Recent Advances in Inverted Perovskite Solar Cells: Designing and Fabrication
Abstract
1. Introduction
2. Basics of Inverted Perovskite Solar Cells
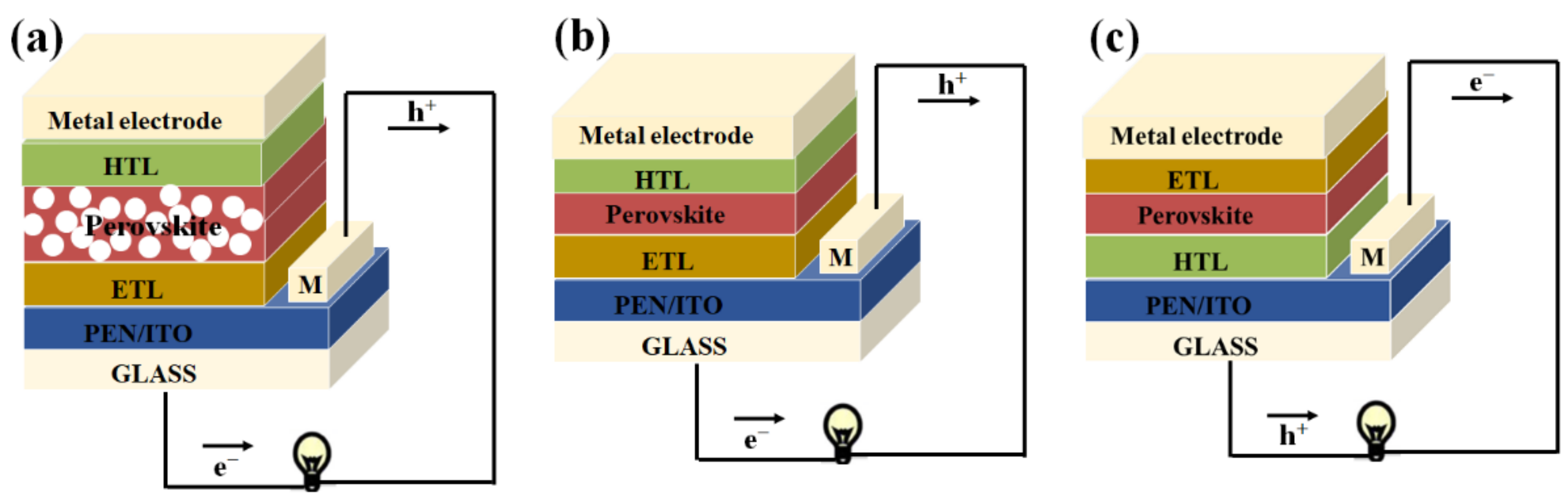
2.1. Architecture of Inverted PSCs
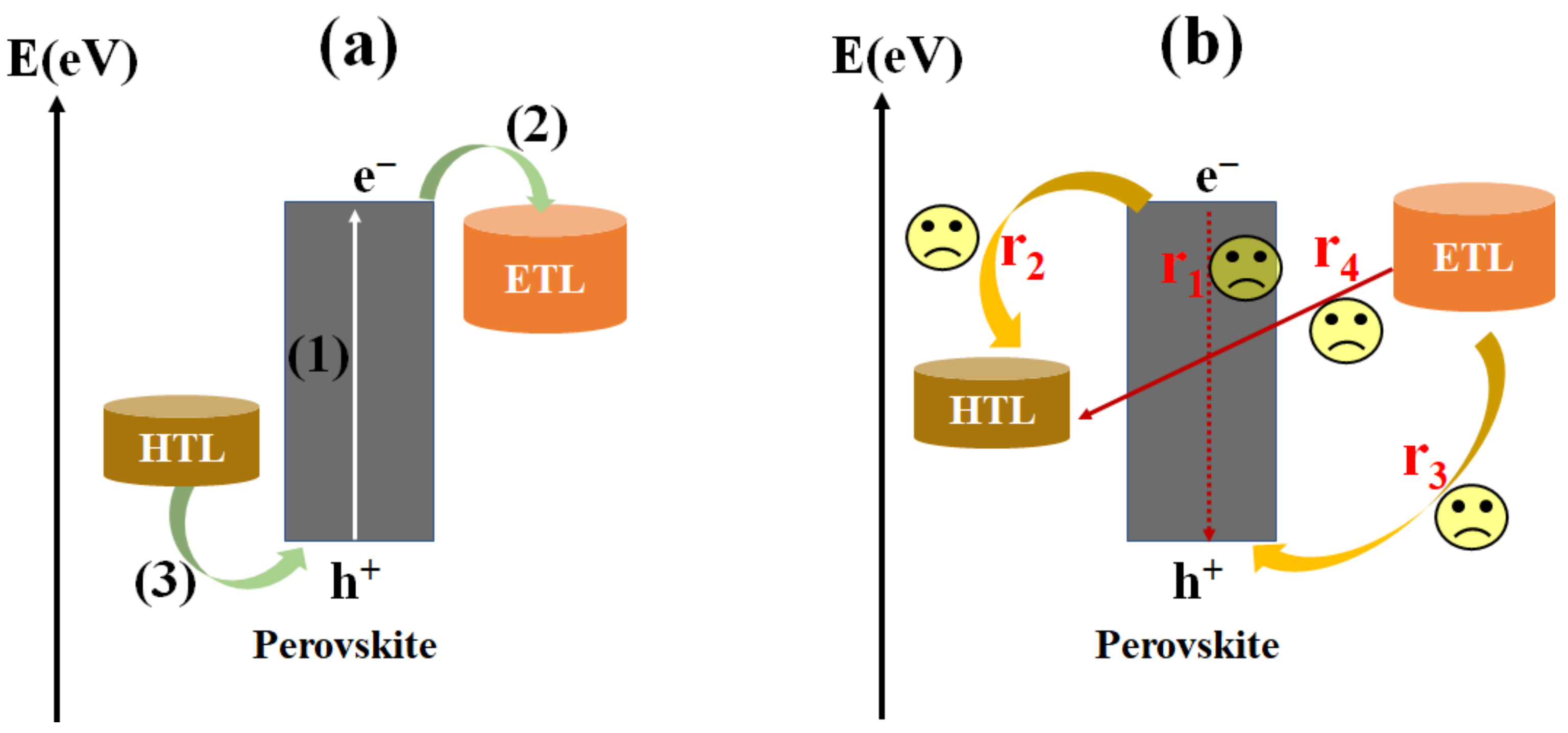
2.2. Working Principle of Inverted PSCs
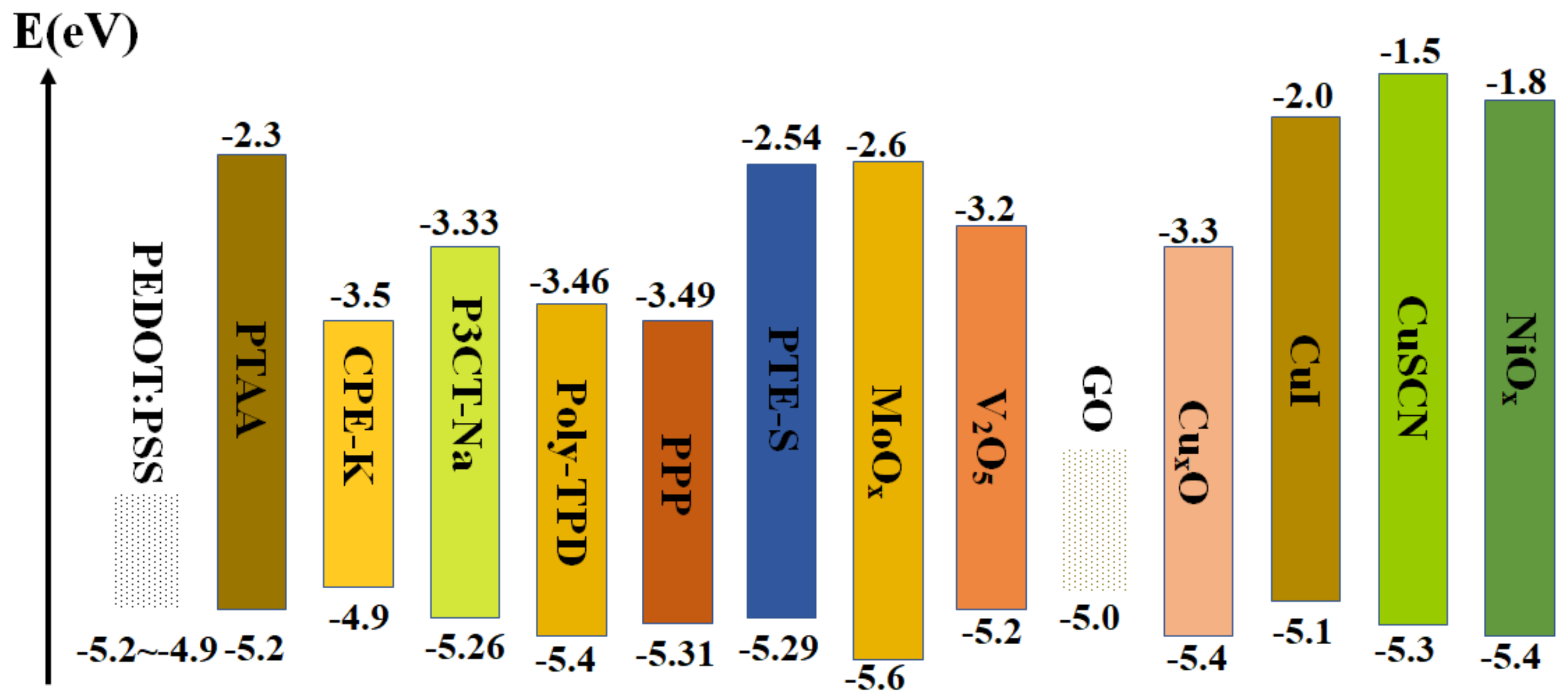
3. Hole−Transport Layer (HTL)
3.1. Conductive Polymers Materials
3.1.1. Poly(3,4−Ethylenedioxythiophene):Poly(Styrenesulfonate) (PEDOT:PSS)
- (1)
- Doping PEDOT:PSS
- (2)
- Modified PEDOT:PSS
- (3)
- Interface engineering
- (4)
- Composite HTL materials
3.1.2. Poly(Bis(4−Phenyl)(2,4,6−Trimethylphenyl)Amine) (PTAA)
3.1.3. Conjugated Polyelectrolyte (CPE)
3.1.4. Polyelectrolyte
3.1.5. Poly[N,N′−Bis(4−Butylphenyl)−N,N′−Bis(Phenyl)Benzidine] (Poly−TPD)
3.1.6. Other Conductive Polymer Materials
3.2. Organic Small−Molecule Materials
3.3. Inorganic Semiconductor Materials
3.3.1. NiOx
- (1)
- Doping NiOx
- (2)
- Interface modification
3.3.2. Cu−Based Materials (CuX)
3.3.3. Other Inorganic Semiconductor Materials
4. Perovskite Absorption Layer
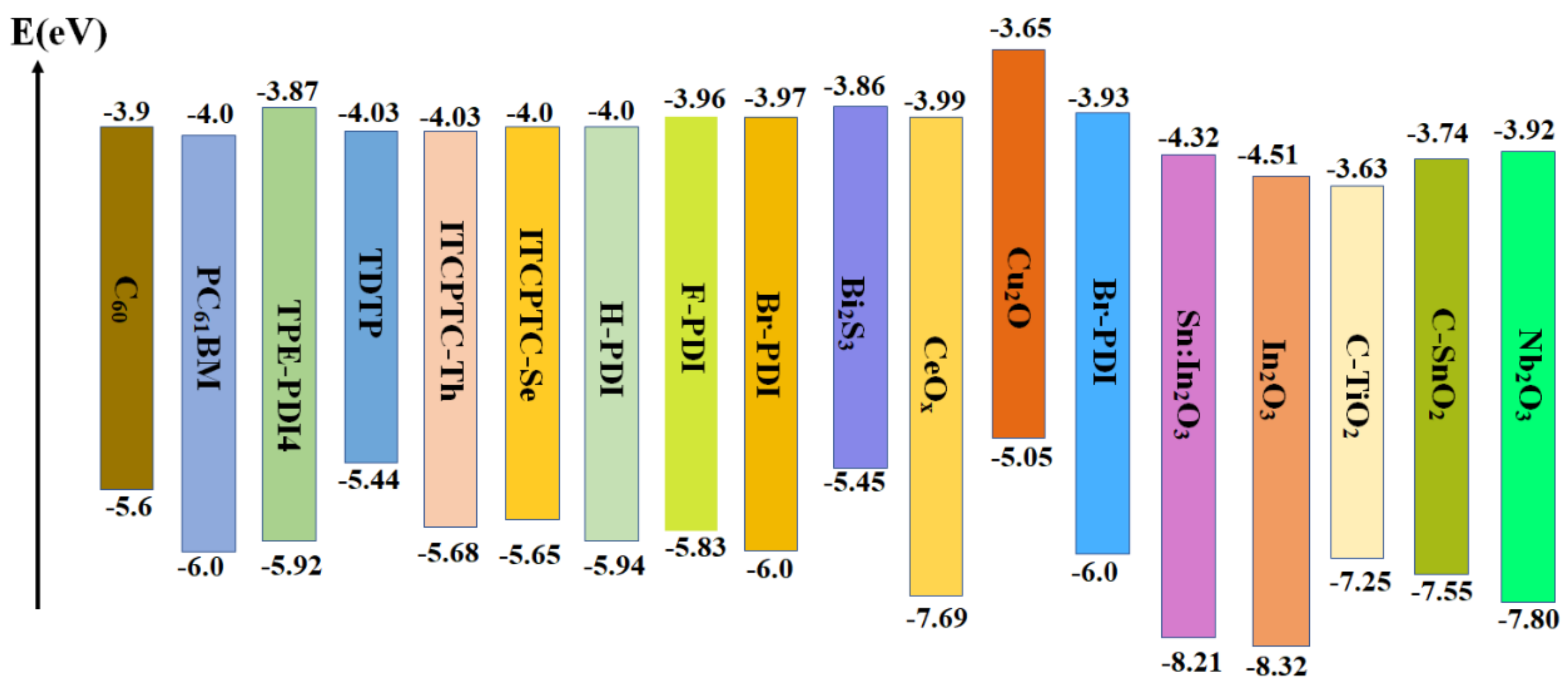
5. Electron−Transport Layer (ETL)
5.1. PCBM
5.2. Organic Small−Molecule Materials
5.3. Inorganic Electron Transport Materials
6. All−Inorganic Perovskite Solar Cells
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Graetzel, M.; Janssen, R.A.J.; Mitzi, D.B.; Sargent, E.H. Materials Interface Engineering for Solution-Processed Photovoltaics. Nature 2012, 488, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhu, P.; Liu, Y.; Qiu, Q.; Li, J.; Liang, T.; Xie, T. Enhanced Photocatalytic Degradation Activity Via a Stable Perovskite-Type LaFeO3/In2S3 Z-Scheme Heterostructured Photocatalyst: Unobstructed Photoexcited Charge Behavior of Z-Scheme Photocatalytic System Exploration. J. Alloys Compd. 2022, 901, 163628. [Google Scholar] [CrossRef]
- Alivisatos, P.; Buchanan, M. Basic Research Needs for Carbon Capture: Beyond 2020; USDOE Office of Science: Washington, DC, USA, 2010; 196p. [Google Scholar]
- Vakulchuk, R.; Overland, I.; Scholten, D. Renewable Energy and Geopolitics: A Review. Renew. Sust. Energ. Rev. 2020, 122, 109547. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhu, P.; Liu, Y.; Liang, T.; Xie, T.; Lin, Y. Highly Efficient In2S3/WO3 Photocatalysts: Z-Scheme Photocatalytic Mechanism for Enhanced Photocatalytic Water Pollutant Degradation under Visible Light Irradiation. RSC Adv. 2021, 11, 3333–3341. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.; Kumar, P.; Kumar, S.; Adelodun, A.A.; Kim, K.-H. Solar Energy: Potential and Future Prospects. Renew. Sust. Energ. Rev. 2018, 82, 894–900. [Google Scholar] [CrossRef]
- Qiu, Q.; Li, S.; Jiang, J.; Wang, D.; Lin, Y.; Xie, T. Improved Electron Transfer between Tio2 and Fto Interface by N-Doped Anatase Tio2 Nanowires and Its Applications in Quantum Dot-Sensitized Solar Cells. J. Phys. Chem. C 2017, 121, 21560–21570. [Google Scholar] [CrossRef]
- Tai, Q.; Tang, K.-C.; Yan, F. Recent Progress of Inorganic Perovskite Solar Cells. Energy Environ. Sci. 2019, 12, 2375–2405. [Google Scholar] [CrossRef]
- Qiu, Q.; Wang, P.; Xu, L.; Wang, D.; Lin, Y.; Xie, T. Photoelectrical Properties of Cds/Cdse Core/Shell Qds Modified Anatase Tio2 Nanowires and Their Application for Solar Cells. Phys. Chem. Chem. Phys. 2017, 19, 15724–15733. [Google Scholar] [CrossRef]
- Green, M.A.; Hishikawa, Y.; Warta, W.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Ho-Baillie, A.W.H. Solar Cell Efficiency Tables (Version 50). Prog. Photovolt. 2017, 25, 668–676. [Google Scholar] [CrossRef]
- Guerrero-Lemus, R.; Shephard, L.E. (Eds.) Photovoltaics. In Low-Carbon Energy in Africa and Latin America: Renewable Technologies, Natural Gas and Nuclear Energy; Springer International Publishing: Cham, Switzerland, 2017; pp. 149–173. [Google Scholar]
- Chao, L.; Niu, T.; Gao, W.; Ran, C.; Song, L.; Chen, Y.; Huang, W. Solvent Engineering of the Precursor Solution toward Large-Area Production of Perovskite Solar Cells. Adv. Mater. 2021, 33, 2005410. [Google Scholar] [CrossRef]
- Kang, D.-H.; Park, N.-G. On the Current–Voltage Hysteresis in Perovskite Solar Cells: Dependence on Perovskite Composition and Methods to Remove Hysteresis. Adv. Mater. 2019, 31, 1805214. [Google Scholar] [CrossRef]
- Son, D.-Y.; Lee, J.-W.; Choi, Y.J.; Jang, I.-H.; Lee, S.; Yoo, P.J.; Shin, H.; Ahn, N.; Choi, M.; Kim, D.; et al. Self-Formed Grain Boundary Healing Layer for Highly Efficient Ch3nh3pbi3 Perovskite Solar Cells. Nat. Energy 2016, 1, 16081. [Google Scholar] [CrossRef]
- Fang, Y.; Bi, C.; Wang, D.; Huang, J. The Functions of Fullerenes in Hybrid Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 782–794. [Google Scholar] [CrossRef]
- Cho, A.-N.; Park, N.-G. Impact of Interfacial Layers in Perovskite Solar Cells. ChemSusChem 2017, 10, 3687–3704. [Google Scholar] [CrossRef]
- Ye, M.; He, C.; Iocozzia, J.; Liu, X.; Cui, X.; Meng, X.; Rager, M.; Hong, X.; Liu, X.; Lin, Z. Recent Advances in Interfacial Engineering of Perovskite Solar Cells. J. Phys. D Appl. Phys. 2017, 50, 373002. [Google Scholar] [CrossRef]
- Bai, Y.; Meng, X.; Yang, S. Interface Engineering for Highly Efficient and Stable Planar P-I-N Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1701883. [Google Scholar] [CrossRef]
- Wei, Q.; Bi, H.; Yan, S.; Wang, S. Morphology and Interface Engineering for Organic Metal Halide Perovskite–Based Photovoltaic Cells. Adv. Mater. Interfaces 2018, 5, 1800248. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, X.; Yang, J.; Qiu, Q.; Xie, T.; Liang, T. Application of Quantum Dot Interface Modification Layer in Perovskite Solar Cells: Progress and Perspectives. Nanomaterials 2022, 12, 2012. [Google Scholar] [CrossRef]
- Zhao, P.; Yin, W.; Kim, M.; Han, M.; Song, Y.J.; Ahn, T.K.; Jung, H.S. Improved Carriers Injection Capacity in Perovskite Solar Cells by Introducing a-Site Interstitial Defects. J. Mater. Chem. A 2017, 5, 7905–7911. [Google Scholar] [CrossRef]
- Tang, Z.; Bessho, T.; Awai, F.; Kinoshita, T.; Maitani, M.M.; Jono, R.; Murakami, T.N.; Wang, H.; Kubo, T.; Uchida, S.; et al. Hysteresis-Free Perovskite Solar Cells Made of Potassium-Doped Organometal Halide Perovskite. Sci. Rep. 2017, 7, 12183. [Google Scholar] [CrossRef]
- Muzammal uz Zaman, M.; Imran, M.; Saleem, A.; Kamboh, A.H.; Arshad, M.; Khan, N.A.; Akhter, P. Potassium Doped Methylammonium Lead Iodide (Mapbi3) Thin Films as a Potential Absorber for Perovskite Solar Cells; Structural, Morphological, Electronic and Optoelectric Properties. Phys. B Condens. Matter 2017, 522, 57–65. [Google Scholar] [CrossRef]
- Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Cacovich, S.; Stavrakas, C.; Philippe, B.; Richter, J.M.; Alsari, M.; Booker, E.P.; Hutter, E.M.; Pearson, A.J.; et al. Maximizing and Stabilizing Luminescence from Halide Perovskites with Potassium Passivation. Nature 2018, 555, 497–501. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, J.; Luo, X.; Li, Y.; Qiu, Q.; Xie, T. Selection, Preparation and Application of Quantum Dots in Perovskite Solar Cells. Int. J. Mol. Sci. 2022, 23, 9482. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Wang, Y.-C.; Zhang, W.; Wang, H.-Q.; Fang, J. In-Situ Cross-Linking Strategy for Efficient and Operationally Stable Methylammoniun Lead Iodide Solar Cells. Nat. Commun. 2018, 9, 3806. [Google Scholar] [CrossRef]
- Meng, L.; You, J.; Guo, T.-F.; Yang, Y. Recent Advances in the Inverted Planar Structure of Perovskite Solar Cells. Acc. Chem. Res. 2016, 49, 155–165. [Google Scholar] [CrossRef]
- Lian, X.; Chen, J.; Fu, R.; Lau, T.-K.; Zhang, Y.; Wu, G.; Lu, X.; Fang, Y.; Yang, D.; Chen, H. An Inverted Planar Solar Cell with 13% Efficiency and a Sensitive Visible Light Detector Based on Orientation Regulated 2d Perovskites. J. Mater. Chem. A 2018, 6, 24633–24640. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, S.-G.; Bae, S.-H.; Lee, D.-K.; Lin, O.; Yang, Y.; Park, N.-G. The Interplay between Trap Density and Hysteresis in Planar Heterojunction Perovskite Solar Cells. Nano Lett. 2017, 17, 4270–4276. [Google Scholar] [CrossRef]
- Jeng, J.-Y.; Chiang, Y.-F.; Lee, M.-H.; Peng, S.-R.; Guo, T.-F.; Chen, P.; Wen, T.-C. Ch3nh3pbi3 Perovskite/Fullerene Planar-Heterojunction Hybrid Solar Cells. Adv. Mater. 2013, 25, 3727–3732. [Google Scholar] [CrossRef]
- Bai, S.; Wu, Z.; Wu, X.; Jin, Y.; Zhao, N.; Chen, Z.; Mei, Q.; Wang, X.; Ye, Z.; Song, T.; et al. High-Performance Planar Heterojunction Perovskite Solar Cells: Preserving Long Charge Carrier Diffusion Lengths and Interfacial Engineering. Nano Res. 2014, 7, 1749–1758. [Google Scholar] [CrossRef]
- Chen, K.; Hu, Q.; Liu, T.; Zhao, L.; Luo, D.; Wu, J.; Zhang, Y.; Zhang, W.; Liu, F.; Russell, T.P.; et al. Charge-Carrier Balance for Highly Efficient Inverted Planar Heterojunction Perovskite Solar Cells. Adv. Mater. 2016, 28, 10718–10724. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, B.; Dai, J.; Fang, Y.; Bai, Y.; Lin, Y.; Wei, H.; Zeng, X.C.; Huang, J. Defect Passivation in Hybrid Perovskite Solar Cells Using Quaternary Ammonium Halide Anions And cations. Nat. Energy 2017, 2, 17102. [Google Scholar] [CrossRef]
- Luo, D.; Yang, W.; Wang, Z.; Sadhanala, A.; Hu, Q.; Su, R.; Shivanna, R.; Trindade, G.F.; Watts, J.F.; Xu, Z.; et al. Enhanced Photovoltage for Inverted Planar Heterojunction Perovskite Solar Cells. Science 2018, 360, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Deng, X.; Qi, F.; Li, Z.; Liu, D.; Shen, D.; Qin, M.; Wu, S.; Lin, F.; Jang, S.-H.; et al. Regulating Surface Termination for Efficient Inverted Perovskite Solar Cells with Greater Than 23% Efficiency. J. Am. Chem. Soc. 2020, 142, 20134–20142. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, W.; Guo, X.; Lu, C.; Wei, J.; Fang, J. Constructing Heterojunctions by Surface Sulfidation for Efficient Inverted Perovskite Solar Cells. Science 2022, 375, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Han, H.J.; Kim, D.; Ahn, T.K.; Im, S.H. Hysteresis-Less Inverted Ch3nh3pbi3 Planar Perovskite Hybrid Solar Cells with 18.1% Power Conversion Efficiency. Energy Environ. Sci. 2015, 8, 1602–1608. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, J.; Li, Z.; Liu, D.; Qin, M.; Cheung, S.H.; Lu, X.; Lei, D.; So, S.K.; Zhu, Z.; et al. Modulation of Defects and Interfaces through Alkylammonium Interlayer for Efficient Inverted Perovskite Solar Cells. Joule 2020, 4, 1248–1262. [Google Scholar] [CrossRef]
- Zaban, A.; Greenshtein, M.; Bisquert, J. Determination of the Electron Lifetime in Nanocrystalline Dye Solar Cells by Open-Circuit Voltage Decay Measurements. ChemPhysChem 2003, 4, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; You, P.; Liu, Z.; Li, L.; Yan, F. Ultrasensitive Broadband Phototransistors Based on Perovskite/Organic-Semiconductor Vertical Heterojunctions. Light Sci. Appl. 2017, 6, e17023. [Google Scholar] [CrossRef]
- Marchioro, A.; Teuscher, J.; Friedrich, D.; Kunst, M.; van de Krol, R.; Moehl, T.; Grätzel, M.; Moser, J.-E. Unravelling the Mechanism of Photoinduced Charge Transfer Processes in Lead Iodide Perovskite Solar Cells. Nat. Photonics 2014, 8, 250–255. [Google Scholar] [CrossRef]
- Liu, F.; Li, Q.; Li, Z. Hole-Transporting Materials for Perovskite Solar Cells. Asian J. Org. Chem. 2018, 7, 2182–2200. [Google Scholar] [CrossRef]
- Kahn, A. Fermi Level, Work Function and Vacuum Level. Mater. Horiz. 2016, 3, 7–10. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, C.; Lin, F.; Chen, T.; Pan, D.; Guo, X. Research Progress of Inverted Perovskite Solar Cells. Acta Chim. Sin. 2019, 77, 964–976. [Google Scholar] [CrossRef]
- Liu, T.; Chen, K.; Hu, Q.; Zhu, R.; Gong, Q. Inverted Perovskite Solar Cells: Progresses and Perspectives. Adv. Energy Mater. 2016, 6, 1600457. [Google Scholar] [CrossRef]
- Mohamad, D.K.; Griffin, J.; Bracher, C.; Barrows, A.T.; Lidzey, D.G. Spray-Cast Multilayer Organometal Perovskite Solar Cells Fabricated in Air. Adv. Energy Mater. 2016, 6, 1600994. [Google Scholar] [CrossRef]
- Wang, Q.; Chueh, C.-C.; Eslamian, M.; Jen, A.K.Y. Modulation of Pedot:Pss Ph for Efficient Inverted Perovskite Solar Cells with Reduced Potential Loss and Enhanced Stability. ACS Appl. Mater. Interfaces 2016, 8, 32068–32076. [Google Scholar] [CrossRef]
- Yi, M.; Jang, W.; Wang, D.H. Controlled Ph of Pedot:Pss for Reproducible Efficiency in Inverted Perovskite Solar Cells: Independent of Active Area and Humidity. ACS Sustain. Chem. Eng. 2019, 7, 8245–8254. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.; Yang, K.; Xiong, Z.; Yang, B.; Wang, M.; Tang, X.; Zang, Z.; Liu, X.; Li, B.; et al. Pedot:Pss Monolayers to Enhance the Hole Extraction and Stability of Perovskite Solar Cells. J. Mater. Chem. A 2018, 6, 16583–16589. [Google Scholar] [CrossRef]
- Lim, K.-G.; Kim, H.-B.; Jeong, J.; Kim, H.; Kim, J.Y.; Lee, T.-W. Boosting the Power Conversion Efficiency of Perovskite Solar Cells Using Self-Organized Polymeric Hole Extraction Layers with High Work Function. Adv. Mater. 2014, 26, 6461–6466. [Google Scholar] [CrossRef]
- Li, D.; Cui, J.; Li, H.; Huang, D.; Wang, M.; Shen, Y. Graphene Oxide Modified Hole Transport Layer for Ch3nh3pbi3 Planar Heterojunction Solar Cells. Sol. Energy 2016, 131, 176–182. [Google Scholar] [CrossRef]
- Niu, J.; Yang, D.; Ren, X.; Yang, Z.; Liu, Y.; Zhu, X.; Zhao, W.; Liu, S. Graphene-Oxide Doped Pedot:Pss as a Superior Hole Transport Material for High-Efficiency Perovskite Solar Cell. Org. Electron. 2017, 48, 165–171. [Google Scholar] [CrossRef]
- Tang, H.; Shang, Y.; Zhou, W.; Peng, Z.; Ning, Z. Energy Level Tuning of Pedot:Pss for High Performance Tin-Lead Mixed Perovskite Solar Cells. Sol. RRL 2019, 3, 1800256. [Google Scholar] [CrossRef]
- Liu, X.; Li, B.; Zhang, N.; Yu, Z.; Sun, K.; Tang, B.; Shi, D.; Yao, H.; Ouyang, J.; Gong, H. Multifunctional Rbcl Dopants for Efficient Inverted Planar Perovskite Solar Cell with Ultra-High Fill Factor, Negligible Hysteresis and Improved Stability. Nano Energy 2018, 53, 567–578. [Google Scholar] [CrossRef]
- Jiang, K.; Wu, F.; Zhang, G.; Chow, P.C.Y.; Ma, C.; Li, S.; Wong, K.S.; Zhu, L.; Yan, H. Inverted Planar Perovskite Solar Cells Based on Csi-Doped Pedot:Pss with Efficiency Beyond 20% and Small Energy Loss. J. Mater. Chem. A 2019, 7, 21662–21667. [Google Scholar] [CrossRef]
- Hu, W.; Xu, C.Y.; Niu, L.B.; Elseman, A.M.; Wang, G.; Liu, D.B.; Yao, Y.Q.; Liao, L.P.; Zhou, G.D.; Song, Q.L. High Open-Circuit Voltage of 1.134 V for Inverted Planar Perovskite Solar Cells with Sodium Citrate-Doped Pedot:Pss as a Hole Transport Layer. ACS Appl. Mater. Interfaces 2019, 11, 22021–22027. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Zhang, C.; Liu, Y.; Zheng, C.; Lv, W.; Li, M.; Chen, Y.; Huang, W.; Chen, R. Improving the Efficiency and Stability of Inverted Perovskite Solar Cells by Cuscn-Doped Pedot:Pss. Sol. Energy Mater. Sol. Cells 2020, 206, 110316. [Google Scholar] [CrossRef]
- Wang, Z.-K.; Li, M.; Yuan, D.-X.; Shi, X.-B.; Ma, H.; Liao, L.-S. Improved Hole Interfacial Layer for Planar Perovskite Solar Cells with Efficiency Exceeding 15%. ACS Appl. Mater. Interfaces 2015, 7, 9645–9651. [Google Scholar] [CrossRef]
- Qian, M.; Li, M.; Shi, X.-B.; Ma, H.; Wang, Z.-K.; Liao, L.-S. Planar Perovskite Solar Cells with 15.75% Power Conversion Efficiency by Cathode and Anode Interfacial Modification. J. Mater. Chem. A 2015, 3, 13533–13539. [Google Scholar] [CrossRef]
- Huang, X.; Wang, K.; Yi, C.; Meng, T.; Gong, X. Efficient Perovskite Hybrid Solar Cells by Highly Electrical Conductive Pedot:Pss Hole Transport Layer. Adv. Energy Mater. 2016, 6, 1501773. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Yuan, J.; Hong, Q.; Shi, G.; Yuan, D.; Wei, J.; Huang, C.; Tang, J.; Fung, M.-K. Improved Performance of Inverted Planar Perovskite Solar Cells with F4-Tcnq Doped Pedot:Pss Hole Transport Layers. J. Mater. Chem. A 2017, 5, 5701–5708. [Google Scholar] [CrossRef]
- Hu, L.; Sun, K.; Wang, M.; Chen, W.; Yang, B.; Fu, J.; Xiong, Z.; Li, X.; Tang, X.; Zang, Z.; et al. Inverted Planar Perovskite Solar Cells with a High Fill Factor and Negligible Hysteresis by the Dual Effect of Nacl-Doped Pedot:Pss. ACS Appl. Mater. Interfaces 2017, 9, 43902–43909. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Obispo, C.; Ripolles, T.S.; Cortijo-Campos, S.; Álvarez, A.L.; Climent-Pascual, E.; de Andrés, A.; Coya, C. Enhanced Stability and Efficiency in Inverted Perovskite Solar Cells through Graphene Doping of Pedot:Pss Hole Transport Layer. Mater. Des. 2020, 191, 108587. [Google Scholar] [CrossRef]
- Xu, L.; Qian, M.; Zhang, C.; Lv, W.; Jin, J.; Zhang, J.; Zheng, C.; Li, M.; Chen, R.; Huang, W. In Situ Construction of Gradient Heterojunction Using Organic Vox Precursor for Efficient and Stable Inverted Perovskite Solar Cells. Nano Energy 2020, 67, 104244. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, K.; Chang, J.; Ouyang, J. Effects of Organic Inorganic Hybrid Perovskite Materials on the Electronic Properties and Morphology of Poly(3,4-Ethylenedioxythiophene):Poly(Styrenesulfonate) and the Photovoltaic Performance of Planar Perovskite Solar Cells. J. Mater. Chem. A 2015, 3, 15897–15904. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.; Ma, Y.; Li, Z.; Xu, Y.; Mai, Y. Homogenizing the Sulfonic Acid Distribution of Dmf-Modified Pedot:Pss Films and Perovskite Solar Cells. J. Energy Chem. 2019, 32, 71–77. [Google Scholar] [CrossRef]
- Huang, D.; Goh, T.; Kong, J.; Zheng, Y.; Zhao, S.; Xu, Z.; Taylor, A.D. Perovskite Solar Cells with a Dmso-Treated Pedot:Pss Hole Transport Layer Exhibit Higher Photovoltaic Performance and Enhanced Durability. Nanoscale 2017, 9, 4236–4243. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, M.; Liu, C.; Zhang, L.; Zhong, X.; Li, X.; Tian, Y.; Cheng, C.; Xu, B. Synergistic Effects of Multiple Functional Ionic Liquid-Treated Pedot:Pss and Less-Ion-Defects S-Acetylthiocholine Chloride-Passivated Perovskite Surface Enabling Stable and Hysteresis-Free Inverted Perovskite Solar Cells with Conversion Efficiency over 20%. Nano Energy 2019, 63, 103866. [Google Scholar]
- Huang, J.; Wang, K.-X.; Chang, J.-J.; Jiang, Y.-Y.; Xiao, Q.-S.; Li, Y. Improving the Efficiency and Stability of Inverted Perovskite Solar Cells with Dopamine-Copolymerized Pedot:Pss as a Hole Extraction Layer. J. Mater. Chem. A 2017, 5, 13817–13822. [Google Scholar] [CrossRef]
- Xue, Q.; Liu, M.; Li, Z.; Yan, L.; Hu, Z.; Zhou, J.; Li, W.; Jiang, X.-F.; Xu, B.; Huang, F.; et al. Efficient and Stable Perovskite Solar Cells Via Dual Functionalization of Dopamine Semiquinone Radical with Improved Trap Passivation Capabilities. Adv. Funct. Mater. 2018, 28, 1707444. [Google Scholar] [CrossRef]
- Hu, L.; Fu, J.; Yang, K.; Xiong, Z.; Wang, M.; Yang, B.; Wang, H.; Tang, X.; Zang, Z.; Li, M.; et al. Inhibition of in-Plane Charge Transport in Hole Transfer Layer to Achieve High Fill Factor for Inverted Planar Perovskite Solar Cells. Sol. RRL 2019, 3, 1900104. [Google Scholar] [CrossRef]
- Dong, H.; Zheng, E.; Niu, Z.; Zhang, X.; Lin, Y.-Y.; Jain, P.; Yu, Q. Hydroxymethyl-Functionalized Pedot-Meoh:Pss for Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 17571–17582. [Google Scholar] [CrossRef]
- Elbohy, H.; Bahrami, B.; Mabrouk, S.; Reza, K.M.; Gurung, A.; Pathak, R.; Liang, M.; Qiao, Q.; Zhu, K. Tuning Hole Transport Layer Using Urea for High-Performance Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1806740. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Hu, X.; Cai, W.; Zhang, C.; Wang, M.; Zang, Z. Sodium Benzenesulfonate Modified Poly (3,4-Ethylenedioxythiophene): Polystyrene Sulfonate with Improved Wettability and Work Function for Efficient and Stable Perovskite Solar Cells. Sol. RRL 2021, 5, 2000573. [Google Scholar] [CrossRef]
- Jhuo, H.-J.; Yeh, P.-N.; Liao, S.-H.; Li, Y.-L.; Sharma, S.; Chen, S.-A. Inverted Perovskite Solar Cells with Inserted Cross-Linked Electron-Blocking Interlayers for Performance Enhancement. J. Mater. Chem. A 2015, 3, 9291–9297. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Xie, F.; Yang, X.; Han, L. Improving the Performance of Inverted Formamidinium Tin Iodide Perovskite Solar Cells by Reducing the Energy-Level Mismatch. ACS Energy Lett. 2018, 3, 1116–1121. [Google Scholar] [CrossRef]
- Zhang, R.; Ling, H.; Lu, X.; Xia, J. The Facile Modification of Pedot:Pss Buffer Layer by Polyethyleneglycol and Their Effects on Inverted Perovskite Solar Cell. Sol. Energy 2019, 186, 398–403. [Google Scholar] [CrossRef]
- Ma, S.; Liu, X.; Wu, Y.; Tao, Y.; Ding, Y.; Cai, M.; Dai, S.; Liu, X.; Alsaedi, A.; Hayat, T. Efficient and Flexible Solar Cells with Improved Stability through Incorporation of a Multifunctional Small Molecule at Pedot:Pss/Perovskite Interface. Sol. Energy Mater. Sol. Cells 2020, 208, 110379. [Google Scholar] [CrossRef]
- Gu, Z.; Zuo, L.; Larsen-Olsen, T.T.; Ye, T.; Wu, G.; Krebs, F.C.; Chen, H. Interfacial Engineering of Self-Assembled Monolayer Modified Semi-Roll-to-Roll Planar Heterojunction Perovskite Solar Cells on Flexible Substrates. J. Mater. Chem. A 2015, 3, 24254–24260. [Google Scholar] [CrossRef]
- Luo, H.; Lin, X.; Hou, X.; Pan, L.; Huang, S.; Chen, X. Efficient and Air-Stable Planar Perovskite Solar Cells Formed on Graphene-Oxide-Modified Pedot:Pss Hole Transport Layer. Nano-Micro Lett. 2017, 9, 39. [Google Scholar] [CrossRef]
- Peng, H.; Sun, W.; Li, Y.; Ye, S.; Rao, H.; Yan, W.; Zhou, H.; Bian, Z.; Huang, C. Solution Processed Inorganic V2oxas Interfacial Function Materials for Inverted Planar-Heterojunction Perovskite Solar Cells with Enhanced Efficiency. Nano Res. 2016, 9, 2960–2971. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zhou, X.; Zhang, L.; Xu, B. Facile Phthalocyanine Doping into Pedot Leads to Highly Efficient and Stable Inverted Metal Halide Perovskite Solar Cells. J. Mater. Chem. A 2018, 6, 12515–12522. [Google Scholar] [CrossRef]
- Yoon, S.; Ha, S.R.; Moon, T.; Jeong, S.M.; Ha, T.-J.; Choi, H.; Kang, D.-W. Carbon Nanotubes Embedded Poly(3,4-Ethylenedioxythiophene): Poly(Styrenesulfonate) Hybrid Hole Collector for Inverted Planar Perovskite Solar Cells. J. Power Sources 2019, 435, 226765. [Google Scholar] [CrossRef]
- Li, Z.; Dong, J.; Han, W.; Ren, G.; Liu, C.; Cui, H.; Shen, L.; Guo, W. Overcoming Intrinsic Defects of the Hole Transport Layer with Optimized Carbon Nanorods for Perovskite Solar Cells. Nanoscale 2019, 11, 8776–8784. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Liu, Z.; Yuan, L.; Zhu, H.; Luo, H.; Yan, K. Pedot:Pss-Metal Oxide Composite Electrode with Regulated Wettability and Work Function for High-Performance Inverted Perovskite Solar Cells. Adv. Opt. Mater. 2020, 8, 2000216. [Google Scholar] [CrossRef]
- Song, J.; Hu, W.; Li, Z.; Wang, X.-F.; Tian, W. A Double Hole-Transport Layer Strategy toward Efficient Mixed Tin-Lead Iodide Perovskite Solar Cell. Sol. Energy Mater. Sol. Cells 2020, 207, 110351. [Google Scholar] [CrossRef]
- Li, W.; Cheng, N.; Cao, Y.; Zhao, Z.; Xiao, Z.; Zi, W.; Sun, Z. Boost the Performance of Inverted Perovskite Solar Cells with Pedot:Pss/Graphene Quantum Dots Composite Hole Transporting Layer. Org. Electron. 2020, 78, 105575. [Google Scholar] [CrossRef]
- Wang, M.; Wang, H.; Li, W.; Hu, X.; Sun, K.; Zang, Z. Defect Passivation Using Ultrathin PTAA Layers for Efficient and Stable Perovskite Solar Cells with a High Fill Factor and Eliminated Hysteresis. J. Mater. Chem. A 2019, 7, 26421–26428. [Google Scholar] [CrossRef]
- Bi, C.; Wang, Q.; Shao, Y.; Yuan, Y.; Xiao, Z.; Huang, J. Non-Wetting Surface-Driven High-Aspect-Ratio Crystalline Grain Growth for Efficient Hybrid Perovskite Solar Cells. Nat. Commun. 2015, 6, 7747. [Google Scholar] [CrossRef]
- Serpetzoglou, E.; Konidakis, I.; Kakavelakis, G.; Maksudov, T.; Kymakis, E.; Stratakis, E. Improved Carrier Transport in Perovskite Solar Cells Probed by Femtosecond Transient Absorption Spectroscopy. ACS Appl. Mater. Interfaces 2017, 9, 43910–43919. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Bi, C.; Huang, J. Doped Hole Transport Layer for Efficiency Enhancement in Planar Heterojunction Organolead Trihalide Perovskite Solar Cells. Nano Energy 2015, 15, 275–280. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.-R.; Yang, F.; Yang, J.-E.; Song, J.; Li, M.; Li, Z.; Tsoi, W.C.; Chinweokwu Eze, M.; Liu, Z.-Y.; et al. Π-Conjugated Small Molecules Enable Efficient Perovskite Growth and Charge-Extraction for High-Performance Photovoltaic Devices. J. Power Sources 2020, 448, 227420. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Lee, E.-C. High-Performance Inverted Perovskite Solar Cells Using Doped Poly(Triarylamine) as the Hole Transport Layer. ACS Appl. Energy Mater. 2019, 2, 1932–1942. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Cai, M.; Xie, F.; Wu, Y.; Lan, Z.; Yang, X.; Qiang, Y.; Islam, A.; Han, L. Stable Inverted Planar Perovskite Solar Cells with Low-Temperature-Processed Hole-Transport Bilayer. Adv. Energy Mater. 2017, 7, 1700763. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, Q.; Li, H.; Niu, Y.; Han, D.; Yang, Q.; Yang, Y.; Yi, S.; Zhou, D.-Y.; Feng, L. Dopant Free Mixture of Spiro-Ometad and PTAA with Tunable Wettability as Hole Transport Layer Enhancing Performance of Inverted Cspbi2br Perovskite Solar Cells. Org. Electron. 2020, 86, 105873. [Google Scholar] [CrossRef]
- Oo, A.M.; Fan, P.; Zhang, X.; Yu, J. Efficiency Improvement of Planar Inverted Perovskite Solar Cells by Introducing Poly 9,9-Dioctyfluorene-Co-Benzothiazole into Polytriarylamine as Mixed Hole-Transport Layer. Energy Technol. 2020, 8, 1901042. [Google Scholar] [CrossRef]
- Chen, K.; Kong, W.; Ali, N.; Song, W.; Wang, Z.; Wang, A.; Yu, Z.; Tao, J.; Yang, S.; Fu, G. Facile Physical Modifications of Polymer Hole Transporting Layers for Efficient and Reproducible Perovskite Solar Cells with Fill Factor Exceeding 80%. Sol. RRL 2020, 4, 2000365. [Google Scholar] [CrossRef]
- Jung, E.D.; Harit, A.K.; Kim, D.H.; Jang, C.H.; Park, J.H.; Cho, S.; Song, M.H.; Woo, H.Y. Multiply Charged Conjugated Polyelectrolytes as a Multifunctional Interlayer for Efficient and Scalable Perovskite Solar Cells. Adv. Mater. 2020, 32, 2002333. [Google Scholar] [CrossRef]
- Zhang, S.; Stolterfoht, M.; Armin, A.; Lin, Q.; Zu, F.; Sobus, J.; Jin, H.; Koch, N.; Meredith, P.; Burn, P.L.; et al. Interface Engineering of Solution-Processed Hybrid Organohalide Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 21681–21687. [Google Scholar] [CrossRef]
- Li, Y.; Liang, C.; Wang, G.; Li, J.; Chen, S.; Yang, S.; Xing, G.; Pan, H. Two-Step Solvent Post-Treatment on PTAA for Highly Efficient and Stable Inverted Perovskite Solar Cells. Photonics Res. 2020, 8, A39–A49. [Google Scholar] [CrossRef]
- Bagheri, Z.; Matteocci, F.; Lamanna, E.; di Girolamo, D.; Marrani, A.G.; Zanoni, R.; di Carlo, A.; Moshaii, A. Light-Induced Improvement of Dopant-Free PTAA on Performance of Inverted Perovskite Solar Cells. Sol. Energy Mater. Sol. Cells 2020, 215, 110606. [Google Scholar] [CrossRef]
- Xu, C.Y.; Hu, W.; Wang, G.; Niu, L.; Elseman, A.M.; Liao, L.; Yao, Y.; Xu, G.; Luo, L.; Liu, D.; et al. Coordinated Optical Matching of a Texture Interface Made from Demixing Blended Polymers for High-Performance Inverted Perovskite Solar Cells. ACS Nano 2020, 14, 196–203. [Google Scholar] [CrossRef]
- Petrović, M.; Maksudov, T.; Panagiotopoulos, A.; Serpetzoglou, E.; Konidakis, I.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Limitations of a Polymer-Based Hole Transporting Layer for Application in Planar Inverted Perovskite Solar Cells. Nanoscale Adv. 2019, 1, 3107–3118. [Google Scholar] [CrossRef] [PubMed]
- Petridis, K.; Kakavelakis, G.; Stylianakis, M.M.; Kymakis, E. Graphene-Based Inverted Planar Perovskite Solar Cells: Advancements, Fundamental Challenges, and Prospects. Chem. Asian J. 2018, 13, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Mai, C.-K.; Kim, H.-B.; Jeong, J.; Song, S.; Bazan, G.C.; Kim, J.Y.; Heeger, A.J. Conjugated Polyelectrolyte Hole Transport Layer for Inverted-Type Perovskite Solar Cells. Nat. Commun. 2015, 6, 7348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, X.; Zhong, X.; Cheng, C.; Tian, Y.; Xu, B. Hole-Transporting Layer Based on a Conjugated Polyelectrolyte with Organic Cations Enables Efficient Inverted Perovskite Solar Cells. Nano Energy 2019, 57, 248–255. [Google Scholar] [CrossRef]
- Liu, P.-H.; Chuang, C.-H.; Zhou, Y.-L.; Wang, S.-H.; Jeng, R.-J.; Rwei, S.-P.; Liau, W.-B.; Wang, L. Conjugated Polyelectrolytes as Promising Hole Transport Materials for Inverted Perovskite Solar Cells: Effect of Ionic Groups. J. Mater. Chem. A 2020, 8, 25173–25177. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, X.; Xie, J.; Chen, S.; Bae, S.; Kim, J.; Xu, B. Conjugated Polyelectrolyte with Potassium Cations Enables Inverted Perovskite Solar Cells with an Efficiency over 20%. J. Mater. Chem. A 2020, 8, 8238–8243. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, J.; Kim, G.; Shin, D.; Jeong, S.Y.; Lee, J.; Hong, S.; Choi, J.W.; Lee, C.-L.; Kim, H.; et al. Introducing Paired Electric Dipole Layers for Efficient and Reproducible Perovskite Solar Cells. Energy Environ. Sci. 2018, 11, 1742–1751. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.-C.; Zhu, L.; Zhang, W.; Wang, H.-Q.; Fang, J. Improving Efficiency and Reproducibility of Perovskite Solar Cells through Aggregation Control in Polyelectrolytes Hole Transport Layer. ACS Appl. Mater. Interfaces 2017, 9, 31357–31361. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Wang, X.; Zhao, L.; Jiu, T.; Fang, J. Polyelectrolyte Based Hole-Transporting Materials for High Performance Solution Processed Planar Perovskite Solar Cells. J. Mater. Chem. A 2015, 3, 15024–15029. [Google Scholar] [CrossRef]
- Li, J.; Zhao, M.; Zhao, C.; Jian, H.; Wang, N.; Yao, L.; Huang, C.; Zhao, Y.; Jiu, T. Graphdiyne-Doped P3ct-K as an Efficient Hole-Transport Layer for Mapbi3 Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 2626–2631. [Google Scholar] [CrossRef]
- Li, S.; He, B.; Xu, J.; Lu, H.; Jiang, J.; Zhu, J.; Kan, Z.; Zhu, L.; Wu, F. Highly Efficient Inverted Perovskite Solar Cells Incorporating P3ct-Rb as a Hole Transport Layer to Achieve a Large Open Circuit Voltage of 1.144 V. Nanoscale 2020, 12, 3686–3691. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Chen, S.; Chen, H.; Liu, L.; Ding, W.; Wang, J.; Zhang, A.; Pang, S.; Guo, X.; et al. Improved Interfacial Property by Small Molecule Ethanediamine for High Performance Inverted Planar Perovskite Solar Cells. J. Energy Chem. 2021, 54, 467–474. [Google Scholar] [CrossRef]
- Zhao, D.; Sexton, M.; Park, H.-Y.; Baure, G.; Nino, J.C.; So, F. High-Efficiency Solution-Processed Planar Perovskite Solar Cells with a Polymer Hole Transport Layer. Adv. Energy Mater. 2015, 5, 1401855. [Google Scholar] [CrossRef]
- Xu, X.; Ma, C.; Cheng, Y.; Xie, Y.-M.; Yi, X.; Gautam, B.; Chen, S.; Li, H.-W.; Lee, C.-S.; So, F.; et al. Ultraviolet-Ozone Surface Modification for Non-Wetting Hole Transport Materials Based Inverted Planar Perovskite Solar Cells with Efficiency Exceeding 18%. J. Power Sources 2017, 360, 157–165. [Google Scholar] [CrossRef]
- You, J.; Guo, F.; Qiu, S.; He, W.; Wang, C.; Liu, X.; Xu, W.; Mai, Y. The Fabrication of Homogeneous Perovskite Films on Non-Wetting Interfaces Enabled by Physical Modification. J. Energy Chem. 2019, 38, 192–198. [Google Scholar] [CrossRef]
- Lee, H.K.H.; Barbé, J.; Meroni, S.M.P.; Du, T.; Lin, C.-T.; Pockett, A.; Troughton, J.; Jain, S.M.; de Rossi, F.; Baker, J.; et al. Outstanding Indoor Performance of Perovskite Photovoltaic Cells—Effect of Device Architectures and Interlayers. Sol. RRL 2019, 3, 1800207. [Google Scholar] [CrossRef]
- Li, B.; Xiang, Y.; Jayawardena, K.D.G.I.; Luo, D.; Watts, J.F.; Hinder, S.; Li, H.; Ferguson, V.; Luo, H.; Zhu, R.; et al. Tailoring Perovskite Adjacent Interfaces by Conjugated Polyelectrolyte for Stable and Efficient Solar Cells. Sol. RRL 2020, 4, 2000060. [Google Scholar] [CrossRef]
- Yan, W.; Li, Y.; Li, Y.; Ye, S.; Liu, Z.; Wang, S.; Bian, Z.; Huang, C. Stable High-Performance Hybrid Perovskite Solar Cells with Ultrathin Polythiophene as Hole-Transporting Layer. Nano Res. 2015, 8, 2474–2480. [Google Scholar] [CrossRef]
- Yan, W.; Li, Y.; Li, Y.; Ye, S.; Liu, Z.; Wang, S.; Bian, Z.; Huang, C. High-Performance Hybrid Perovskite Solar Cells with Open Circuit Voltage Dependence on Hole-Transporting Materials. Nano Energy 2015, 16, 428–437. [Google Scholar] [CrossRef]
- Yang, L.; Yan, Y.; Cai, F.; Li, J.; Wang, T. Poly(9-Vinylcarbazole) as a Hole Transport Material for Efficient and Stable Inverted Planar Heterojunction Perovskite Solar Cells. Sol. Energy Mater. Sol. Cells 2017, 163, 210–217. [Google Scholar] [CrossRef]
- Liu, N.; Zong, X.; Wang, Z.; Cui, T.; Liang, M.; Zhang, Y.; Xue, S. Litfsi/Tbp-Free Hole Transport Materials with Nonlinear Π-Conjugation for Efficient Inverted Perovskite Solar Cells. Electrochim. Acta 2019, 296, 283–293. [Google Scholar] [CrossRef]
- Shao, J.-Y.; Yu, B.; Wang, Y.-D.; Lan, Z.-R.; Li, D.; Meng, Q.; Zhong, Y.-W. In-Situ Electropolymerized Polyamines as Dopant-Free Hole-Transporting Materials for Efficient and Stable Inverted Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 5058–5066. [Google Scholar] [CrossRef]
- Rodríguez-Seco, C.; Cabau, L.; Vidal-Ferran, A.; Palomares, E. Advances in the Synthesis of Small Molecules as Hole Transport Materials for Lead Halide Perovskite Solar Cells. Acc. Chem. Res. 2018, 51, 869–880. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Zhao, S.; Qiao, B.; Huang, D.; Zhao, L.; Zhao, J.; Wang, P.; Zhu, Y.; Li, X.; et al. Highly Efficient P-I-N Perovskite Solar Cells Utilizing Novel Low-Temperature Solution-Processed Hole Transport Materials with Linear Π-Conjugated Structure. Small 2016, 12, 4902–4908. [Google Scholar] [CrossRef]
- Yang, L.; Cai, F.; Yan, Y.; Li, J.; Liu, D.; Pearson, A.J.; Wang, T. Conjugated Small Molecule for Efficient Hole Transport in High-Performance P-I-N Type Perovskite Solar Cells. Adv. Funct. Mater. 2017, 27, 1702613. [Google Scholar] [CrossRef]
- Zhang, Y.; Kou, C.; Zhang, J.; Liu, Y.; Li, W.; Bo, Z.; Shao, M. Crosslinked and Dopant Free Hole Transport Materials for Efficient and Stable Planar Perovskite Solar Cells. J. Mater. Chem. A 2019, 7, 5522–5529. [Google Scholar] [CrossRef]
- Li, Y.; Cole, M.D.; Gao, Y.; Emrick, T.; Xu, Z.; Liu, Y.; Russell, T.P. High-Performance Perovskite Solar Cells with a Non-Doped Small Molecule Hole Transporting Layer. ACS Appl. Energy Mater. 2019, 2, 1634–1641. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.; Morrissey, T.; Lam, B.; Patrick, B.O.; Dvorak, D.J.; Xia, Z.; Kelly, T.L.; Berlinguette, C.P. Dopant-Free Molecular Hole Transport Material That Mediates a 20% Power Conversion Efficiency in a Perovskite Solar Cell. Energy Environ. Sci. 2019, 12, 3502–3507. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Wang, L.; Tu, B.; Chen, T.; Liu, B.; Yang, K.; Koh, C.W.; Zhang, X.; Sun, H.; et al. Dopant-Free Small-Molecule Hole-Transporting Material for Inverted Perovskite Solar Cells with Efficiency Exceeding 21%. Adv. Mater. 2019, 31, 1902781. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, J.; Wu, F.; Xue, Q.; Yao, Q.; Zhang, J.; Chen, Y.; Zhang, G.; Zhu, Z.; Yan, H.; et al. Dopant-Free Organic Hole-Transporting Material for Efficient and Stable Inverted All-Inorganic and Hybrid Perovskite Solar Cells. Adv. Mater. 2020, 32, 1908011. [Google Scholar] [CrossRef]
- Li, B.; Li, Z.; Xing, J.; Zhu, M.; Zhou, Z. Fused Furan-Based Organic Small Molecules as Dopant-Free Hole Transporting Material for Inverted Perovskite Solar Cells. Sol. RRL 2020, 4, 2000536. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, W.; Sun, H.; Wang, D.; Chen, P.; Djurišić, A.B.; Zhu, Y.; Tu, B.; Guo, X.; Tang, B.-Z.; et al. Efficient Perovskite Solar Cells with a Novel Aggregation-Induced Emission Molecule as Hole-Transport Material. Sol. RRL 2020, 4, 1900189. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, X.; Liu, X.; Ghadari, R.; Cai, M.; Ding, Y.; Mateen, M.; Dai, S. Pyridine-Triphenylamine Hole Transport Material for Inverted Perovskite Solar Cells. J. Energy Chem. 2021, 54, 395–402. [Google Scholar] [CrossRef]
- Yang, X.; Xi, J.; Sun, Y.; Zhang, Y.; Zhou, G.; Wong, W.-Y. A Dopant-Free Twisted Organic Small-Molecule Hole Transport Material for Inverted Planar Perovskite Solar Cells with Enhanced Efficiency and Operational Stability. Nano Energy 2019, 64, 103946. [Google Scholar] [CrossRef]
- Chen, R.; Bu, T.; Li, J.; Li, W.; Zhou, P.; Liu, X.; Ku, Z.; Zhong, J.; Peng, Y.; Huang, F.; et al. Efficient and Stable Inverted Planar Perovskite Solar Cells Using a Triphenylamine Hole-Transporting Material. ChemSusChem 2018, 11, 1467–1473. [Google Scholar] [CrossRef]
- Park, S.J.; Jeon, S.; Lee, I.K.; Zhang, J.; Jeong, H.; Park, J.-Y.; Bang, J.; Ahn, T.K.; Shin, H.-W.; Kim, B.-G.; et al. Inverted Planar Perovskite Solar Cells with Dopant Free Hole Transporting Material: Lewis Base-Assisted Passivation and Reduced Charge Recombination. J. Mater. Chem. A 2017, 5, 13220–13227. [Google Scholar] [CrossRef]
- Docampo, P.; Ball, J.M.; Darwich, M.; Eperon, G.E.; Snaith, H.J. Efficient Organometal Trihalide Perovskite Planar-Heterojunction Solar Cells on Flexible Polymer Substrates. Nat. Commun. 2013, 4, 2761. [Google Scholar] [CrossRef]
- Zhu, Z.; Bai, Y.; Zhang, T.; Liu, Z.; Long, X.; Wei, Z.; Wang, Z.; Zhang, L.; Wang, J.; Yan, F.; et al. High-Performance Hole-Extraction Layer of Sol–Gel-Processed Nio Nanocrystals for Inverted Planar Perovskite Solar Cells. Angew. Chem. Int. Ed. 2014, 53, 12571–12575. [Google Scholar]
- Park, J.H.; Seo, J.; Park, S.; Shin, S.S.; Kim, Y.C.; Jeon, N.J.; Shin, H.-W.; Ahn, T.K.; Noh, J.H.; Yoon, S.C.; et al. Efficient Ch3nh3pbi3 Perovskite Solar Cells Employing Nanostructured P-Type Nio Electrode Formed by a Pulsed Laser Deposition. Adv. Mater. 2015, 27, 4013–4019. [Google Scholar] [CrossRef]
- Yin, X.; Que, M.; Xing, Y.; Que, W. High Efficiency Hysteresis-Less Inverted Planar Heterojunction Perovskite Solar Cells with a Solution-Derived Niox Hole Contact Layer. J. Mater. Chem. A 2015, 3, 24495–24503. [Google Scholar] [CrossRef]
- Islam, M.B.; Yanagida, M.; Shirai, Y.; Nabetani, Y.; Miyano, K. Niox Hole Transport Layer for Perovskite Solar Cells with Improved Stability and Reproducibility. ACS Omega 2017, 2, 2291–2299. [Google Scholar] [CrossRef]
- Tang, L.J.; Chen, X.; Wen, T.Y.; Yang, S.; Zhao, J.J.; Qiao, H.W.; Hou, Y.; Yang, H.G. A Solution-Processed Transparent Nio Hole-Extraction Layer for High-Performance Inverted Perovskite Solar Cells. Chem. Eur. J. 2018, 24, 2845–2849. [Google Scholar] [CrossRef]
- Mali, S.S.; Kim, H.; Kim, H.H.; Shim, S.E.; Hong, C.K. Nanoporous P-Type Niox Electrode for P-I-N Inverted Perovskite Solar Cell toward Air Stability. Mater. Today 2018, 21, 483–500. [Google Scholar] [CrossRef]
- Kim, J.H.; Liang, P.-W.; Williams, S.T.; Cho, N.; Chueh, C.-C.; Glaz, M.S.; Ginger, D.S.; Jen, A.K.Y. High-Performance and Environmentally Stable Planar Heterojunction Perovskite Solar Cells Based on a Solution-Processed Copper-Doped Nickel Oxide Hole-Transporting Layer. Adv. Mater. 2015, 27, 695–701. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Yue, Y.; Liu, J.; Zhang, W.; Yang, X.; Chen, H.; Bi, E.; Ashraful, I.; Grätzel, M.; et al. Efficient and Stable Large-Area Perovskite Solar Cells with Inorganic Charge Extraction Layers. Science 2015, 350, 944–948. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F.-Z.; Feng, X.-Y.; Djurišić, A.B.; Chan, W.K.; He, Z.-B. Cesium Doped Niox as an Efficient Hole Extraction Layer for Inverted Planar Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1700722. [Google Scholar] [CrossRef]
- Wei, Y.; Yao, K.; Wang, X.; Jiang, Y.; Liu, X.; Zhou, N.; Li, F. Improving the Efficiency and Environmental Stability of Inverted Planar Perovskite Solar Cells Via Silver-Doped Nickel Oxide Hole-Transporting Layer. Appl. Surf. Sci. 2018, 427, 782–790. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, D.; Yang, P.; Yang, L.; Qin, L.; Huang, Y.; Zhao, X. Sol-Gel-Processed Yttrium-Doped Nio as Hole Transport Layer in Inverted Perovskite Solar Cells for Enhanced Performance. Appl. Surf. Sci. 2018, 441, 258–264. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Y.; Wang, L.; Wu, Y.; Tu, B.; Yu, B.; Liu, F.; Tam, H.-W.; Wang, G.; Djurišić, A.B.; et al. Molecule-Doped Nickel Oxide: Verified Charge Transfer and Planar Inverted Mixed Cation Perovskite Solar Cell. Adv. Mater. 2018, 30, 1800515. [Google Scholar] [CrossRef]
- Chen, P.-C.; Yang, S.-H. Potassium-Doped Nickel Oxide as the Hole Transport Layer for Efficient and Stable Inverted Perovskite Solar Cells. ACS Appl. Energy Mater. 2019, 2, 6705–6713. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Liu, J.; Qin, C.; Yang, X.; Islam, A.; Cheng, Y.-B.; Han, L. Hybrid Interfacial Layer Leads to Solid Performance Improvement of Inverted Perovskite Solar Cells. Energy Environ. Sci. 2015, 8, 629–640. [Google Scholar] [CrossRef]
- Du, Y.; Xin, C.; Huang, W.; Shi, B.; Ding, Y.; Wei, C.; Zhao, Y.; Li, Y.; Zhang, X. Polymeric Surface Modification of Niox-Based Inverted Planar Perovskite Solar Cells with Enhanced Performance. ACS Sustain. Chem. Eng. 2018, 6, 16806–16812. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Y.; Chen, G.; Wu, Y.; Tu, B.; Liu, F.-Z.; Huang, L.; Ng, A.M.C.; Djurišić, A.B.; He, Z. Alkali Chlorides for the Suppression of the Interfacial Recombination in Inverted Planar Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1803872. [Google Scholar] [CrossRef]
- Lian, X.; Chen, J.; Shan, S.; Wu, G.; Chen, H. Polymer Modification on the Niox Hole Transport Layer Boosts Open-Circuit Voltage to 1.19 V for Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 46340–46347. [Google Scholar] [CrossRef]
- Ru, P.; Bi, E.; Zhang, Y.; Wang, Y.; Kong, W.; Sha, Y.; Tang, W.; Zhang, P.; Wu, Y.; Chen, W.; et al. High Electron Affinity Enables Fast Hole Extraction for Efficient Flexible Inverted Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 1903487. [Google Scholar] [CrossRef]
- Subbiah, A.S.; Halder, A.; Ghosh, S.; Mahuli, N.; Hodes, G.; Sarkar, S.K. Inorganic Hole Conducting Layers for Perovskite-Based Solar Cells. J. Phys. Chem. Lett. 2014, 5, 1748–1753. [Google Scholar] [CrossRef]
- Ye, S.; Sun, W.; Li, Y.; Yan, W.; Peng, H.; Bian, Z.; Liu, Z.; Huang, C. Cuscn-Based Inverted Planar Perovskite Solar Cell with an Average Pce of 15.6%. Nano Lett. 2015, 15, 3723–3728. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Deng, L.-L.; Dai, S.-M.; Wang, X.; Tian, C.-B.; Zhan, X.-X.; Xie, S.-Y.; Huang, R.-B.; Zheng, L.-S. Low-Cost Solution-Processed Copper Iodide as an Alternative to Pedot:Pss Hole Transport Layer for Efficient and Stable Inverted Planar Heterojunction Perovskite Solar Cells. J. Mater. Chem. A 2015, 3, 19353–19359. [Google Scholar] [CrossRef]
- Sun, W.; Ye, S.; Rao, H.; Li, Y.; Liu, Z.; Xiao, L.; Chen, Z.; Bian, Z.; Huang, C. Room-Temperature and Solution-Processed Copper Iodide as the Hole Transport Layer for Inverted Planar Perovskite Solar Cells. Nanoscale 2016, 8, 15954–15960. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Jiang, X.; Li, J.; Cai, B.; Yang, X.; Sun, L. Efficient and Stable Inverted Planar Perovskite Solar Cells Employing Cui as Hole-Transporting Layer Prepared by Solid–Gas Transformation. Energy Technol. 2017, 5, 1836–1843. [Google Scholar] [CrossRef]
- Ye, S.; Rao, H.; Zhao, Z.; Zhang, L.; Bao, H.; Sun, W.; Li, Y.; Gu, F.; Wang, J.; Liu, Z.; et al. A Breakthrough Efficiency of 19.9% Obtained in Inverted Perovskite Solar Cells by Using an Efficient Trap State Passivator Cu(Thiourea)I. J. Am. Chem. Soc. 2017, 139, 7504–7512. [Google Scholar] [CrossRef]
- Yu, W.; Li, F.; Wang, H.; Alarousu, E.; Chen, Y.; Lin, B.; Wang, L.; Hedhili, M.N.; Li, Y.; Wu, K.; et al. Ultrathin Cu2o as an Efficient Inorganic Hole Transporting Material for Perovskite Solar Cells. Nanoscale 2016, 8, 6173–6179. [Google Scholar] [CrossRef]
- Yu, Z.-K.; Fu, W.-F.; Liu, W.-Q.; Zhang, Z.-Q.; Liu, Y.-J.; Yan, J.-L.; Ye, T.; Yang, W.-T.; Li, H.-Y.; Chen, H.-Z. Solution-Processed Cuox as an Efficient Hole-Extraction Layer for Inverted Planar Heterojunction Perovskite Solar Cells. Chin. Chem. Lett. 2017, 28, 13–18. [Google Scholar] [CrossRef]
- Rao, H.; Sun, W.; Ye, S.; Yan, W.; Li, Y.; Peng, H.; Liu, Z.; Bian, Z.; Huang, C. Solution-Processed Cus Nps as an Inorganic Hole-Selective Contact Material for Inverted Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 7800–7805. [Google Scholar] [CrossRef]
- Yin, X.; McClary, S.A.; Song, Z.; Zhao, D.; Graeser, B.; Wang, C.; Shrestha, N.; Wang, X.; Chen, C.; Li, C.; et al. A Cu3ps4 Nanoparticle Hole Selective Layer for Efficient Inverted Perovskite Solar Cells. J. Mater. Chem. A 2019, 7, 4604–4610. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Lai, J.; Song, X.; Yang, X.; Hagfeldt, A.; Sun, L. One Plus One Greater Than Two: High-Performance Inverted Planar Perovskite Solar Cells Based on a Composite Cui/Cuscn Hole-Transporting Layer. J. Mater. Chem. A 2018, 6, 21435–21444. [Google Scholar] [CrossRef]
- Javaid, H.; Duzhko, V.V.; Venkataraman, D. Hole Transport Bilayer for Highly Efficient and Stable Inverted Perovskite Solar Cells. ACS Appl. Energy Mater. 2021, 4, 72–80. [Google Scholar] [CrossRef]
- Wu, Z.; Bai, S.; Xiang, J.; Yuan, Z.; Yang, Y.; Cui, W.; Gao, X.; Liu, Z.; Jin, Y.; Sun, B. Efficient Planar Heterojunction Perovskite Solar Cells Employing Graphene Oxide as Hole Conductor. Nanoscale 2014, 6, 10505–10510. [Google Scholar] [CrossRef]
- Yeo, J.-S.; Kang, R.; Lee, S.; Jeon, Y.-J.; Myoung, N.; Lee, C.-L.; Kim, D.-Y.; Yun, J.-M.; Seo, Y.-H.; Kim, S.-S.; et al. Highly Efficient and Stable Planar Perovskite Solar Cells with Reduced Graphene Oxide Nanosheets as Electrode Interlayer. Nano Energy 2015, 12, 96–104. [Google Scholar] [CrossRef]
- Chen, H.; Hou, Y.; Halbig, C.E.; Chen, S.; Zhang, H.; Li, N.; Guo, F.; Tang, X.; Gasparini, N.; Levchuk, I.; et al. Extending the Environmental Lifetime of Unpackaged Perovskite Solar Cells through Interfacial Design. J. Mater. Chem. A 2016, 4, 11604–11610. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Han, D.; Yuan, Q.; Cao, T.; Chen, N.; Zhou, D.; Cong, H.; Feng, L. Ammonia-Treated Graphene Oxide and Pedot:Pss as Hole Transport Layer for High-Performance Perovskite Solar Cells with Enhanced Stability. Org. Electron. 2019, 70, 63–70. [Google Scholar] [CrossRef]
- Pang, S.; Li, X.; Dong, H.; Chen, D.; Zhu, W.; Chang, J.; Lin, Z.; Xi, H.; Zhang, J.; Zhang, C.; et al. Efficient Bifacial Semitransparent Perovskite Solar Cells Using Ag/V2o5 as Transparent Anodes. ACS Appl. Mater. Interfaces 2018, 10, 12731–12739. [Google Scholar] [CrossRef]
- Guo, C.X.; Sun, K.; Ouyang, J.; Lu, X. Layered V2o5/Pedot Nanowires and Ultrathin Nanobelts Fabricated with a Silk Reelinglike Process. Chem. Mater. 2015, 27, 5813–5819. [Google Scholar] [CrossRef]
- Duan, C.; Zhao, M.; Zhao, C.; Wang, Y.; Li, J.; Han, W.; Hu, Q.; Yao, L.; Jian, H.; Lu, F.; et al. Inverted Ch3nh3pbi3 Perovskite Solar Cells Based on Solution-Processed V2o5 Film Combined with P3ct Salt as Hole Transport Layer. Mater. Today Energy 2018, 9, 487–495. [Google Scholar] [CrossRef]
- Tseng, Z.-L.; Chen, L.-C.; Chiang, C.-H.; Chang, S.-H.; Chen, C.-C.; Wu, C.-G. Efficient Inverted-Type Perovskite Solar Cells Using Uv-Ozone Treated Moox and Wox as Hole Transporting Layers. Sol. Energy 2016, 139, 484–488. [Google Scholar] [CrossRef]
- Shalan, A.E.; Oshikiri, T.; Narra, S.; Elshanawany, M.M.; Ueno, K.; Wu, H.-P.; Nakamura, K.; Shi, X.; Diau, E.W.-G.; Misawa, H. Cobalt Oxide (Coox) as an Efficient Hole-Extracting Layer for High-Performance Inverted Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 33592–33600. [Google Scholar] [CrossRef]
- Castriotta, L.A.; Matteocci, F.; Vesce, L.; Cinà, L.; Agresti, A.; Pescetelli, S.; Ronconi, A.; Löffler, M.; Stylianakis, M.M.; di Giacomo, F.; et al. Air-Processed Infrared-Annealed Printed Methylammonium-Free Perovskite Solar Cells and Modules Incorporating Potassium-Doped Graphene Oxide as an Interlayer. ACS Appl. Mater. Interfaces 2021, 13, 11741–11754. [Google Scholar] [CrossRef]
- Anizelli, H.S.; Stoichkov, V.; Fernandes, R.V.; Duarte, J.L.; Laureto, E.; Kettle, J.; Visoly-Fisher, I.; Katz, E.A. Application of Luminescence Downshifting Materials for Enhanced Stability of Ch3nh3pbi3(1-X)Cl3x Perovskite Photovoltaic Devices. Org. Electron. 2017, 49, 129–134. [Google Scholar] [CrossRef]
- Guo, R.; Khenkin, M.V.; Arnaoutakis, G.E.; Samoylova, N.A.; Barbé, J.; Lee, H.K.H.; Tsoi, W.C.; Katz, E.A. Initial Stages of Photodegradation of Mapbi3 Perovskite: Accelerated Aging with Concentrated Sunlight. Sol. RRL 2020, 4, 1900270. [Google Scholar] [CrossRef]
- Mahon, N.S.; Korolik, O.V.; Khenkin, M.V.; Arnaoutakis, G.E.; Galagan, Y.; Soriūtė, V.; Litvinas, D.; Ščajev, P.; Katz, E.A.; Mazanik, A.V. Photoluminescence Kinetics for Monitoring Photoinduced Processes in Perovskite Solar Cells. Sol. Energy 2020, 195, 114–120. [Google Scholar] [CrossRef]
- Han, G.; Koh, T.M.; Lim, S.S.; Goh, T.W.; Guo, X.; Leow, S.W.; Begum, R.; Sum, T.C.; Mathews, N.; Mhaisalkar, S. Facile Method to Reduce Surface Defects and Trap Densities in Perovskite Photovoltaics. ACS Appl. Mater. Interfaces 2017, 9, 21292–21297. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional Engineering of Perovskite Materials for High-Performance Solar Cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.-Y.; Ummadisingu, A.; Zakeeruddin Shaik, M.; Correa-Baena, J.-P.; Tress Wolfgang, R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of Rubidium Cations into Perovskite Solar Cells Improves Photovoltaic Performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Noel, N.K.; Stranks, S.D.; Abate, A.; Wehrenfennig, C.; Guarnera, S.; Haghighirad, A.-A.; Sadhanala, A.; Eperon, G.E.; Pathak, S.K.; Johnston, M.B.; et al. Lead-Free Organic–Inorganic Tin Halide Perovskites for Photovoltaic Applications. Energy Environ. Sci. 2014, 7, 3061–3068. [Google Scholar] [CrossRef]
- Stranks Samuel, D.; Eperon Giles, E.; Grancini, G.; Menelaou, C.; Alcocer Marcelo, J.P.; Leijtens, T.; Herz Laura, M.; Petrozza, A.; Snaith Henry, J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical Management for Colorful, Efficient, and Stable Inorganic–Organic Hybrid Nanostructured Solar Cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef]
- Yu, W.; Li, F.; Yu, L.; Niazi, M.R.; Zou, Y.; Corzo, D.; Basu, A.; Ma, C.; Dey, S.; Tietze, M.L.; et al. Single Crystal Hybrid Perovskite Field-Effect Transistors. Nat. Commun. 2018, 9, 5354. [Google Scholar] [CrossRef]
- Cui, P.; Wei, D.; Ji, J.; Huang, H.; Jia, E.; Dou, S.; Wang, T.; Wang, W.; Li, M. Planar P–N Homojunction Perovskite Solar Cells with Efficiency Exceeding 21.3%. Nat. Energy 2019, 4, 150–159. [Google Scholar] [CrossRef]
- Tan, H.; Jain, A.; Voznyy, O.; Lan, X.; García de Arquer, F.P.; Fan James, Z.; Quintero-Bermudez, R.; Yuan, M.; Zhang, B.; Zhao, Y.; et al. Efficient and Stable Solution-Processed Planar Perovskite Solar Cells Via Contact Passivation. Science 2017, 355, 722–726. [Google Scholar] [CrossRef]
- Niu, T.; Lu, J.; Munir, R.; Li, J.; Barrit, D.; Zhang, X.; Hu, H.; Yang, Z.; Amassian, A.; Zhao, K.; et al. Stable High-Performance Perovskite Solar Cells Via Grain Boundary Passivation. Adv. Mater. 2018, 30, 1706576. [Google Scholar] [CrossRef]
- You, J.; Hong, Z.; Yang, Y.; Chen, Q.; Cai, M.; Song, T.-B.; Chen, C.-C.; Lu, S.; Liu, Y.; Zhou, H.; et al. Low-Temperature Solution-Processed Perovskite Solar Cells with High Efficiency and Flexibility. ACS Nano 2014, 8, 1674–1680. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Tseng, Z.-L.; Wu, C.-G. Planar Heterojunction Perovskite/Pc71bm Solar Cells with Enhanced Open-Circuit Voltage Via a (2/1)-Step Spin-Coating Process. J. Mater. Chem. A 2014, 2, 15897–15903. [Google Scholar] [CrossRef]
- Wang, K.-C.; Jeng, J.-Y.; Shen, P.-S.; Chang, Y.-C.; Diau, E.W.-G.; Tsai, C.-H.; Chao, T.-Y.; Hsu, H.-C.; Lin, P.-Y.; Chen, P.; et al. P-Type Mesoscopic Nickel Oxide/Organometallic Perovskite Heterojunction Solar Cells. Sci. Rep. 2014, 4, 4756. [Google Scholar] [CrossRef]
- Cui, J.; Meng, F.; Zhang, H.; Cao, K.; Yuan, H.; Cheng, Y.; Huang, F.; Wang, M. Ch3nh3pbi3-Based Planar Solar Cells with Magnetron-Sputtered Nickel Oxide. ACS Appl. Mater. Interfaces 2014, 6, 22862–22870. [Google Scholar] [CrossRef]
- Ren, L.; Li, H.; Xie, Z.; Ai, D.; Zhou, Y.; Liu, Y.; Zhang, S.; Yang, L.; Zhao, X.; Peng, Z.; et al. High-Temperature High-Energy-Density Dielectric Polymer Nanocomposites Utilizing Inorganic Core–Shell Nanostructured Nanofillers. Adv. Energy Mater. 2021, 11, 2101297. [Google Scholar] [CrossRef]
- Ji, S.; Chen, Y.; Wang, X.; Zhang, Z.; Wang, D.; Li, Y. Chemical Synthesis of Single Atomic Site Catalysts. Chem. Rev. 2020, 120, 11900–11955. [Google Scholar] [CrossRef]
- Eftekhari, A.; Jian, Z.; Ji, X. Potassium Secondary Batteries. ACS Appl. Mater. Interfaces 2017, 9, 4404–4419. [Google Scholar] [CrossRef]
- Bin, Z.; Li, J.; Wang, L.; Duan, L. Efficient N-Type Dopants with Extremely Low Doping Ratios for High Performance Inverted Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 3424–3428. [Google Scholar] [CrossRef]
- Chen, S.; Yang, S.; Sun, H.; Zhang, L.; Peng, J.; Liang, Z.; Wang, Z.-S. Enhanced Interfacial Electron Transfer of Inverted Perovskite Solar Cells by Introduction of Cose into the Electron-Transporting-Layer. J. Power Sources 2017, 353, 123–130. [Google Scholar] [CrossRef]
- Kakavelakis, G.; Maksudov, T.; Konios, D.; Paradisanos, I.; Kioseoglou, G.; Stratakis, E.; Kymakis, E. Efficient and Highly Air Stable Planar Inverted Perovskite Solar Cells with Reduced Graphene Oxide Doped PCBM Electron Transporting Layer. Adv. Energy Mater. 2017, 7, 1602120. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, C.; Li, J.; Han, W.; Zhao, M.; Yao, L.; Wang, Y.; Yan, C.; Jiu, T. Performance Enhancement of Inverted Perovskite Solar Cells Based on Smooth and Compact Pc61bm:Sno2 Electron Transport Layers. ACS Appl. Mater. Interfaces 2018, 10, 20128–20135. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, X.; Wang, K.; Wu, C.; Yang, R.; Hou, Y.; Jiang, Y.; Liu, S.; Priya, S. Stable Efficiency Exceeding 20.6% for Inverted Perovskite Solar Cells through Polymer-Optimized Pcbm Electron-Transport Layers. Nano Lett. 2019, 19, 3313–3320. [Google Scholar] [CrossRef] [PubMed]
- Tsikritzis, D.; Rogdakis, K.; Chatzimanolis, K.; Petrović, M.; Tzoganakis, N.; Najafi, L.; Martín-García, B.; Oropesa-Nuñez, R.; Bellani, S.; del Rio Castillo, A.E.; et al. A Two-Fold Engineering Approach Based on Bi2te3 Flakes Towards Efficient and Stable Inverted Perovskite Solar Cells. Mater. Adv. 2020, 1, 450–462. [Google Scholar] [CrossRef]
- Rueda-Delgado, D.; Hossain, I.M.; Jakoby, M.; Schwenzer, J.A.; Abzieher, T.; Howard, I.A.; Richards, B.S.; Lemmer, U.; Paetzold, U.W. Solution-Processed and Evaporated C60 Interlayers for Improved Charge Transport in Perovskite Photovoltaics. Org. Electron. 2020, 77, 105526. [Google Scholar] [CrossRef]
- Wu, J.-l.; Huang, W.-K.; Chang, Y.-C.; Tsai, B.-C.; Hsiao, Y.-C.; Chang, C.-Y.; Chen, C.-T.; Chen, C.-T. Correction: Simple Mono-Halogenated Perylene Diimides as Non-Fullerene Electron Transporting Materials in Inverted Perovskite Solar Cells with Zno Nanoparticle Cathode Buffer Layers. J. Mater. Chem. A 2017, 5, 13229. [Google Scholar] [CrossRef]
- Gu, P.-Y.; Wang, N.; Wang, C.; Zhou, Y.; Long, G.; Tian, M.; Chen, W.; Sun, X.W.; Kanatzidis, M.G.; Zhang, Q. Pushing up the Efficiency of Planar Perovskite Solar Cells to 18.2% with Organic Small Molecules as the Electron Transport Layer. J. Mater. Chem. A 2017, 5, 7339–7344. [Google Scholar] [CrossRef]
- Jiang, K.; Wu, F.; Yu, H.; Yao, Y.; Zhang, G.; Zhu, L.; Yan, H. A Perylene Diimide-Based Electron Transport Layer Enabling Efficient Inverted Perovskite Solar Cells. J. Mater. Chem. A 2018, 6, 16868–16873. [Google Scholar] [CrossRef]
- Wu, F.; Gao, W.; Yu, H.; Zhu, L.; Li, L.; Yang, C. Efficient Small-Molecule Non-Fullerene Electron Transporting Materials for High-Performance Inverted Perovskite Solar Cells. J. Mater. Chem. A 2018, 6, 4443–4448. [Google Scholar] [CrossRef]
- Said, A.A.; Xie, J.; Zhang, Q. Recent Progress in Organic Electron Transport Materials in Inverted Perovskite Solar Cells. Small 2019, 15, 1900854. [Google Scholar] [CrossRef]
- You, J.; Meng, L.; Song, T.-B.; Guo, T.-F.; Yang, Y.; Chang, W.-H.; Hong, Z.; Chen, H.; Zhou, H.; Chen, Q.; et al. Improved Air Stability of Perovskite Solar Cells Via Solution-Processed Metal Oxide Transport Layers. Nat. Nanotechnol. 2016, 11, 75–81. [Google Scholar] [CrossRef]
- Li, D.-B.; Hu, L.; Xie, Y.; Niu, G.; Liu, T.; Zhou, Y.; Gao, L.; Yang, B.; Tang, J. Low-Temperature-Processed Amorphous Bi2s3 Film as an Inorganic Electron Transport Layer for Perovskite Solar Cells. ACS Photonics 2016, 3, 2122–2128. [Google Scholar] [CrossRef]
- Tan, F.; Xu, W.; Hu, X.; Yu, P.; Zhang, W. Highly Efficient Inverted Perovskite Solar Cells with Cdse Qds/Lif Electron Transporting Layer. Nanoscale Res. Lett. 2017, 12, 614. [Google Scholar] [CrossRef]
- Hu, T.; Xiao, S.; Yang, H.; Chen, L.; Chen, Y. Cerium Oxide as an Efficient Electron Extraction Layer for P–I–N Structured Perovskite Solar Cells. Chem. Commun. 2018, 54, 471–474. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, W.; Wu, S.; Chen, R.; Huang, Y.; Yang, Z.; Li, J.; Han, L.; Chen, W. A General Strategy to Prepare High-Quality Inorganic Charge-Transporting Layers for Efficient and Stable All-Layer-Inorganic Perovskite Solar Cells. J. Mater. Chem. A 2019, 7, 18603–18611. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Chen, L.-C.; Chang, L.-B.; Wu, G.-M.; Feng, W.-S.; Jeng, M.-J.; Chen, D.W.; Lee, K.-L. Cu2o-Htm/Sio2-Etm Assisted for Synthesis Engineering Improving Efficiency and Stability with Heterojunction Planar Perovskite Thin-Film Solar Cells. Sol. Energy 2020, 204, 270–279. [Google Scholar] [CrossRef]
- Jiang, J.; Lang, X.; Zeng, Q.; Faheem, M.B.; Rong, S.; Zhao, H.; Li, Y. Polyacetylene Derivatives in Perovskite Solar Cells: From Defect Passivation to Moisture Endurance. J. Mater. Chem. A 2021, 9, 13220–13230. [Google Scholar] [CrossRef]
- Yang, B.; Ma, R.; Wang, Z.; Ouyang, D.; Huang, Z.; Lu, J.; Duan, X.; Yue, L.; Xu, N.; Choy, W.C.H. Efficient Gradient Potential Top Electron Transport Structures Achieved by Combining an Oxide Family for Inverted Perovskite Solar Cells with High Efficiency and Stability. ACS Appl. Mater. Interfaces 2021, 13, 27179–27187. [Google Scholar] [CrossRef]
- Chiang, Y.-H.; Shih, C.-K.; Sie, A.-S.; Li, M.-H.; Peng, C.-C.; Shen, P.-S.; Wang, Y.-P.; Guo, T.-F.; Chen, P. Highly Stable Perovskite Solar Cells with All-Inorganic Selective Contacts from Microwave-Synthesized Oxide Nanoparticles. J. Mater. Chem. A 2017, 5, 25485–25493. [Google Scholar] [CrossRef]
- Jia, J.; Wu, J.; Dong, J.; Fan, L.; Huang, M.; Lin, J.; Lan, Z. Cadmium Sulfide as an Efficient Electron Transport Material for Inverted Planar Perovskite Solar Cells. Chem. Commun. 2018, 54, 3170–3173. [Google Scholar] [CrossRef]
- Kohnehpoushi, S.; Nazari, P.; Nejand, B.A.; Eskandari, M. Mos2: A Two-Dimensional Hole-Transporting Material for High-Efficiency, Low-Cost Perovskite Solar Cells. Nanotechnology 2018, 29, 205201. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, W.; Wu, S.; Chen, R.; Liu, Z.; Huang, Y.; Yang, Z.; Zhu, H.; Li, J.; Han, L.; et al. Hybrid Inorganic Electron-Transporting Layer Coupled with a Halogen-Resistant Electrode in Cspbi2br-Based Perovskite Solar Cells to Achieve Robust Long-Term Stability. ACS Appl. Mater. Interfaces 2019, 11, 43303–43311. [Google Scholar] [CrossRef]
- Guo, T.; Yun, S.; Li, Y.; Huang, A.; Kang, L. Magnetron Sputtered All-Metal-Oxide Layers with Balanced Charge Carrier Transport Efficiency for Long-Term Stable Perovskite Solar Cells. Sol. Energy 2020, 208, 652–658. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Wu, G.; Chang, L.-B.; Jeng, M.-J.; Feng, W.-S.; Chen, D.W.; Chen, L.-C.; Lee, K.-L. Effects of Annealing on Characteristics of Cu2znsnse4/Ch3nh3pbi3/Zns/Izo Nanostructures for Enhanced Photovoltaic Solar Cells. Nanomaterials 2020, 10, 521. [Google Scholar] [CrossRef]
- Hultqvist, A.; Jacobsson, T.J.; Svanström, S.; Edoff, M.; Cappel, U.B.; Rensmo, H.; Johansson, E.M.J.; Boschloo, G.; Törndahl, T. Snox Atomic Layer Deposition on Bare Perovskite—An Investigation of Initial Growth Dynamics, Interface Chemistry, and Solar Cell Performance. ACS Appl. Energy Mater. 2021, 4, 510–522. [Google Scholar] [CrossRef]
- Hossain, I.M.; Hudry, D.; Mathies, F.; Abzieher, T.; Moghadamzadeh, S.; Rueda-Delgado, D.; Schackmar, F.; Bruns, M.; Andriessen, R.; Aernouts, T.; et al. Scalable Processing of Low-Temperature Tio2 Nanoparticles for High-Efficiency Perovskite Solar Cells. ACS Appl. Energy Mater. 2019, 2, 47–58. [Google Scholar] [CrossRef]
- Eliwi, A.A.; Malekshahi Byranvand, M.; Fassl, P.; Khan, M.R.; Hossain, I.M.; Frericks, M.; Ternes, S.; Abzieher, T.; Schwenzer, J.A.; Mayer, T.; et al. Optimization of Sno2 Electron Transport Layer for Efficient Planar Perovskite Solar Cells with Very Low Hysteresis. Mater. Adv. 2022, 3, 456–466. [Google Scholar] [CrossRef]
- Shivarudraiah, S.B.; Ng, M.; Li, C.H.A.; Halpert, J.E. All-Inorganic, Solution-Processed, Inverted Cspbi3 Quantum Dot Solar Cells with a Pce of 13.1% Achieved Via a Layer-by-Layer Fai Treatment. ACS Appl. Energy Mater. 2020, 3, 5620–5627. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Chen, J.; Fan, J.; Mai, Y.; Schropp, R.E.I. Ultra-Thin Moox as Cathode Buffer Layer for the Improvement of All-Inorganic Cspbibr2 Perovskite Solar Cells. Nano Energy 2017, 41, 75–83. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L.; Gao, L.; Cao, J.; Han, Q.; Yu, F.; Kamata, Y.; Zhang, C.; Fan, M.; Wei, G.; et al. Excellent Moisture Stability and Efficiency of Inverted All-Inorganic Cspbibr2 Perovskite Solar Cells through Molecule Interface Engineering. ACS Appl. Mater. Interfaces 2020, 12, 13931–13940. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Zhang, C.; Ma, Y.; Fan, J.; Mai, Y. All-Inorganic Cspbi2br Perovskite Solar Cells with High Efficiency Exceeding 13%. J. Am. Chem. Soc. 2018, 140, 3825–3828. [Google Scholar] [CrossRef]
- Pan, L.; Liu, C.; Zhu, H.; Wan, M.; Li, Y.; Mai, Y. Fine Modification of Reactively Sputtered Niox Hole Transport Layer for Application in All-Inorganic Cspbi2br Perovskite Solar Cells. Sol. Energy 2020, 196, 521–529. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Qian, Z.; Ren, G.; Wu, J.; Zhang, H. Optimal Intermediate Adducts Regulate Low-Temperature Cspbi2br Crystallization for Efficient Inverted All-Inorganic Perovskite Solar Cells. J. Mater. Chem. A 2020, 8, 25336–25344. [Google Scholar] [CrossRef]
- Han, D.; Yuan, Q.; Slanina, Z.; Tang, X.; Yi, S.; Zhou, D.-Y.; Uhlik, F.; Feng, L. Enhancing Built-in Electric Field and Defect Passivation through Gradient Doping in Inverted Cspbi2br Perovskite Solar Cells. Sol. RRL 2021, 5, 2000629. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, W.; Wu, Y.; Yuan, R.; Yang, J.; Shan, W.; Zhang, S.; Zhang, W.-H. Multilayer Cascade Charge Transport Layer for High-Performance Inverted Mesoscopic All-Inorganic and Hybrid Wide-Bandgap Perovskite Solar Cells. Sol. RRL 2020, 4, 2000344. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Zhou, Y.; Liu, H.; Xue, Q.; Li, X.; Chueh, C.-C.; Yip, H.-L.; Zhu, Z.; Jen, A.K.Y. Highly Efficient All-Inorganic Perovskite Solar Cells with Suppressed Non-Radiative Recombination by a Lewis Base. Nat. Commun. 2020, 11, 177. [Google Scholar] [CrossRef]
- Akhil, S.; Akash, S.; Pasha, A.; Kulkarni, B.; Jalalah, M.; Alsaiari, M.; Harraz, F.A.; Balakrishna, R.G. Review on Perovskite Silicon Tandem Solar Cells: Status and Prospects 2t, 3t and 4t for Real World Conditions. Mater. Des. 2021, 211, 110138. [Google Scholar] [CrossRef]
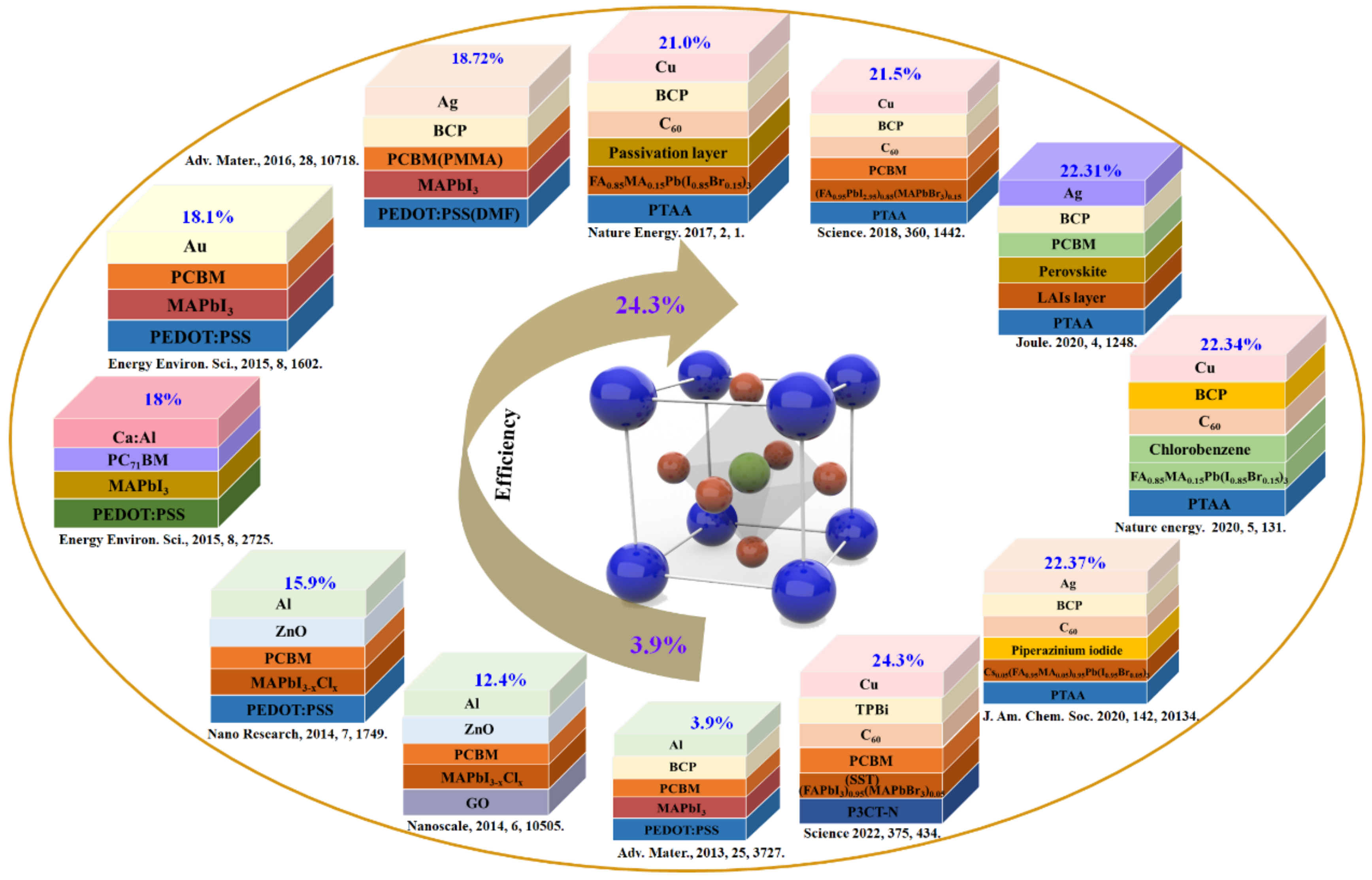
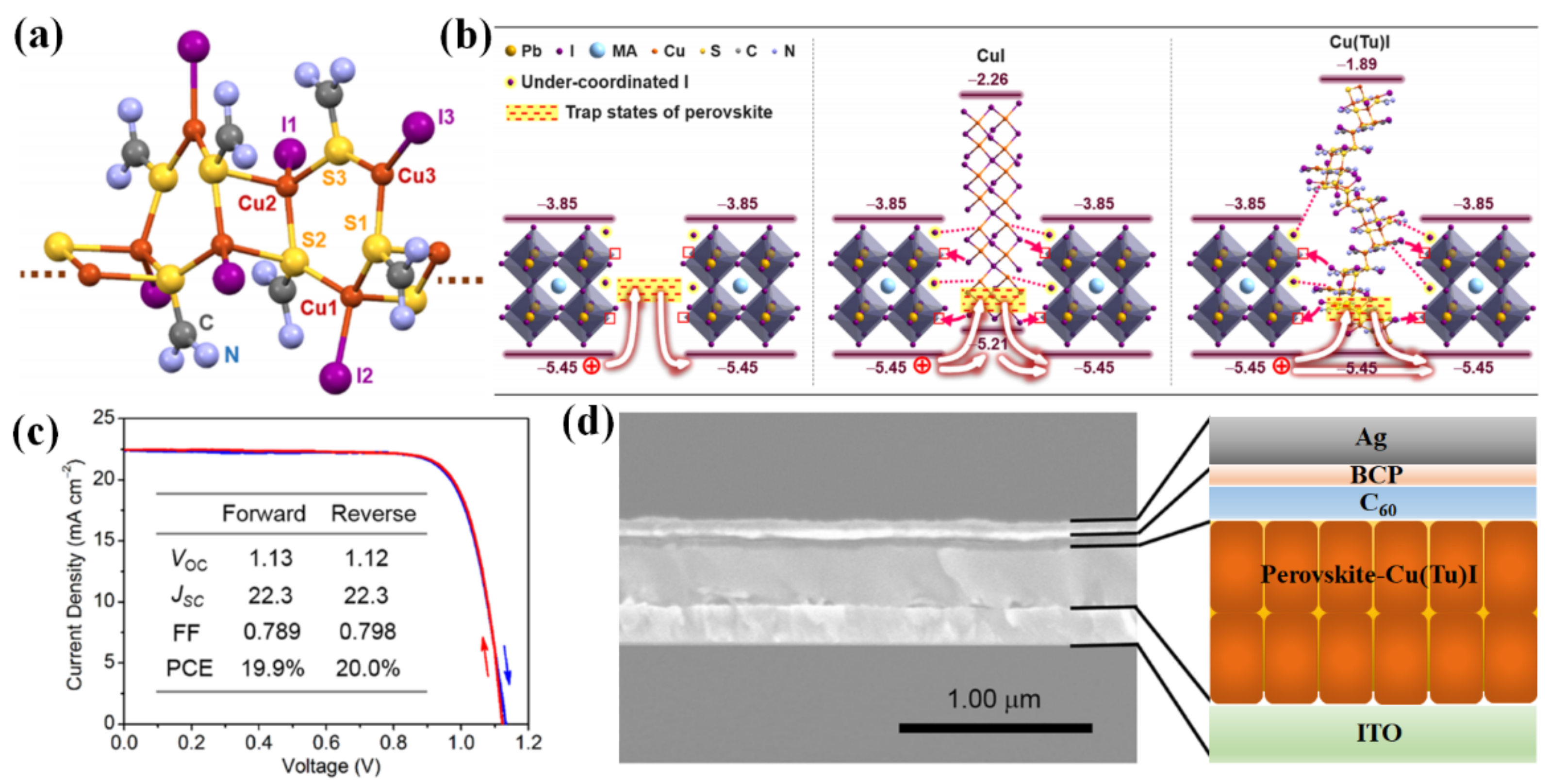

| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| PEDOT:PSS/MAPbI3/PCBM/BCP/Al | 0.06 cm2 | 0.60 | 10.32 | 0.63 | 3.9 | [30] |
| PEDOT:PSS/MAPbI3/PCBM/LiF/Al | 25 cm2 | 0.93 | 18.0 | 0.77 | 12.8 | [46] |
| PEDOT:PSS/MAPbI3/PCBM/Bis−C60/Ag | 3.14 mm2 | 1.06 | 19.1 | 0.77 | 15.7 | [47] |
| PEDOT:PSS/MAPbI3/PCBM/TiO2/Al | 11.8 mm2 | 0.994 | 19.8 | 0.72 | 14.25 | [48] |
| PEDOT:PSS/MAPbI3−xClx/PCBM/ RhB101/LiF/Ag | 0.11 cm2 | 1.11 | 20.11 | 0.81 | 18.0 | [49] |
| PEDOT:PSS/MAPbI3/PCBM/Au | 0.16 cm2 | 1.1 | 20.9 | 0.79 | 18.1 | [37] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| PEDOT:PSS−PEI/MAPbI3/PCBM/Al | 0.06 cm2 | 0.982 | 16.7 | 0.705 | 11.7 | [50] |
| PEDOT:PSS−GO/MAPbI3/PCBM/Al | ~ | 0.96 | 17.96 | 0.76 | 13.1 | [51] |
| PEDOT:PSS−GO/MAPbI3/PCBM/BCP/Ag | 0.09 cm2 | 0.90 | 20.01 | 0.79 | 14.2 | [52] |
| PEDOT:PSS−PFI/FA0.6MA0.4Sn0.6Pb0.4I3/PCBM/BCP/Ag | ~ | 0.783 | 27.22 | 0.744 | 15.85 | [53] |
| PEDOT:PSS−RbCl/MA0.7FA0.3Sn0.6Pb(I0.9Br0.1)3/PCBM/C60/BCP/Ag | ~ | 1.0 | 22.41 | 0.824 | 18.3 | [54] |
| PEDOT:PSS−CsI/MAPbI3/PCBM/Ag | ~ | 1.084 | 22.58 | 0.83 | 20.22 | [55] |
| PEDOT:PSS−Sodium Citrate/MAPbI3(Cl)/PCBM/BCP/Ag | 0.09 cm2 | 1.134 | 21.62 | 0.75 | 18.39 | [56] |
| PEDOT:PSS−CuSCN/MAPbI3/ PCBM/C60/LiF/Al | ~ | 1.02 | 19.10 | 0.785 | 15.3 | [57] |
| PEDOT:PSS−GeO2/MAPbI3−xClx/ PCBM/Ag | 7.25 mm2 | 0.96 | 21.55 | 0.74 | 15.15 | [58] |
| PEDOT:PSS−Ag/MAPbI3−xClx/PCBM/ Bphen/Ag | 7.25 mm2 | 0.93 | 21.51 | 0.79 | 15.75 | [59] |
| PEDOT:PSS−PEO/MAPbI3/PCBM/Al | 0.16 cm2 | 0.88 | 23.42 | 0.801 | 16.52 | [60] |
| PEDOT:PSS−F4−TCNQ/MAPbI3−xClx /PCBM/BCP/Ag | 7.25 mm2 | 1.02 | 21.93 | 0.77 | 17.22 | [61] |
| PEDOT:PSS−NaCl/MAPbI3−xClx/PCBM/RhB101/LiF/Ag | 0.11 cm2 | 1.08 | 20.5 | 0.819 | 18.1 | [62] |
| GHJ/MAPbI3/PCBM/C60/LiF/Al | ~ | 1.02 | 22.98 | 0.77 | 18.0 | [64] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| DMF−PEDOT:PSS/MAPbI3−xClx/PCBM/RhB101/LiF/Ag | 0.11 cm2 | 1.08 | 17.44 | 0.68 | 12.9 | [65] |
| DMF−PEDOT:PSS /MAPbI3/PCBM(PMMA)/BCP/Ag | 0.09 cm2 | 1.02 | 22.38 | 0.82 | 18.72 | [32] |
| DMF−PEDOT:PSS/MAPbI3/PCBM/BCP/Ag | 9 mm2 | 1.048 | 21.1 | 0.76 | 16.8 | [66] |
| DMSO−PEDOT:PSS/MAPbI3/PCBM/ BCP/Ag | 1.8 mm2 | 0.92 | 22.76 | 0.80 | 16.7 | [67] |
| EMIC−PEDOT:PSS/MAPbI3/Passivation layer/C60/BCP/Ag | 1 cm2 | 1.08 | 23.81 | 0.78 | 20.06 | [68] |
| DA−PEDOT:PSS/MAPbI3−xClx/PCBM/BCP/Ag | ~ | 1.08 | 19.4 | 0.78 | 16.4 | [69] |
| DA−PEDOT:PSS/MAxFA3−xPbI3−xBrx/PCBM/PN4N/Ag | ~ | 1.08 | 22.0 | 0.775 | 18.5 | [70] |
| Oxidized PEDOT:PSS/MAPbI3−xClx/PCBM/ RhB 101/LiF/Ag | 11 mm2 | 1.07 | 21.6 | 0.82 | 18.8 | [71] |
| PEDOT−MeOH:PSS/MAPbI3/PCBM/ BCP/Ag | 3.14 mm2 | 1.01 | 15.83 | 0.60 | 9.56 | [72] |
| Urea−PEDOT:PSS/MAPbI3/PCBM/ RhB 101/Ag | 0.16 cm2 | 1.03 | 22.57 | 0.809 | 18.8 | [73] |
| SBS−PEDOT:PSS/MA0.8FA0.2PbI3−xClx/PCBM/Ag | ~ | 1.08 | 21.57 | 0.833 | 19.41 | [74] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| PEDOT:PSS/Cross−linked interlayer/MAPbI3−xClx/PCBM/Al | 7.5 mm2 | 0.99 | 18.07 | 0.73 | 13.06 | [75] |
| PEG−PEDOT:PSS/FASnI3/PCBM/BCP/Ag | ~ | 0.37 | 22.06 | 0.627 | 5.12 | [76] |
| PEDOT:PSS/PEG/MAPbI3/PCBM/Ag | 0.06 cm2 | 0.79 | 23.02 | 0.61 | 12.56 | [77] |
| PEDOT:PSS/NPB/MAPbI3/PCBM/ BCP/Ag | 0.1225 cm2 | 1.05 | 22.46 | 0.78 | 18.4 | [78] |
| PEDOT:PSS/C3−SAM/MAPbI3−xClx/ PCBM/ZnO NPs/Ag | 0.055 cm2 | 0.89 | 18.9 | 0.69 | 11.6 | [79] |
| PEDOT:PSS/GO/MAPbI3/PCBM/Ag | 1 cm2 | 0.985 | 21.9 | 0.71 | 15.34 | [80] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| PEDOT:PSS/V2O5/MAPbI3/C60/ BCP/Ag | 0.10 cm2 | 1.05 | 0.212 | 0.785 | 17.5 | [81] |
| NiPcS4−PEDOT:PSS/MAPbI3/C60/PCBM/BCP/Ag | 0.08 cm2 | 1.08 | 23.01 | 0.77 | 18.9 | [82] |
| CNTs−PEDOT:PSS/MAPbI3/PCBM/Ag | ~ | 1.04 | 20.35 | 0.754 | 16.0 | [83] |
| OCNRs−PEDOT:PSS/MAPbI3/ PCBM/BCP/Ag | ~ | 1.01 | 22.76 | 0.804 | 19.02 | [84] |
| MoOx−PEDOT:PSS/MAPbI3/PCBM/ ZnO/Al | 0.06 cm2 | 1.08 | 22.78 | 0.804 | 19.64 | [85] |
| CuI/PEDOT:PSS/(FASnI3)0.6(MAPbI3)0.4/C60/BCP/Cu | 0.1 cm2 | 0.75 | 28.5 | 0.737 | 15.75 | [86] |
| PEDOT:PSS/Graphene quantum dots/MAPbI3/PCBM/Ag | 0.1 cm2 | 1.00 | 21.41 | 0.753 | 16.16 | [87] |
| PEDOT:PSS/PTAA/MAPbI3−xClx/ PCBM/Ag | 0.11 cm2 | 1.07 | 21.38 | 0.826 | 19.04 | [88] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| Pure PTAA | PTAA/MAPbI3/PCBM/C60/ BCP/Al | 7.25 mm2 | 1.07 | 22.0 | 0.768 | 18.1 | [89] |
| PTAA/MAPbI3/PCBM/PFN/Al | 4 mm2 | 1.01 | 20.24 | 0.767 | 15.67 | [90] | |
| PTAA/(FA0.95PbI2.95)0.85(MAPbBr3)0.5/PCBM/C60/BCP/Cu | ~ | 1.21 | 22.5 | 0.790 | 21.5 | [34] | |
| Doping PTAA | PTAA−F4−TCNQ/MAPbI3/PCBM/C60/BCP/Al | ~ | 1.09 | 21.6 | 0.740 | 17.5 | [91] |
| PTAA−NPB/MAPbI3/PCBM/Ag | 6.25 mm2 | 1.14 | 22.6 | 0.784 | 20.15 | [92] | |
| PTAA−CuSCN/MAPbI3/PCBM/ BCP/Ag | 0.1 cm2 | 1.12 | 21.92 | 0.750 | 18.16 | [93] | |
| Modified PTAA | rGO/PTAA/MAPbI3/PCBM/ BCP/Ag | 1.02 cm2 | 1.09 | 20.3 | 0.777 | 17.20 | [94] |
| Spiro−OMeTAD−PTAA/CsPbI2Br/ZnO:C60/Ag | 4 mm2 | 1.14 | 14.3 | 0.764 | 12.52 | [95] | |
| PTAA/F8BT/MAPbI3/PCBM/Bphen/Ag | 0.045 cm2 | 0.98 | 21.27 | 0.717 | 14.88 | [96] | |
| PTAA/F4−TCNQ/MAPbI3/ PCBM/BCP/Ag | 0.09 cm2 | 1.10 | 22.6 | 0.811 | 19.7 | [97] | |
| PTAA/CPEs/(FAPbI3)0.83(MAPbBr3)0.17/LiF/C60/Ag | 1 cm2 | 1.11 | 22.18 | 0.747 | 18.38 | [98] | |
| Interface engineering | PTAA/MAPbI3/C60/BCP/Cu | 6 mm2 | 1.05 | 22.7 | 0.80 | 19.0 | [99] |
| PTAA/(FAPbI3)0.9(MAPbBr3)0.1/ PCBM/BCP/Ag | 0.05 cm2 | 1.09 | 23.87 | 0.801 | 19.51 | [100] | |
| PTAA/Cs0.05(MA0.17FA0.83)0.95 Pb(I0.83Br0.17)3/PCBM/BCP/Cu | 0.09 cm2 | 1.08 | 22.74 | 0.78 | 19.17 | [101] | |
| PTAA/MAPbI3/PCBM/BCP/Ag | 6 mm2 | 1.14 | 23.26 | 0.815 | 21.6 | [102] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| CPE−K/MAPbI3−xClx/PCBM/Al | 3.30 mm2 | 0.89 | 20.1 | 0.77 | 12.51 | [105] |
| TB(MA)/MAPbI3/PCBM/C60/BCP/Ag | 0.10 cm2 | 1.08 | 23.45 | 0.78 | 19.76 | [106] |
| BF−NH3/MAPbI3/PC61BM/PEI/Ag | ~ | 1.05 | 20.1 | 0.84 | 17.71 | [107] |
| TB(K)/FA0.85MA0.15Pb(Br0.15I0.85)3/ PCBM/C60/BCP/Ag | 0.06 cm2 | 1.1 | 22.72 | 0.80 | 20.1 | [108] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| P3CT−Na/MAPbCl3−xIx/PC61BM/C60 | ~ | 1.07 | 21.14 | 73.0 | 16.6 | [111] |
| P3CT−Na(GD)/MAPbI3/PCBM/ZnO/Al | ~ | 1.06 | 22.8 | 80.8 | 19.5 | [112] |
| P3CT−Rb/MAPbCl3−xIx/C60/BCP/Ag | ~ | 1.14 | 21.67 | 82.8 | 20.52 | [113] |
| P3CT−ED/MAPbI3/PC61BM/ZnO/Al | 0.06 cm2 | 1.08 | 23.3 | 80.9 | 20.5 | [114] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| Poly−TPD/MAPbI3/PCBM/C60/BCP/Ag | ~ | 1.1 | 22.0 | 0.697 | 15.3 | [115] |
| Poly−TPD/MAPbI3/C60/BCP/Ag | 0.1 cm2 | 1.04 | 23.2 | 0.754 | 18.19 | [116] |
| Poly−TPD/MAPbI3/PCBM/BCP/Ag | 9 mm2 | 1.07 | 20.08 | 0.75 | 16.11 | [117] |
| Poly−TPD/MAPbI3/PCBM/LiF/Al | 0.045 cm2 | 1.07 | 21.8 | 0.737 | 17.2 | [118] |
| Poly−TPD/PFN−I/Cs0.05FA0.79MA0.16PbI2.4Br0.6/PFN−I/PC61BM/BCP/Ag | ~ | 1.13 | 22.47 | 0.81 | 20.47 | [119] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| ITO/polythiophene/MAPbI3/C60/BCP/Ag | ~ | 0.99 | 20.3 | 0.774 | 15.4 | [120] |
| ITO/PPP/MAPbI3/C60/BCP/Ag | ~ | 1.03 | 21.6 | 0.75 | 16.5 | [121] |
| PVK/MAPbI3/PCBM/Ag | 4 mm2 | 0.96 | 21.9 | 0.75 | 15.8 | [122] |
| XSln847/MAPbI3/PCBM/Ag | ~ | 1.08 | 22.34 | 0.71 | 17.16 | [123] |
| poly−1/MAPbI3/PC61BM/BCP/Ag | 0.1 cm2 | 1.01 | 23.2 | 0.71 | 16.5 | [124] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| TPASB/MAPbI3/PCBM/Al | 4 mm2 | 1.05 | 20.8 | 0.80 | 17.6 | [126] |
| TAPC/MAPbI3/PCBM/Ag | 4 mm2 | 1.04 | 22.32 | 0.81 | 18.8 | [127] |
| VB−MeO−FDPA/MAPbI3−xClx/ PCBM/Ag | 0.045 cm2 | 1.15 | 20.89 | 0.78 | 18.7 | [128] |
| NPB/MAPbI3/PCBM/PDI−Br/Ag | ~ | 1.11 | 22.92 | 0.78 | 19.96 | [129] |
| DFH/MA0.9FA0.1PbI3−xClx/C60/ BCP/Ag | ~ | 1.10 | 22.6 | 0.83 | 20.6 | [130] |
| MPA−BTTI/CsFAMA/C60/BCP/Ag | ~ | 1.12 | 23.23 | 0.814 | 21.17 | [131] |
| TPE−S/CsPbI2Br/PCBM/ZnO/Ag | ~ | 1.26 | 15.6 | 0.785 | 15.4 | [132] |
| C8−DPNDF/MAPbI3/C60/BCP/Ag | 9 mm2 | 1.06 | 21.05 | 0.784 | 17.5 | [133] |
| TFM/CsFAMA/C60/BCP/Ag | 7.5 mm2 | 22.7 | 0.97 | 0.73 | 16.03 | [134] |
| H−Pyr/MAPbI3/PCBM/Ag | 0.1225 cm2 | 1.04 | 22.26 | 0.741 | 17.09 | [135] |
| XY1/CsFAMA/C60/BCP/Cu | 1 cm2 | 1.11 | 22.2 | 0.762 | 18.78 | [136] |
| m−MTDATA/Cs0.05(FA0.85MA0.15)0.95 Pb(I0.85Br0.15)3/C60/BCP/Cu | 0.16 cm2 | 1.04 | 22.5 | 0.78 | 18.12 | [137] |
| TPAC3M/MAPbI3/PC61BM/ZnO/Al | 4 mm2 | 1.00 | 22.11 | 0.78 | 17.54 | [138] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| Pure NiOx | NiOx/MAPbI3/PCBM/Au | ~ | 0.882 | 16.27 | 0.635 | 9.11 | [140] |
| NiOx/MAPbI3/PCBM/LiF/Al | ~ | 1.06 | 20.2 | 0.813 | 17.3 | [141] | |
| NiOx/MAPbI3/PCBM/Ag | ~ | 1.09 | 17.93 | 0.738 | 14.42 | [142] | |
| NiOx/MAPbI3−xClx/PC61BM/ AZO/Ag | 0.19 cm2 | 1.08 | 20.33 | 0.69 | 15.2 | [143] | |
| NiOx/MAI/PCBM/BCP/Ag | ~ | 0.99 | 22.92 | 0.803 | 18.15 | [144] | |
| np−NiOx/(FAPbI3)0.85−(MAPbBr3)0.15/PCBM/ZnO/Ag | 0.09 cm2 | 1.076 | 22.76 | 0.78 | 19.1 | [145] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| Doping NiOx | Cu:NiOx/MAPb(I0.8Br0.2)3/ PC61BM/C60/Ag | 0.314 cm2 | 1.12 | 18.83 | 0.73 | 15.4 | [146] |
| Li0.05Mg0.15Ni0.8O/MAPbI3/ PCBM/Ti(Nb)Ox/Ag | 1.02 cm2 | 1.08 | 20.4 | 0.827 | 18.3 | [147] | |
| Cs:NiOx/MAPbI3/PC61BM/ ZrAcac/Ag | 10 mm2 | 1.12 | 21.77 | 0.793 | 19.35 | [148] | |
| Ag:NiOx/MAPbI3/PCBM/ BCP/Ag | 4 mm2 | 1.07 | 19.42 | 0.79 | 16.4 | [149] | |
| Y:NiOx/MAPbI3/PC61BM/Au | 0.08 cm2 | 1.0 | 23.82 | 0.68 | 16.31 | [150] | |
| F6TCNNQ:NiOx/CsFAMA/ PCBM/ZrAcac/Ag | ~ | 1.12 | 23.18 | 0.803 | 20.86 | [151] | |
| K:NiOx/Cs0.05FA0.81MA0.14Pb (Br0.15I0.85)3/PC61BM/TIPD/Ag | 4 mm2 | 1.13 | 20.53 | 0.74 | 17.05 | [152] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| Interface modification | NiO/meso−Al2O3/MAPbI3/PCBM/BCP/Ag | 0.09 cm2 | 1.04 | 18.0 | 0.72 | 13.5 | [153] |
| NiOx/PTAA/FA1−xMAxPb (I3−yBry)/PCBM/BCP/Au | ~ | 1.02 | 20.8 | 0.783 | 16.7 | [154] | |
| NiOx@KCl/CsFAMA/ PCBM/ZrAcac/Ag | 0.1 cm2 | 1.15 | 22.21 | 0.795 | 20.96 | [155] | |
| NiOx/PTAA/(MAPbI3)0.95(MAPbBr2Cl)0.05/PCBM/BCP/Ag | ~ | 1.19 | 22.23 | 0.817 | 21.56 | [156] | |
| NiOx/F2HCNQ/CsFAMA/PCBM/BCP/Ag | 36.1 cm2 | 1.14 | 23.44 | 0.828 | 22.13 | [157] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| CuSCN | CuSCN/MAPbI3−xClx/PCBM/Ag | 0.07 cm2 | 0.68 | 8.8 | 0.635 | 3.8 | [158] |
| CuSCN/MAPbI3/C60/BCP/ Ag | 0.10 cm2 | 1.0 | 21.9 | 0.758 | 16.6 | [159] | |
| CuI | CuI/MAPbI3/PCBM/Al | 0.06 cm2 | 1.04 | 21.6 | 0.62 | 13.58 | [160] |
| CuI/MAPbI3/C60/BCP/Ag | >1 cm2 | 0.97 | 22.7 | 0.738 | 16.8 | [161] | |
| CuI/MAPbI3/PCBM/PEI/Ag | 0.05 cm2 | 1.04 | 20.9 | 0.68 | 14.7 | [162] | |
| PTAA−CuSCN/MAPbI3/PCBM/BCP/Ag | 0.10 cm2 | 1.13 | 22.3 | 0.789 | 19.9 | [163] | |
| CuxO | CuOx/MAPbI3/PCBM/Ag | ~ | 0.952 | 17.5 | 0.662 | 11.0 | [164] |
| CuOx/MAPbI3/PCBM/ZnO/Al | 5.5 mm2 | 1.03 | 22.42 | 0.76 | 17.43 | [165] | |
| CuS | CuS/MAPbI3/C60/BCP/Ag | 0.10 cm2 | 1.02 | 22.3 | 0.712 | 16.2 | [166] |
| Cu3PS4 | Cu3PS4/MA0.7FA0.3PbI3/ PCBM/BCP/Ag | 0.08 cm2 | 1.069 | 20.83 | 0.816 | 18.17 | [167] |
| Modification | CuI/CuSCN/MAPbI3−xClx/PC61BM/PEI/Ag | 0.09 cm2 | 1.11 | 22.33 | 0.76 | 18.76 | [168] |
| PTAA/CuI/FA0.05MA0.95PbI3/PCBM/C60−N/Ag | 5.2 mm2 | 1.057 | 24.8 | 0.774 | 20.34 | [169] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| GO | GO/MAPbI3−xClx/PCBM/ZnO/Al | 7.25 mm2 | 1.0 | 17.46 | 0.71 | 12.4 | [170] |
| rGO/MAPbI3/PC61BM/BCP/Ag | 0.09 cm2 | 0.98 | 15.4 | 0.716 | 10.8 | [171] | |
| oxo−G1/MAPbI3/PCBM/ZnO/Al | ~ | 1.08 | 18.06 | 0.777 | 15.2 | [172] | |
| a−GO/MAPbI3−xClx/PCBM/BCP/Ag | 4 mm2 | 1.0 | 18.4 | 0.768 | 14.14 | [173] | |
| V2O5 | V2O5/PEDOT/MAPbI3−xClx/PCBM/ RhB101/LiF/Ag | 0.11 cm2 | 1.05 | 13.59 | 0.59 | 8.4 | [175] |
| V2O5/P3CT−K/MAPbI3/PC61BM/ ZnO/Ag | 0.06 cm2 | 1.09 | 23.24 | 0.779 | 19.7 | [176] | |
| MoOx | MoOx/MAPbI3/PCBM/Ag | ~ | 0.99 | 18.8 | 0.71 | 13.1 | [177] |
| CoOx | CoOx/MAPbI3/PCBM/Ag | 0.02 cm2 | 0.95 | 20.28 | 0.755 | 14.5 | [178] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| Pure PCBM | PEDOT:PSS/MAPbI3−xClx/PC61BM/Al | 0.1 cm2 | 0.87 | 18.5 | 0.72 | 11.5 | [193] |
| PEDOT:PSS/MAPbI3/ PCBM/Al | 0.1 cm2 | 1.05 | 19.98 | 0.78 | 16.3 | [194] | |
| Interface modification | NiOx/NiOnc/MAPbI3/ PCBM/BCP/Al | 0.06 cm2 | 1.04 | 13.2 | 0.69 | 9.51 | [195] |
| P3CT−Na/MAPbI3/PCBM/C60/Al | ~ | 1.07 | 21.1 | 0.73 | 16.6 | [196] | |
| PTAA/MAPbI3/PCBM/C60/BCP/Al | 7.25 mm2 | 1.07 | 22.0 | 0.768 | 18.1 | [89] | |
| Poly−TPD/MAPbI3/PCBM/LiF/Al | 0.045 cm2 | 1.07 | 21.8 | 0.737 | 17.2 | [118] | |
| Li0.05Mg0.15Ni0.8O/MAPbI3/PCBM/Ti(Nb)Ox/Ag | 1.02 cm2 | 1.083 | 20.4 | 0.827 | 18.3 | [147] | |
| PEDOT:PSS/MAPbI3/ PCBM/π−PFEs/Ag | ~ | 1.04 | 22.11 | 0.799 | 18.46 | [197] | |
| Doping modification | PEDOT:PSS/MAPbI3/ PCBM:GD/C60/Al | 0.06 cm2 | 0.969 | 23.4 | 0.654 | 14.8 | [198] |
| PEDOT:PSS/MAPbI3−xClx/Oleamide:PCBM/Ag | 14.0 mm2 | 0.98 | 18.76 | 0.693 | 12.69 | [199] | |
| NiMgLiO/MAPbI3/H3:PCBM/BCP/Ag | ~ | 1.06 | 21.5 | 0.793 | 18.1 | [200] | |
| NiO/MAPbI3/PCBM:CoSe/Ag | 0.07 cm2 | 1.073 | 19.85 | 0.70 | 14.91 | [201] | |
| PEDOT:PSS/MAPbI3/rGO:PCBM/Ag | 4 mm2 | 0.942 | 23.52 | 0.655 | 14.51 | [202] | |
| P3CT−K/MAPbI3/PC61BM:SnO2/Ag | 0.06 cm2 | 1.12 | 23.15 | 0.76 | 19.7 | [203] | |
| PEDOT:PSS/MAPbI3/ PCBM:F8TBT/Ag | 6.25 cm2 | 1.12 | 22.43 | 0.82 | 20.6 | [204] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| PEDOT:PSS/MAPbI3/Br−PDI/ZnO/Ag | 0.12 cm2 | 0.83 | 18.9 | 0.669 | 10.5 | [207] |
| PEDOT:PSS/MAPbI3/TDTP/LiF/Ag | ~ | 1.05 | 22.4 | 0.777 | 18.2 | [208] |
| P3CT−Na/MAPbI3/TPE−PDI4/ Rhodamine 101/LiF/Ag | ~ | 1.052 | 21.98 | 0.81 | 18.78 | [209] |
| P3CT−Na/MAPbI3/ITCPTC−Th/ Rhodamine 101/LiF/Ag | ~ | 1.029 | 21.77 | 0.76 | 17.11 | [210] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| NiOx/MAPbI3/ZnO/Al | 0.1 cm2 | 1.01 | 21.0 | 0.760 | 16.1 | [212] |
| NiO/MAPbI3/Bi2S3/Au | ~ | 0.949 | 18.6 | 0.742 | 13.1 | [213] |
| PEDOT:PSS/MAPbI3/ CdSe QDs/LiF/Ag | 0.04 cm2 | 0.985 | 21.8 | 0.703 | 15.1 | [214] |
| NiOx/MAPbI3/CeOx/Ag | ~ | 1.047 | 20.43 | 0.797 | 17.1 | [215] |
| NiMgLiO/CsPbI2Br/C−TiO2/ Bi/Ag | 0.09 cm2 | 1.26 | 14.72 | 0.76 | 14.0 | [216] |
| Cu2O/MAPbI3/SiO2/GZO/Ag | 0.1 cm2 | 1.12 | 20.9 | 0.786 | 18.4 | [217] |
| NiOx/P1:FA0.85MA0.15PbI2.55Br0.45/ Nb2O5/Ag | 0.16 cm2 | 1.098 | 23.59 | 0.788 | 20.41 | [218] |
| NiOx/MAPbI3/Sn:In2O3/In2O3/Ag | ~ | 1.10 | 23.22 | 0.809 | 20.65 | [219] |
| c−NiOx/mp−NiOx/ Cs0.05(FA0.83MA0.17Pb(I0.83Br0.17))0.95/ IZO/Al | 0.2 cm2 | 1.02 | 22.86 | 0.694 | 16.2 | [220] |
| Cu:NiOx/MAPbI3/CdS/Au | ~ | 1.012 | 19.74 | 0.668 | 13.36 | [221] |
| MoS2/MAPbI3/TiO2/Ag | ~ | 0.93 | 26.24 | 0.83 | 20.43 | [222] |
| NiMgLiO/CsPbI2Br/C−TiO2/Sb | 0.09 cm2 | 1.28 | 15.0 | 0.77 | 14.8 | [223] |
| Cu:NiOx/MAPbI3/ZnO/Ag | 0.2 cm2 | 1.03 | 23.05 | 0.64 | 16.51 | [224] |
| Mo/Cu2ZnSnSe4/MAPbI3/ZnS/ IZO/Ag | 0.1 cm2 | 1.1 | 20.8 | 0.763 | 17.4 | [225] |
| NiOx/Cs0.05FA0.79MA0.16PbBr0.51I2.49/ SnOx/Al | 0.126 cm2 | 1.12 | 18.1 | 0.661 | 13.5 | [226] |
| Structure of PSCs | Area | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| NiO/CsPbI3/C60/ZnO/Ag | ~ | 1.19 | 14.25 | 0.776 | 13.1 | [229] |
| NiOx/CsPbIBr2/MoOx/Au | 9 mm2 | 0.85 | 10.56 | 0.62 | 5.52 | [230] |
| Cs−NiOx/N749/CsPbIBr2/ PCBM/BCP/Ag | ~ | 1.19 | 11.49 | 0.69 | 9.49 | [231] |
| NiOx/CsPbI2Br/ZnO@C60/Ag | ~ | 1.14 | 15.2 | 0.77 | 13.1 | [232] |
| NiMgLiO/CsPbI2Br/ C−TiO2/Sb | 0.09 cm2 | 1.28 | 15.0 | 0.77 | 14.8 | [223] |
| NiOx/CsPbI2Br/ZnO@C60/Ag | 0.09 cm2 | 1.1 | 15.1 | 0.756 | 12.6 | [233] |
| NiOx/CsPbI2Br/PCBM/Ag | ~ | 1.12 | 15.6 | 0.761 | 13.3 | [234] |
| NiOx/CsPbI2Br/ZnO/C60/Ag | 0.09 cm2 | 1.15 | 16.12 | 0.776 | 14.38 | [235] |
| NiOx/Zn:CuGaO2/F−CsPbI2Br/TiO2/PC61BM/ZnO | 0.09 cm2 | 1.17 | 15.98 | 0.807 | 15.1 | [236] |
| NiOx/CsPbI2Br/6TIC−4F/ ZnO/C60/Ag | 0.0672 cm2 | 1.16 | 17.7 | 0.786 | 16.1 | [237] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Luo, X.; Zhou, Y.; Li, Y.; Qiu, Q.; Xie, T. Recent Advances in Inverted Perovskite Solar Cells: Designing and Fabrication. Int. J. Mol. Sci. 2022, 23, 11792. https://doi.org/10.3390/ijms231911792
Yang J, Luo X, Zhou Y, Li Y, Qiu Q, Xie T. Recent Advances in Inverted Perovskite Solar Cells: Designing and Fabrication. International Journal of Molecular Sciences. 2022; 23(19):11792. https://doi.org/10.3390/ijms231911792
Chicago/Turabian StyleYang, Jiayan, Xingrui Luo, Yankai Zhou, Yingying Li, Qingqing Qiu, and Tengfeng Xie. 2022. "Recent Advances in Inverted Perovskite Solar Cells: Designing and Fabrication" International Journal of Molecular Sciences 23, no. 19: 11792. https://doi.org/10.3390/ijms231911792
APA StyleYang, J., Luo, X., Zhou, Y., Li, Y., Qiu, Q., & Xie, T. (2022). Recent Advances in Inverted Perovskite Solar Cells: Designing and Fabrication. International Journal of Molecular Sciences, 23(19), 11792. https://doi.org/10.3390/ijms231911792





