The TRPV1 Receptor Is Up-Regulated by Sphingosine 1-Phosphate and Is Implicated in the Anandamide-Dependent Regulation of Mitochondrial Activity in C2C12 Myoblasts
Abstract
1. Introduction
2. Results
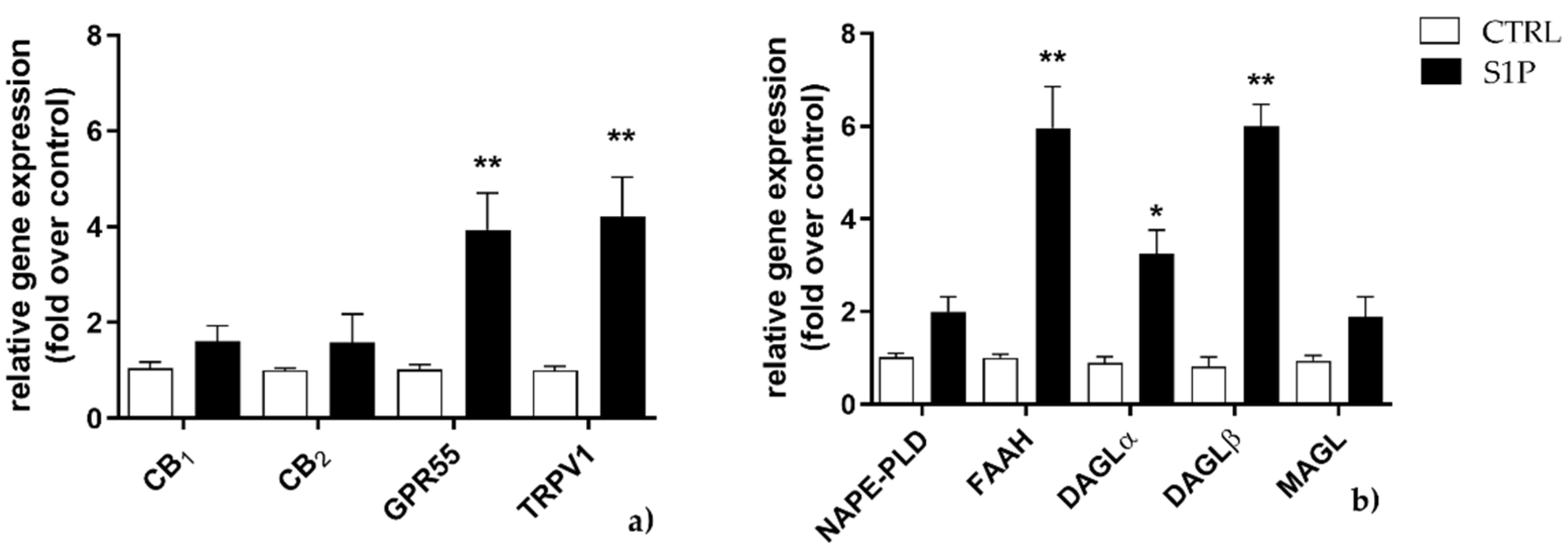
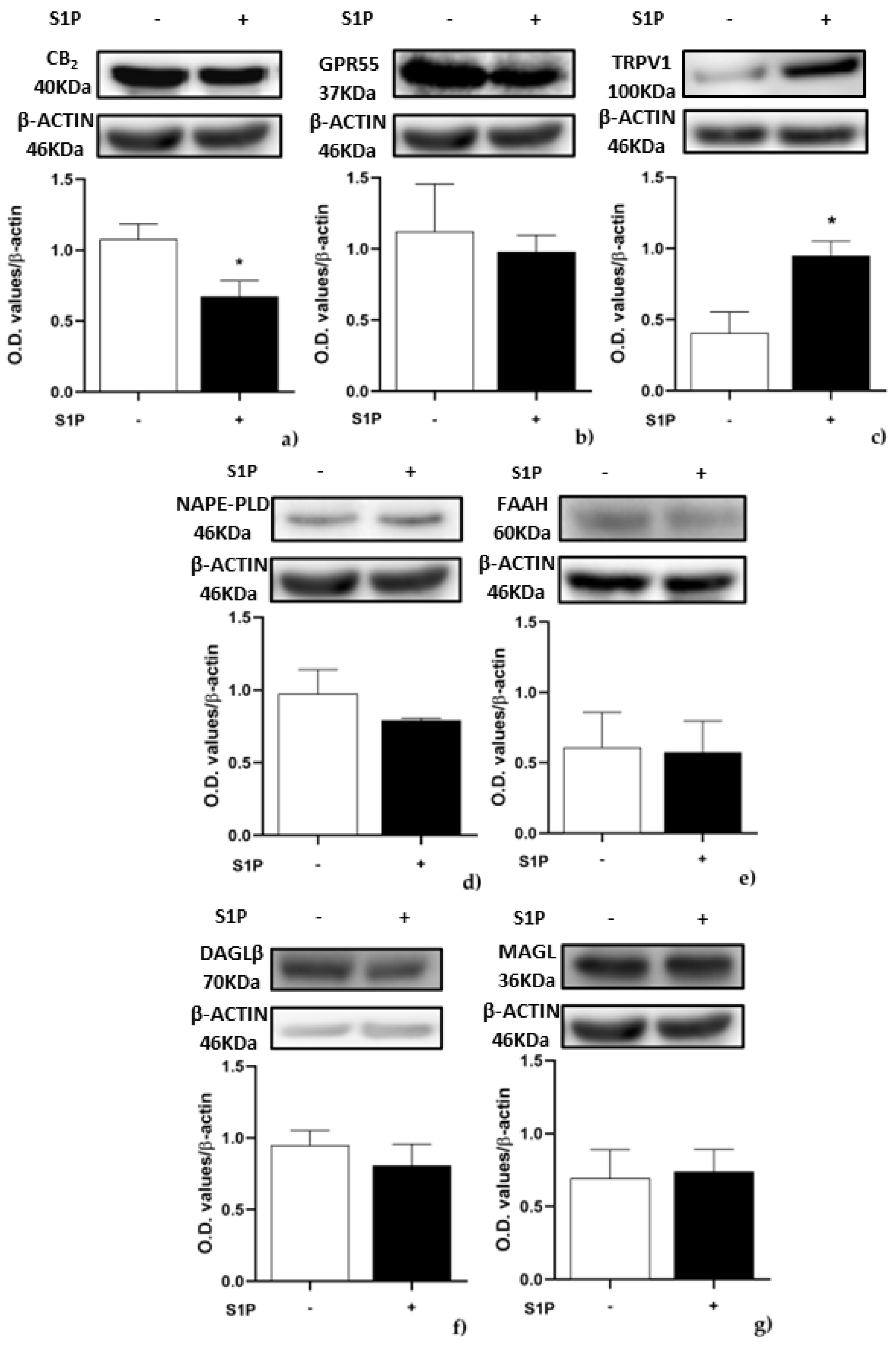
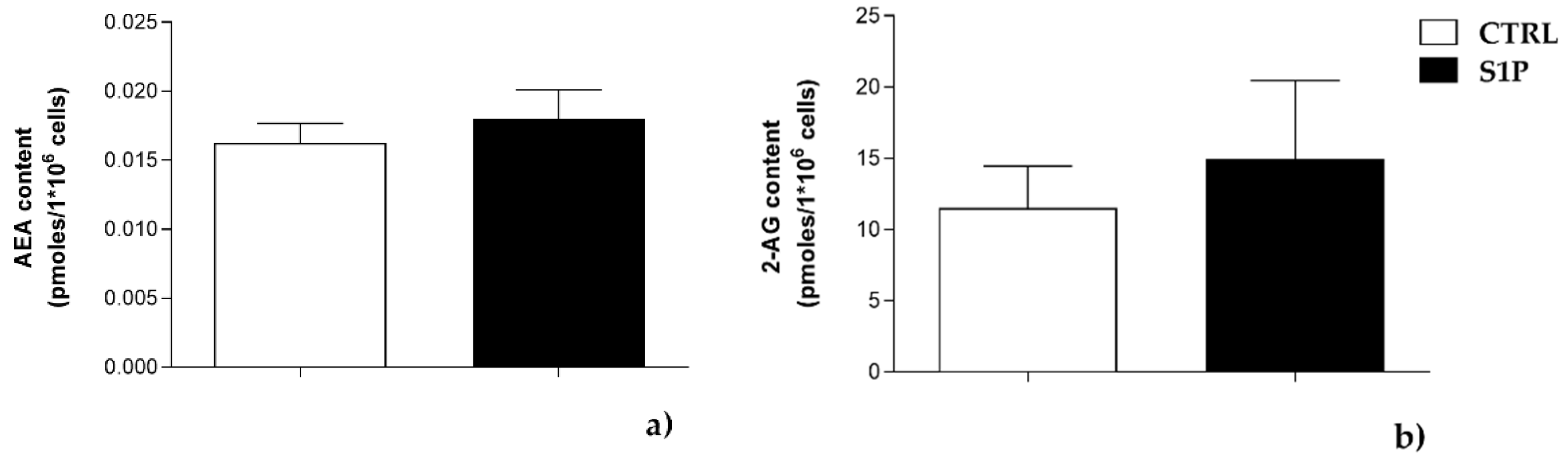
2.1. Effect of S1P on the Expression of ECS Elements
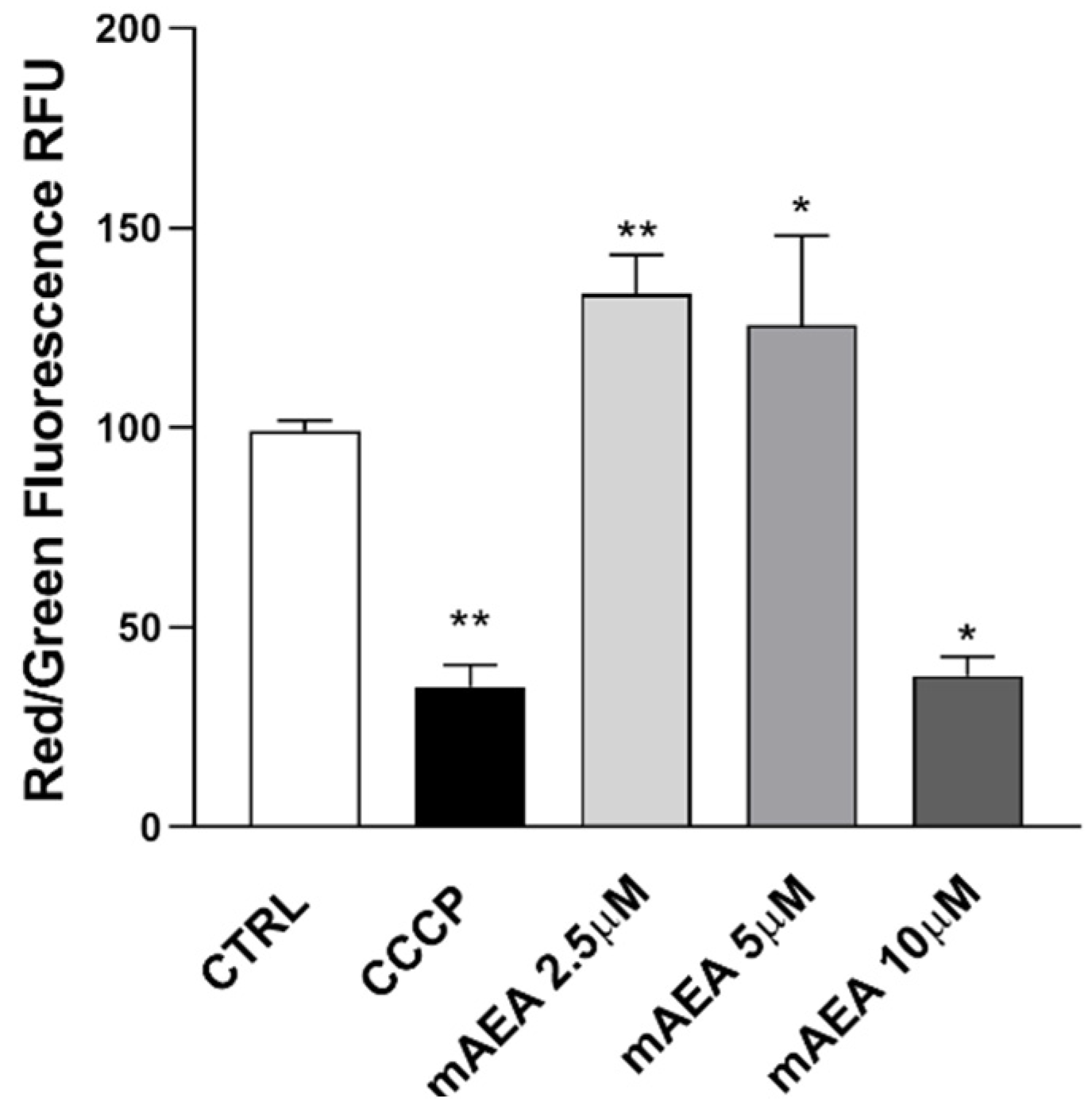
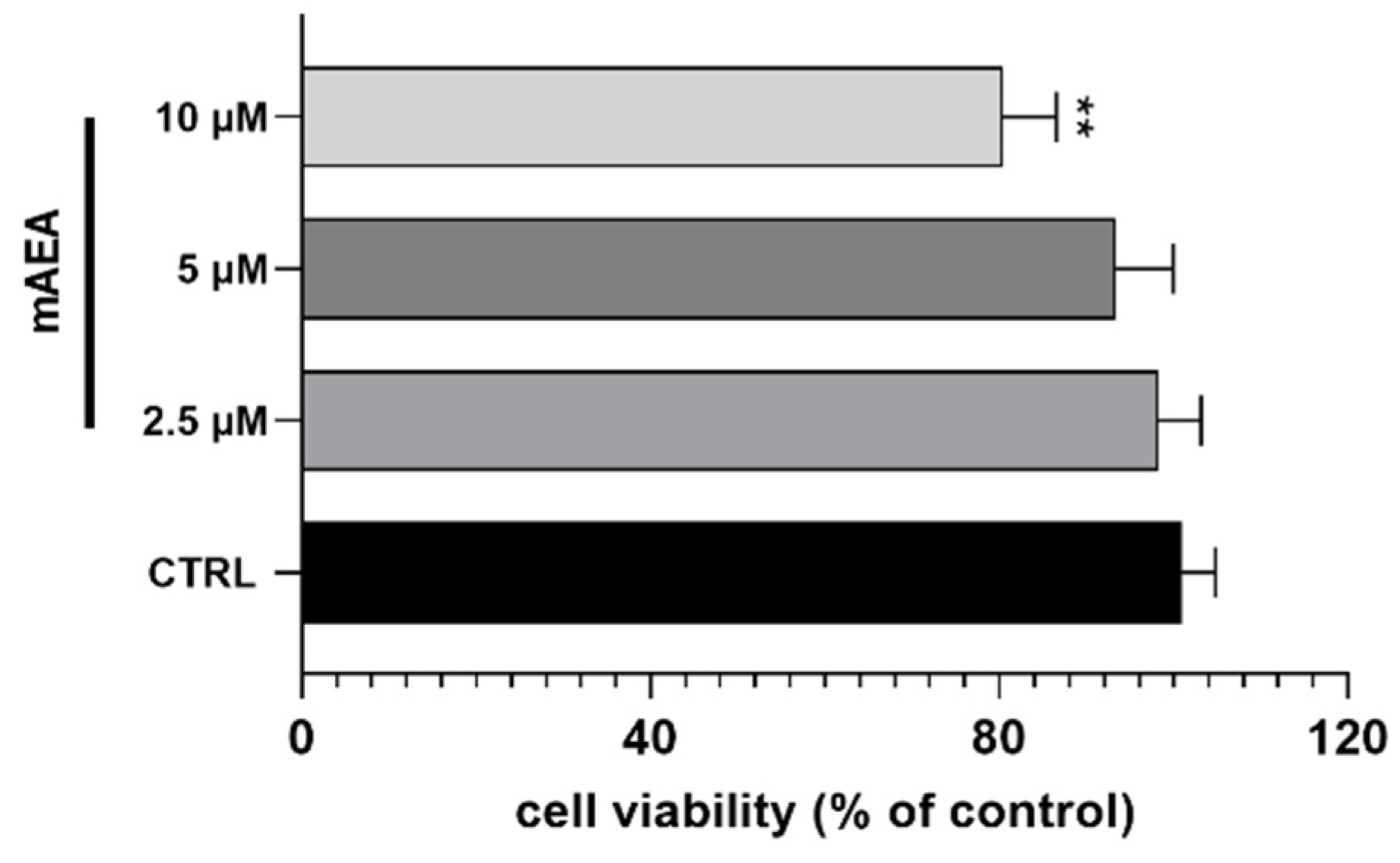
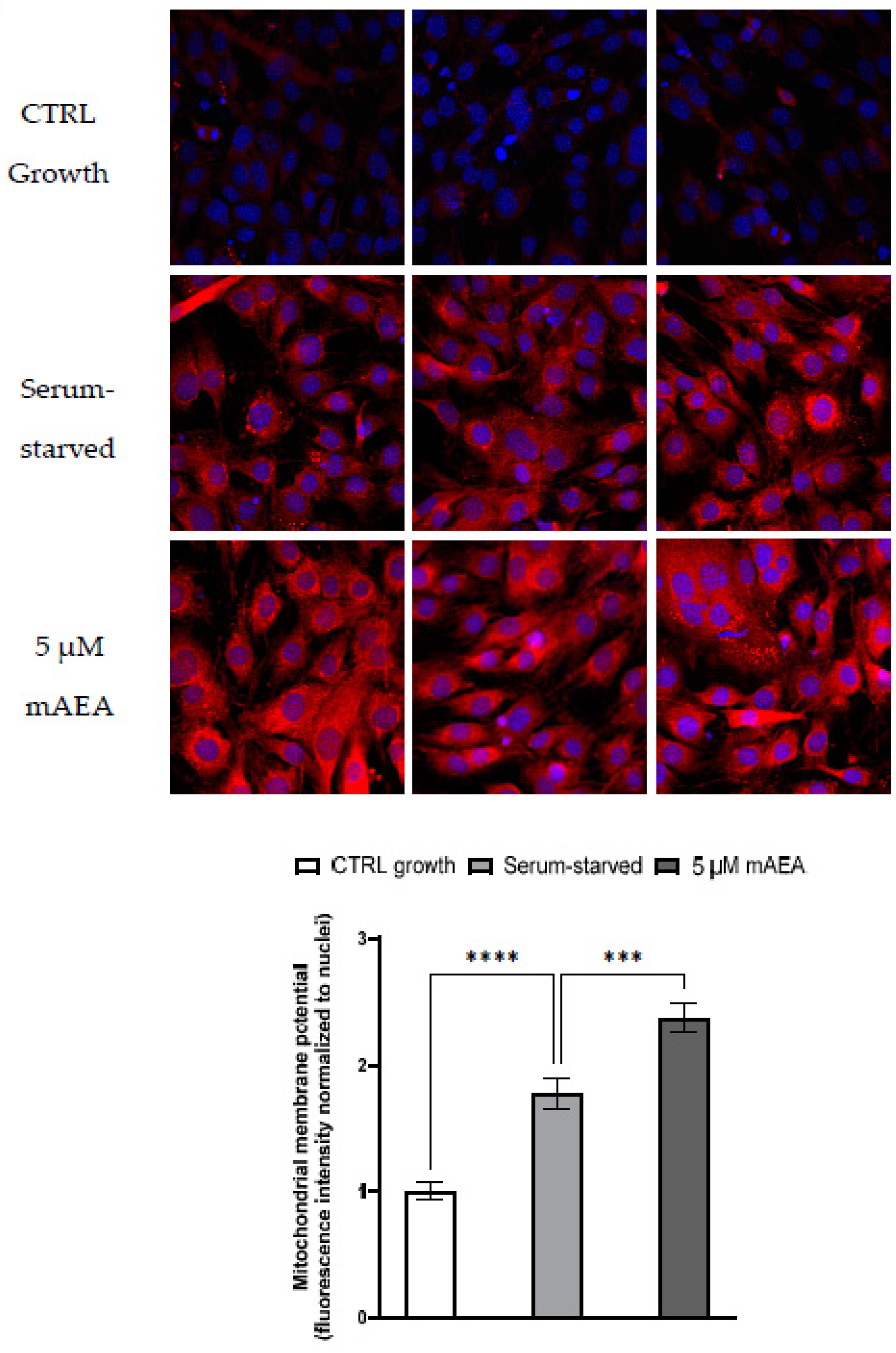
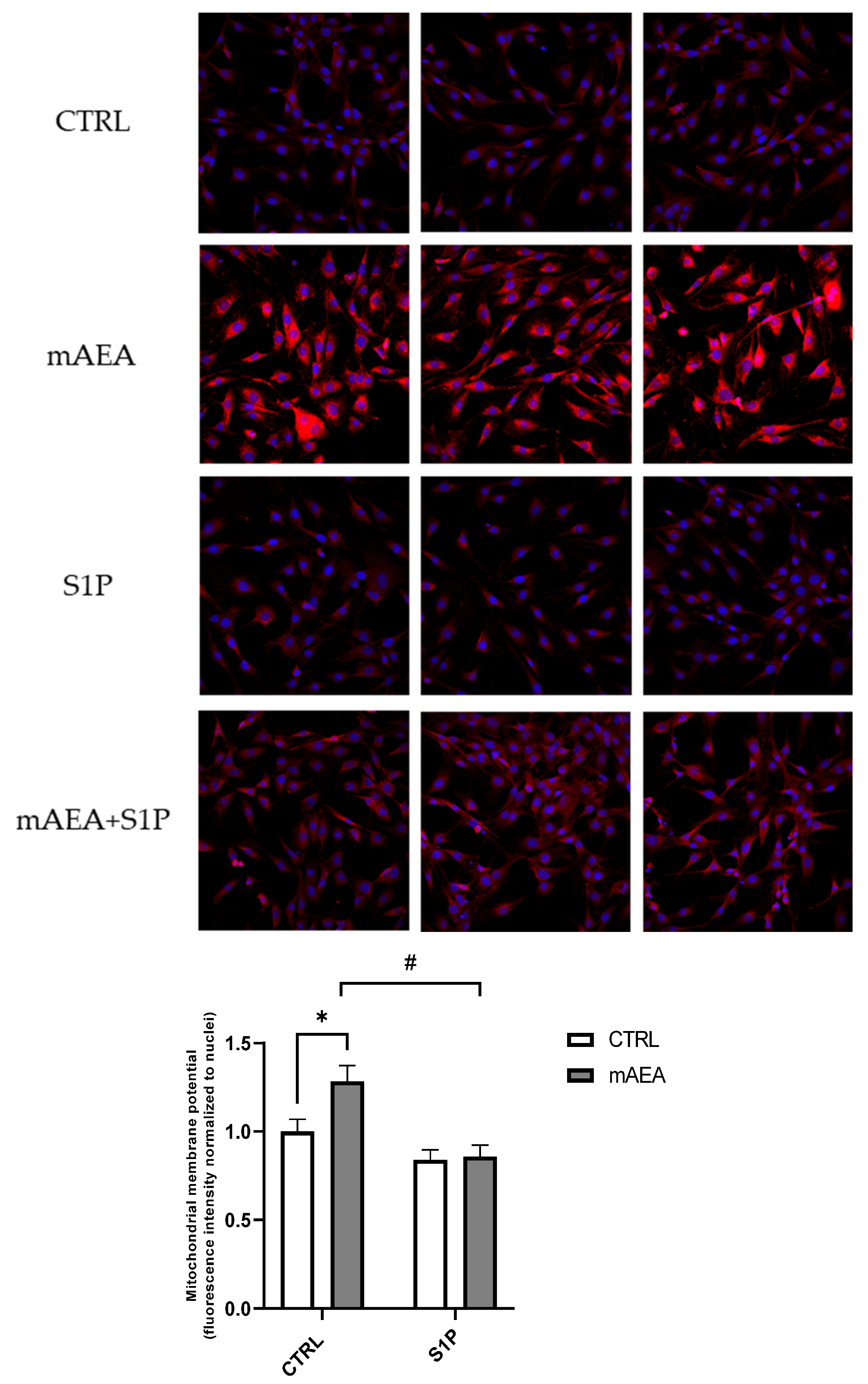
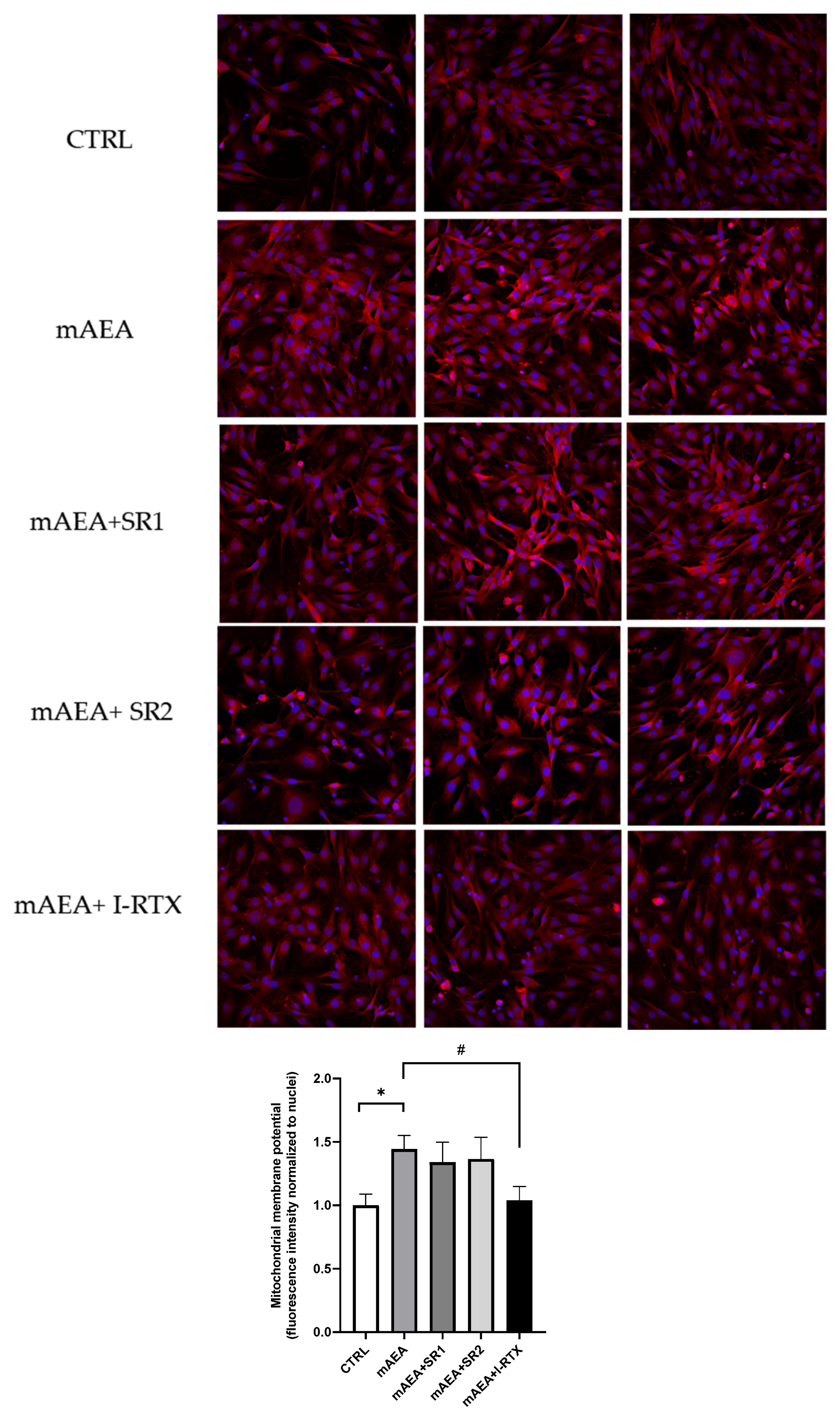
2.2. Effect of Methanandamide on Mitochondrial Membrane potential
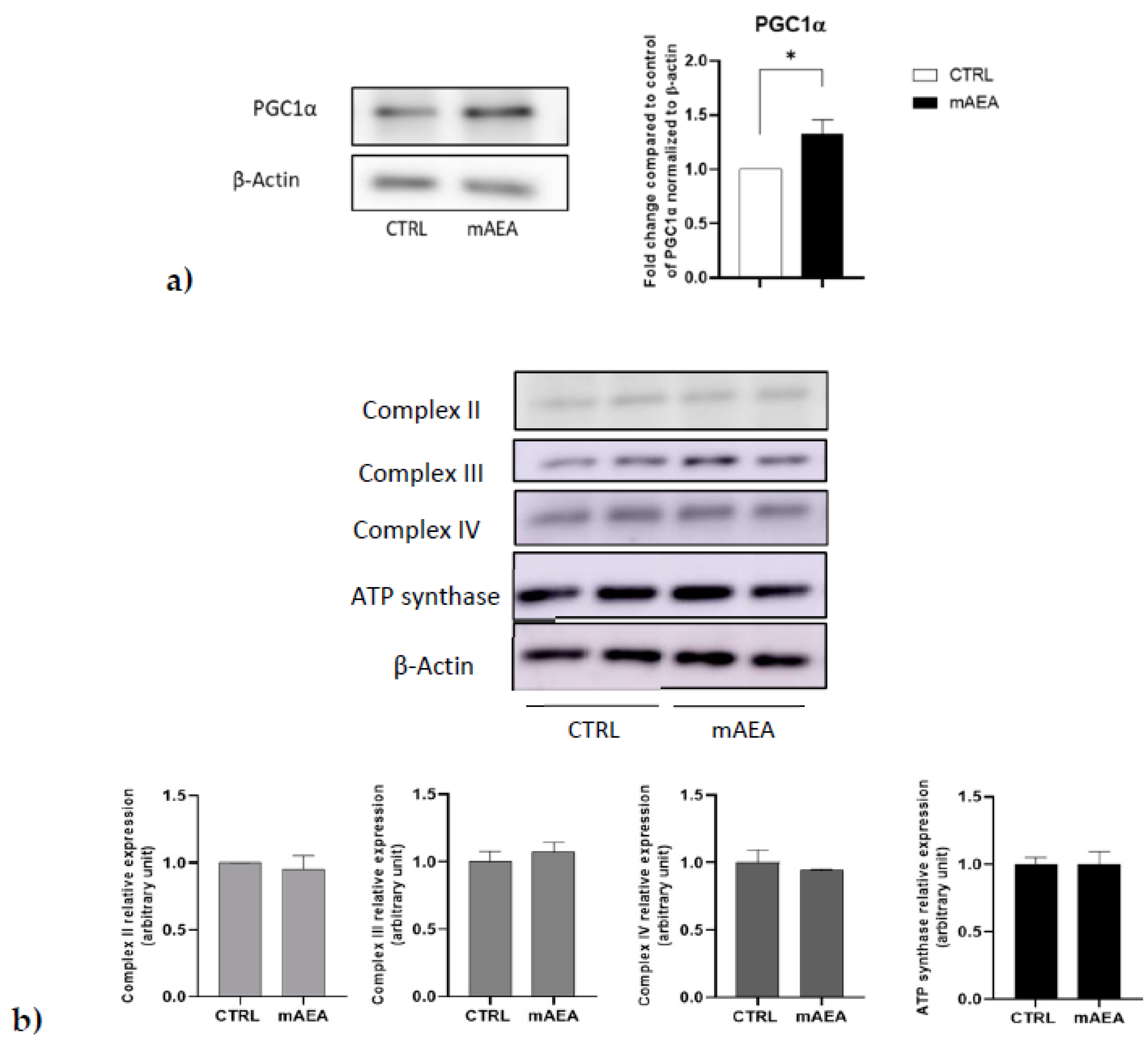
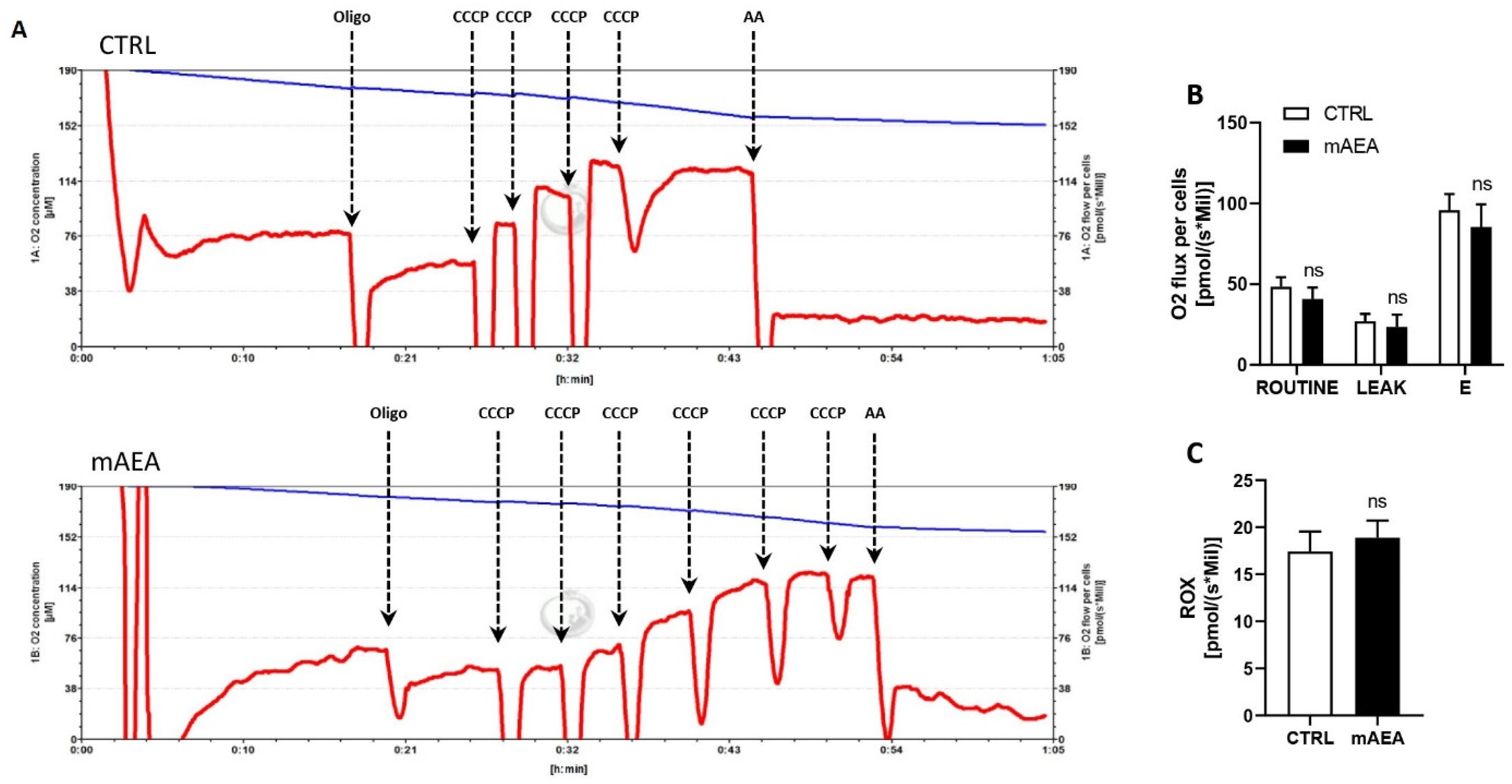
2.3. Effect of Methanandamide on PGC1α, Respiratory Chain Complex Expression and Oxygen Consumption
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Cell Culture and Treatment
4.3. Quantitative Real Time-Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR) Analyses
4.4. Western Blotting
4.5. LC/MS Analysis
4.6. Mitochondrial Membrane Potential Assays
4.7. MTT Assay
4.8. Intact Myoblast Respiration Using High-Resolution Respirometry
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Bryan, L.; Kordula, T.; Spiegel, S.; Milstien, S. Regulation and functions of sphingosine kinases in the brain. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2008, 1781, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Peest, U.; Sensken, S.C.; Andréani, P.; Hänel, P.; van Veldhoven, P.P.; Gräler, M.H. S1P-lyase independent clearance of extracellular sphingosine 1-phosphate after dephosphorylation and cellular uptake. J. Cell Biochem. 2008, 104, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Takabe, K.; Paugh, S.W.; Milstien, S.; Spiegel, S. Inside-out signaling of sphingosine-1-phosphate: Therapeutic targets. Pharmacol. Rev. 2008, 60, 181–195. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kawasaki-Nishi, S.; Otsuka, M.; Hisano, Y.; Yamaguchi, A.; Nishi, T. MFSD2B is a sphingosine 1-phosphate transporter in erythroid cells. Sci. Rep. 2018, 8, 4969. [Google Scholar] [CrossRef]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Cosot, O.A.; Gutkind, J.S.; Spiegel, S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef]
- Rosen, H.; Goetzl, E.J. Sphingosine 1-phosphate and its receptors: An autocrine and paracrine network. Nat. Rev. Immunol. 2005, 5, 560–570. [Google Scholar] [CrossRef]
- Cartier, A.; Hla, T. Sphingosine 1-phosphate: Lipid signaling in pathology and therapy. Science 2019, 366, eaar5551. [Google Scholar] [CrossRef]
- Donati, C.; Cencetti, F.; Bruni, P. Sphingosine 1-phosphate axis: A new leader actor in skeletal muscle biology. Front. Physiol. 2013, 4, 338. [Google Scholar] [CrossRef]
- Friedman, D.; French, J.A.; Maccarrone, M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol. 2019, 18, 504–512. [Google Scholar] [CrossRef]
- Maccarrone, M.; Guzmán, M.; Mackie, K.; Doherty, P.; Harkany, T. Programming of neural cells by (endo)cannabinoids: From physiological rules to emerging therapies. Nat. Rev. Neurosci. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Rapino, C.; Francavilla, F.; Barbonetti, A. Cannabinoid signalling and effects of cannabis on the male reproductive system. Nat. Rev. Urol. 2020, 18, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Bisogno, T.; di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Luchicchi, A.; Pistis, M. Anandamide and 2-arachidonoylglycerol: Pharmacological properties, functional features, and emerging specificities of the two major endocannabinoids. Mol. Neurobiol. 2012, 46, 374–392. [Google Scholar] [CrossRef]
- Maccarrone, M.; Dainese, E.; Oddi, S. Intracellular trafficking of anandamide: New concepts for signaling. Trends Biochem. Sci. 2010, 35, 601–608. [Google Scholar] [CrossRef]
- Murataeva, N.; Straiker, A.; MacKie, K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br. J. Pharmacol. 2014, 171, 1379–1391. [Google Scholar] [CrossRef]
- Lu, H.C.; MacKie, K. An introduction to the endogenous cannabinoid system. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor gpr55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- Gasperi, V.; Dainese, E.; Oddi, S.; Sabatucci, A.; Maccarrone, M. GPR55 and its interaction with membrane lipids: Comparison with other endocannabinoid-binding receptors. Curr. Med. Chem. 2012, 20, 64–78. [Google Scholar] [CrossRef]
- Ross, R.A. The enigmatic pharmacology of GPR55. Trends Pharmacol. Sci. 2009, 30, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, T.; Sydney, A.S. TRPV1 receptors and signal transduction. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Howlett, A.C.; Shim, J. Cannabinoid Receptors and Signal Transduction; Landes Bioscience: Austin, TX, USA, 2011. [Google Scholar]
- Greig, F.H.; Nather, K.; Ballantyne, M.D.; Kazi, Z.H.; Alganga, H.; Ewart, M.A.; Zaborska, K.E.; Fertig, B.; Pyne, N.J.; Pyne, S.; et al. Requirement for sphingosine kinase 1 in mediating phase 1 of the hypotensive response to anandamide in the anaesthetised mouse. Eur. J. Pharmacol. 2019, 842, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, T.; Hla, T. Structural and functional characteristics of S1P receptors. J. Cell Biochem. 2004, 92, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Galve-Roperh, I.; Sánchez, C.; Cortés, M.L.; del Pulgar, T.G.; Izquierdo, M.; Guzmán, M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000, 6, 313–319. [Google Scholar] [CrossRef]
- Mair, K.M.; Robinson, E.; Kane, K.A.; Pyne, S.; Brett, R.R.; Pyne, N.J.; Kennedy, S. Interaction between anandamide and sphingosine-1-phosphate in mediating vasorelaxation in rat coronary artery. Br. J. Pharmacol. 2010, 161, 176–192. [Google Scholar] [CrossRef]
- Paugh, S.W.; Cassidy, M.P.; He, H.; Milstien, S.; Sim-Selley, L.J.; Spiegel, S.; Selley, D.E. Sphingosine and its analog, the immunosuppressant 2-amino-2-(2-[4-octylphenyl] ethyl)-1,3-propanediol, interact with the CB1 cannabinoid receptor. Mol. Pharmacol. 2006, 70, 41–50. [Google Scholar] [CrossRef]
- Zhao, D.; Pond, A.; Watkins, B.; Gerrard, D.; Wen, Y.; Kuang, S.; Hannon, K. Peripheral endocannabinoids regulate skeletal muscle development and maintenance. Eur. J. Transl. Myol. 2010, 20, 167–180. [Google Scholar] [CrossRef]
- Kim, J.; Carlson, M.E.; Watkins, B.A. Docosahexaenoyl ethanolamide improves glucose uptake and alters endocannabinoid system gene expression in proliferating and differentiating C2C12 myoblasts. Front. Physiol. 2014, 5, 100. [Google Scholar] [CrossRef]
- Bernacchioni, C.; Ghini, V.; Squecco, R.; Idrizaj, E.; Garella, R.; Puliti, E.; Cencetti, F.; Bruni, P.; Donati, C. Role of sphingosine 1-phosphate signalling axis in muscle atrophy induced by Tnfα in C2c12 myotubes. Int. J. Mol. Sci. 2021, 22, 1280. [Google Scholar] [CrossRef]
- Donati, C.; Meacci, E.; Nuti, F.; Becciolini, L.; Farnararo, M.; Bruni, P. Sphingosine 1-phosphate regulates myogenic differentiation: A major role for S1P2 receptor. FASEB J. 2005, 19, 1–22. [Google Scholar] [CrossRef]
- Cencetti, F.; Bernacchioni, C.; Nincheri, P.; Donati, C.; Bruni, P. Transforming growth factor-1 induces transdifferentiation of myoblasts into myofibroblasts via up-regulation of sphingosine kinase-1/S1P 3 axis. Mol. Biol. Cell 2010, 21, 1111–1124. [Google Scholar] [CrossRef]
- Bernacchioni, C.; Cencetti, F.; Blescia, S.; Donati, C.; Bruni, P. Sphingosine kinase/sphingosine 1-phosphate axis: A new player for insulin-like growth factor-1-induced myoblast differentiation. Skelet. Muscle 2012, 2, 15. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Silvestri, C.; Mazzarella, E.; Martella, A.; Calvigioni, D.; Piscitelli, F.; Ambrosino, P.; Petrosino, S.; Czifra, G.; Bíŕo, T.; et al. The endocannabinoid 2-AG controls skeletal muscle cell differentiation via CB1 receptor-dependent inhibition of Kv7 channels. Proc. Natl. Acad. Sci. USA 2014, 111, E2472–E2481. [Google Scholar] [CrossRef] [PubMed]
- Pagano, E.; Orlando, P.; Finizio, S.; Rossi, A.; Buono, L.; Iannotti, F.A.; Piscitelli, F.; Izzo, A.A.; di Marzo, V.; Borrelli, F. Role of the endocannabinoid system in the control of mouse myometrium contractility during the menstrual cycle. Biochem. Pharmacol. 2017, 124, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Kunos, G.; Osei-Hyiaman, D.; Liu, J.; Godlewski, G.; Bátkai, S. Endocannabinoids and the control of energy homeostasis. J. Biol. Chem. 2008, 283, 33021–33025. [Google Scholar] [CrossRef] [PubMed]
- Pasquariello, N.; Catanzaro, G.; Marzano, V.; Amadio, D.; Barcaroli, D.; Oddi, S.; Federici, G.; Urbani, A.; Agrò, A.F.; Maccarrone, M. Characterization of the endocannabinoid system in human neuronal cells and proteomic analysis of anandamide-induced apoptosis. J. Biol. Chem. 2009, 284, 29413–29426. [Google Scholar] [CrossRef]
- Silvestri, C.; di Marzo, V. The Endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013, 17, 475–490. [Google Scholar] [CrossRef]
- Abadji, V.; Lin, S.; Taha, G.; Griffin, G.; Stevenson, L.A.; Pertwee, R.G.; Makriyannis, A. (R)-methanandamide: A chiral novel anandamide possessing higher potency and metabolic stability. J. Med. Chem. 1994, 37, 1889–1893. [Google Scholar] [CrossRef]
- Huang, T.Y.; Zheng, D.; Houmard, J.A.; Brault, J.J.; Hickner, R.C.; Cortright, R.N. Overexpression of PGC-1α increases peroxisomal activity and mitochondrial fatty acid oxidation in human primary myotubes. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E253–E263. [Google Scholar] [CrossRef]
- Catanzaro, G.; Rapino, C.; Oddi, S.; Maccarrone, M. Anandamide increases swelling and reduces calcium sensitivity of mitochondria. Biochem. Biophys. Res. Commun. 2009, 388, 439–442. [Google Scholar] [CrossRef]
- Gasperi, V.; Fezza, F.; Pasquariello, N.; Bari, M.; Oddi, S.; Finazzi Agrò, A.; Maccarrone, M. Endocannabinoids in adipocytes during differentiation and their role in glucose uptake. Cell. Mol. Life Sci. 2007, 64, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe. Bio-Protocol 2019, 9, e3128. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr. Med. Chem. 2010, 17, 1360–1381. [Google Scholar] [CrossRef]
- Kotsikorou, E.; Sharir, H.; Shore, D.M.; Hurst, D.P.; Lynch, D.L.; Madrigal, K.E.; Heynen-Genel, S.; Milan, L.B.; Chung, T.D.Y.; Seltzman, H.H.; et al. Identification of the GPR55 antagonist binding site using a novel set of high-potency GPR55 selective ligands. Biochemistry 2013, 52, 9456–9469. [Google Scholar] [CrossRef] [PubMed]
- van der Stelt, M.; Trevisani, M.; Vellani, V.; de Petrocellis, L.; Moriello, A.S.; Campi, B.; McNaughton, P.; Geppetti, P.; di Marzo, V. Anandamide acts as an intracellular messenger amplifying Ca2+ influx via TRPV1 channels. EMBO J. 2005, 24, 3026–3037. [Google Scholar] [CrossRef]
- Rigoni, M.; Trevisani, M.; Gazzieri, D.; Nadaletto, R.; Tognetto, M.; Creminon, C.; Davis, J.B.; Campi, B.; Amadesi, S.; Geppetti, P.; et al. Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affnity antagonist, iodo-resiniferatoxin. Br. J. Pharmacol. 2003, 138, 977–985. [Google Scholar] [CrossRef]
- Calise, S.; Blescia, S.; Cencetti, F.; Bernacchioni, C.; Donati, C.; Bruni, P. Sphingosine 1-phosphate stimulates proliferation and migration of satellite cells. role of s1p receptors. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 439–450. [Google Scholar] [CrossRef]
- Loh, K.C.; Leong, W.I.; Carlson, M.E.; Oskouian, B.; Kumar, A.; Fyrst, H.; Zhang, M.; Proia, R.L.; Hoffman, E.P.; Saba, J.D. Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through a S1PR2/STAT3 signaling pathway. PLoS ONE 2012, 7, e37218. [Google Scholar] [CrossRef]
- Nagata, Y.; Partridge, T.A.; Matsuda, R.; Zammit, P.S. Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling. J. Cell Biol. 2006, 174, 245–253. [Google Scholar] [CrossRef]
- Rapizzi, E.; Taddei, M.L.; Fiaschi, T.; Donati, C.; Bruni, P.; Chiarugi, P. Sphingosine 1-phosphate increases glucose uptake through trans-activation of insulin receptor. Cell. Mol. Life Sci. 2009, 66, 3207–3218. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Pagano, E.; Guardiola, O.; Adinolfi, S.; Saccone, V.; Consalvi, S.; Piscitelli, F.; Gazzerro, E.; Busetto, G.; Carrella, D.; et al. Genetic and pharmacological regulation of the endocannabinoid CB1 receptor in duchenne muscular dystrophy. Nat. Commun. 2018, 9, 3950. [Google Scholar] [CrossRef]
- Nguyen-Tran, D.H.; Hait, N.C.; Sperber, H.; Qi, J.; Fischer, K.; Ieronimakis, N.; Pantoja, M.; Hays, A.; Allegood, J.; Reyes, M.; et al. Molecular mechanism of sphingosine-1-phosphate action in duchenne muscular dystrophy. DMM Dis. Models Mech. 2014, 7, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.J.; et al. Cloning of the first Sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 2003, 163, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Glanemann, C.; Loos, A.; Gorret, N.; Willis, L.B.; O’brien, X.M.; Lessard, P.A.; Sinskey, A.J. Disparity between changes in MRNA abundance and enzyme activity in corynebacterium glutamicum: Implications for dna microarray analysis. Appl. Microbiol. Biotechnol. 2003, 61, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Rusconi, F.; Rubino, T.; Cattaneo, A.; Martegani, E.; Parolaro, D.; Bachi, A.; Zippel, R.; Rubino, T.; Parolaro, D.; et al. Transcriptomic and proteomic analyses of mouse cerebellum reveals alterations in RasGRF1 expression following in vivo chronic treatment with delta 9-tetrahydrocannabinol. J. Mol. Neurosci. 2009, 37, 111–122. [Google Scholar] [CrossRef]
- Bari, M.; Paradisi, A.; Pasquariello, N.; Maccarrone, M. Cholesterol-dependent modulation of type 1 cannabinoid receptors in nerve cells. J. Neurosci. Res. 2005, 81, 275–283. [Google Scholar] [CrossRef]
- Pasquariello, N.; Oddi, S.; Malaponti, M.; Maccarrone, M. Chapter 17 regulation of gene transcription and keratinocyte differentiation by anandamide. Vitam. Horm. 2009, 81, 441–467. [Google Scholar] [CrossRef]
- Hong, H.; Yoon, B.; Ghil, S. Interactions between lysophosphatidylinositol receptor GPR55 and sphingosine-1-phosphate receptor S1P5 in live cells. Biochem. Biophys. Res. Commun. 2021, 570, 53–59. [Google Scholar] [CrossRef]
- Bénard, G.; Massa, F.; Puente, N.; Lourenço, J.; Bellocchio, L.; Soria-Gómez, E.; Matias, I.; Delamarre, A.; Metna-Laurent, M.; Cannich, A.; et al. Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nat. Neurosci. 2012, 15, 558–564. [Google Scholar] [CrossRef]
- Melser, S.; Zottola, A.C.P.; Serrat, R.; Puente, N.; Grandes, P.; Marsicano, G.; Hebert-Chatelain, E. Functional analysis of mitochondrial CB1 cannabinoid receptors (MtCB1) in the brain. Methods Enzymol. 2017, 593, 143–174. [Google Scholar] [CrossRef]
- Movsesyan, V.A.; Stoica, B.A.; Yakovlev, A.G.; Knoblach, S.M.; Lea, P.M.; Cernak, I.; Vink, R.; Faden, A.I. Anandamide-induced cell death in primary neuronal cultures: Role of calpain and caspase pathways. Cell Death Differ. 2004, 11, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Bartke, N.; Hannun, Y.A. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 2009, 50, S91–S96. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Oskeritzian, C.A.; Paugh, S.W.; Milstien, S.; Spiegel, S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta Biomembr. 2006, 1758, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Fugio, L.B.; Coeli-Lacchini, F.B.; Leopoldino, A.M. Sphingolipids and mitochondrial dynamic. Cells 2020, 9, 581. [Google Scholar] [CrossRef]
- di Marzo, V.; Iannotti, F.A.; Pagano, E.; Schiano Moriello, A.; Grazia Alvino, F.; Sorrentino, N.C.; Gazzerro, E.; Capasso, R.; de Leonibus, E.; de Petrocellis, L.; et al. Effects of non-euphoric plant cannabinoids on muscle quality and performance of dystrophic mdx mice. Cannabinoid Res. Br. J. Pharmacol. Br. J. Pharmacol. 2019, 176, 1568–1584. [Google Scholar] [CrossRef]
- Rapizzi, E.; Donati, C.; Cencetti, F.; Nincheri, P.; Bruni, P. Sphingosine 1-phosphate differentially regulates proliferation of C2C12 reserve cells and myoblasts. Mol. Cell Biochem. 2008, 314, 193–199. [Google Scholar] [CrossRef]
- Becciolini, L.; Meacci, E.; Donati, C.; Cencetti, F.; Rapizzi, E.; Bruni, P. Sphingosine 1-phosphate inhibits cell migration in C2C12 myoblasts. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2006, 1761, 43–51. [Google Scholar] [CrossRef]
- Kittaka, H.; DeBrecht, J.; Mishra, S.K. Differential contribution of sensory transient receptor potential channels in response to the bioactive lipid sphingosine-1-phosphate. Mol. Pain 2020, 16, 1744806920903515. [Google Scholar] [CrossRef]
- Meacci, E.; Cencetti, F.; Formigli, L.; Squecco, R.; Donati, C.; Tiribilli, B.; Quercioli, F.; Zecchi Orlandini, S.; Francini, F.; Bruni, P. Sphingosine 1-phosphate evokes calcium signals in C2C12 myoblasts via Edg3 and Edg5 receptors. Biochem. J. 2002, 362, 349–357. [Google Scholar] [CrossRef]
- Duchen, M.R. Mitochondria and calcium: From cell signalling to cell death. J. Physiol. 2000, 529, 57–68. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, L.; Zhao, Z.; He, H.; Yang, D.; Feng, X.; Ma, S.; Chen, X.; Zhu, T.; Cao, T.; et al. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1α upregulation in mice. Cell Res. 2012, 22, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, A.; Clarke, A.B.; Turner, A.E.; Kumaran, N.M.; Vakilpour, S.; Smith, P.A.; Bagiokou, D.; Bradshaw, T.D.; Westwell, A.D.; Fang, L.; et al. Cannabinoid receptor agonists are mitochondrial inhibitors: A unified hypothesis of how cannabinoids modulate mitochondrial function and induce cell death. Biochem. Biophys. Res. Commun. 2007, 364, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Pasquariello, N.; Battista, N.; di Tommaso, M.; Rapino, C.; Fezza, F.; Zuccolo, M.; Jourdain, R.; Agrò, A.F.; Breton, L.; et al. Endocannabinoids stimulate human melanogenesis via type-1 cannabinoid receptor. J. Biol. Chem. 2012, 287, 15466–15478. [Google Scholar] [CrossRef]
- Bruno, G.; Cencetti, F.; Bernacchioni, C.; Donati, C.; Blankenbach, K.V.; Thomas, D.; Meyer zu Heringdorf, D.; Bruni, P. Bradykinin mediates myogenic differentiation in murine myoblasts through the involvement of SK1/Spns2/S1P 2 axis. Cell Signal. 2018, 45, 110–121. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sergi, M.; Montesano, C.; Napoletano, S.; Pizzoni, D.; Manetti, C.; Colistro, F.; Curini, R.; Compagnone, D. Analysis of bile acids profile in human serum by ultrafiltration clean-up and LC-MS/MS. Chromatographia 2012, 75, 479–489. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Osycka-Salut, C.; Caballero, J.; Vazquez-Levin, M.; Pereyra, E.; Billi, S.; Franchi, A.; Perez-Martinez, S. Anandamide capacitates bull spermatozoa through CB1 and TRPV1 activation. PLoS ONE 2011, 6, e16993. [Google Scholar] [CrossRef] [PubMed]
- Maccarone, R.; Rapino, C.; Zerti, D.; di Tommaso, M.; Battista, N.; di Marco, S.; Bisti, S.; Maccarrone, M. Modulation of type-1 and type-2 cannabinoid receptors by saffron in a rat model of retinal neurodegeneration. PLoS ONE 2016, 11, e0166827. [Google Scholar] [CrossRef]
- Pertwee, R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997, 74, 129–180. [Google Scholar] [CrossRef]
- Heynen-Genel, S.; Dahl, R.; Shi, S.; Milan, L.; Hariharan, S.; Sergienko, E.; Hedrick, M.; Dad, S.; Stonich, D.; Su, Y.; et al. Probe report title: Screening for selective ligands for GPR55-agonists. In Probe Reports from the NIH Molecular Libraries Program; National Center for Biotechnology Information: Bethesda, MD, USA, 2011. [Google Scholar]
- Liao, C.; Xu, D.; Liu, X.; Fang, Y.; Yi, J.; Li, X.; Guo, B. Iridium (III) complex-loaded liposomes as a drug delivery system for lung cancer through mitochondrial dysfunction. Int. J. Nanomed. 2018, 13, 4417–4431. [Google Scholar] [CrossRef]
- Ye, F.; Hoppel, C.L. Measuring oxidative phosphorylation in human skin fibroblasts. Anal. Biochem. 2013, 437, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Pesta Dominik, G.E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar] [CrossRef]


| Forward Primer (5′->3′) | Reverse Primer (5′->3′) | |
|---|---|---|
| β-ACTIN | TGTTACCAACTGGGACGA | GTCTCAAACATGATCTGGGTC |
| GAPDH | AACGGGAAGCTCACTGGCAT | GCTTCACCACCTTCTTGATG |
| CB1 | CCAAGAAAAGATGACGGCAG | AGGATGACACATAGCACCAG |
| CB2 | TCGCTTACATCCTTCAGACAG | TCTTCCCTCCCAACTCCTTC |
| GPR55 | ATTCGATTCCGTGGATAAGC | ATGCTGATGAAGTAGAGGC |
| TRPV1 | TGAACTGGACTACCTGGAAC | TCCTTGAAGACCTCAGCATC |
| NAPE-PLD | AAGTGTGTCTTCTAGGTTCTCC | TTGTCAAGTTCCTCTTTGGAACC |
| FAAH | AGATTGAGATGTATCGCCAG | CTTCAGAATGTTGTCCCAC |
| DAGLα | AATGGCTATCATCTGGCTGAGC | TTCCGAGGGTGACATTCTTAGC |
| DAGLβ | TGTCAGCATGAGAGGAACCAT | CGCCAGGCGGATATAGAGC |
| MAGL | TTGTAGATACTGGAAGCCC | ATGGTGTCCACGTGTTGCAGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Standoli, S.; Pecchioli, S.; Tortolani, D.; Di Meo, C.; Fanti, F.; Sergi, M.; Bacci, M.; Seidita, I.; Bernacchioni, C.; Donati, C.; et al. The TRPV1 Receptor Is Up-Regulated by Sphingosine 1-Phosphate and Is Implicated in the Anandamide-Dependent Regulation of Mitochondrial Activity in C2C12 Myoblasts. Int. J. Mol. Sci. 2022, 23, 11103. https://doi.org/10.3390/ijms231911103
Standoli S, Pecchioli S, Tortolani D, Di Meo C, Fanti F, Sergi M, Bacci M, Seidita I, Bernacchioni C, Donati C, et al. The TRPV1 Receptor Is Up-Regulated by Sphingosine 1-Phosphate and Is Implicated in the Anandamide-Dependent Regulation of Mitochondrial Activity in C2C12 Myoblasts. International Journal of Molecular Sciences. 2022; 23(19):11103. https://doi.org/10.3390/ijms231911103
Chicago/Turabian StyleStandoli, Sara, Sara Pecchioli, Daniel Tortolani, Camilla Di Meo, Federico Fanti, Manuel Sergi, Marina Bacci, Isabelle Seidita, Caterina Bernacchioni, Chiara Donati, and et al. 2022. "The TRPV1 Receptor Is Up-Regulated by Sphingosine 1-Phosphate and Is Implicated in the Anandamide-Dependent Regulation of Mitochondrial Activity in C2C12 Myoblasts" International Journal of Molecular Sciences 23, no. 19: 11103. https://doi.org/10.3390/ijms231911103
APA StyleStandoli, S., Pecchioli, S., Tortolani, D., Di Meo, C., Fanti, F., Sergi, M., Bacci, M., Seidita, I., Bernacchioni, C., Donati, C., Bruni, P., Maccarrone, M., Rapino, C., & Cencetti, F. (2022). The TRPV1 Receptor Is Up-Regulated by Sphingosine 1-Phosphate and Is Implicated in the Anandamide-Dependent Regulation of Mitochondrial Activity in C2C12 Myoblasts. International Journal of Molecular Sciences, 23(19), 11103. https://doi.org/10.3390/ijms231911103










