Safety of Tepotinib Challenge after Capmatinib-Induced Pneumonitis in a Patient with Non-Small Cell Lung Cancer Harboring MET Exon 14 Skipping Mutation: A Case Report
Abstract
- Drug-induced pneumonitis is an uncommon but life-threatening adverse effect of target therapies. The safety of using another MET inhibitor after discontinuing the first remains unknown and such patients have rarely been reported.
- Given the lack of targeted therapies for MET inhibitors, rechallenge with tepotinib after capmatinib-induced pneumonitis could be an option for patients with NSCLC harboring METex14.
1. Introduction
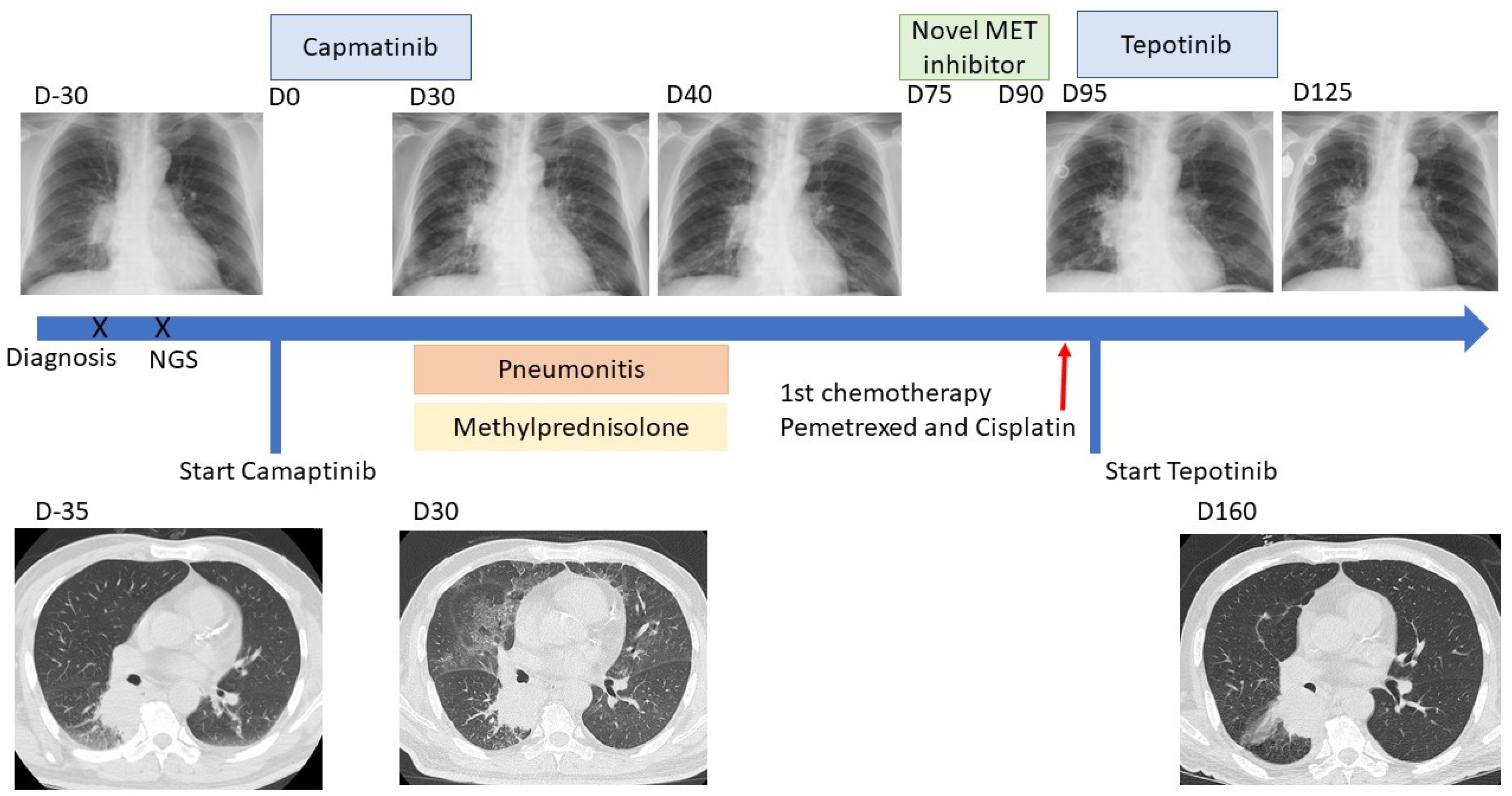
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Awad, M.M.; Oxnard, G.R.; Jackman, D.M.; Savukoski, D.O.; Hall, D.; Shivdasani, P.; Heng, J.C.; Dahlberg, S.E.; Jänne, P.A.; Verma, S.; et al. MET Exon 14 Mutations in Non–Small-Cell Lung Cancer Are Associated with Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. J. Clin. Oncol. 2016, 34, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.H.; Yeung, S.F.; Chan, A.W.H.; Chung, L.Y.; Chau, S.L.; Lung, R.W.M.; Tong, C.Y.; Chow, C.; Tin, E.K.Y.; Yu, Y.H.; et al. MET Amplification and Exon 14 Splice Site Mutation Define Unique Molecular Subgroups of Non–Small Cell Lung Carcinoma with Poor Prognosis. Clin. Cancer Res. 2016, 22, 3048–3056. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Seto, T.; Han, J.Y.; Reguart, N.; Garon, E.B.; Groen, H.J.; Tan, D.S.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, T.; Yamaoka, T.; Ando, K.; Kusumoto, S.; Kishino, Y.; Manabe, R.; Sagara, H. Molecular and Clinical Features of EGFR-TKI-Associated Lung Injury. Int. J. Mol. Sci. 2021, 22, 792. [Google Scholar] [CrossRef] [PubMed]
- Kanemura, H.; Takeda, M.; Shimizu, S.; Nakagawa, K. Interstitial lung disease associated with capmatinib therapy in a patient with non-small cell lung cancer harboring a skipping mutation of MET exon 14. Thorac. Cancer 2021, 12, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, M.H.; Sato, T.; Yamamoto, H.; Watanabe, R.; Kagyo, J.; Domoto, H.; Shiomi, T. Successful Tepotinib Challenge After Capmatinib-Induced Interstitial Lung Disease in a Patient with Lung Adenocarcinoma Harboring MET Exon 14 Skipping Mutation: Case Report. JTO Clin. Res. Rep. 2022, 3, 100271. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Sakai, H.; Felip, E.; Veillon, R.; Garassino, M.C.; Raskin, J.; Cortot, A.B.; Viteri, S.; Mazieres, J.; Smit, E.F.; et al. Tepotinib Efficacy and Safety in Patients with MET Exon 14 Skipping NSCLC: Outcomes in Patient Subgroups from the VISION Study with Relevance for Clinical Practice. Clin. Cancer Res. 2022, 28, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, T.; Mangeshkar, S.; Rathmann, J. Successful Rechallenge with Osimertinib after Very Acute Onset of Drug-Induced Pneumonitis. Case Rep. Oncol. 2021, 14, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Wakuda, K.; Yabe, M.; Nishioka, N.; Miyawaki, E.; Miyawaki, T.; Mamesaya, N.; Kawamura, T.; Kobayashi, H.; Omori, S.; et al. Retrospective analysis of osimertinib re-challenge after osimertinib-induced interstitial lung disease in patients with EGFR-mutant non-small cell lung carcinoma. Investig. N. Drugs 2021, 39, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhong, W.; Li, A.; Qiu, Z.; Xie, R.; Shi, H.; Lu, S. Successful treatment of EGFR T790M-mutant non-small cell lung cancer with almonertinib after osimertinib-induced interstitial lung disease: A case report and literature review. Ann. Transl. Med. 2021, 9, 950. [Google Scholar] [CrossRef] [PubMed]
- Myall, N.J.; Lei, A.Q.; Wakelee, H.A. Safety of lorlatinib following alectinib-induced pneumonitis in two patients with ALK-rearranged non-small cell lung cancer: A case series. Transl. Lung Cancer Res. 2021, 10, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-C.; Li, S.-H.; Yang, C.-T. Recurrent Pneumonitis Induced by Atezolizumab (Anti–Programmed Death Ligand 1) in NSCLC Patients Who Previously Experienced Anti-Programmed Death 1 Immunotherapy-Related Pneumonitis. J. Thorac. Oncol. 2018, 13, e227–e230. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, L.-W.; Chang, J.W.-C.; Wu, C.-E. Safety of Tepotinib Challenge after Capmatinib-Induced Pneumonitis in a Patient with Non-Small Cell Lung Cancer Harboring MET Exon 14 Skipping Mutation: A Case Report. Int. J. Mol. Sci. 2022, 23, 11809. https://doi.org/10.3390/ijms231911809
Tseng L-W, Chang JW-C, Wu C-E. Safety of Tepotinib Challenge after Capmatinib-Induced Pneumonitis in a Patient with Non-Small Cell Lung Cancer Harboring MET Exon 14 Skipping Mutation: A Case Report. International Journal of Molecular Sciences. 2022; 23(19):11809. https://doi.org/10.3390/ijms231911809
Chicago/Turabian StyleTseng, Liang-Wei, John Wen-Cheng Chang, and Chiao-En Wu. 2022. "Safety of Tepotinib Challenge after Capmatinib-Induced Pneumonitis in a Patient with Non-Small Cell Lung Cancer Harboring MET Exon 14 Skipping Mutation: A Case Report" International Journal of Molecular Sciences 23, no. 19: 11809. https://doi.org/10.3390/ijms231911809
APA StyleTseng, L.-W., Chang, J. W.-C., & Wu, C.-E. (2022). Safety of Tepotinib Challenge after Capmatinib-Induced Pneumonitis in a Patient with Non-Small Cell Lung Cancer Harboring MET Exon 14 Skipping Mutation: A Case Report. International Journal of Molecular Sciences, 23(19), 11809. https://doi.org/10.3390/ijms231911809




