Novel In Vivo CometChip Reveals NDMA-Induced DNA Damage and Repair in Multiple Mouse Tissues
Abstract
1. Introduction
2. Results
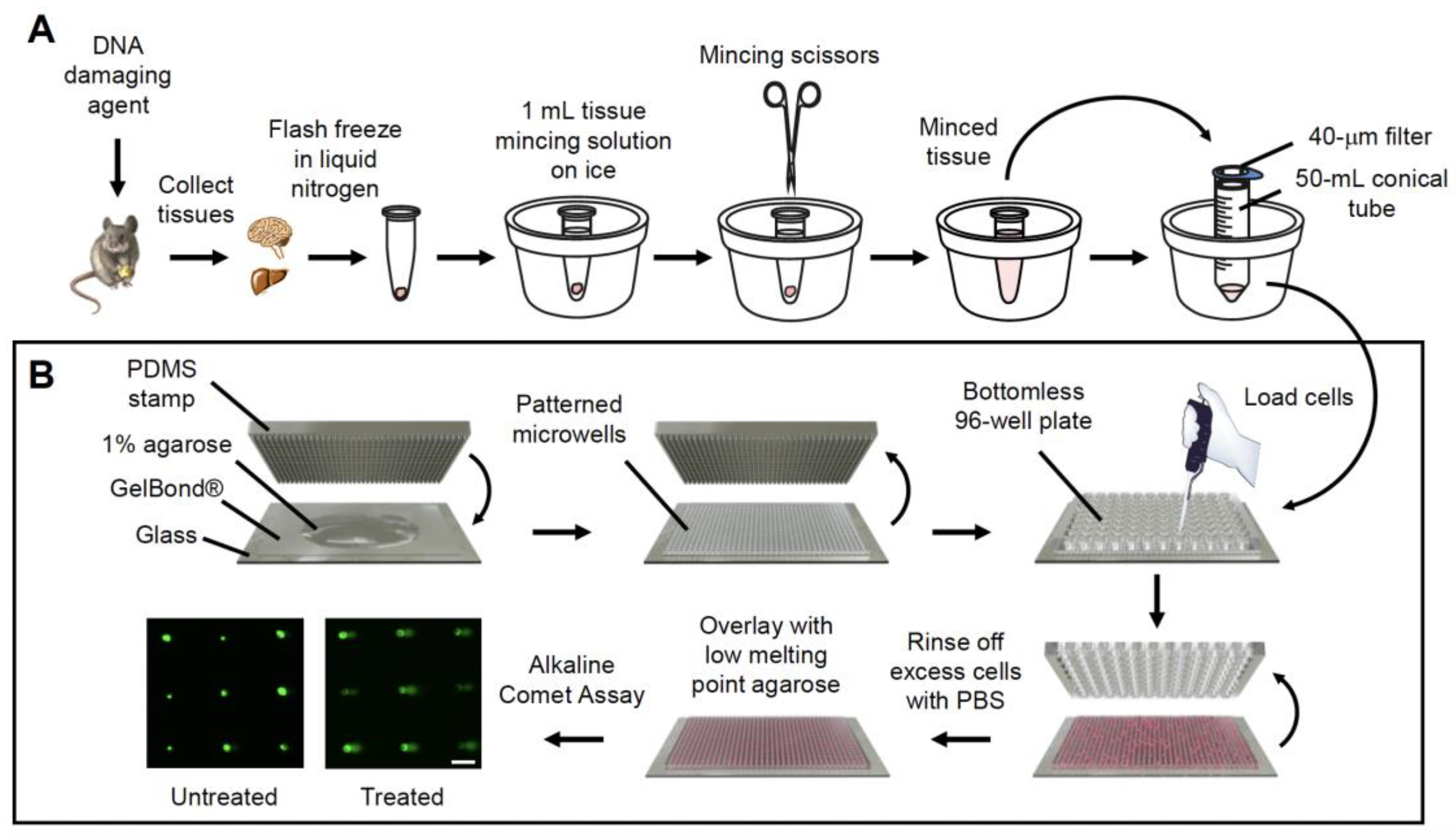
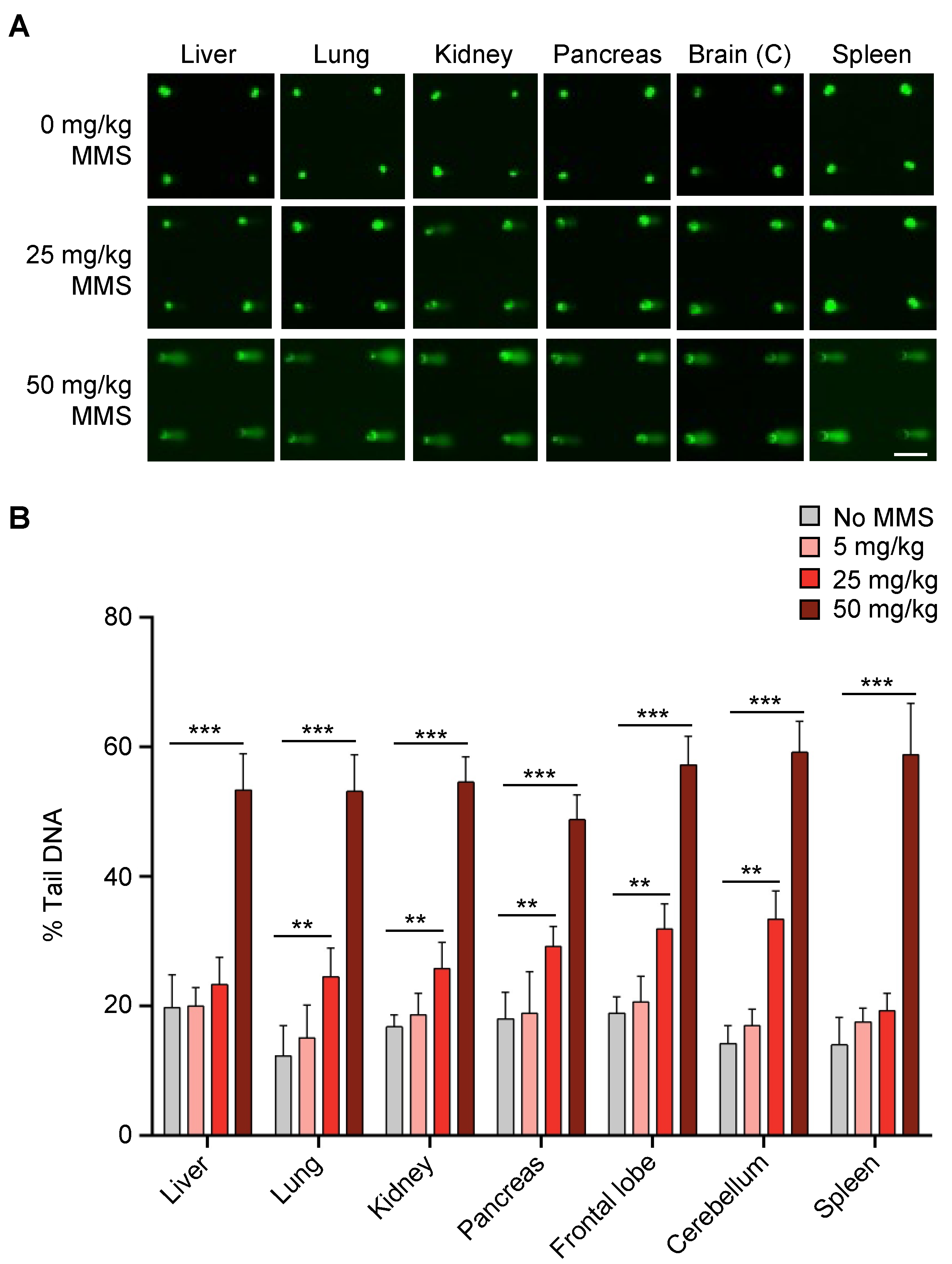
2.1. In Vivo CometChip Can Detect MMS-Induced DNA Damage in Multiple Mouse Tissues
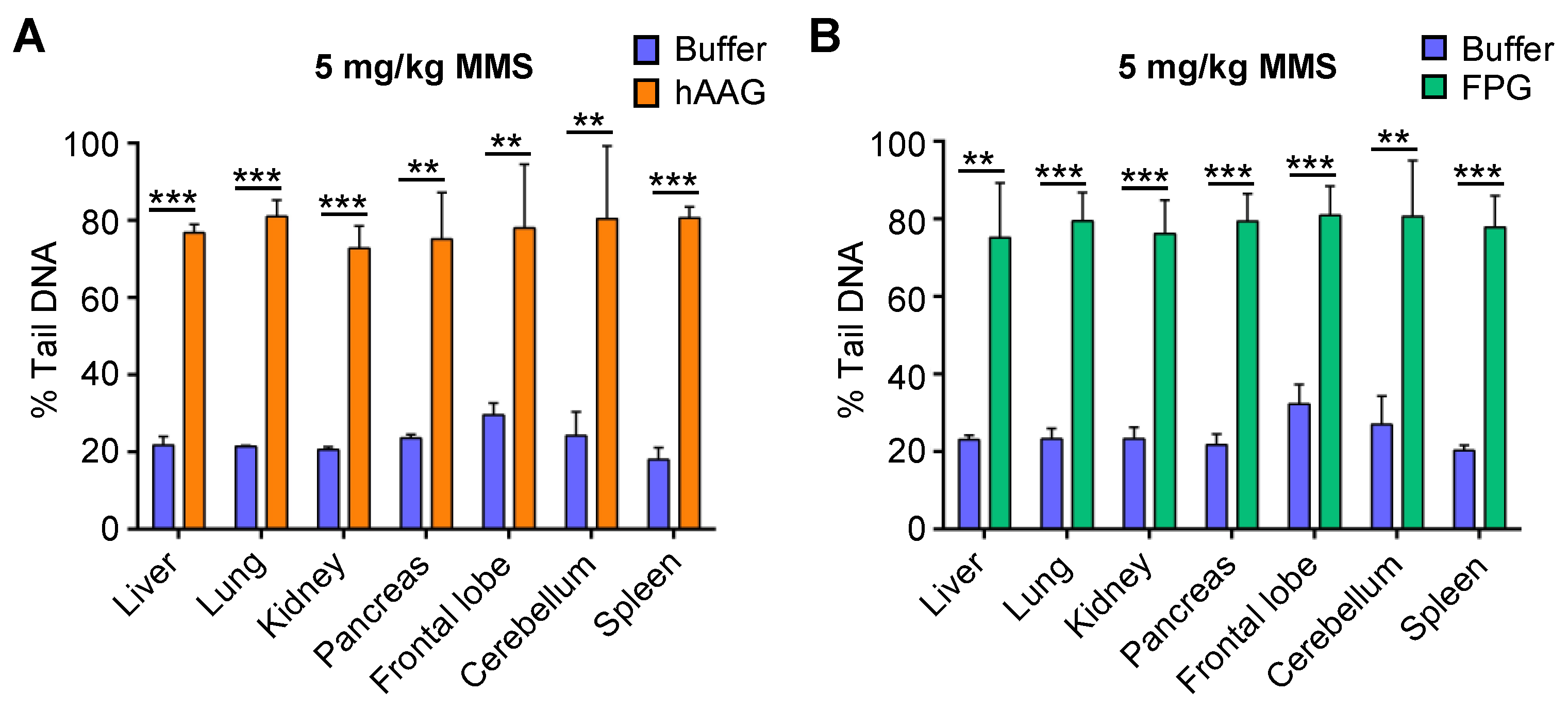
2.2. Undetectable Base Damage Can Be Revealed Using DNA Glycosylases
2.3. Effect of Freezing Tissues on DNA Damage Levels
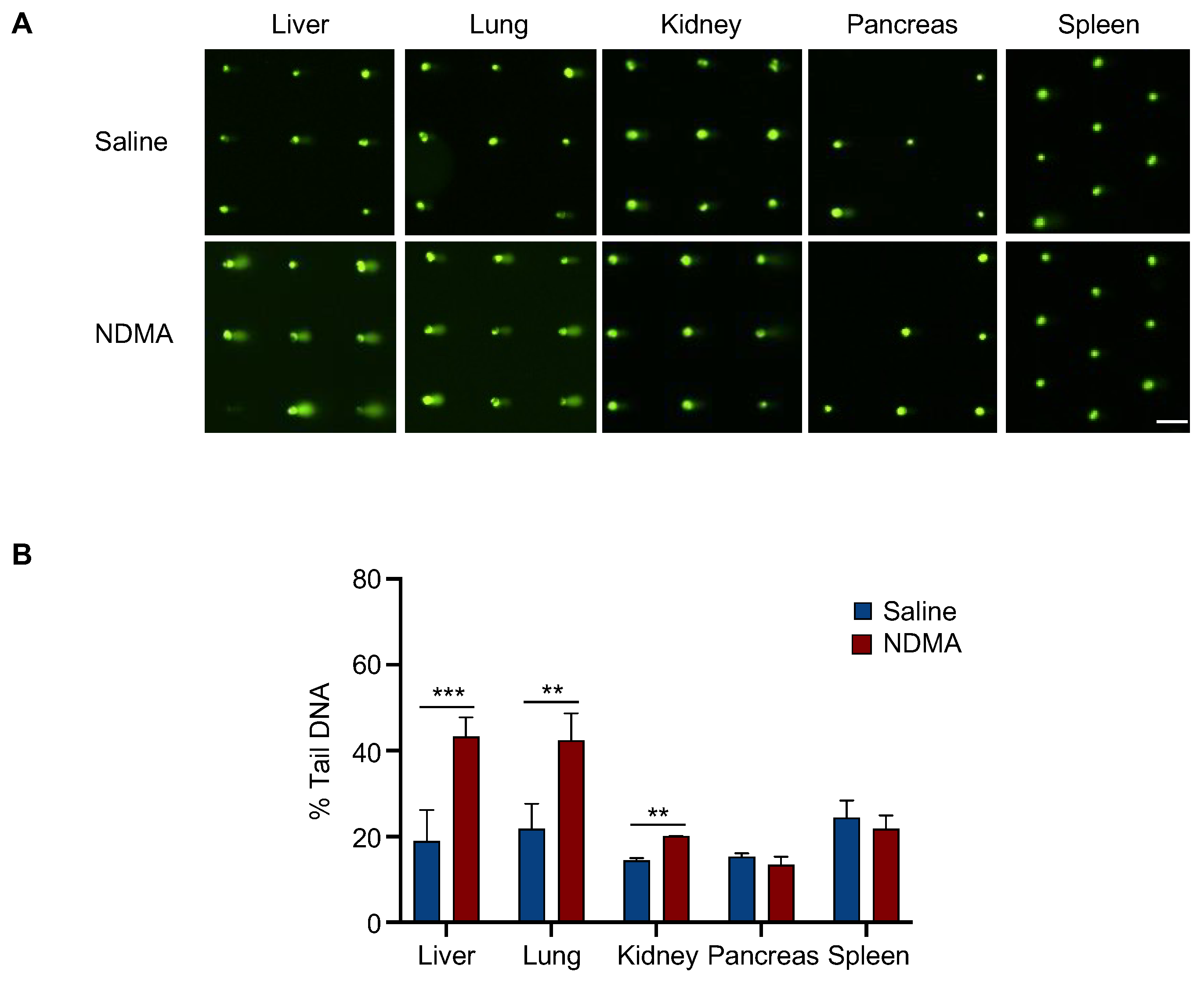
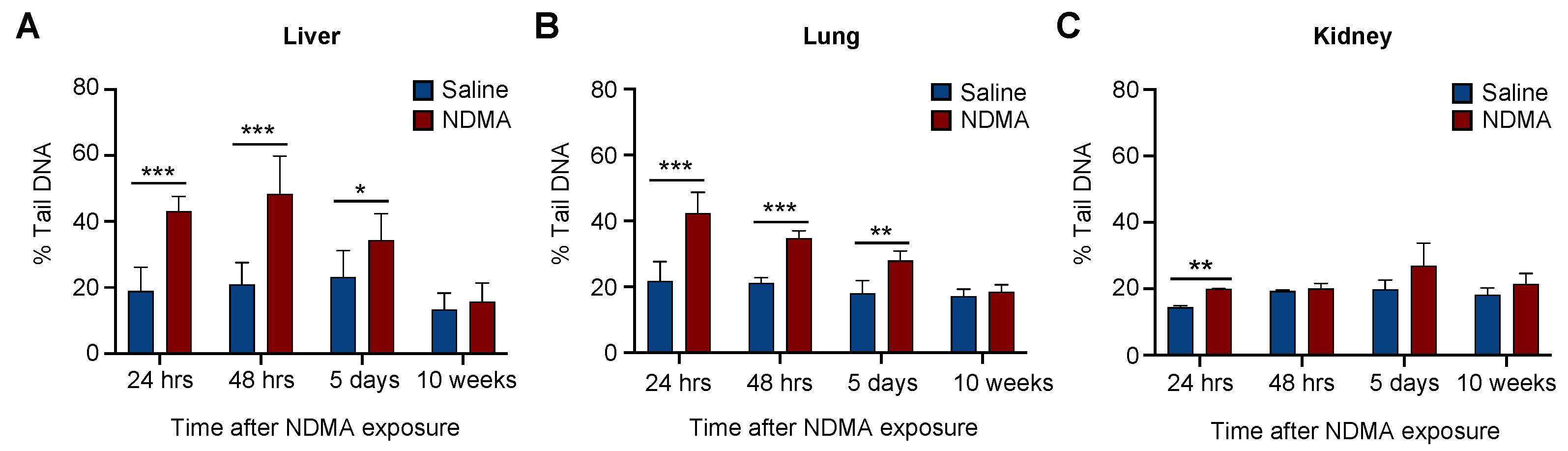
2.4. The In Vivo CometChip Can Be Utilized to Detect DNA Damage Present in NDMA-Exposed Mice
2.5. Evaluating Repair Using CometChip in Different Tissues Following NDMA Exposure
2.6. Base Damage Present in Livers of NDMA-Exposed Mice Can Be Revealed Using hAAG and Fpg
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. MMS Treatment of MEFs and HepG2 Cells
4.4. Animals
4.5. MMS Treatments of Mice
4.6. NDMA Treatments of Mice
4.7. Tissue Sample Processing
4.8. Alkaline Comet Assay Using the CometChip
4.9. Fpg Enzyme Incubation
4.10. hAAG Enzyme Incubation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tubbs, A.; Nussenzweig, A. Endogenous DNA Damage as a Source of Genomic Instability in Cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, E.C.; Walker, G.C.; Siede, W.; Wood, R.D.; Schultz, R.A.; Ellenberger, T. DNA Repair and Mutagenesis, 2nd ed.; ASM Press: Washington, DC, USA, 2006. [Google Scholar]
- Beranek, D.T. Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1990, 231, 11–30. [Google Scholar] [CrossRef]
- Souliotis, V. DNA adducts, mutant frequencies and mutation spectra in lambda lacZ transgenic mice treated with N-nitrosodimethylamine. Carcinogenesis 1998, 19, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Calvo, J.A.; Samson, L.D. Balancing repair and tolerance of DNA damage caused by alkylating agents. Nat. Rev. Cancer 2012, 12, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.E.; Corrigan, J.J.; Armijo, A.L.; Nazari, I.S.; Kohale, I.N.; Torous, D.K.; Avlasevich, S.L.; Croy, R.G.; Wadduwage, D.N.; Carrasco, S.E.; et al. Excision of mutagenic replication-blocking lesions suppresses cancer but promotes cytotoxicity and lethality in nitrosamine-exposed mice. Cell Rep. 2021, 34, 108864. [Google Scholar] [CrossRef]
- Fahrer, J.; Frisch, J.; Nagel, G.; Kraus, A.; Dörsam, B.; Thomas, A.D.; Reißig, S.; Waisman, A.; Kaina, B. DNA repair by MGMT, but not AAG, causes a threshold in alkylation-induced colorectal carcinogenesis. Carcinogenesis 2015, 36, 1235–1244. [Google Scholar] [CrossRef]
- Haggerty, H.G.; Holsapple, M.P. Role of metabolism in dimethylnitrosamine-induced immunosuppression: A review. Toxicology 1990, 63, 1–23. [Google Scholar] [CrossRef]
- Lee, V.M.; Keefer, L.K.; Archer, M.C. An Evaluation of the Roles of Metabolic Denitrosation and α-Hydroxylation in the Hepatotoxicity of N-Nitrosodimethylamine. Chem. Res. Toxicol. 1996, 9, 1319–1324. [Google Scholar] [CrossRef]
- Sohn, O.S.; Ishizaki, H.; Yang, C.S.; Fiala, E.S. Metabolism of azoxymethane, methylazoxymethanol and N-nitrosodimethylamine by cytochrome P450IIE1. Carcinogenesis 1991, 12, 127–131. [Google Scholar] [CrossRef]
- Li, Y.; Hecht, S.S. Metabolic Activation and DNA Interactions of Carcinogenic N-Nitrosamines to Which Humans Are Commonly Exposed. Int. J. Mol. Sci. 2022, 23, 4559. [Google Scholar] [CrossRef]
- Richardson, S. Disinfection by-products and other emerging contaminants in drinking water. TrAC Trends Anal. Chem. 2003, 22, 666–684. [Google Scholar] [CrossRef]
- Sedlak, D.L.; Deeb, R.A.; Hawley, E.L.; Mitch, W.A.; Durbin, T.D.; Mowbray, S.; Carr, S. Sources and Fate of Nitrosodimethylamine and its Precursors in Municipal Wastewater Treatment Plants. Water Environ. Res. 2005, 77, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.A.; Sedlak, D.L. Characterization and Fate of N-Nitrosodimethylamine Precursors in Municipal Wastewater Treatment Plants. Environ. Sci. Technol. 2004, 38, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Tuesuwan, B.; Vongsutilers, V. Nitrosamine Contamination in Pharmaceuticals: Threat, Impact, and Control. J. Pharm. Sci. 2021, 110, 3118–3128. [Google Scholar] [CrossRef] [PubMed]
- Sedlo, I.; Kolonic, T.; Tomic, S. Presence of nitrosamine impurities in medicinal products. Arh Hig Rada Toksikol 2021, 72, 1–5. [Google Scholar] [CrossRef]
- MA-DPH. The Wilmington Childhood Cancer Study. United States Bureau of Environmental Health, 2021. Available online: https://www.mass.gov/doc/full-study-report/download (accessed on 5 May 2022).
- Conti, K. State Study Suggests Link Between Elevated Rates of Childhood Cancer in Wilmington in the 1990s and Formerly Contaminated Public Water Supply. MA Department of Public Health 2021. Available online: https://www.mass.gov/news/state-study-suggests-link-between-elevated-rates-of-childhood-cancer-in-wilmington-in-the-1990s-and-formerly-contaminated-public-water-supply (accessed on 5 May 2022).
- Singh, N. A simple technique for quantification of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Ostling, O.; Johanson, K.J. Microelectrophoretic study of radiation-induced DNA damage in individual mammalian cells. Biochem. Biophys. Res. Commun. 1984, 123, 291–298. [Google Scholar] [CrossRef]
- Olive, P.L.; Banath, J.P. The comet assay: A method to measure DNA damage in individual cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef]
- Milic, M.; Ceppi, M.; Bruzzone, M.; Azqueta, A.; Brunborg, G.; Godschalk, R.; Koppen, G.; Langie, S.; Moller, P.; Teixeira, J.P.; et al. The hCOMET project: International database comparison of results with the comet assay in human biomonitoring. Baseline frequency of DNA damage and effect of main confounders. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108371. [Google Scholar] [CrossRef]
- Azqueta, A.; Collins, A.R. The essential comet assay: A comprehensive guide to measuring DNA damage and repair. Arch. Toxicol. 2013, 87, 949–968. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Env. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Hartmann, A.; Agurell, E.; Beevers, C.; Brendler-Schwaab, S.; Burlinson, B.; Clay, P.; Collins, A.; Smith, A.; Speit, G.; Thybaud, V.; et al. Recommendations for conducting the in vivo alkaline Comet assay. Mutagenesis 2003, 18, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Burlinson, B.; Tice, R.R.; Speit, G.; Agurell, E.; Brendler-Schwaab, S.Y.; Collins, A.R.; Escobar, P.; Honma, M.; Kumaravel, T.S.; Nakajima, M.; et al. Fourth International Workgroup on Genotoxicity testing: Results of the in vivo Comet assay workgroup. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 627, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R. Measuring oxidative damage to DNA and its repair with the comet assay. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Koppen, G.; Valdiglesias, V.; Dusinska, M.; Kruszewski, M.; Møller, P.; Rojas, E.; Dhawan, A.; Benzie, I.; Coskun, E.; et al. The comet assay as a tool for human biomonitoring studies: The ComNet Project. Mutat. Res. Rev. Mutat. Res. 2014, 759, 27–39. [Google Scholar] [CrossRef]
- Dusinska, M.; Collins, A.R. The comet assay in human biomonitoring: Gene-environment interactions. Mutagenesis 2008, 23, 191–205. [Google Scholar] [CrossRef]
- Bonassi, S.; Ceppi, M.; Moller, P.; Azqueta, A.; Milic, M.; Neri, M.; Brunborg, G.; Godschalk, R.; Koppen, G.; Langie, S.A.S.; et al. DNA damage in circulating leukocytes measured with the comet assay may predict the risk of death. Sci. Rep. 2021, 11, 16793. [Google Scholar] [CrossRef]
- Trzeciak, A.R.; Mohanty, J.G.; Jacob, K.D.; Barnes, J.; Ejiogu, N.; Lohani, A.; Zonderman, A.B.; Rifkind, J.M.; Evans, M.K. Oxidative damage to DNA and single strand break repair capacity: Relationship to other measures of oxidative stress in a population cohort. Mutat. Res. 2012, 736, 93–103. [Google Scholar] [CrossRef]
- Dixon, D.R. Marine invertebrate eco-genotoxicology: A methodological overview. Mutagenesis 2002, 17, 495–507. [Google Scholar] [CrossRef]
- Jha, A.N. Genotoxicological studies in aquatic organisms: An overview. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004, 552, 1–17. [Google Scholar] [CrossRef]
- Uno, Y.; Kojima, H.; Omori, T.; Corvi, R.; Honma, M.; Schechtman, L.M.; Tice, R.R.; Beevers, C.; Boeck, M.D.; Burlinson, B.; et al. JaCVAM-organized international validation study of the in vivo rodent alkaline comet assay for detection of genotoxic carcinogens: II. Summary of definitive validation study results. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 786–788, 45–76. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 489: In Vivo Mammalian Alkaline Comet Assay. 2016. Available online: https://www.oecd.org/env/test-no-489-in-vivo-mammalian-alkaline-comet-assay-9789264264885-en.htm (accessed on 10 April 2021).
- Wood, D.K.; Weingeist, D.M.; Bhatia, S.N.; Engelward, B.P. Single cell trapping and DNA damage analysis using microwell arrays. Proc. Natl. Acad. Sci. USA 2010, 107, 10008–10013. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Chow, D.N.; Fessler, J.L.; Weingeist, D.M.; Wood, D.K.; Engelward, B.P. Micropatterned comet assay enables high throughput and sensitive DNA damage quantification. Mutagenesis 2015, 30, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Koczor, C.A.; Haider, A.J.; Saville, K.M.; Li, J.; Andrews, J.F.; Beiser, A.V.; Sobol, R.W. Live Cell Detection of Poly(ADP-Ribose) for Use in Genetic and Genotoxic Compound Screens. Cancers 2022, 14, 3676. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Sánchez, C.; Encarnación-Medina, J.; Park, J.Y.; Moreno, N.; Ruiz-Deya, G.; Matta, J. Reduced DNA Repair Capacity in Prostate Cancer Patients: A Phenotypic Approach Using the CometChip. Cancers 2022, 14, 3117. [Google Scholar] [CrossRef]
- Seo, J.-E. Evaluation of an in vitro three-dimensional HepaRG spheroid model for genotoxicity testing using the high-throughput CometChip platform. Altex 2022. [Google Scholar] [CrossRef]
- Boyadzhiev, A.; Solorio-Rodriguez, S.A.; Wu, D.; Avramescu, M.-L.; Rasmussen, P.; Halappanavar, S. The High-Throughput In Vitro CometChip Assay for the Analysis of Metal Oxide Nanomaterial Induced DNA Damage. Nanomaterials 2022, 12, 1844. [Google Scholar] [CrossRef]
- Barranger, A.; Le Hégarat, L. Towards better prediction of xenobiotic genotoxicity: CometChip technology coupled with a 3D model of HepaRG human liver cells. Arch. Toxicol. 2022, 96, 2087–2095. [Google Scholar] [CrossRef]
- Seo, J.-E.; Davis, K.; Malhi, P.; He, X.; Bryant, M.; Talpos, J.; Burks, S.; Mei, N.; Guo, X. Genotoxicity evaluation using primary hepatocytes isolated from rhesus macaque (Macaca mulatta). Toxicology 2021, 462, 152936. [Google Scholar] [CrossRef]
- Buick, J.K.; Williams, A.; Meier, M.J.; Swartz, C.D.; Recio, L.; Gagné, R.; Ferguson, S.S.; Engelward, B.P.; Yauk, C.L. A Modern Genotoxicity Testing Paradigm: Integration of the High-Throughput CometChip® and the TGx-DDI Transcriptomic Biomarker in Human HepaRG™ Cell Cultures. Front. Public Health 2021, 9, 694834. [Google Scholar] [CrossRef]
- Ge, J.; Ngo, L.P.; Kaushal, S.; Tay, I.J.; Thadhani, E.; Kay, J.E.; Mazzucato, P.; Chow, D.N.; Fessler, J.L.; Weingeist, D.M.; et al. CometChip enables parallel analysis of multiple DNA repair activities. DNA Repair 2021, 106, 103176. [Google Scholar] [CrossRef] [PubMed]
- Muruzabal, D.; Collins, A.; Azqueta, A. The enzyme-modified comet assay: Past, present and future. Food Chem. Toxicol. 2021, 147, 111865. [Google Scholar] [CrossRef] [PubMed]
- Muruzabal, D.; Sanz-Serrano, J.; Sauvaigo, S.; Treillard, B.; Olsen, A.K.; Lopez de Cerain, A.; Vettorazzi, A.; Azqueta, A. Validation of the in vitro comet assay for DNA cross-links and altered bases detection. Arch. Toxicol. 2021, 95, 2825–2838. [Google Scholar] [CrossRef] [PubMed]
- Azqueta, A.; Arbillaga, L.; Lopez de Cerain, A.; Collins, A. Enhancing the sensitivity of the comet assay as a genotoxicity test, by combining it with bacterial repair enzyme FPG. Mutagenesis 2013, 28, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Azqueta, A.; Enciso, J.M.; Pastor, L.; López de Cerain, A.; Vettorazzi, A. Applying the comet assay to fresh vs frozen animal solid tissues: A technical approach. Food Chem. Toxicol. 2019, 132, 110671. [Google Scholar] [CrossRef]
- Jackson, P.; Pedersen, L.M.; Kyjovska, Z.O.; Jacobsen, N.R.; Saber, A.T.; Hougaard, K.S.; Vogel, U.; Wallin, H. Validation of freezing tissues and cells for analysis of DNA strand break levels by comet assay. Mutagenesis 2013, 28, 699–707. [Google Scholar] [CrossRef]
- Forsberg, K.; Aalling, N.; Wörtwein, G.; Loft, S.; Møller, P.; Hau, J.; Hageman, I.; Jørgensen, M.B.; Jørgensen, A. Dynamic regulation of cerebral DNA repair genes by psychological stress. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 778, 37–43. [Google Scholar] [CrossRef]
- Sasaki, Y.F.; Sekihashi, K.; Izumiyama, F.; Nishidate, E.; Saga, A.; Ishida, K.; Tsuda, S. The comet assay with multiple mouse organs: Comparison of comet assay results and carcinogenicity with 208 chemicals selected from the IARC monographs and U.S. NTP Carcinogenicity Database. Crit. Rev. Toxicol. 2000, 30, 629–799. [Google Scholar] [CrossRef]
- Pant, K.; Springer, S.; Bruce, S.; Lawlor, T.; Hewitt, N.; Aardema, M.J. Vehicle and positive control values from the in vivo rodent comet assay and biomonitoring studies using human lymphocytes: Historical database and influence of technical aspects. Environ. Mol. Mutagen. 2014, 55, 633–642. [Google Scholar] [CrossRef]
- Collins, A. Investigating oxidative DNA damage and its repair using the comet assay. Mutat. Res. 2009, 681, 24–32. [Google Scholar] [CrossRef]
- Smith, C.C.; O’Donovan, M.R.; Martin, E.A. hOGG1 recognizes oxidative damage using the comet assay with greater specificity than FPG or ENDOIII. Mutagenesis 2006, 21, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, M.D.; Pittman, D.L. Methylating Agents and DNA Repair Responses: Methylated Bases and Sources of Strand Breaks. Chem. Res. Toxicol. 2006, 19, 1580–1594. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, C.; Sueyoshi, Y.; Ema, M.; Mori, Y.; Takaishi, K.; Hisatomi, H. Induction of oxidative stress by anticancer drugs in the presence and absence of cells. Oncol. Lett. 2017, 14, 6066–6070. [Google Scholar] [CrossRef] [PubMed]
- Tse, A.K.-W.; Chen, Y.-J.; Fu, X.-Q.; Su, T.; Li, T.; Guo, H.; Zhu, P.-L.; Kwan, H.-Y.; Cheng, B.C.-Y.; Cao, H.-H.; et al. Sensitization of melanoma cells to alkylating agent-induced DNA damage and cell death via orchestrating oxidative stress and IKKβ inhibition. Redox. Biol. 2017, 11, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C.A.; Recio, L.; Winters, J.; Witt, K.L. Use of Frozen Tissue in the Comet Assay for the Evaluation of DNA Damage. J. Vis. Exp. 2020, 157, e59955. [Google Scholar] [CrossRef]
- Vieira, I.; Pasanen, M.; Raunio, H.; Cresteil, T. Expression ofCYP2E1in Human Lung and Kidney during Development and in Full-Term Placenta: A Differential Methylation of the Gene is Involved in the Regulation Process. Pharmacol. Toxicol. 1998, 83, 183–187. [Google Scholar] [CrossRef]
- Gonzalez, F.J. Role of cytochromes P450 in chemical toxicity and oxidative stress: Studies with CYP2E1. Mutat. Res. /Fundam. Mol. Mech. Mutagen. 2005, 569, 101–110. [Google Scholar] [CrossRef]
- Liteplo, R.G.; Meek, M.E.; Windle, W. Concise International Chemical Assessment Document 38—N-nitrosodimethylamine. 2002. Available online: https://apps.who.int/iris/bitstream/handle/10665/42422/a73568.pdf (accessed on 10 June 2022).
- Meira, L.B.; Bugni, J.M.; Green, S.L.; Lee, C.-W.; Pang, B.; Borenshtein, D.; Rickman, B.H.; Rogers, A.B.; Moroski-Erkul, C.A.; McFaline, J.L.; et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Investig. 2008, 118, 2516–2525. [Google Scholar] [CrossRef]
- Ge, J.; Prasongtanakij, S.; Wood, D.K.; Weingeist, D.M.; Fessler, J.; Navasummrit, P.; Ruchirawat, M.; Engelward, B.P. CometChip: A high-throughput 96-well platform for measuring DNA damage in microarrayed human cells. J. Vis. Exp. 2014, 92, e50607. [Google Scholar] [CrossRef]
- Chaim, I.A.; Gardner, A.; Wu, J.; Iyama, T.; Wilson, D.M.; Samson, L.D. A novel role for transcription-coupled nucleotide excision repair for thein vivorepair of 3,N4-ethenocytosine. Nucleic Acids Res. 2017, 45, 3242–3252. [Google Scholar] [CrossRef][Green Version]
- He, M.; Croy, R.G.; Essigmann, J.M.; Swager, T.M. Chemiresistive Carbon Nanotube Sensors for N-Nitrosodialkylamines. ACS Sens. 2019, 4, 2819–2824. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owiti, N.A.; Corrigan, J.J.; Pribyl, L.J.; Kay, J.E.; Engelward, B.P. Novel In Vivo CometChip Reveals NDMA-Induced DNA Damage and Repair in Multiple Mouse Tissues. Int. J. Mol. Sci. 2022, 23, 11776. https://doi.org/10.3390/ijms231911776
Owiti NA, Corrigan JJ, Pribyl LJ, Kay JE, Engelward BP. Novel In Vivo CometChip Reveals NDMA-Induced DNA Damage and Repair in Multiple Mouse Tissues. International Journal of Molecular Sciences. 2022; 23(19):11776. https://doi.org/10.3390/ijms231911776
Chicago/Turabian StyleOwiti, Norah A., Joshua J. Corrigan, Lee J. Pribyl, Jennifer E. Kay, and Bevin P. Engelward. 2022. "Novel In Vivo CometChip Reveals NDMA-Induced DNA Damage and Repair in Multiple Mouse Tissues" International Journal of Molecular Sciences 23, no. 19: 11776. https://doi.org/10.3390/ijms231911776
APA StyleOwiti, N. A., Corrigan, J. J., Pribyl, L. J., Kay, J. E., & Engelward, B. P. (2022). Novel In Vivo CometChip Reveals NDMA-Induced DNA Damage and Repair in Multiple Mouse Tissues. International Journal of Molecular Sciences, 23(19), 11776. https://doi.org/10.3390/ijms231911776





