The Change in Paradigm for NSCLC Patients with EML4–ALK Translocation
Abstract
1. Introduction
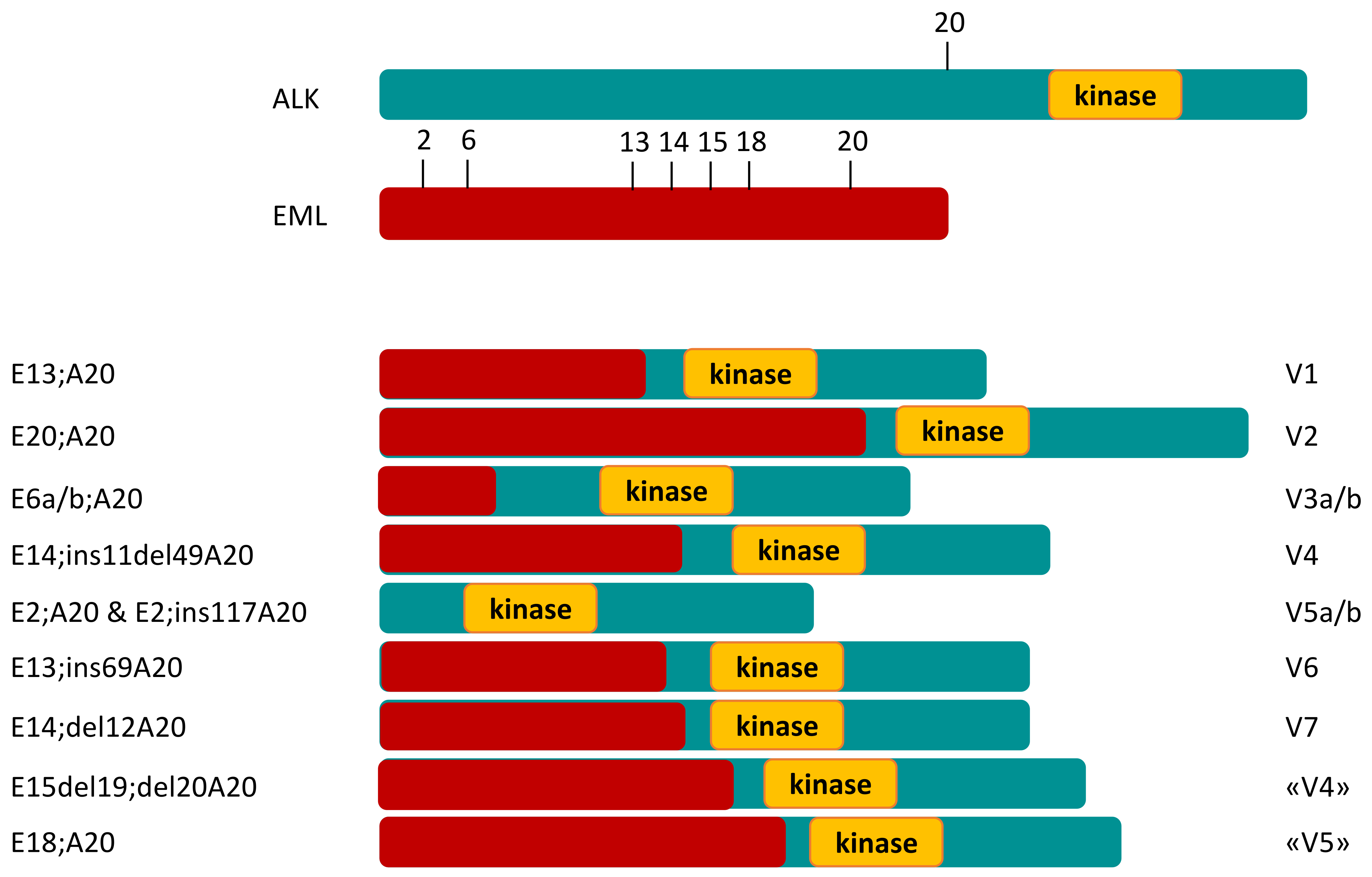
Disease Biology
2. Historical Treatment of ALK-Positive NSCLC
- In advanced ALK-positive NSCLC, the long expectancy of survival makes the ALK model unique;
- The treatment of intracranial disease is available without radiotherapy, and the prediction of survival is irrespective of brain disease.
3. ALK Inhibitors and the Expectancy of Survival
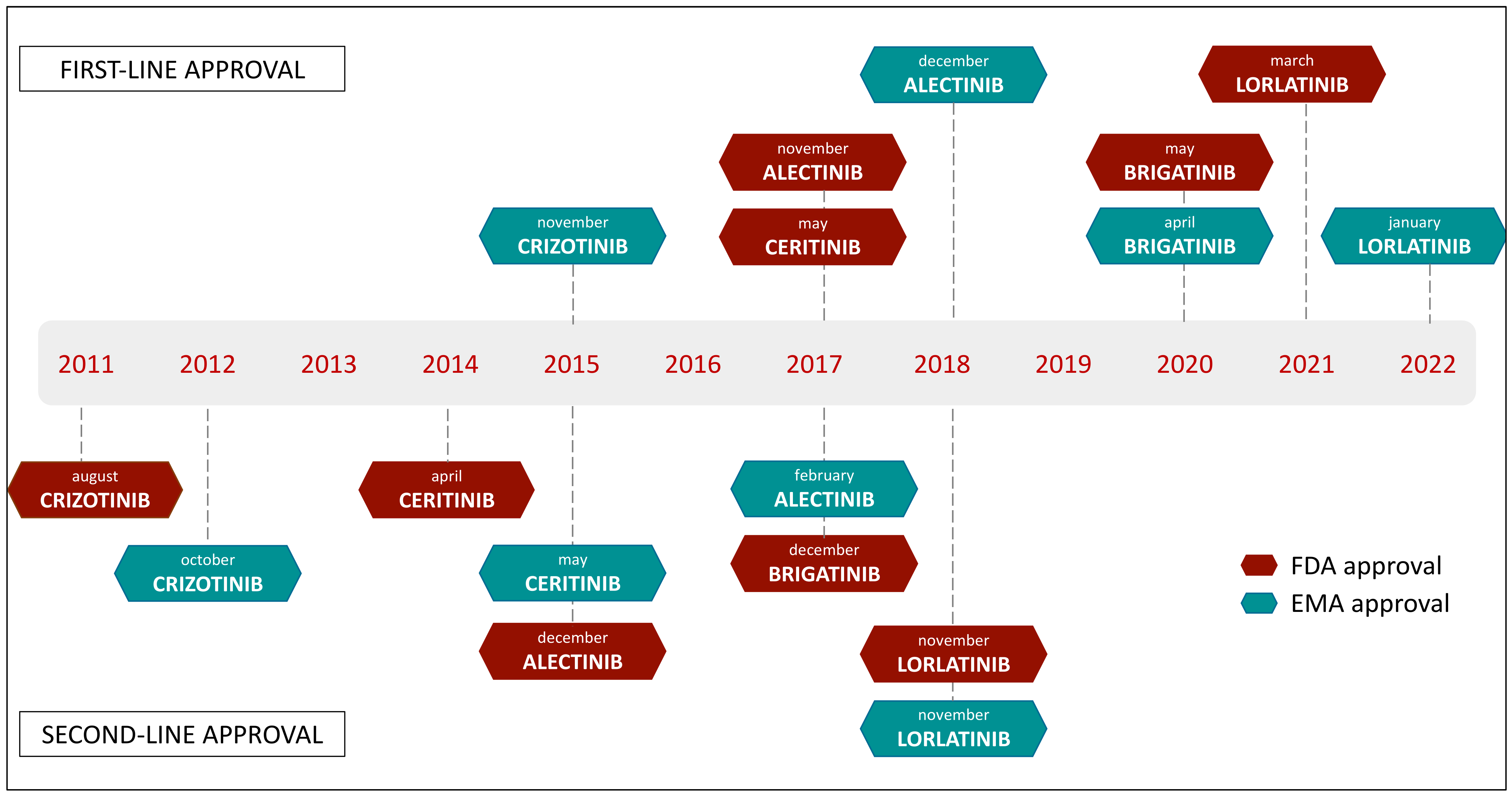
3.1. First-Generation ALK Inhibitor
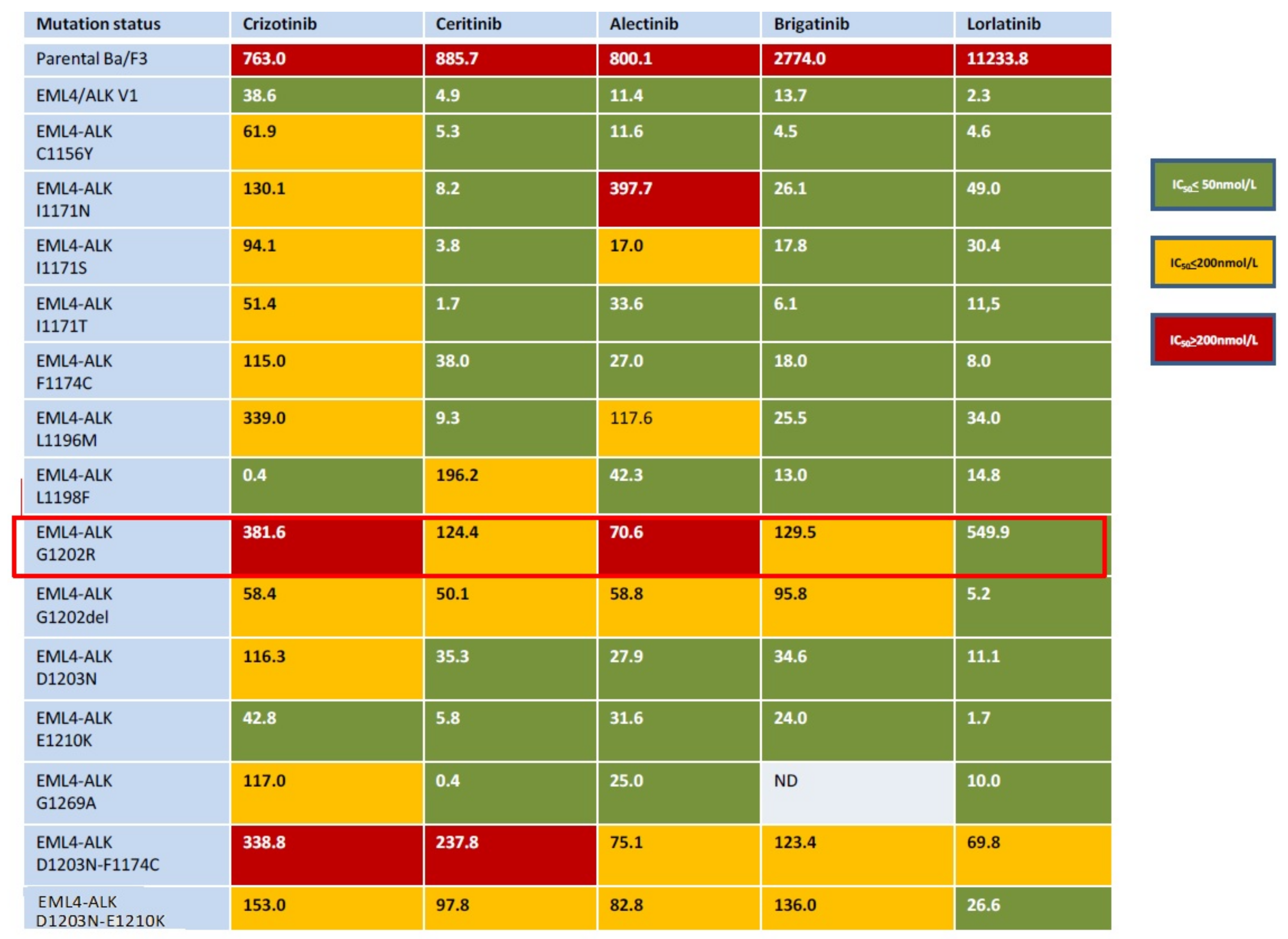
3.2. Next-Generation ALK Inhibitors
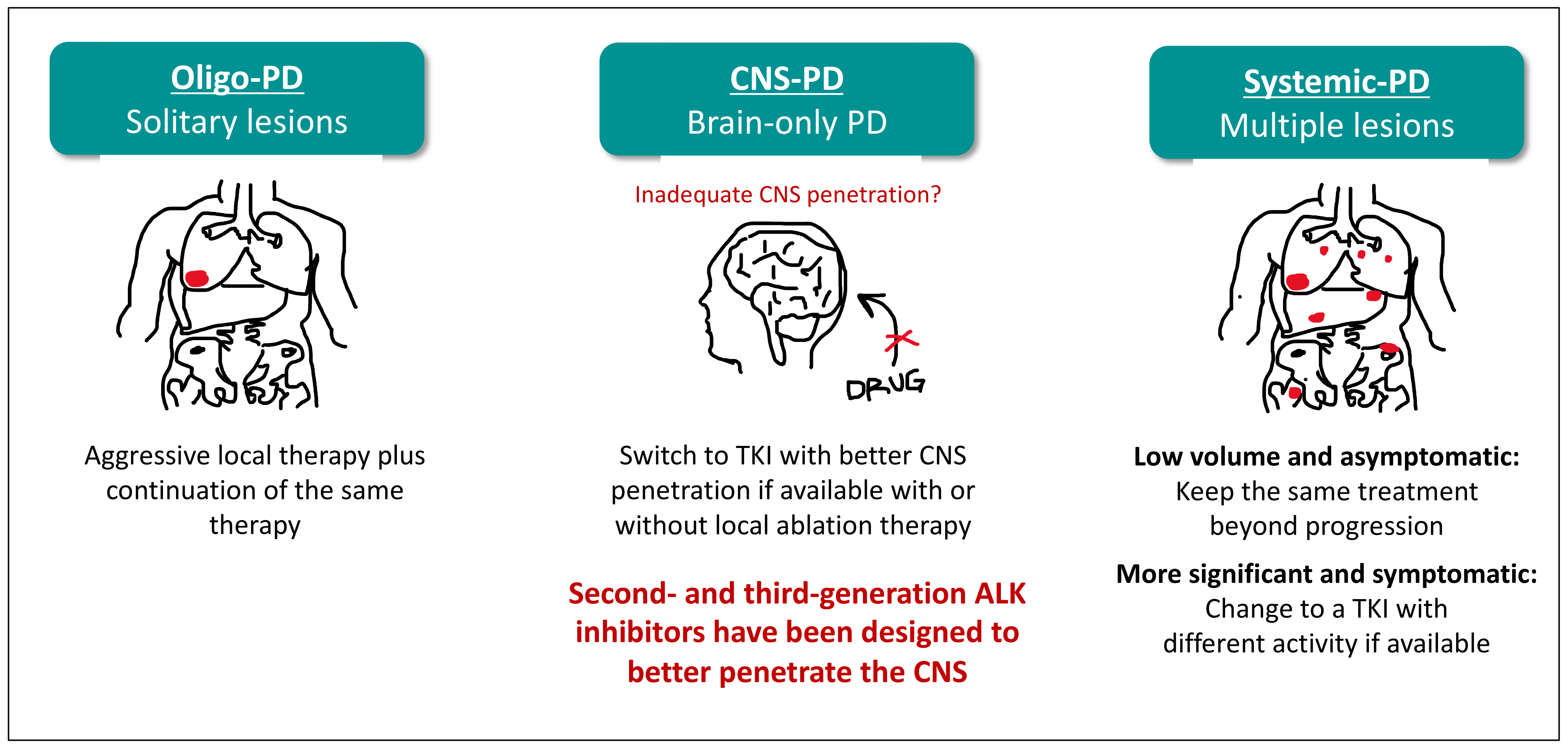
4. The Second Paradigmatic Shift in Lung Cancer: Management of Brain Metastases in ALK-Positive NSCLC
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Allen, M.S.; Aubry, M.C.; Wampfler, J.A.; Marks, R.S.; Edell, E.S.; Thibodeau, S.; Adjei, A.A.; Jett, J.; Deschamps, C. Clinical Features of 5,628 Primary Lung Cancer Patients: Experience at Mayo Clinic From 1997 to 2003. Yearb. Pulm. Dis. 2007, 2007, 102–104. [Google Scholar] [CrossRef]
- Society, A.C. Lung Cancer Survival Rates. Available online: https://www.Cancer.Org/Cancer/Lung-Cancer/Detection-Diagnosis-Staging/Survival-Rates.Html (accessed on 1 May 2022).
- Wild, C.P.; Weiderpass, E.; Stewart, B.V. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for research on cancer: Lyon, France, 2020. [Google Scholar]
- Travis, W.T. Lung cancer Pathology: Current concepts. Clin. Chest Med. 2020, 41, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Nokihara, H.; Kondo, M.; Kim, Y.H.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F.; et al. Alectinib versus Crizotinib in Patients with ALK -Positive Non-Small-Cell Lung Cancer (J-ALEX): An Open-Label, Randomised Phase 3 Trial. Lancet 2017, 390, 29–39. [Google Scholar] [CrossRef]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.-W.; Ou, S.-H.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK -Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Pacheco, J.M.; Gao, D.; Smith, D.; Purcell, T.; Hancock, M.; Bunn, P.; Robin, T.; Liu, A.; Karam, S.; Gaspar, L.; et al. Natural History and Factors Associated with Overall Survival in Stage IV ALK-Rearranged Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2019, 14, 691–700. [Google Scholar] [CrossRef]
- Mano, H. ALKoma: A Cancer Subtype with a Shared Target. Cancer Discov. 2012, 2, 495–502. [Google Scholar] [CrossRef]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the Transforming EML4–ALK Fusion Gene in Non-Small-Cell Lung Cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef]
- Sabir, S.R.; Yeoh, S.; Jackson, G.; Bayliss, R. EML4-ALK Variants: Biological and Molecular Properties, and the Implications for Patients. Cancers 2017, 9, 118. [Google Scholar] [CrossRef]
- Wang, S.; Luo, R.; Shi, Y.; Han, K. The impact of the ALK fusion variant on clinical outcomes in EML4-ALK patients with NSCLC: A systematic review and meta-analysis. Future Oncol. 2022, 18, 385–402. [Google Scholar] [CrossRef]
- Chia, P.L.; John, T.; Dobrovic, A.; Mitchell, P. Prevalence and Natural History of ALK Positive Non-Small-Cell Lung Cancer and the Clinical Impact of Targeted Therapy with ALK Inhibitors. Clin. Epidemiol. 2014, 6, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Kim, D.-W.; Nakagawa, K.; Seto, T.; Crinó, L.; Ahn, M.-J.; De Pas, T.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib versus Chemotherapy in Advanced ALK -Positive Lung Cancer. N. Engl. J. Med. 2013, 368, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Gristina, V.; La Mantia, M.; Iacono, F.; Galvano, A.; Russo, A.; Bazan, V. The Emerging Therapeutic Landscape of ALK Inhibitors in Non-Small Cell Lung Cancer. Pharmaceuticals 2020, 13, 474. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.; Camidge, D.R.; Gadgeel, S.M.; Rosell, R.; Dziadziuszko, R.; Kim, D.-W.; Pérol, M.; Ou, S.-H.I.; Ahn, J.S.; Shaw, A.T.; et al. Updated Overall Survival and Final Progression-Free Survival Data for Patients with Treatment-Naive Advanced ALK-Positive Non-Small-Cell Lung Cancer in the ALEX Study. Ann. Oncol. 2020, 31, 1056–1064. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.H.; Han, J.-Y.; Hochmair, M.J.; Lee, K.H.; Delmonte, A.; García Campelo, M.R.; Kim, D.-W.; et al. Brigatinib Versus Crizotinib in Advanced ALK Inhibitor–Naive ALK-Positive Non–Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA-1L Trial. J. Clin. Oncol. 2020, 38, 3592–3603. [Google Scholar] [CrossRef]
- Solomon, B.J.; Besse, B.; Bauer, T.M.; Felip, E.; Soo, R.A.; Camidge, D.R.; Chiari, R.; Bearz, A.; Lin, C.-C.; Gadgeel, S.M.; et al. Lorlatinib in Patients with ALK-Positive Non-Small-Cell Lung Cancer: Results from a Global Phase 2 Study. Lancet Oncol. 2018, 19, 1654–1667. [Google Scholar] [CrossRef]
- Shaw, A.T.; Solomon, B.J.; Besse, B.; Bauer, T.M.; Lin, C.-C.; Soo, R.A.; Riely, G.J.; Ou, S.-H.I.; Clancy, J.S.; Li, S.; et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2019, 37, 1370–1379. [Google Scholar] [CrossRef]
- Lin, J.J.; Schoenfeld, A.J.; Zhu, V.W.; Yeap, B.Y.; Chin, E.; Rooney, M.; Plodkowski, A.J.; Digumarthy, S.R.; Dagogo-Jack, I.; Gainor, J.F.; et al. Efficacy of Platinum/Pemetrexed Combination Chemotherapy in ALK-Positive NSCLC Refractory to Second-Generation ALK Inhibitors. J. Thorac. Oncol. 2020, 15, 258–265. [Google Scholar] [CrossRef]
- Shaw, A.T.; Bauer, T.M.; de Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.-W.; Mok, T.; Polli, A.; et al. First-Line Lorlatinib or Crizotinib in Advanced ALK -Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef]
- Reif, M.S.; Rivera, M.P. Evidence-Based Outcomes for Patients with Advanced Non-Small Cell Lung Cancer. Semin. Respir. Crit. Care Med. 2000, 21, 443–450. [Google Scholar] [CrossRef]
- Schiller, J.H.; Harrington, D.; Belani, C.P.; Langer, C.; Sandler, A.; Krook, J.; Zhu, J.; Johnson, D.H. Comparison of Four Chemotherapy Regimens for Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2002, 346, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Heigener, D.F.; Mok, T.; Soria, J.-C.; Rabe, K.F. Management of Non-Small-Cell Lung Cancer: Recent Developments. Lancet 2013, 382, 709–719. [Google Scholar] [CrossRef]
- Paz-Ares, L.G.; de Marinis, F.; Dediu, M.; Thomas, M.; Pujol, J.-L.; Bidoli, P.; Molinier, O.; Sahoo, T.P.; Laack, E.; Reck, M.; et al. PARAMOUNT: Final Overall Survival Results of the Phase III Study of Maintenance Pemetrexed Versus Placebo Immediately After Induction Treatment With Pemetrexed Plus Cisplatin for Advanced Nonsquamous Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2013, 31, 2895–2902. [Google Scholar] [CrossRef]
- Reck, M.; Kaiser, R.; Mellemgaard, A.; Douillard, J.-Y.; Orlov, S.; Krzakowski, M.; von Pawel, J.; Gottfried, M.; Bondarenko, I.; Liao, M.; et al. Docetaxel plus Nintedanib versus Docetaxel plus Placebo in Patients with Previously Treated Non-Small-Cell Lung Cancer (LUME-Lung 1): A Phase 3, Double-Blind, Randomised Controlled Trial. Lancet Oncol. 2014, 15, 143–155. [Google Scholar] [CrossRef]
- Zer, A.; Leighl, N.B. Second-Line Therapy in Non–Small-Cell Lung Cancer: The DELTA Between Different Genotypes Widens. J. Clin. Oncol. 2014, 32, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Suda, K.; Wiens, J.; Bunn, P.A. New and Emerging Targeted Treatments in Advanced Non-Small-Cell Lung Cancer. Lancet 2016, 388, 1012–1024. [Google Scholar] [CrossRef]
- Kris, M.G.; Johnson, B.E.; Berry, L.D.; Kwiatkowski, D.J.; Iafrate, A.J.; Wistuba, I.I.; Varella-Garcia, M.; Franklin, W.A.; Aronson, S.L.; Su, P.-F.; et al. Using Multiplexed Assays of Oncogenic Drivers in Lung Cancers to Select Targeted Drugs. JAMA 2014, 311, 1998. [Google Scholar] [CrossRef]
- Cameron, L.B.; Hitchen, N.; Chandran, E.; Morris, T.; Manser, R.; Solomon, B.J.; Jordan, V. Targeted Therapy for Advanced Anaplastic Lymphoma Kinase (ALK)-Rearranged Non-Small Cell Lung Cancer. Cochrane Database Syst. Rev. 1. 2022. EBM Reviews—Cochrane Database of Systematic Reviews. Available online: http://Ovidsp.Ovid.Com/ (accessed on 9 May 2022).
- Popat, S.; Kim, H.R.; Ahn, M. Intracranial Efficacy of Brigatinib (BRG) vs. Crizotinib (CRZ): Updated Results from the ALTA-1L Trial. Ann. Oncol. 2020, 31 (Suppl. 4), S754–S840. [Google Scholar] [CrossRef]
- Soria, J.-C.; Tan, D.S.W.; Chiari, R.; Wu, Y.-L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.-J.; et al. First-Line Ceritinib versus Platinum-Based Chemotherapy in Advanced ALK -Rearranged Non-Small-Cell Lung Cancer (ASCEND-4): A Randomised, Open-Label, Phase 3 Study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef]
- Horn, L.; Wang, Z.; Wu, G.; Poddubskaya, E.; Mok, T.; Reck, M.; Wakelee, H.; Chiappori, A.A.; Lee, D.H.; Breder, V.; et al. Ensartinib versus crizotinib in patients with anaplastic lymphoma kinase-positive non-small cell lung cancer: A randomized clinical trial. JAMA Oncol. 2021, 7, 1715–1725. [Google Scholar] [CrossRef]
- Solomon, B.; Bauer, T.M.; De Marinis, F.; Felip, E.; Goto, Y.; Lie, G. Lorlatinib versus Crizotinib in the First-Line Treatment of Patients (Pts) with Advanced ALK-Positive Non-Small Cell Lung Cancer (NSCLC): Results of the Phase III CROWN Study. Ann. Oncol. 2020, 31 (Suppl. 4), S1180–S1181. [Google Scholar] [CrossRef]
- Solomon, B.J.; Kim, D.W.; Wu, Y.L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; Tang, Y.; et al. Final overall survival analysis from a study comparing first-line crizotinib versus chemotherapy in ALK mutation-positive non-small cell lung cancer. J. Clin. Oncol 2018, 36, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Mok, T.; Kim, D.W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.B.; Kobayashi, S.; Pandya, S.S.; Yeo, W.-L.; Shen, Z.; Tan, W.; Wilner, K.D. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J. Clin. Oncol. 2011, 29, e443–e445. [Google Scholar] [CrossRef]
- Gainor, J.F.; Dardaei, L.; Yoda, S. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov. 2016, 6, 1118–1133. [Google Scholar] [CrossRef]
- Metro, G.; Lunardi, G.; Floridi, P.; Pascali, J.P.; Marcomigni, L.; Chiari, R.; Ludovini, V.; Crinò, L.; Gori, S. CSF concentration of crizotinib in two ALK-positive non-small cell lung cancer patients with CNS metastases deriving clinical benefit from treatment. J. Thorac. Oncol. 2015, 10, e26–e27. [Google Scholar] [CrossRef]
- Tang, S.C.; Nguyen, L.N.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Increased oral availability and brain accumulation of the ALK inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int. J. Cancer. 2014, 134, 1484–1494. [Google Scholar] [CrossRef]
- Friboulet, L.; Li, N.; Katayama, R.; Lee, C.C.; Gainor, J.F.; Crystal, A.S.; Michellys, P.-Y.; Awad, M.M.; Yanagitani, N.; Kim, S.; et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014, 4, 662–663. [Google Scholar] [CrossRef]
- Cho, B.C.; Kim, D.W.; Bearz, A.; Laurie, S.A.; McKeage, M.; Borra, G.; Park, K.; Kim, S.-W.; Ghosn, M.; Ardizzoni, A. ASCEND-8: A randomized phase 1 study of Ceritinib 450 mg or 600 mg taken with a low-fat meal versus 750 ng in fasted state in patients with anaplastic lymphoma kinase (ALK)-rearranged metastatic Non-small cell lung cancer (NSCLC). J. Thorac. Oncol. 2017, 12, 1357–1367. [Google Scholar] [CrossRef]
- Seto, T.; Nishio, M.; Hida, T.; Kim, Y.H.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F.; et al. Final PFS analysis and safety data from the phase III J-ALEX study of alectinib (ALC) vs. crizotinib (CRZ) in ALK-inhibitor naive ALK-positive non-small cell lung cancer (ALK1 NSCLC). J. Clin. Oncol. 2019, 37, 9052. [Google Scholar]
- Serritella, A.V.; Bestvina, C.M. Anaplastic lymphoma kinase mutation-positive non-small cell lung cancer. Thorac. Surg. Clin. 2020, 30, 137–146. [Google Scholar] [CrossRef]
- Zhang, S.; Anjum, R.; Squillace, R.; Eichinger, L.; Das, B.; Ye, E.Y.; Hodgson, J.G.; Rivera, V.M. The potent ALK inhibitor AP26113 can overcome mechanisms of resistance to first- and second-generation ALK TKIs in preclinical models. Clin. Cancer Res. 2016, 22, 5527–5538. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Pabani, A.; Miller, R.M.; Rizvi, N.A.; Bazhenova, L. Management strategies for early onset pulmonary events associated with brigatinib. J. Thorac. Oncol. 2019, 14, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.M.; Hansen, K.H.; Paz-Ares, L.; West, H.L.; Reckamp, K.L.; Leighl, N.B.; Tiseo, M.; Smit, E.F.; Kim, D.-W.; Gettinger, S.N.; et al. Brigatinib in crizotinib-refractory ALK+ NSCLC:2-year follow-up on systemic and intracranial outcomes in the phase 2 ALTA trial. J. Thorac. Oncol. 2020, 3, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.W.; Zhai, D.; Deng, W.; Zhang, X.; Ung, J.; Nguyen, V.; Zhang, H.; Barrera, M.; Parra, A.; Cowell, J.; et al. TPX-0131, a Potent CNS-Penetrant, Next-Generation Inhibitor of Wild-Type ALK and ALK-Resistant Mutations. Mol. Cancer Ther. 2021, 20, 1499–1507. [Google Scholar] [CrossRef]
- Camidge, D.R.; Dziadziuszko, R.; Peters, S.; Mok, T.; Noe, J.; Nowicka, M.; Gadgeel, S.M.; Cheema, P.; Pavlakis, N.; de Marinis, F.; et al. Updated Efficacy and Safety Data and Impact of the EML4-ALK Fusion Variant on the Efficacy of Alectinib in Untreated ALK-Positive Advanced Non–Small Cell Lung Cancer in the Global Phase III ALEX Study. J. Thorac. Oncol. 2019, 14, 1233–1243. [Google Scholar] [CrossRef]
- De Carlo, E.; Schiappacassi, M.; Stanzione, B.; Del Conte, A.L.; Bearz, A. Combination of chemotherapy and ALK-inhibitors in ALK positive oatients. J. Thor. Oncol. 2021, 16, e31–e32. [Google Scholar] [CrossRef]
- Ou, S.-H.I.; Solomon, B.J.; Shaw, A.T.; Gadgeel, S.M.; Besse, B.; Soo, R.A.; Abbattista, A.; Toffalorio, F.; Wiltshire, R.; Bearz, A. Continuation of Lorlatinib in ALK-Positive NSCLC Beyond Progressive Disease. J. Thorac. Oncol. 2022, 17, 568–577. [Google Scholar] [CrossRef]
- Chang, G.-C.; Yang, T.-Y.; Chen, K.-C.; Hsu, K.-H.; Huang, Y.-H.; Su, K.-Y.; Yu, S.-L.; Tseng, J.-S. ALK Variants, PD-L1 Expression, and Their Association with Outcomes in ALK-Positive NSCLC Patients. Sci. Rep. 2020, 10, 21063. [Google Scholar] [CrossRef]
- Budczies, J.; Kirchner, M.; Kluck, K.; Kazdal, D.; Glade, J.; Allgäuer, M.; Kriegsmann, M.; Heußel, C.-P.; Herth, F.J.; Winter, H.; et al. Deciphering the Immunosuppressive Tumor Microenvironment in ALK- and EGFR-Positive Lung Adenocarcinoma. Cancer Immunol. Immunother. 2022, 71, 251–265. [Google Scholar] [CrossRef]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune Checkpoint Inhibitors for Patients with Advanced Lung Cancer and Oncogenic Driver Alterations: Results from the IMMUNOTARGET Registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- De Carlo, E.; Del Savio, M.C.; Polesel, J.; Da Ros, V.; Berto, E.; Santarossa, S.; Chimienti, E.; Fratino, L.; Bearz, A. Outcomes of ALK Positive Lung Cancer Patients Treated with Crizotinib or Second-Generation ALK Inhibitor: A Monoinstitutional Experience. Oncotarget 2018, 9, 15340–15349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duruisseaux, M.; Besse, B.; Cadranel, J.; Pérol, M.; Mennecier, B.; Bigay-Game, L.; Descourt, R.; Dansin, E.; Audigier-Valette, C.; Moreau, L.; et al. Overall Survival with Crizotinib and Next-Generation ALK Inhibitors in ALK -Positive Non-Small-Cell Lung Cancer (IFCT-1302 CLINALK): A French Nationwide Cohort Retrospective Study. Oncotarget 2017, 8, 21903–21917. [Google Scholar] [CrossRef] [PubMed]
- Petrovich, Z.; Yu, C.; Giannotta, S.L.; O’day, S.; Apuzzo, M.L.J. Survival and Pattern of Failure in Brain Metastasis Treated with Stereotactic Gamma Knife Radiosurgery. J. Neurosurg. 2002, 97, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.B.; Shaw, A.T.; Ou, S.-H.I.; Solomon, B.J.; Riely, G.J.; Ahn, M.-J.; Zhou, C.; Shreeve, S.M.; Selaru, P.; Polli, A.; et al. Clinical Experience With Crizotinib in Patients With Advanced ALK -Rearranged Non–Small-Cell Lung Cancer and Brain Metastases. J. Clin. Oncol. 2015, 33, 1881–1888. [Google Scholar] [CrossRef]
- Magnuson, W.J.; Lester-Coll, N.H.; Wu, A.J.; Yang, T.J.; Lockney, N.A.; Gerber, N.K.; Beal, K.; Amini, A.; Patil, T.; Kavanagh, B.D.; et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor–Naïve Epidermal Growth Factor Receptor–Mutant Non–Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J. Clin. Oncol. 2017, 35, 1070–1077. [Google Scholar] [CrossRef]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR -Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Lin, J.J.; Jiang, G.Y.; Joshipura, N.; Ackil, J.; Digumarthy, S.R.; Rincon, S.P.; Yeap, B.Y.; Gainor, J.F.; Shaw, A.T. Efficacy of Alectinib in Patients with ALK-Positive NSCLC and Symptomatic or Large CNS Metastases. J. Thorac. Oncol. 2019, 14, 683–690. [Google Scholar] [CrossRef]
- Tallet, A.V.; Azria, D.; Barlesi, F.; Spano, J.-P.; Carpentier, A.F.; Gonçalves, A.; Metellus, P. Neurocognitive Function Impairment after Whole Brain Radiotherapy for Brain Metastases: Actual Assessment. Radiat. Oncol. 2012, 7, 77. [Google Scholar] [CrossRef]
| Drug | Clinical Trial | # pts | CNS Mets at Baseline | ORR (%) (95% CI) | PFS (Months) in ITT (5% CI) | Intracranial Response Rate | Ref. |
|---|---|---|---|---|---|---|---|
| Lorlatinib | Crown | 296 | Lorlatinib: 26% Crizotinib: 27% | Lorlatinib: 76% (68–83) Crizotinib: 58% (49–66) | Lorlatinib: NE Crizotinib: 9.3 (7.6–11.1) HR:0.28 (0.18–0.41) | Lorlatinib 82% (57–96) Crizotinib: 23% (5–54) | [21] |
| Alectinib | ALEX | 303 | Alectinib: 38% Crizotinib: 42% | Alectinib: 82.9% (76.0–88.5) Crizotinib: 75.5% (67.6–822.1) | Alectinib: 34.8 (19.9–NE) Crizotinib: 10.4 (7.7–14.6) HR: 0.50 (0.36–0.70) | Alectinib: 81% (58–95) Crizotinib: 50% (28–72) HR: 0.32 (0.15–0.64) | [16] |
| Brigatinib | ALTA-1 | 275 | Brigatinib: 29% Crizotinib: 30% | Brigatinib: 71% (62–78) Crizotinib: 60% (51–68) | Brigatinib: not reached Crizotinib: 9.8 (9.0–12.9) HR: 0.49 (0.33–0.74) | Brigatinib: 78% (52–94) Crizotinib: 29% (11–52) | [17] |
| Ensartinib | eXalt-3 | 290 | Ensartinib: 33% Crizotinib: 39% | Ensartinib: 75% Crizotinib: 67% | Ensartinib: 25.8 Crizotinib: 12.7 HR: 0.51 (0.35–0.72) | Ensartinib: 64% Crizotinib: 21% | [33] |
| Drug | Serious TRAEs | TRAES Leading to Dose Reduction (% pts) | TRAES Leading to Drug Discontinuation (% pts) | More Common TRAES | Ref. |
|---|---|---|---|---|---|
| Lorlatinib | Lorlatinib: 34% Crizotinib: 27% | Lorlatinib: 49% Crizotinib: 47% | Lorlatinib: 7% Crizotinib: 9% | Hypercholesterolemia Hypertriglyceridemia Weight increase Peripheral neuropathy Cognitive effects | [21] |
| Alectinib | Alectinib: 28% Crizotinib: 29% | Alectinib: 16% Crizotinib: 21% | Alectinib: 11% Crizotinib:13% | Anemia Myalgia Increased bilirubin Weight increase Musculoskeletal pain Photosensitivity | [16] |
| Brigatinib | Brigatinib: 28% Crizotinib: 29% | Brigatinib: 28% Crizotinib: 29% | Brigatinib: 12% Crizotinib: 9% | Increased CK Cough Hypertension Increased lipase Early-onset ILD | [17] |
| Ensartinib | Ensartinib: 24% Crizotinib: 20% | Ensartinib: 24% Crizotinib: 20% | Ensartinib: 9% Crizotinib: 7% | Rash Pruritus Pyrexia Increased transaminase | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bearz, A.; De Carlo, E.; Del Conte, A.; Spina, M.; Da Ros, V.; Bertoli, E.; Revelant, A.; Stanzione, B.; Tirelli, U. The Change in Paradigm for NSCLC Patients with EML4–ALK Translocation. Int. J. Mol. Sci. 2022, 23, 7322. https://doi.org/10.3390/ijms23137322
Bearz A, De Carlo E, Del Conte A, Spina M, Da Ros V, Bertoli E, Revelant A, Stanzione B, Tirelli U. The Change in Paradigm for NSCLC Patients with EML4–ALK Translocation. International Journal of Molecular Sciences. 2022; 23(13):7322. https://doi.org/10.3390/ijms23137322
Chicago/Turabian StyleBearz, Alessandra, Elisa De Carlo, Alessandro Del Conte, Michele Spina, Valentina Da Ros, Elisa Bertoli, Alberto Revelant, Brigida Stanzione, and Umberto Tirelli. 2022. "The Change in Paradigm for NSCLC Patients with EML4–ALK Translocation" International Journal of Molecular Sciences 23, no. 13: 7322. https://doi.org/10.3390/ijms23137322
APA StyleBearz, A., De Carlo, E., Del Conte, A., Spina, M., Da Ros, V., Bertoli, E., Revelant, A., Stanzione, B., & Tirelli, U. (2022). The Change in Paradigm for NSCLC Patients with EML4–ALK Translocation. International Journal of Molecular Sciences, 23(13), 7322. https://doi.org/10.3390/ijms23137322






