Amyloid Aβ25-35 Aggregates Say ‘NO’ to Long-Term Potentiation in the Hippocampus through Activation of Stress-Induced Phosphatase 1 and Mitochondrial Na+/Ca2+ Exchanger
Abstract
1. Introduction
2. Results
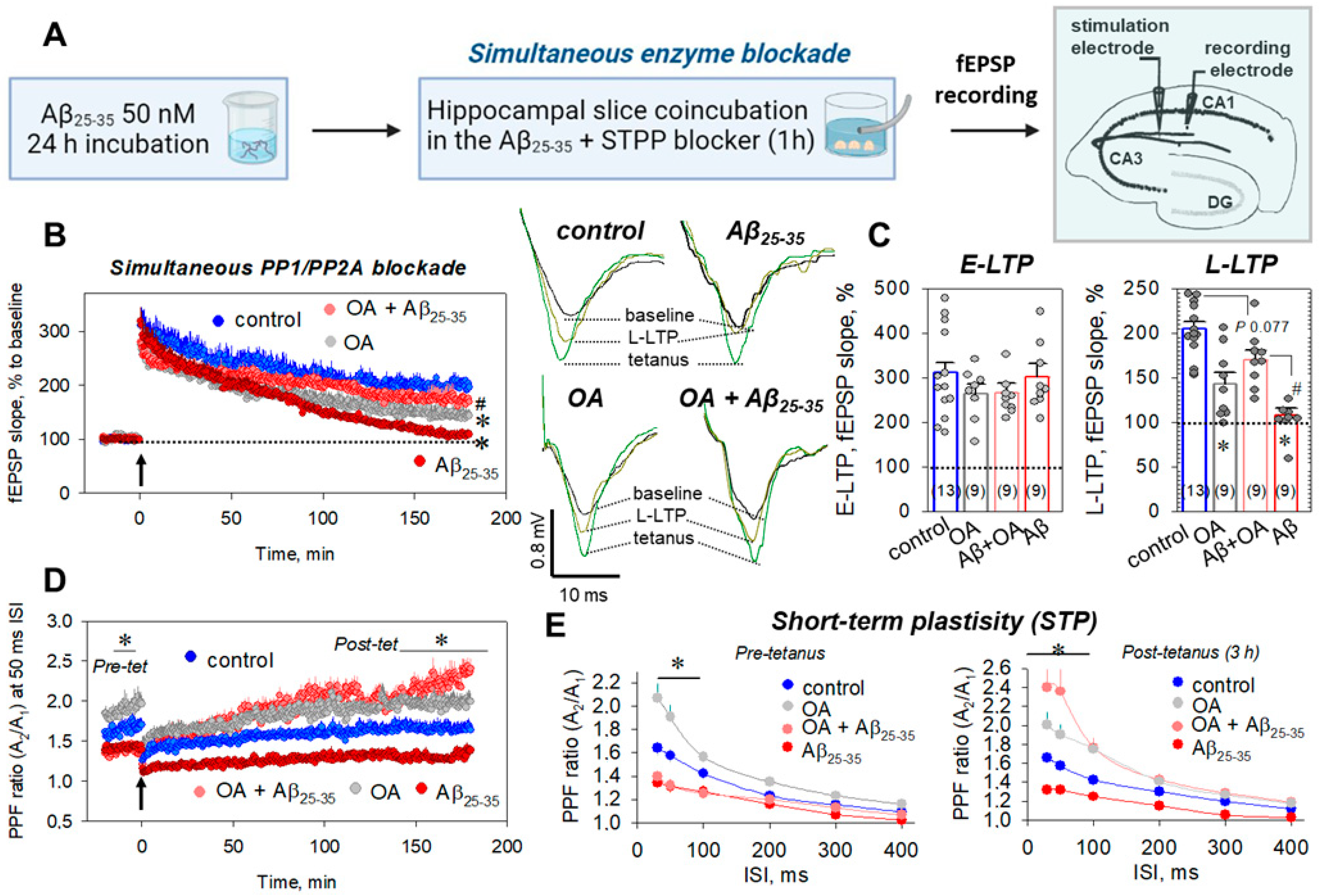
2.1. Aβ25-35 Aggregates Impaired Synaptic Plasticity through PP1/PP2A-Sensitive Mechanism
2.2. PP2B Is Not Involved in the Aβ25-35-Dependent Deteriorations of Synaptic Plasticity
2.3. Direct Measurements of STPP Activity in Hippocampal Slices
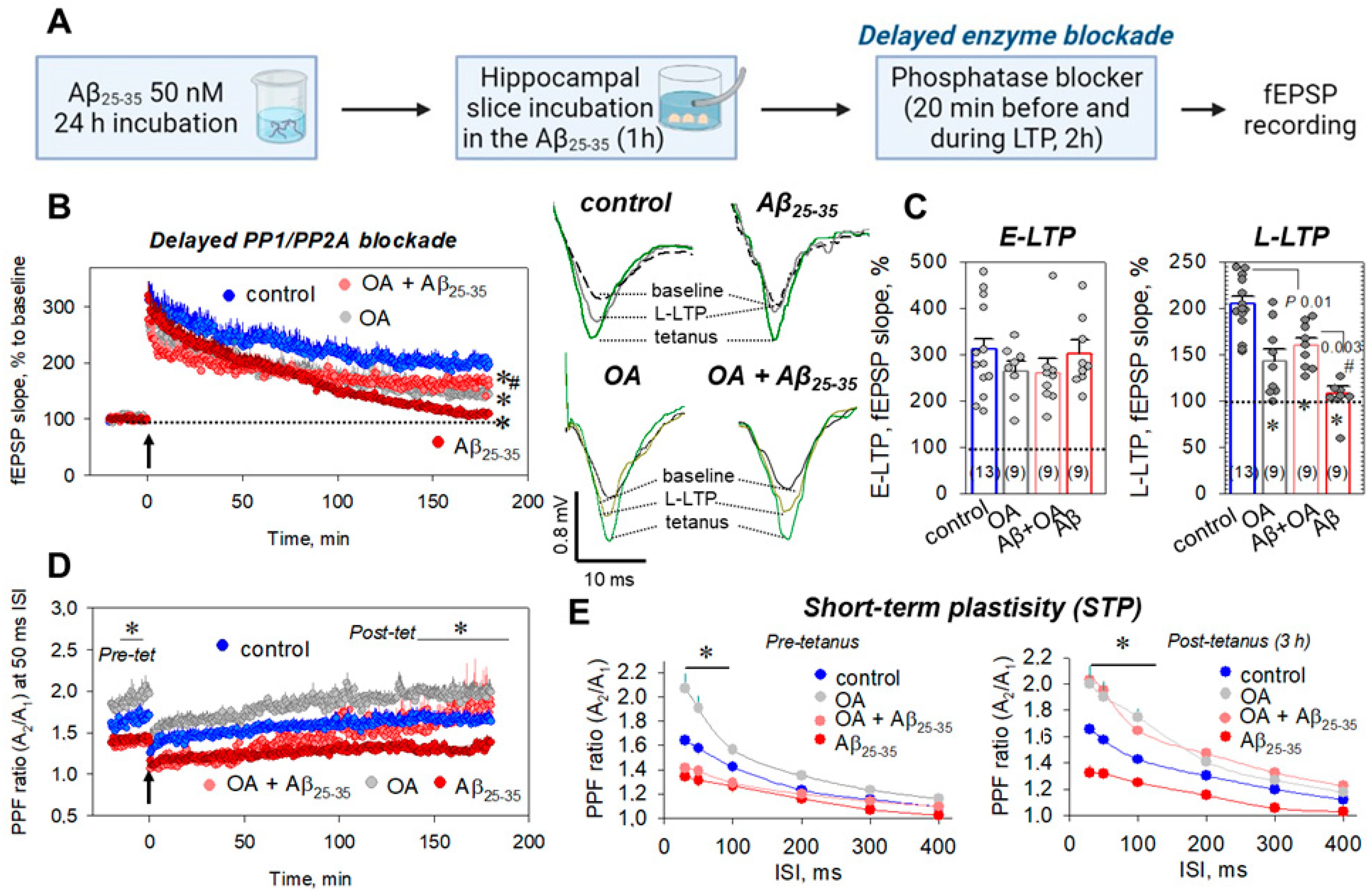
2.4. Delayed Enzyme Blockade Protocol for STPP Influence on Synaptic Transmission
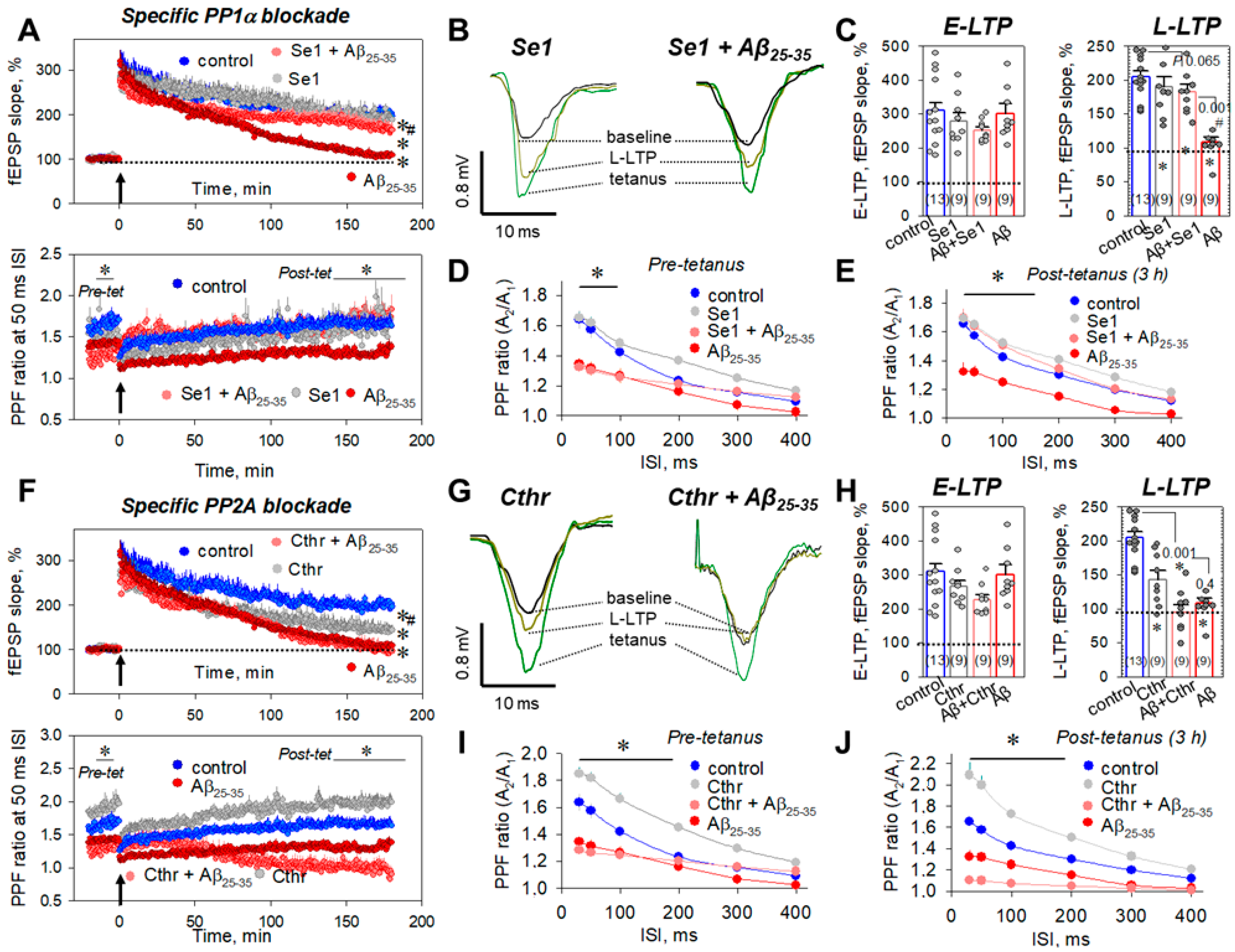
2.5. Which Phosphatase(s) Is Involved in OA-Associated Attenuation of Aβ25-35-Induced LTP Suppression?
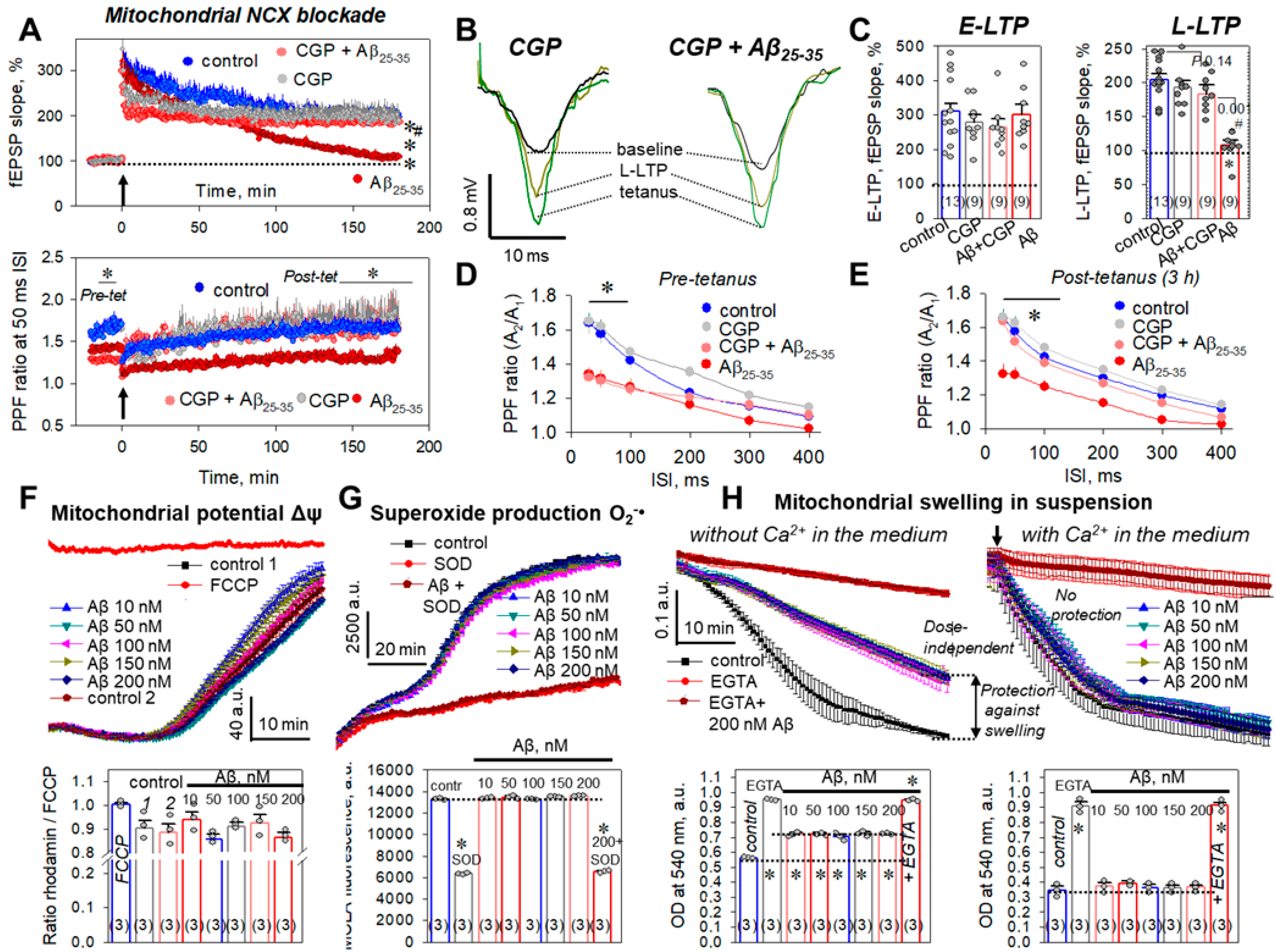
2.6. Mitochondria and Aβ25-35 Aggregates
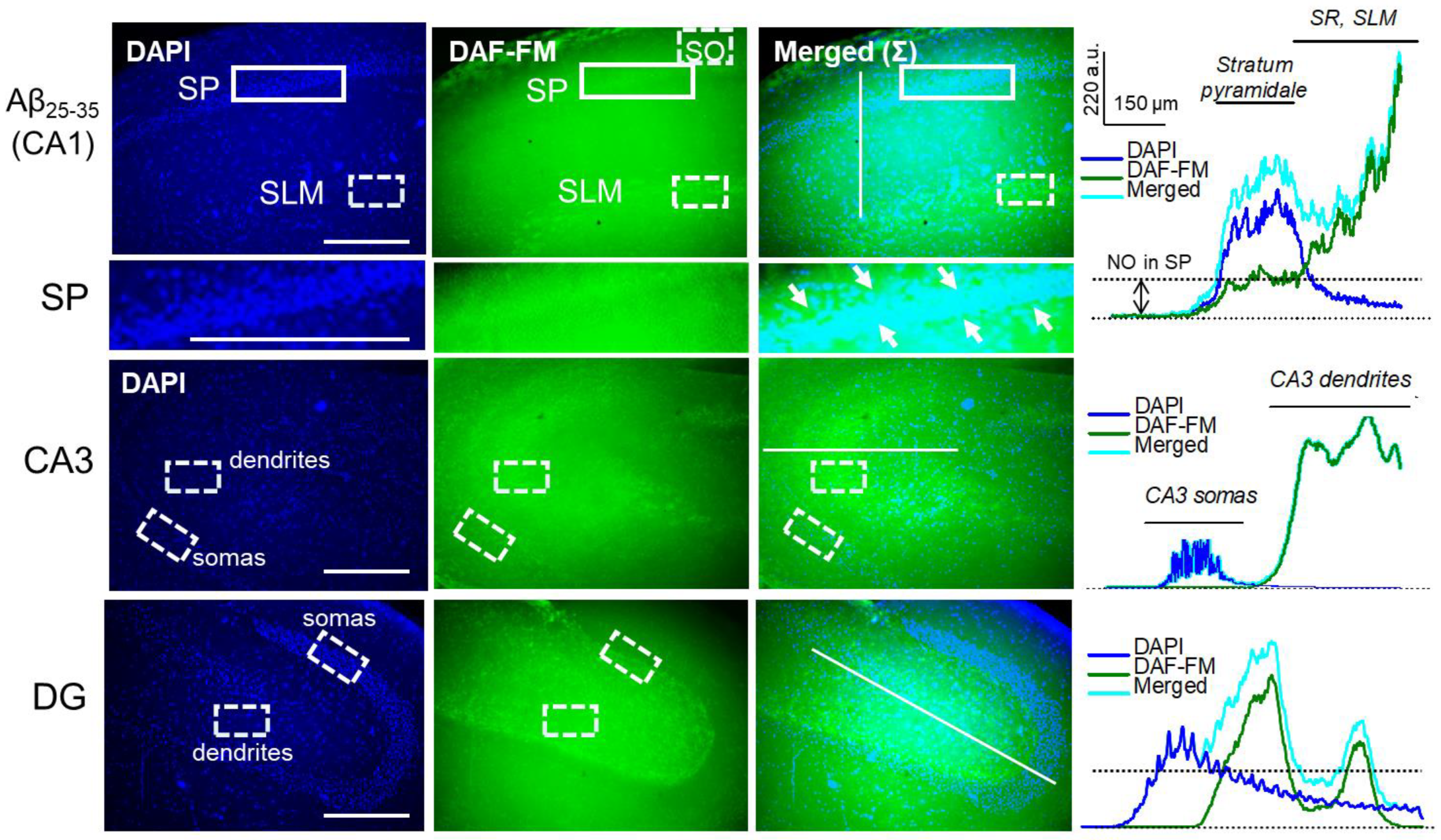
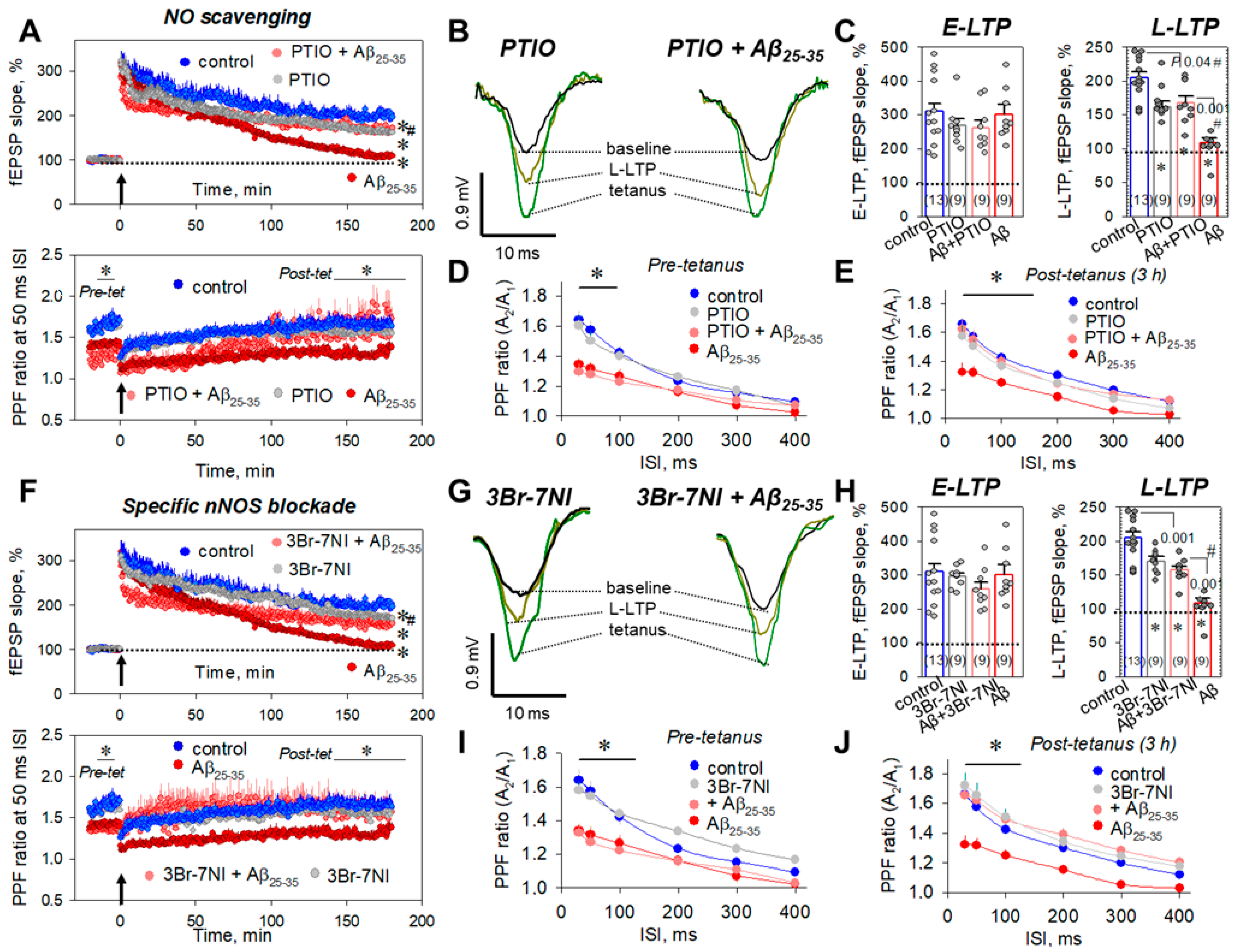
2.7. Aβ25-35 Aggregates ‘Say’ NO to the LTP in CA3–CA1 Synapses
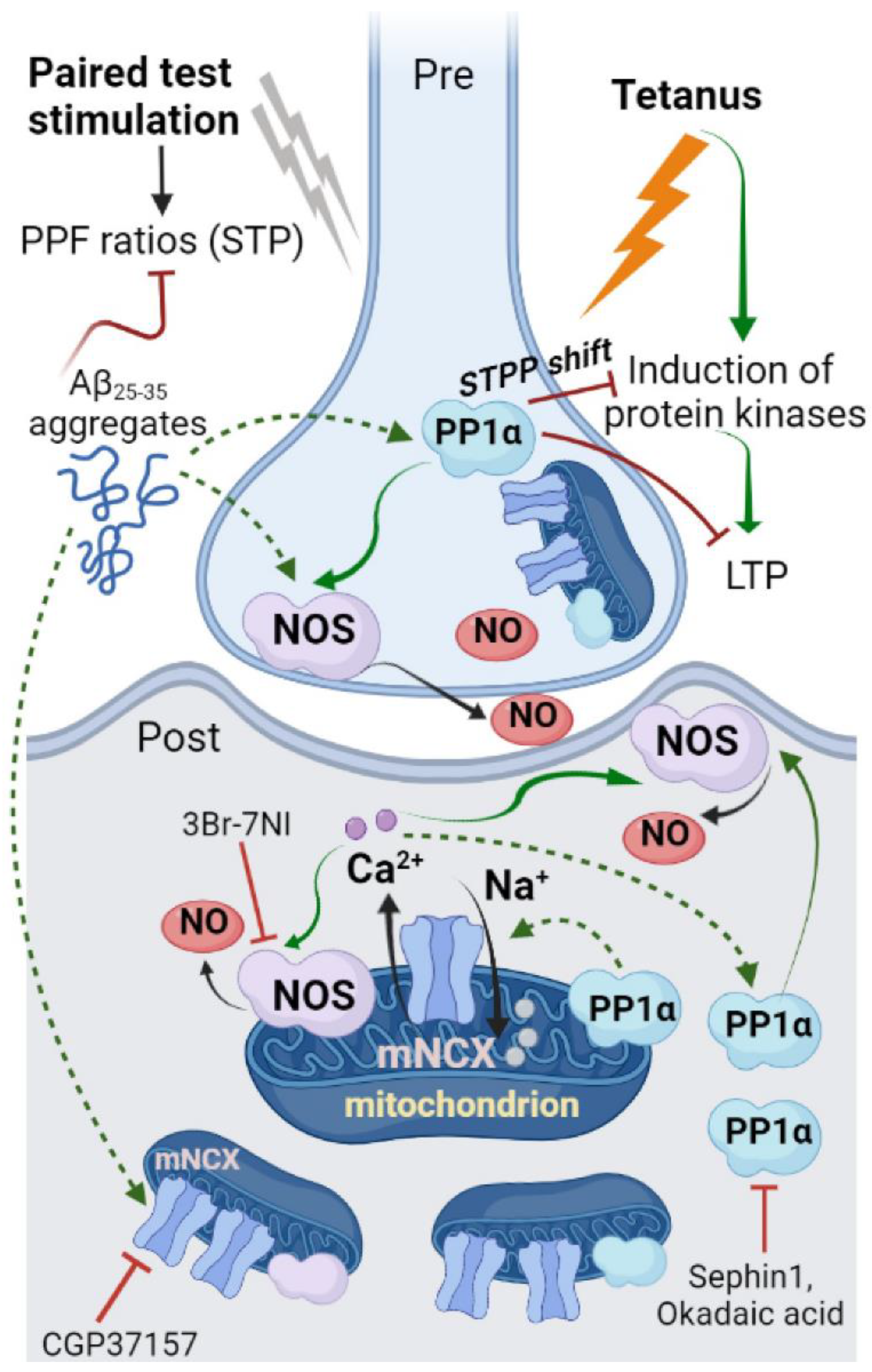
3. Discussion
4. Materials and Methods
4.1. Hippocampal Slice Preparation
4.2. Electrophysiology
4.3. NO Imaging of Hippocampal Slices
4.4. Phosphatase Assay
4.5. Isolation and Purification of Mitochondria
4.6. Ca2+-Dependent Swelling of Mitochondria
4.7. ROS Detection
4.8. Measurements of the Mitochondrial Transmembrane Potential, ΔΨm
4.9. Drugs
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Fonar, G.; Polis, B.; Sams, D.S.; Levi, A.; Malka, A.; Bal, N.; Maltsev, A.; Elliott, E.; Samson, A.O. Modified Snake α-Neurotoxin Averts β-Amyloid Binding to α7 Nicotinic Acetylcholine Receptor and Reverses Cognitive Deficits in Alzheimer’s Disease Mice. Mol. Neurobiol. 2021, 58, 2322–2341. [Google Scholar] [CrossRef]
- Paroni, G.; Bisceglia, P.; Seripa, D. Understanding the Amyloid Hypothesis in Alzheimer’s Disease. J. Alzheimers Dis. 2019, 68, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, Y.H.; Hebisch, M.; Sliwinski, C.; Lee, S.; D’Avanzo, C.; Chen, H.; Hooli, B.; Asselin, C.; Muffat, J.; et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 2014, 515, 274–278. [Google Scholar] [CrossRef]
- Penke, B.; Bogár, F.; Paragi, G.; Gera, J.; Fülöp, L. Key Peptides and Proteins in Alzheimer’s Disease. Curr. Protein Pept. Sci. 2019, 20, 577–599. [Google Scholar] [CrossRef] [PubMed]
- Axenhus, M.; Winblad, B.; Tjernberg, L.O.; Schedin-Weiss, S. Huntingtin Levels are Elevated in Hippocampal Post-Mortem Samples of Alzheimer’s Disease Brain. Curr. Alzheimer Res. 2020, 17, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Samarova, E.I.; Bravarenko, N.I.; Korshunova, T.A.; Gulyaeva, N.V.; Palotás, A.; Balaban, P.M. Effect of beta-amyloid peptide on behavior and synaptic plasticity in terrestrial snail. Brain Res. Bull. 2005, 67, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef]
- Turrel, O.; Goguel, V.; Preat, T. Drosophila Neprilysin 1 Rescues Memory Deficits Caused by Amyloid-β Peptide. J. Neurosci. 2017, 37, 10334–10345. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.W.; Eldadah, B.A.; Faden, A.I. Beta-amyloid-induced apoptosis of cerebellar granule cells and cortical neurons: Exacerbation by selective inhibition of group I metabotropic glutamate receptors. Neuropharmacology 1999, 38, 1243–1252. [Google Scholar] [CrossRef]
- Cheng, L.; Yin, W.J.; Zhang, J.F.; Qi, J.S. Amyloid beta-protein fragments 25-35 and 31-35 potentiate long-term depression in hippocampal CA1 region of rats in vivo. Synapse 2009, 63, 206–214. [Google Scholar] [CrossRef]
- Wang, X.J.; Song, X.J.; Gao, P.H.; Zhou, X.Y.; Zhou, S.N. Estradiol prevents Aβ 25 35-inhibited long-term potentiation induction through enhancing survival of newborn neurons in the dentate gyrus. Int. J. Neurosci. 2016, 126, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Pavía, J.; Alberch, J.; Alvárez, I.; Toledano, A.; de Ceballos, M.L. Repeated intracerebroventricular administration of beta-amyloid(25-35) to rats decreases muscarinic receptors in cerebral cortex. Neurosci. Lett. 2000, 278, 69–72. [Google Scholar] [CrossRef]
- Yi, J.H.; Whitcomb, D.J.; Park, S.J.; Martinez-Perez, C.; Barbati, S.A.; Mitchell, S.J.; Cho, K. M1 muscarinic acetylcholine receptor dysfunction in moderate Alzheimer’s disease pathology. Brain Commun. 2020, 2, e058. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Maskos, U. Role of the nicotinic acetylcholine receptor in Alzheimer’s disease pathology and treatment. Neuropharmacology 2015, 96 Pt B, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Shemer, I.; Holmgren, C.; Min, R.; Fülöp, L.; Zilberter, M.; Sousa, K.M.; Farkas, T.; Härtig, W.; Penke, B.; Burnashev, N.; et al. Non-fibrillar beta-amyloid abates spike-timing-dependent synaptic potentiation at excitatory synapses in layer 2/3 of the neocortex by targeting postsynaptic AMPA receptors. Eur. J. Neurosci. 2006, 23, 2035–2047. [Google Scholar] [CrossRef]
- Guntupalli, S.; Widagdo, J.; Anggono, V. Amyloid-β-Induced Dysregulation of AMPA Receptor Trafficking. Neural. Plast. 2016, 2016, e3204519. [Google Scholar] [CrossRef] [PubMed]
- Tanqueiro, S.R.; Ramalho, R.M.; Rodrigues, T.M.; Lopes, L.V.; Sebastião, A.M.; Diógenes, M.J. Inhibition of NMDA Receptors Prevents the Loss of BDNF Function Induced by Amyloid β. Front. Pharmacol. 2018, 9, e237. [Google Scholar] [CrossRef]
- Lai, C.C.; Lo, H.; Lin, H.G.; Lin, H.H. Potentiation of NMDA-Mediated Responses by Amyloid-β Peptide 1-40 in Rat Sympathetic Preganglionic Neurons. J. Alzheimers Dis. 2019, 67, 1291–1303. [Google Scholar] [CrossRef]
- Anekonda, T.S.; Quinn, J.F.; Harris, C.; Frahler, K.; Wadsworth, T.L.; Woltjer, R.L. L-type voltage-gated calcium channel blockade with isradipine as a therapeutic strategy for Alzheimer’s disease. Neurobiol. Dis. 2011, 41, 62–70. [Google Scholar] [CrossRef]
- Ishii, M.; Hiller, A.J.; Pham, L.; McGuire, M.J.; Iadecola, C.; Wang, G. Amyloid-Beta Modulates Low-Threshold Activated Voltage-Gated L-Type Calcium Channels of Arcuate Neuropeptide Y Neurons Leading to Calcium Dysregulation and Hypothalamic Dysfunction. J. Neurosci. 2019, 39, 8816–8825. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Lee, J.; Joe, E.H.; Uhm, D.Y. Beta-amyloid peptide induces the expression of voltage dependent outward rectifying K+ channels in rat microglia. Neurosci. Lett. 2001, 300, 67–70. [Google Scholar] [CrossRef]
- Ye, C.P.; Selkoe, D.J.; Hartley, D.M. Protofibrils of amyloid beta-protein inhibit specific K+ currents in neocortical cultures. Neurobiol. Dis. 2003, 13, 177–190. [Google Scholar] [CrossRef]
- Liu, J.; Supnet, C.; Sun, S.; Zhang, H.; Good, L.; Popugaeva, E.; Bezprozvanny, I. The role of ryanodine receptor type 3 in a mouse model of Alzheimer disease. Channels 2014, 8, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Zhao, F.; Wang, C.F.; Zhang, X.M.; Xiao, Y.; Zhou, F.; Wu, M.N.; Zhang, J.; Qi, J.S.; Yang, W. Xestospongin C, a Reversible IP3 Receptor Antagonist, Alleviates the Cognitive and Pathological Impairments in APP/PS1 Mice of Alzheimer’s Disease. J. Alzheimers Dis. 2019, 72, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Casley, C.S.; Canevari, L.; Land, J.M.; Clark, J.B.; Sharpe, M.A. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J. Neurochem. 2002, 80, 91–100. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Reddy, P.H. Synaptic basis of Alzheimer’s disease: Focus on synaptic amyloid beta, P-tau and mitochondria. Ageing Res. Rev. 2021, 65, e101208. [Google Scholar] [CrossRef] [PubMed]
- Ricke, K.M.; Cruz, S.A.; Qin, Z.; Farrokhi, K.; Sharmin, F.; Zhang, L.; Zasloff, M.A.; Stewart, A.F.R.; Chen, H.H. Neuronal Protein Tyrosine Phosphatase 1B Hastens Amyloid β-Associated Alzheimer’s Disease in Mice. J. Neurosci. 2020, 40, 1581–1593. [Google Scholar] [CrossRef]
- Nassif, M.; Hoppe, J.; Santin, K.; Frozza, R.; Zamin, L.L.; Simão, F.; Horn, A.P.; Salbego, C. Beta-amyloid peptide toxicity in organotypic hippocampal slice culture involves Akt/PKB, GSK-3beta, and PTEN. Neurochem. Int. 2007, 50, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kurup, P.; Xu, J.; Anderson, G.M.; Greengard, P.; Nairn, A.C.; Lombroso, P.J. Reduced levels of the tyrosine phosphatase STEP block β amyloid-mediated GluA1/GluA2 receptor internalization. J. Neurochem. 2011, 119, 664–672. [Google Scholar] [CrossRef]
- Shentu, Y.P.; Hu, W.T.; Liang, J.W.; Liuyang, Z.Y.; Wei, H.; Qun, W.; Wang, X.C.; Wang, J.Z.; Westermarck, J.; Liu, R. Genistein Decreases APP/tau Phosphorylation and Ameliorates Aβ Overproduction Through Inhibiting CIP2A. Curr. Alzheimer Res. 2019, 16, 732–740. [Google Scholar] [CrossRef]
- Wang, X.; Takata, T.; Bai, X.; Ou, F.; Yokono, K.; Sakurai, T. Pyruvate prevents the inhibition of the long-term potentiation induced by amyloid-β through protein phosphatase 2A inactivation. J. Alzheimers Dis. 2012, 30, 665–673. [Google Scholar] [CrossRef]
- Swerdlow, R.H.; Khan, S.M. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med. Hypotheses 2004, 63, 8–20. [Google Scholar] [CrossRef]
- Swerdlow, R.H. The mitochondrial hypothesis: Dysfunction, bioenergetic defects, and the metabolic link to Alzheimer’s disease. Int. Rev. Neurobiol. 2020, 154, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Haapasalo, A.; Kauppinen, A.; Kaarniranta, K.; Soininen, H.; Hiltunen, M. Impaired mitochondrial energy metabolism in Alzheimer’s disease: Impact on pathogenesis via disturbed epigenetic regulation of chromatin landscape. Prog. Neurobiol. 2015, 131, 1–20. [Google Scholar] [CrossRef]
- Sierra, A.; Gottfried-Blackmore, A.C.; McEwen, B.S.; Bulloch, K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 2007, 55, 412–424. [Google Scholar] [CrossRef]
- Kilbride, S.M.; Telford, J.E.; Tipton, K.F.; Davey, G.P. Partial inhibition of complex I activity increases Ca-independent glutamate release rates from depolarized synaptosomes. J. Neurochem. 2008, 106, 826–834. [Google Scholar] [CrossRef]
- Bell, S.M.; Barnes, K.; De Marco, M.; Shaw, P.J.; Ferraiuolo, L.; Blackburn, D.J.; Venneri, A.; Mortiboys, H. Mitochondrial Dysfunction in Alzheimer’s Disease: A Biomarker of the Future? Biomedicines 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Leuner, K.; Schütt, T.; Kurz, C.; Eckert, S.H.; Schiller, C.; Occhipinti, A.; Mai, S.; Jendrach, M.; Eckert, G.P.; Kruse, S.E.; et al. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxid. Redox Signal. 2012, 16, 1421–1433. [Google Scholar] [CrossRef]
- Chaturvedi, R.K.; Beal, M.F. Mitochondria targeted therapeutic approaches in Parkinson’s and Huntington’s diseases. Mol. Cell Neurosci. 2013, 55, 101–114. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Beal, M.F. Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med. 2008, 14, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, A.V.; Balaban, P.M. PP1/PP2A phosphatase inhibition-induced metaplasticity in protein synthesis blocker-treated hippocampal slices: LTP and LTD; or There and Back again. Biochem. Biophys. Res. Commun. 2021, 558, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, A.V.; Bal, N.V.; Balaban, P.M. LTP suppression by protein synthesis inhibitors is NO dependent. Neuropharmacology 2019, 146, 276–288. [Google Scholar] [CrossRef]
- Lal, R.; Lin, H.; Quist, A.P. Amyloid beta ion channel: 3D structure and relevance to amyloid channel paradigm. Biochim. Biophys. Acta 2007, 1768, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Supnet, C.; Bezprozvanny, I. Neuronal calcium signaling, mitochondrial dysfunction, and Alzheimer’s disease. J. Alzheimers Dis. 2010, 20 (Suppl. S2), S487–S498. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.; Hérault, K.; Oheim, M.; Maldera, S.; Vialou, V.; Cauli, B.; Li, D. Astrocytes respond to a neurotoxic Aβ fragment with state-dependent Ca2+ alteration and multiphasic transmitter release. Acta Neuropathol. Commun. 2021, 9, e44. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.P.; Lal, V.; Bowser, D.; Cappai, R.; Masters, C.L.; Ciccotosto, G.D. Stimulus pattern dependence of the Alzheimer’s disease amyloid-beta 42 peptide’s inhibition of long term potentiation in mouse hippocampal slices. Brain Res. 2009, 1269, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.W.; Freir, D.B.; Herron, C.E. Inhibition of LTP in vivo by beta-amyloid peptide in different conformational states. Brain Res. 2008, 1197, 135–142. [Google Scholar] [CrossRef]
- Fagiani, F.; Lanni, C.; Racchi, M.; Pascale, A.; Govoni, S. Amyloid-β and Synaptic Vesicle Dynamics: A Cacophonic Orchestra. J. Alzheimers Dis. 2019, 72, 1–14. [Google Scholar] [CrossRef]
- Walsh, D.M.; Klyubin, I.; Fadeeva, J.V.; Cullen, W.K.; Anwyl, R.; Wolfe, M.S.; Rowan, M.J.; Selkoe, D.J. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 2002, 416, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Bisel, B.E.; Henkins, K.M.; Parfitt, K.D. Alzheimer amyloid beta-peptide A-beta25-35 blocks adenylate cyclase-mediated forms of hippocampal long-term potentiation. Ann. N. Y. Acad. Sci. 2007, 1097, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Mayordomo-Cava, J.; Iborra-Lázaro, G.; Djebari, S.; Temprano-Carazo, S.; Sánchez-Rodríguez, I.; Jeremic, D.; Gruart, A.; Delgado-García, J.M.; Jiménez-Díaz, L.; Navarro-López, J.D. Impairments of Synaptic Plasticity Induction Threshold and Network Oscillatory Activity in the Hippocampus Underlie Memory Deficits in a Non-Transgenic Mouse Model of Amyloidosis. Biology 2020, 9, 175. [Google Scholar] [CrossRef]
- Paschen, W.; Proud, C.G.; Mies, G. Shut-down of translation, a global neuronal stress response: Mechanisms and pathological relevance. Curr. Pharm. Des. 2007, 13, 1887–1902. [Google Scholar] [CrossRef]
- Gambardella, G.; Staiano, L.; Moretti, M.N.; De Cegli, R.; Fagnocchi, L.; Di Tullio, G.; Polletti, S.; Braccia, C.; Armirotti, A.; Zippo, A.; et al. GADD34 is a modulator of autophagy during starvation. Sci. Adv. 2020, 6, eabb0205. [Google Scholar] [CrossRef]
- Zhou, W.; Brush, M.H.; Choy, M.S.; Shenolikar, S. Association with endoplasmic reticulum promotes proteasomal degradation of GADD34 protein. J. Biol. Chem. 2011, 286, 21687–21696. [Google Scholar] [CrossRef]
- Brush, M.H.; Weiser, D.C.; Shenolikar, S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol. Cell Biol. 2003, 23, 1292–1303. [Google Scholar] [CrossRef]
- Chen, Y.; Podojil, J.R.; Kunjamma, R.B.; Jones, J.; Weiner, M.; Lin, W.; Miller, S.D.; Popko, B. Sephin1, which prolongs the integrated stress response, is a promising therapeutic for multiple sclerosis. Brain 2019, 142, 344–361. [Google Scholar] [CrossRef]
- Pernin, F.; Luo, J.; Cui, Q.L.; Blain, M.; Fernandes, M.G.F.; Yaqubi, M.; Srour, M.; Hall, J.; Dudley, R.; Jamann, H.; et al. Diverse injury responses of human oligodendrocyte to mediators implicated in multiple sclerosis. Brain 2022, awac075. [Google Scholar] [CrossRef]
- Farook, J.M.; Shields, J.; Tawfik, A.; Markand, S.; Sen, T.; Smith, S.B.; Brann, D.; Dhandapani, K.M.; Sen, N. GADD34 induces cell death through inactivation of Akt following traumatic brain injury. Cell Death Dis. 2013, 4, e754. [Google Scholar] [CrossRef]
- Ho, K.H.; Lee, Y.T.; Chen, P.H.; Shih, C.M.; Cheng, C.H.; Chen, K.C. Guanabenz Sensitizes Glioblastoma Cells to Sunitinib by Inhibiting GADD34-Mediated Autophagic Signaling. Neurotherapeutics 2021, 18, 1371–1392. [Google Scholar] [CrossRef]
- Yang, T.; He, R.; Li, G.; Liang, J.; Zhao, L.; Zhao, X.; Li, L.; Wang, P. Growth arrest and DNA damage-inducible protein 34 (GADD34) contributes to cerebral ischemic injury and can be detected in plasma exosomes. Neurosci. Lett. 2021, 758, e136004. [Google Scholar] [CrossRef]
- Honjo, Y.; Ayaki, T.; Tomiyama, T.; Horibe, T.; Ito, H.; Mori, H.; Takahashi, R.; Kawakami, K. Increased GADD34 in oligodendrocytes in Alzheimer’s disease. Neurosci. Lett. 2015, 602, 50–55. [Google Scholar] [CrossRef]
- Xu, N.; Xiao, Z.; Zou, T.; Huang, Z. Induction of GADD34 Regulates the Neurotoxicity of Amyloid β. Am. J. Alzheimers Dis. Other Demen. 2015, 30, 313–319. [Google Scholar] [CrossRef]
- Abel, T.; Kandel, E. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res. Brain Res. Rev. 1998, 26, 360–378. [Google Scholar] [CrossRef]
- Soderling, T.R.; Derkach, V.A. Postsynaptic protein phosphorylation and LTP. Trends Neurosci. 2000, 23, 75–80. [Google Scholar] [CrossRef]
- Baltaci, S.B.; Mogulkoc, R.; Baltaci, A.K. Molecular Mechanisms of Early and Late LTP. Neurochem. Res. 2019, 44, 281–296. [Google Scholar] [CrossRef]
- Maltsev, A.V.; Bal, N.V.; Balaban, P.M. Serine/Threonine Phosphatases in LTP: Two B or Not to Be the Protein Synthesis Blocker-Induced Impairment of Early Phase. Int. J. Mol. Sci. 2021, 22, 4857. [Google Scholar] [CrossRef]
- Hölscher, C. Nitric oxide, the enigmatic neuronal messenger: Its role in synaptic plasticity. Trends Neurosci. 1997, 20, 298–303. [Google Scholar] [CrossRef]
- Hardingham, N.; Dachtler, J.; Fox, K. The role of nitric oxide in pre-synaptic plasticity and homeostasis. Front. Cell Neurosci. 2013, 7, e190. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, D.Y. Hippocampus and nitric oxide. Vitam. Horm. 2014, 96, 127–160. [Google Scholar] [CrossRef]
- Ivanova, V.O.; Balaban, P.M.; Bal, N.V. Modulation of AMPA Receptors by Nitric Oxide in Nerve Cells. Int. J. Mol. Sci. 2020, 21, 981. [Google Scholar] [CrossRef]
- Rameau, G.A.; Chiu, L.Y.; Ziff, E.B. Bidirectional regulation of neuronal nitric-oxide synthase phosphorylation at serine 847 by the N-methyl-D-aspartate receptor. J. Biol. Chem. 2004, 279, 14307–14314. [Google Scholar] [CrossRef]
- Song, T.; Hatano, N.; Kume, K.; Sugimoto, K.; Yamaguchi, F.; Tokuda, M.; Watanabe, Y. Inhibition of neuronal nitric-oxide synthase by phosphorylation at Threonine1296 in NG108-15 neuronal cells. FEBS Lett. 2005, 579, 5658–5662. [Google Scholar] [CrossRef]
- Xu, Y.; Krukoff, T.L. Adrenomedullin stimulates nitric oxide production from primary rat hypothalamic neurons: Roles of calcium and phosphatases. Mol. Pharmacol. 2007, 72, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Navia-Pelaez, J.M.; Campos, G.P.; Araujo-Souza, J.C.; Stergiopulos, N.; Capettini, L.S.A. Modulation of nNOSser852 phosphorylation and translocation by PKA/PP1 pathway in endothelial cells. Nitric Oxide 2018, 72, 52–58. [Google Scholar] [CrossRef]
- Katakam, P.V.; Dutta, S.; Sure, V.N.; Grovenburg, S.M.; Gordon, A.O.; Peterson, N.R.; Rutkai, I.; Busija, D.W. Depolarization of mitochondria in neurons promotes activation of nitric oxide synthase and generation of nitric oxide. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1097–H1106. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Osuka, K.; Watanabe, Y.; Usuda, N.; Fukasawa, M.; Araki, Y.; Okamoto, S.; Wakabayashi, T. Subarachnoid hemorrhage induces neuronal nitric oxide synthase phosphorylation at Ser1412 in the dentate gyrus of the rat brain. Nitric Oxide 2018, 81, 67–74. [Google Scholar] [CrossRef]
- Guerra, D.D.; Bok, R.; Cari, E.L.; Nicholas, C.; Orlicky, D.J.; Johnson, J.; Hurt, K.J. Effect of neuronal nitric oxide synthase serine-1412 phosphorylation on hypothalamic-pituitary-ovarian function and leptin response. Biol. Reprod. 2020, 102, 1281–1289. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef]
- Esteras, N.; Abramov, A.Y. Mitochondrial Calcium Deregulation in the Mechanism of Beta-Amyloid and Tau Pathology. Cells 2020, 9, 2135. [Google Scholar] [CrossRef]
- Carafoli, E. The interplay of mitochondria with calcium: An historical appraisal. Cell Calcium 2012, 52, 1–8. [Google Scholar] [CrossRef]
- Sekler, I. Standing of giants shoulders the story of the mitochondrial Na(+)Ca(2+) exchanger. Biochem. Biophys. Res. Commun. 2015, 460, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Carafoli, E. Calcium signalling: A historical account, recent developments and future perspectives. Cell Mol. Life Sci. 2000, 57, 354–370. [Google Scholar] [CrossRef]
- Palty, R.; Hershfinkel, M.; Sekler, I. Molecular identity and functional properties of the mitochondrial Na+/Ca2+ exchanger. J. Biol. Chem. 2012, 287, 31650–31657. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Stull, J.T. Calmodulin-dependent regulation of inducible and neuronal nitric-oxide synthase. J. Biol. Chem. 1998, 273, 27430–27437. [Google Scholar] [CrossRef]
- Zhou, N.; Rungta, R.L.; Malik, A.; Han, H.; Wu, D.C.; MacVicar, B.A. Regenerative glutamate release by presynaptic NMDA receptors contributes to spreading depression. J. Cereb. Blood Flow Metab. 2013, 33, 1582–1594. [Google Scholar] [CrossRef] [PubMed]
- Luccini, E.; Romei, C.; Di Prisco, S.; Raiteri, M.; Raiteri, L. Ionic dysregulations typical of ischemia provoke release of glycine and GABA by multiple mechanisms. J. Neurochem. 2010, 114, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Jadiya, P.; Kolmetzky, D.W.; Tomar, D.; Di Meco, A.; Lombardi, A.A.; Lambert, J.P.; Luongo, T.S.; Ludtmann, M.H.; Praticò, D.; Elrod, J.W. Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer’s disease. Nat. Commun. 2019, 10, e3885. [Google Scholar] [CrossRef] [PubMed]
- Bastioli, G.; Piccirillo, S.; Castaldo, P.; Magi, S.; Tozzi, A.; Amoroso, S.; Calabresi, P. Selective inhibition of mitochondrial sodium-calcium exchanger protects striatal neurons from α-synuclein plus rotenone induced toxicity. Cell Death Dis. 2019, 10, e80. [Google Scholar] [CrossRef] [PubMed]
- Sokolow, S.; Luu, S.H.; Headley, A.J.; Hanson, A.Y.; Kim, T.; Miller, C.A.; Vinters, H.V.; Gylys, K.H. High levels of synaptosomal Na(+)-Ca(2+) exchangers (NCX1; NCX2; NCX3) co-localized with amyloid-beta in human cerebral cortex affected by Alzheimer’s disease. Cell Calcium 2011, 49, 208–216. [Google Scholar] [CrossRef][Green Version]
- Pannaccione, A.; Piccialli, I.; Secondo, A.; Ciccone, R.; Molinaro, P.; Boscia, F.; Annunziato, L. The Na+/Ca2+exchanger in Alzheimer’s disease. Cell Calcium 2020, 87, e102190. [Google Scholar] [CrossRef]
- Pannaccione, A.; Secondo, A.; Molinaro, P.; D’Avanzo, C.; Cantile, M.; Esposito, A.; Boscia, F.; Scorziello, A.; Sirabella, R.; Sokolow, S.; et al. A new concept: Aβ1-42 generates a hyperfunctional proteolytic NCX3 fragment that delays caspase-12 activation and neuronal death. J. Neurosci. 2012, 32, 10609–10617. [Google Scholar] [CrossRef] [PubMed]
- Nita, L.I.; Hershfinkel, M.; Sekler, I. Life after the birth of the mitochondrial Na+/Ca2+ exchanger, NCLX. Sci. China Life Sci. 2015, 58, 59–65. [Google Scholar] [CrossRef]
- Wood-Kaczmar, A.; Deas, E.; Wood, N.W.; Abramov, A.Y. The role of the mitochondrial NCX in the mechanism of neurodegeneration in Parkinson’s disease. Adv. Exp. Med. Biol. 2013, 961, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Doutheil, J.; Althausen, S.; Gissel, C.; Paschen, W. Activation of MYD116 (gadd34) expression following transient forebrain ischemia of rat: Implications for a role of disturbances of endoplasmic reticulum calcium homeostasis. Brain Res. Mol. Brain Res. 1999, 63, 225–232. [Google Scholar] [CrossRef]
- Badiola, N.; Penas, C.; Miñano-Molina, A.; Barneda-Zahonero, B.; Fadó, R.; Sánchez-Opazo, G.; Comella, J.X.; Sabriá, J.; Zhu, C.; Blomgren, K.; et al. Induction of ER stress in response to oxygen-glucose deprivation of cortical cultures involves the activation of the PERK and IRE-1 pathways and of caspase-12. Cell Death Dis. 2011, 2, e149. [Google Scholar] [CrossRef]
- Maltsev, A.; Funikov, S.; Burov, A.; Spasskaya, D.; Ignatyuk, V.; Astakhova, T.; Lyupina, Y.; Deikin, A.; Tutyaeva, V.; Bal, N.; et al. Immunoproteasome Inhibitor ONX-0914 Affects Long-Term Potentiation in Murine Hippocampus. J. Neuroimmune Pharmacol. 2021, 16, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, A.V.; Evdokimovskii, E.V.; Kokoz, Y.M. Synergism of myocardial β-adrenoceptor blockade and I1-imidazoline receptor-driven signaling: Kinase-phosphatase switching. Biochem. Biophys. Res. Commun. 2019, 511, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Krestinina, O.; Azarashvili, T.; Baburina, Y.; Galvita, A.; Grachev, D.; Stricker, R.; Reiser, G. In aging, the vulnerability of rat brain mitochondria is enhanced due to reduced level of 2’;3’-cyclic nucleotide-3’-phosphodiesterase (CNP) and subsequently increased permeability transition in brain mitochondria in old animals. Neurochem. Int. 2015, 80, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Kambayashi, Y.; Ogino, K. Reestimation of Cypridina luciferin analogs (MCLA) as a chemiluminescence probe to detect active oxygen species--cautionary note for use of MCLA. J. Toxicol. Sci. 2003, 28, 139–148. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maltsev, A.V.; Nikiforova, A.B.; Bal, N.V.; Balaban, P.M. Amyloid Aβ25-35 Aggregates Say ‘NO’ to Long-Term Potentiation in the Hippocampus through Activation of Stress-Induced Phosphatase 1 and Mitochondrial Na+/Ca2+ Exchanger. Int. J. Mol. Sci. 2022, 23, 11848. https://doi.org/10.3390/ijms231911848
Maltsev AV, Nikiforova AB, Bal NV, Balaban PM. Amyloid Aβ25-35 Aggregates Say ‘NO’ to Long-Term Potentiation in the Hippocampus through Activation of Stress-Induced Phosphatase 1 and Mitochondrial Na+/Ca2+ Exchanger. International Journal of Molecular Sciences. 2022; 23(19):11848. https://doi.org/10.3390/ijms231911848
Chicago/Turabian StyleMaltsev, Alexander V., Anna B. Nikiforova, Natalia V. Bal, and Pavel M. Balaban. 2022. "Amyloid Aβ25-35 Aggregates Say ‘NO’ to Long-Term Potentiation in the Hippocampus through Activation of Stress-Induced Phosphatase 1 and Mitochondrial Na+/Ca2+ Exchanger" International Journal of Molecular Sciences 23, no. 19: 11848. https://doi.org/10.3390/ijms231911848
APA StyleMaltsev, A. V., Nikiforova, A. B., Bal, N. V., & Balaban, P. M. (2022). Amyloid Aβ25-35 Aggregates Say ‘NO’ to Long-Term Potentiation in the Hippocampus through Activation of Stress-Induced Phosphatase 1 and Mitochondrial Na+/Ca2+ Exchanger. International Journal of Molecular Sciences, 23(19), 11848. https://doi.org/10.3390/ijms231911848






