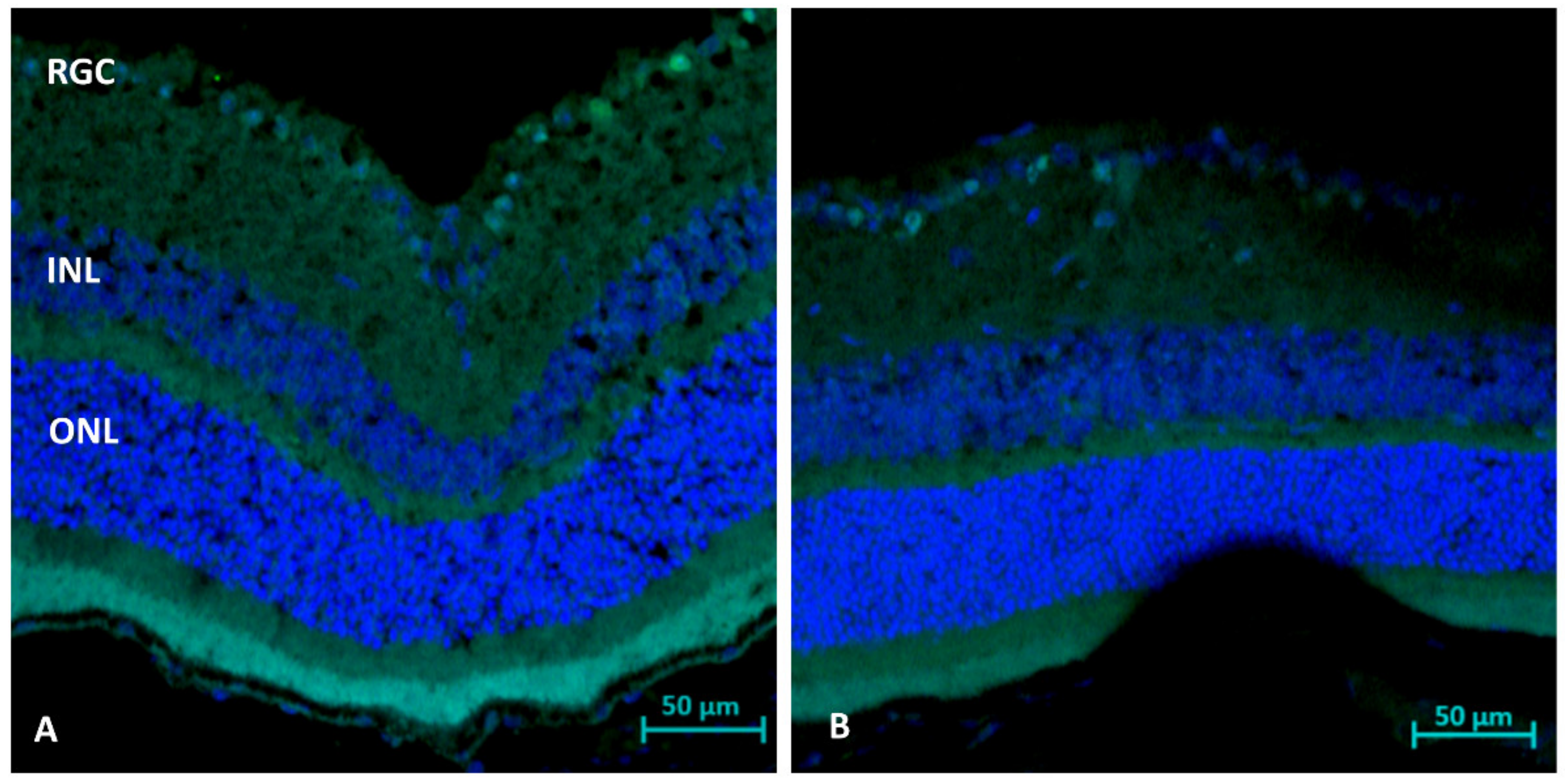
2.2. Immunohistochemistry Analysis (Day 21): Gliosis (GFAP)
2.3. TUNEL Staining (Day 1 and 3): Apoptosis
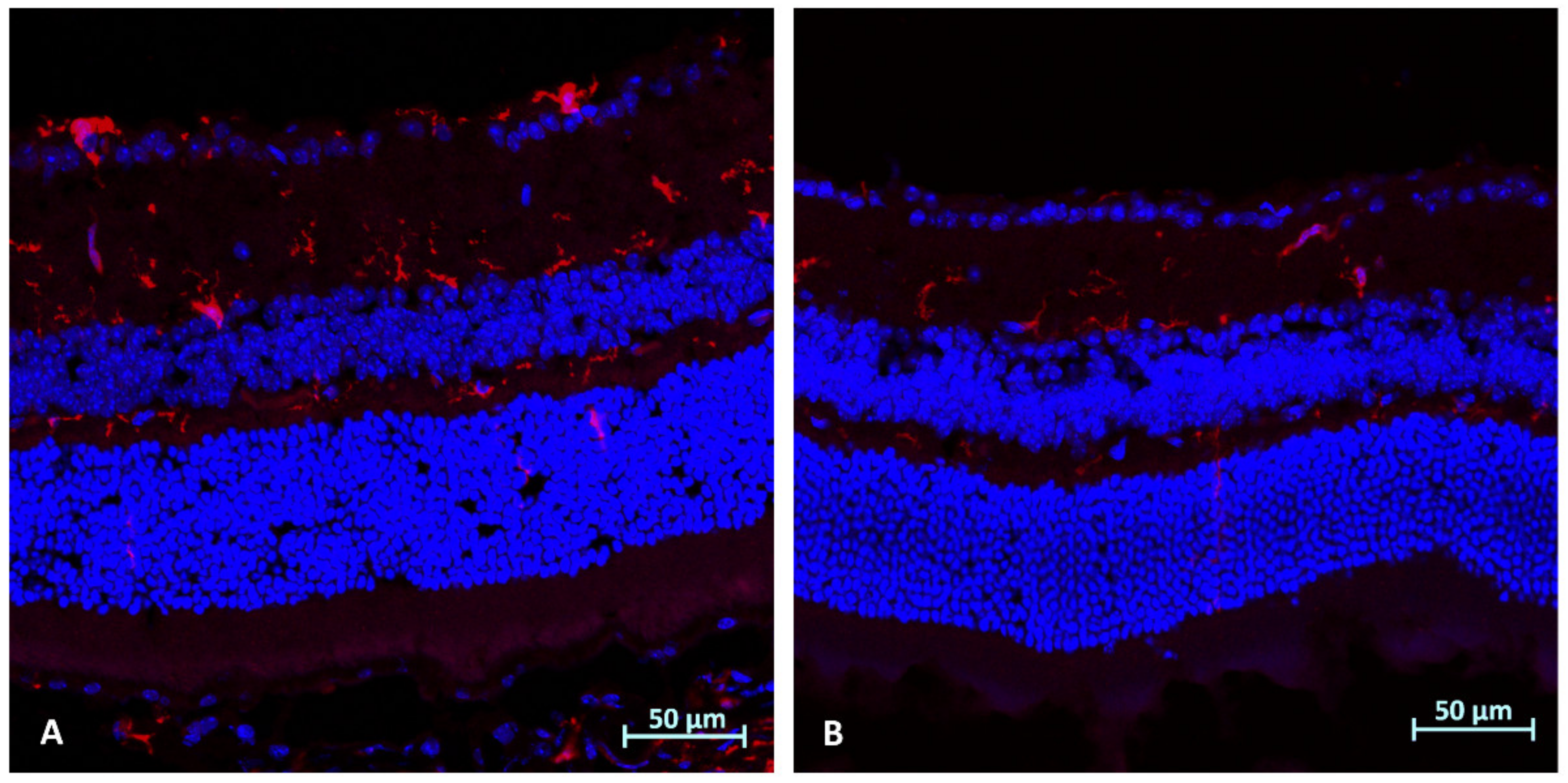
2.4. Immunohistochemistry Analysis (Day 3): Microglial Activation (Iba1)
2.5. Immunohistochemistry Analysis (Day 3): CD45
2.6. Molecular Analysis WT and NSG Mice (Day 3 after ONC): Optic Nerves
2.7. Molecular Analysis WT and NSG Mice (Day 3 after ONC): Retinas
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Tissue Collection
4.4. Molecular Analysis (Day 3)
4.5. Histological Analysis
4.5.1. Hematoxylin and Eosin Staining (Day 21)
4.5.2. Retinal Ganglion Cell (RGC) Count and Retinal Thickness Measurement
4.5.3. GFAP and CD45 Immunostaining
4.5.4. In Situ TdT-Mediated dUTP Nick End-Labeling (TUNEL) Immunostaining
4.5.5. IBA1 Immunostaining
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biousse, V.; Newman, N.J. Ischemic optic neuropathies. N. Engl. J. Med. 2015, 372, 2428–2436. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Song, D.; Shan, G.; Wang, K.; Wang, Y.; Ma, J.; Zhong, Y. The association between diabetes mellitus and nonarteritic anterior ischemic optic neuropathy: A systematic review and meta-analysis. PLoS ONE 2013, 8, e76653. [Google Scholar] [CrossRef]
- Fraser, C.L. Obstructive sleep apnea and optic neuropathy: Is there a link? Curr. Neurol. Neurosci. Rep. 2014, 14, 465. [Google Scholar] [CrossRef]
- Salgado, C.; Vilson, F.; Miller, N.R.; Bernstein, S.L. Cellular inflammation in nonarteritic anterior ischemic optic neuropathy and its primate model. Arch. Ophthalmol. 2011, 129, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Rappoport, D.; Morzaev, D.; Weiss, S.; Vieyra, M.; Nicholson, J.D.; Leiba, H.; Goldenberg-cohen, N. Effect of intravitreal injection of bevacizumab on optic nerve head leakage and retinal ganglion cell survival in a mouse model of optic nerve crush. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8160–8171. [Google Scholar] [CrossRef][Green Version]
- Morzaev, D.; Nicholson, J.D.; Caspi, T.; Weiss, S.; Hochhauser, E.; Goldenberg-Cohen, N. Toll-like receptor-4 knockout mice are more resistant to optic nerve crush damage than wild-type mice. Clin. Exp. Ophthalmol. 2015, 43, 655–665. [Google Scholar] [CrossRef]
- Bosnar, M.; Čužić, S.; Bošnjak, B.; Nujić, K.; Ergović, G.; Marjanović, N.; Pašalić, I.; Hrvačić, B.; Polančec, D.; Glojnarić, I.; et al. Azithromycin inhibits macrophage interleukin-1β production through inhibition of activator protein-1 in lipopolysaccharide-induced murine pulmonary neutrophilia. Int. Immunopharmacol. 2011, 11, 424–434. [Google Scholar] [CrossRef]
- Feola, D.J.; Garvy, B.A.; Cory, T.J.; Birket, S.E.; Hoy, H.; Hayes, D., Jr.; Murphy, B.S. Azithromycin alters macrophage phenotype and pulmonary compartmentalization during lung infection with Pseudomonas. Antimicrob. Agents Chemother. 2010, 54, 2437–2447. [Google Scholar] [CrossRef]
- Parnham, M.J.; Erakovic Haber, V.; Giamarellos-Bourboulis, E.J.; Perletti, G.; Verleden, G.M.; Vos, R. Azithromycin: Mechanisms of action and their relevance for clinical applications. Pharmacol. Ther. 2014, 143, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Geudens, N.; Timmermans, L.; Vanhooren, H.; Vanaudenaerde, B.M.; Vos, R.; Van De Wauwer, C.; Verleden, G.M.; Verbeken, E.; Lerut, T.; Van Raemdonck, D.E. Azithromycin reduces airway inflammation in a murine model of lung ischaemia reperfusion injury. Transpl. Int. 2008, 21, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Amantea, D.; Certo, M.; Petrelli, F.; Tassorelli, C.; Micieli, G.; Corasaniti, M.T.; Puccetti, P.; Fallarino, F.; Bagetta, G. Azithromycin protects mice against ischemic stroke injury by promoting macrophage transition towards M2 phenotype. Exp. Neurol. 2016, 275 Pt 1, 116–125. [Google Scholar] [CrossRef]
- Cercek, B.; Shah, P.K.; Noc, M.; Zahger, D.; Zeymer, U.; Matetzky, S.; Maurer, G.; Mahrer, P.; AZACS Investigators. Effect of short-term treatment with azithromycin on recurrent ischaemic events in patients with acute coronary syndrome in the Azithromycin in Acute Coronary Syndrome (AZACS) trial: A randomised controlled trial. Lancet 2003, 361, 809–813. [Google Scholar] [CrossRef]
- Varano, G.P.; Parisi, V.; Adornetto, A.; Cavaliere, F.; Amantea, D.; Nucci, C.; Corasaniti, M.T.; Morrone, L.A.; Bagetta, G.; Russo, R. Post-ischemic treatment with azithromycin protects ganglion cells against retinal ischemia/reperfusion injury in the rat. Mol. Vis. 2017, 23, 911–921. [Google Scholar] [PubMed]
- Inaba, T.; Katayama, Y.; Ueda, M.; Nito, C. Neuroprotective effects of pretreatment with macrolide antibiotics on cerebral ischemia reperfusion injury. Neurol. Res. 2015, 37, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg-Cohen, N.; Guo, Y.; Margolis, F.; Cohen, Y.; Miller, N.R.; Bernstein, S.L. Oligodendrocyte dysfunction after induction of experimental anterior optic nerve ischemia. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2716–2725. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Y.; Miller, N.R.; Bernstein, S.L. Optic nerve infarction and post-ischemic inflammation in the rodent model of anterior ischemic optic neuropathy (rAION). Brain Res. 2009, 1264, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Mac Nair, C.E.; Fernandes, K.A.; Schlamp, C.L.; Libby, R.T.; Nickells, R.W. Tumor necrosis factor alpha has an early protective effect on retinal ganglion cells after optic nerve crush. J. Neuroinflamm. 2014, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Eleutherio, E.C.A.; Silva Magalhães, R.S.; de Araújo Brasil, A.; Monteiro Neto, J.R.; de Holanda Paranhos, L. SOD1, more than just an antioxidant. Arch. Biochem. Biophys. 2021, 697, 108701. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.A. Superoxide generation explains common features of optic neuropathies associated with cecocentral scotomas. J. Neuroophthalmol. 2015, 35, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, A.; Catrinescu, M.M.; Mahammed, A.; Gross, Z.; Levin, L.A. Neuroprotection against superoxide anion radical by metallocorroles in cellular and murine models of optic neuropathy. J. Neurochem. 2010, 114, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Himori, N.; Maruyama, K.; Yamamoto, K.; Yasuda, M.; Ryu, M.; Omodaka, K.; Shiga, Y.; Tanaka, Y.; Nakazawa, T. Critical neuroprotective roles of heme oxygenase-1 induction against axonal injury-induced retinal ganglion cell death. J. Neurosci. Res. 2014, 92, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Kutty, R.K.; Kutty, G.; Wiggert, B.; Chader, G.J.; Darrow, R.M.; Organisciak, D.T. Induction of heme oxygenase 1 in the retina by intense visible light: Suppression by the antioxidant dimethylthiourea. Proc. Natl. Acad. Sci. USA 1995, 92, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Dratviman-Storobinsky, O.; Hasanreisoglu, M.; Offen, D.; Barhum, Y.; Weinberger, D.; Goldenberg-Cohen, N. Progressive damage along the optic nerve following induction of crush injury or rodent anterior ischemic optic neuropathy in transgenic mice. Mol. Vis. 2008, 14, 2171–2179. [Google Scholar] [PubMed]
- Goldenberg-Cohen, N.; Dadon, S.; Avraham, B.C.; Kramer, M.; Hasanreisoglu, M.; Eldar, I.; Weinberger, D.; Bahar, I. Molecular and histological changes following central retinal artery occlusion in a mouse model. Exp. Eye Res. 2008, 87, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg-Cohen, N.; Dadon-Bar-El, S.; Hasanreisoglu, M.; Avraham-Lubin, B.C.; Dratviman-Storobinsky, O.; Cohen, Y.; Weinberger, D. Possible neuroprotective effect of brimonidine in a mouse model of ischaemic optic neuropathy. Clin. Exp. Ophthalmol. 2009, 37, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Avraham-Lubin, B.C.; Dratviman-Storobinsky, O.; Dadon-Bar El, S.; Hasanreisoglu, M.; Goldenberg-Cohen, N. Neuroprotective effect of hyperbaric oxygen therapy on anterior ischemic optic neuropathy. Front. Neurol. 2011, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Scopim-Ribeiro, R.; Lizardo, M.M.; Zhang, H.F.; Dhez, A.C.; Hughes, C.S.; Sorensen, P.H. NSG mice facilitate ex vivo characterization of Ewing sarcoma lung metastasis using the PuMA model. Front. Oncol. 2021, 11, 645757. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ryu, B.; Kim, U.; Kim, C.H.; Hur, G.H.; Kim, C.Y.; Park, J.H. Improved human hematopoietic reconstitution in HepaRG co-transplanted humanized NSG mice. BMB Rep. 2020, 53, 466–471. [Google Scholar] [CrossRef]
- Skoda, J.; Neradil, J.; Staniczkova Zambo, I.; Nunukova, A.; Macsek, P.; Borankova, K.; Dobrotkova, V.; Nemec, P.; Sterba, J.; Veselska, R. Serial xenotransplantation in NSG mice promotes a hybrid epithelial/mesenchymal gene expression signature and stemness in rhabdomyosarcoma cells. Cancers 2020, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, H.; Su, S.B. Neuroinflammatory responses in diabetic retinopathy. J. Neuroinflamm. 2015, 12, 141. [Google Scholar] [CrossRef]
- Azoulay-Dupuis, E.; Vallée, E.; Bedos, J.P.; Muffat-Joly, M.; Pocidalo, J.J. Prophylactic and therapeutic activities of azithromycin in a mouse model of pneumococcal pneumonia. Antimicrob. Agents Chemother. 1991, 35, 1024–1028. [Google Scholar] [CrossRef] [PubMed]


| Procedure/Study | Analysis Method | C57Bl/6 Mice (n = 24) | NSG Mice (n = 24) |
|---|---|---|---|
| ONC | molecular | 10 | 5 |
| IHC | 11 | 7 | |
| ONC + AZ | molecular | 10 | 5 |
| IHC | 13 | 7 |
| Sod1_F | GCCCGGCGGATGAAGA |
| Sod1_R | CGTCCTTTCCAGCAGTCACA |
| Bax_F | CTGAGCTGACCTTGGAGC |
| Bax_R | GACTCCAGCCACAAAGATG |
| Ho-1_F | CAGGTGTCCAGAGAAGGCT |
| Ho-1_R | TCTTCCAGGGCCGTGTAGAT |
| Il1β _F | TGACAGTGATGAGAATGACCTGTTC |
| Il1β _R | GGACAGCCCAGGTCAAAGG |
| Gapdh_F | TGCCACTCAGAAGACTGTGGATG |
| Gapdh_R | GCCTGCTTCACCACCTTCTTGAT |
| Nfkb1_F | CCTGCAAAGGTTATCGTTCAGTT |
| Nfkb1_R | GCAAAGCCAACCACCATGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zloto, O.; Zahavi, A.; Richard, S.; Friedman-Gohas, M.; Weiss, S.; Goldenberg-Cohen, N. Neuroprotective Effect of Azithromycin Following Induction of Optic Nerve Crush in Wild Type and Immunodeficient Mice. Int. J. Mol. Sci. 2022, 23, 11872. https://doi.org/10.3390/ijms231911872
Zloto O, Zahavi A, Richard S, Friedman-Gohas M, Weiss S, Goldenberg-Cohen N. Neuroprotective Effect of Azithromycin Following Induction of Optic Nerve Crush in Wild Type and Immunodeficient Mice. International Journal of Molecular Sciences. 2022; 23(19):11872. https://doi.org/10.3390/ijms231911872
Chicago/Turabian StyleZloto, Ofira, Alon Zahavi, Stephen Richard, Moran Friedman-Gohas, Shirel Weiss, and Nitza Goldenberg-Cohen. 2022. "Neuroprotective Effect of Azithromycin Following Induction of Optic Nerve Crush in Wild Type and Immunodeficient Mice" International Journal of Molecular Sciences 23, no. 19: 11872. https://doi.org/10.3390/ijms231911872
APA StyleZloto, O., Zahavi, A., Richard, S., Friedman-Gohas, M., Weiss, S., & Goldenberg-Cohen, N. (2022). Neuroprotective Effect of Azithromycin Following Induction of Optic Nerve Crush in Wild Type and Immunodeficient Mice. International Journal of Molecular Sciences, 23(19), 11872. https://doi.org/10.3390/ijms231911872








